Abstract
Background
Oral exposure to food allergens may be limited in infancy and the initial site of antigen exposure likely plays an important role in food allergy induction.
Objective
To examine the impact of different routes of exposure using milk allergens, with and without adjuvant, on sensitization.
Methods
C3H/HeJ mice were repeatedly exposed to the milk allergen α-lactalbumin (ALA), with or without cholera toxin (CT). Sensitization routes used were intragastric, cutaneous, intranasal, and sublingual. Anaphylaxis severity was assessed by symptoms and body temperature in response to oral or systemic challenge. Antigen-specific serum antibodies were measured by ELISA. The mechanism of adjuvant activity of cutaneous CT was also determined.
Results
Sensitization to ALA as measured by allergen-specific IgE occurred by all routes of sensitization, and was maximal in response to cutaneous exposure. Sensitization was dependent on CT and did not occur to antigen alone by any route. Mucosal, but not cutaneous exposure, resulted in a robust allergen-specific IgA response. Anaphylaxis occurred in all sensitized groups when orally challenged with ALA. Topical CT induced migration of langerinneg dermal DCs to the lymph node, resulting in enhanced proliferation and Th2 cytokine production from responder T cells.
Conclusions
Sensitization can occur via all physiologic routes when adjuvant is present. The skin is a potent and likely important physiologic route of sensitization whereby adjuvant induces an efflux of antigen-bearing dermal DCs to the lymph node that generate a pro-allergic Th2 response.
Keywords: Adjuvant, dendritic cell, milk allergy, mucosal, tolerance
INTRODUCTION
Food allergies are increasing in incidence in the industrialized world1–3 and previous recommendations for avoidance of allergen ingestion are theorized to have a role in this increase. Many children have adverse reactions upon their first known ingestion of some allergens4 thus indicating that alternative routes of sensitization may account for the lack of induction of oral tolerance. Results from epidemiologic studies5–7 support the idea that avoidance of food allergen exposure prevents proper oral tolerance induction and perhaps increases the risk of sensitization through cutaneous exposure.
We have previously shown that the site where cow’s milk antigens are initially sampled in the gut influences the degree of sensitization and clinical reactivity to that allergen8. The different trafficking of milk antigens in the gut also results in an alteration of the clinical response, such that anaphylaxis in response to oral challenge only occurs in response to soluble antigens. Emerging evidence suggests that non-intestinal sites of antigen exposure such as the skin may be highly relevant inductive sites of allergic sensitization to food proteins. This is supported by both epidemiologic data5–7 and experimental systems showing that mice can be sensitized to allergens via the skin in an adjuvant-independent manner9–11. Conversely, some routes of exposure have been proposed to be inherently tolerogenic. Oral exposure to antigens has long been known to result in the well-known phenomenon of oral tolerance12, but recent evidence suggests that sublingual exposure may be optimally tolerogenic due to the unique phenotype of sublingual antigen presenting cells13, 14.
Our aims were to directly assess the contribution of physiologic routes of exposure and the need for an adjuvant in the development of allergic sensitization to the milk allergen α-lactalbumin (ALA). Using experimental murine models of food antigen-induced anaphylaxis, we assessed the impact of antigen exposure via intragastric, sublingual, nasal, or cutaneous routes. Although we observed that cutaneous exposure was optimal for the generation of IgE responses, the context in which antigen is delivered (the presence or absence of a pro-allergic adjuvant) was a significantly more important factor than the route of sensitization.
METHODS
Mice
Three-week old female C3H/HeJ and D011.10 transgenic mice were purchased from The Jackson Laboratories (Bar Harbor, ME), and Balb/c mice were purchased from the National Cancer Institute (Frederick, MD). C3H/HeJ mice were used because they are susceptible to develop anaphylactic symptoms after oral challenge. They have a missense mutation in the TLR4 gene, which renders them resistant to endotoxin.15 Animals were kept under specific pathogen-free conditions in filter-top cages and were placed on a milk-free chow, Purina 5LG4. All procedures were approved by the Mount Sinai School of Medicine Institutional Animal Care and Use Committee.
Sensitization of mice
Three-week old C3H/HeJ mice were sensitized once a week for six weeks with ALA via various routes. Mice were sensitized orally with 1mg of ALA (Sigma, St. Louis, MO) in 0.2M bicarbonate buffer ± 10 µg cholera toxin (CT) (List Biologicals, Campbell, CA) in a volume of 200 µl by gavage feeding. Mice sensitized intranasally received 10 µg of ALA in PBS ± 1 µg CT placed on the nares in a volume of 10 µl. Sublingually sensitized mice were given 10 µg of ALA ± 1µg CT in a volume of 5 µl under the tongue. These doses were chosen based on the literature and following a series of preliminary dose titration studies. For cutaneous sensitization, mice were anesthetized and abdominal fur removed with depilatory cream (Veet, Reckitt Benckiser, Parsippany, NJ), followed by application of 100 µg of ALA ± 10 µg CT in a total volume of 50 µl of PBS. The solution was spread on the skin and mice were placed on their back until recovery from anesthesia (20–30 min).
Oral Challenge of mice
On day 42, a graded oral challenge study was performed in all groups of mice as previously described and anaphylaxis severity determined by body temperature and symptom score 8. Briefly, mice were challenged with progressively increasing doses of ALA (5mg, 10mg, 20mg, and 50mg) every 20 minutes. If anaphylaxis occurred as determined by symptoms and a decrease in body temperature, no further doses were given and the dose required for anaphylaxis was defined by that incremental dosage.
Measurement of antigen specific immunoglobulins
ALA-specific IgG1, IgG2a, and IgA were measured by ELISA as previously described8. ALA and CAS-specific IgE were measured by a modified capture ELISA. Briefly, plates were coated with unlabeled goat anti-mouse IgE (Southern Biotech, Birmingham, AL), incubated with diluted sera, and incubated with digoxigenin (DIG)-labeled ALA. DIG labeling of antigen was done with Digoxigenin-3-O-methylcarbonyl-ε-aminocaproic acid-N-hydroxysuccinimide ester (Roche, Indianapolis, IN) according to the manufacturer’s instructions. Detection was performed by HRP-labeled anti-DIG antibody (Roche).
Assessment of skin-draining dendritic cells (DCs)
C3H/HeJ or Balb/c mice were exposed to 10 µg CT or PBS as control on the abdominal skin as described above. After 24 hours, draining (inguinal) lymph nodes were isolated and cells stained using anti-CD11c, CD11b, CD8α, langerin and CD103 mAbs (all from eBioscience, San Diego, CA). To assess DC migration, mice were exposed to 10 mg FITC-dextran (Sigma) in the presence or absence of 10 µg CT, and lymph node cells were analyzed after depletion of B220+ and CD3+ cells (using biotinylated antibodies from eBioscience and avidin-coated dynabeads from Invitrogen). Data was acquired by an LSR II flow cytometer (BD Bioscience), and analysis was performed using FlowJo software (Treestar, Ashland, OR).
Assessment of T cell activation in vivo
The impact of CT on antigen-specific T cell activation and cytokine secretion was performed using T cells from DO11.10 (OVA-specific TCR) transgenic mice as previously described16. Briefly, CD4+ T cells from DO11.10 mice were purified by negative selection (StemCell, Vancouver, Canada), labeled with CFSE, and 3 × 106 cells were injected into Balb/c recipients. 24 hours later, recipient mice were exposed on the abdominal skin to 1mg OVA ± 10 µg CT as described above. Lymph node cells were harvested after 4 days for assessment of proliferation by CFSE dilution, or cytokine secretion after re-stimulation with 1 ug/ml OVA323–339 peptide for 72 h, followed by anti-CD3/CD28 stimulation (eBioscience) for 72 h. Secreted cytokines were measured by ELISA (eBioscience).
Antigen presentation assay
Antigen presentation assays were performed based on modification of a previously described protocol16. BALB/c mice were topically exposed to 10 mg OVA in the presence or absence of 10 µg CT. After 24h, DCs were purified from the inguinal LNs using CD11c microbeads (Miltenyi, Auburn, CA). DCs were cultured at a ratio of 1:5 with DO11.10 CD4+ T cells. After 72 h, cells were re-stimulated with anti-CD3/CD28. After 72h, supernatants were harvested and cytokines measured by ELISA.
Statistics
Differences between sensitization route groups were analyzed by ANOVA, followed by either non-parametric Mann-Whitney U-test or Bonferroni analysis when appropriate. Data analysis was done using Prism software (GraphPad, San Diego, CA). The data are expressed as mean +/− SEM. A value of P<0.05 was considered significant.
RESULTS
Mice can be sensitized to milk allergens via all routes in an adjuvant-dependent manner
We have previously shown that mice can be orally sensitized to the milk protein ALA when it is co-administered with the adjuvant CT to break oral tolerance8. Since physiologic exposure during infancy may occur through multiple routes, we assessed whether site of exposure played a role in the development of sensitization to milk allergens. Exposure of C3H/HeJ mice to ALA (Fig 1) plus CT by intragastric, sublingual, intranasal, or cutaneous routes led to significant production of allergen-specific IgE that was maximal in the group exposed via the cutaneous route. Mice were exposed to ALA in the absence of CT, but this did not result in any detectable IgE or other immunoglobulin production by any route. Exposure to ALA plus CT induced a significant allergen-specific systemic IgA response by all mucosal routes, but was notably absent in response to cutaneous exposure. Allergen-specific IgG1 and IgG2a responses were induced by all routes.
Figure 1. Antigen-specific serum antibody profile.
C3H/HeJ mice were exposed to ALA plus CT by the intragastric (oral) (n=9), sublingual (SL) (n=9), intranasal (IN) (n=5) or cutaneous (skin) (n=5) route for 6 weeks prior to measurement of antigen-specific antibodies in the serum by ELISA. *p<.05, **p<.01, ***p<.001 vs. none, ¶p<.05 vs oral, IN, SL
Mice become primed for systemic anaphylaxis via all routes in an adjuvant-dependent manner
We have previously shown that mice orally sensitized with ALA plus the adjuvant CT will undergo anaphylaxis when orally re-challenged with ALA8. Consistent with the IgE responses, C3H/HeJ mice sensitized to ALA by the intragastric, intranasal, or cutaneous routes all responded to oral ALA re-challenge with systemic anaphylaxis as measured by symptom score (Fig 2A) and drop in body temperature (Fig 2B). Interestingly, mice sensitized by the sublingual route responded to oral re-challenge but had minimal IgE production. The route of sensitization did not significantly influence the dose required to induce anaphylaxis (median of 20 mg in all groups). Body temperature correlated well with symptom score. Cutaneous sensitization resulted in a greater severity of anaphylaxis compared to orally sensitized mice, as measured by higher symptom scores and lower body temperature (p<.05). In contrast, mice repeatedly exposed to ALA without CT developed no anaphylactic symptoms or drop in body temperature after allergen challenge, independent of the route of allergen exposure.
Figure 2. Symptom score and body temperature.
C3H/HeJ mice were exposed to ALA ± CT by the intragastric (oral), sublingual (SL), intranasal (IN) or cutaneous (skin) route. Mice were then challenged with progressively increasing oral ALA and 30 min after the first dose eliciting symptoms (A) and a decrease in body temperature (B), a symptom score was assigned and body temperature measured. *p<.05, **p<.01, ***p<.001 vs. non-exposed (None). (n are the same as in fig 1)
Route of sensitization influences the cytokine profile
Production of cytokines derived from splenocytes of ALA sensitized C3H/HeJ mice revealed patterns that differed depending on the route of sensitization (Supplemental Fig 1). However, these showed no correlation with the serum specific antibody response or the clinical responses described for all routes except the cutaneous route. The cutaneous route induced a Th2 response with increases in IL-4 and IL-13 with no effect on IL-10 and IFN-γ.
CT drives the migration of antigen-carrying dermal DCs to the draining lymph node
Our results show that the epicutaneous route is optimal for sentization and that CT must be present for sensitization to ALA to occur. We hypothesize that this is due to CT-induced changes in the phenotype of the antigen presenting cells. Therefore we next assessed the impact of CT on DCs within the skin-draining lymph node in both C3H/HeJ and Balb/c mice. We first examined the impact of topical CT exposure on the DC populations present in the draining lymph nodes. After gating on the CD11c+ MHC Class II-high migratory population of DCs, we identified DC subsets based on the expression of langerin and CD103. CT did not induce any global changes in the total number of migratory DCs or DC subsets (Langerhans cells, Langerin+ dermal DCs, or Langerinneg dermal DCs). (Fig 3A).
Figure 3. Effect of CT on skin-draining DCs.
A: Effect of CT on migratory DCs and their subsets in the draining lymph node. n=6 B: Effect of CT on migration of FITC-dextran-positive DCs to the draining lymph nodes. n=3 experiments of 3 Balb/c mice/group C: Median fluorescent intensity (MFI) of MHC class II of FITC-dextran-positive DCs. n=3 experiments of 3 Balb/c mice/group D: Representative flow cytometry plot showing the phenotype of FITC-dextran-positive DCs (lower panel) compared to the total migratory DC population (upper panel). n=3, *p<.05, **p<.01
We next directly addressed migration of skin DCs by applying FITC-dextran topically to the skin in the presence or absence of CT. We observed a significant increase of FITC-dextran containing migratory DCs in the skin draining LNs of mice exposed to CT as compared to controls exposed to FITC-dextran alone (p<0.05). (Figure 3B, and Supplementary Figure 2A)
In addition to increased DC migration, there was an increased DC maturation in the FITC dextran carrying DCs as measured by MHC class II expression (p<0.01). (Figure 3C) These results indicate that following exposure to CT a greater number of mature DCs are present in the lymph node where they can present acquired antigen to naive T cells. Subset analysis identified the FITC dextran-containing migratory DCs as langerinneg dermal DCs (Figure 3D, and Supplementary Figure 2B).
CT induces the generation of antigen-specific effector T cells in skin-draining lymph nodes
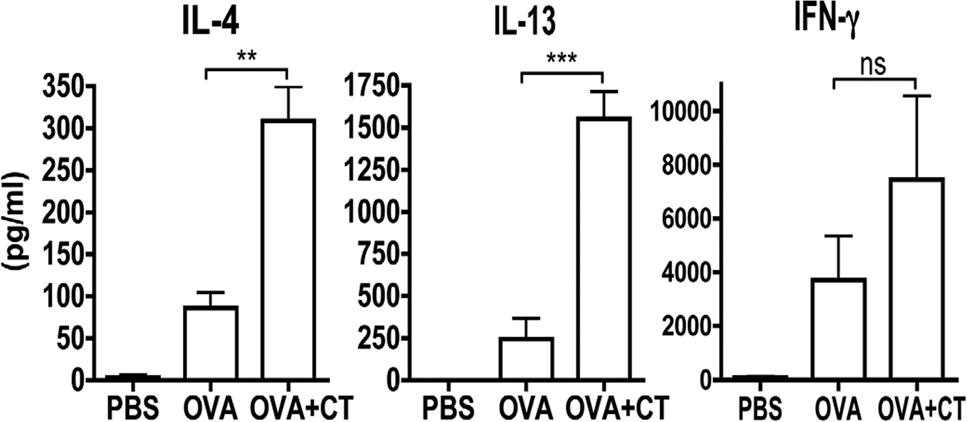
Given the importance of CT and its effect on DCs we sought to determine the impact of CT on naive T cells in response to antigen applied to the skin. CFSE-labeled DO11.10 (OVA-TCR transgenic) CD4+ T cells (Balb/c background) were transferred to naïve Balb/c mice. Exposure of recipient mice to 1mg of OVA in the absence of CT on the skin resulted in an increase in T cell proliferation as measured by CFSE dilution in the draining lymph node 4 days after exposure as compared with mice exposed to PBS alone. However, exposure to OVA in the presence of CT markedly enhanced the degree of proliferation (Figure 4A and B). To assess the resulting T cell cytokine production, draining lymph node cells were re-stimulated in vitro with OVA323–339 peptide, followed by anti-CD3/CD28. The second round of stimulation was required for detectable cytokine secretion. Lymph node cells from mice that had not received DO11.10 cells did not have a detectable cytokine response to OVA + CT within this time period (data not shown). Mice that had received DO11.10 cells and were exposed to OVA alone had a negligible cytokine response despite the fact that proliferation could be detected. In contrast, exposure to OVA in the presence of CT induced a robust effector T cell response characterized by a mixed cytokine response of IL-4, IL-13, and IFN-γ (Figure 4C). Effector T cells were restricted to the skin-draining lymph node, and were not observed in distal lymph nodes after cutaneous antigen exposure.
Figure 4. Effect of CT on the antigen-specific T cell response.
A representative histogram (A) and quantification (B) of proliferation of DO11.10 T cells in the skin-draining lymph node after exposure to PBS, OVA, or OVA + CT. n=5 C: Cytokine secretion from transferred OVA-specific T cells after in vivo exposure to PBS, OVA, or OVA+CT after re-stimulation with OVA peptide and subsequent stimulation with anti-CD3/CD28. n=3 experiments of 3 Balb/c mice/group
*p<.05, **p<.01, ***p<.001
DCs isolated from mice exposed to epicutaneous Ag with CT are able to induce higher production of cytokines by T cells in culture
In order to determine if the T cell effector priming was primarily due to the observed changes in DC phenotype, antigen presentation assays were performed. DCs purified from the draining lymph node of Balb/c mice exposed on the skin with OVA plus CT caused a significant increase in IL-4 and IL-13 production by DO11.10 T cells compared to DCs purified from mice exposed to OVA alone (Figure 5). These results demonstrate that the induction of Th2 priming induced by CT in vivo can be mediated by DCs independent of other mechanisms.
Figure 5. Effect of in vivo treatment with CT on the immunogenicity of DCs.
DCs were purified from the inguinal lymph node of Balb/c mice treated topically with PBS, OVA, or OVA+CT and co-cultured with DO11.10 cells. Secreted cytokines were measured by ELISA after re-stimulation with OVA peptide and subsequent stimulation with anti-CD3/CD28. n=2 experiments of 3 mice/group, **p<.01, ***p<.001
DISCUSSION
The mechanisms responsible for allergic sensitization to food proteins remain unclear. Emerging evidence suggests that the skin may be a highly relevant inductive site for allergic sensitization to food proteins. Children with environmental exposure, likely cutaneous, to peanut had a high probability of developing peanut allergy5, 6. More recent studies also cite epicutaneous sensitization to latex and cross reactivity to multiple foods including kiwi, banana and potato7. Conversely, some routes of exposure have been proposed to be inherently tolerogenic. Oral exposure to antigens has long been known to result in oral tolerance, and recent evidence suggests that sublingual exposure may be optimally tolerogenic due to the unique phenotype of sublingual antigen presenting cells13, 14. We directly tested these routes of allergen exposure for their ability to sensitize mice.
We observed that sensitization to the milk protein ALA could be induced via all sites with a significantly higher symptomatic response in the epicutaneous route as compared with the oral route of sensitization. Our results underscore the importance of the presence of adjuvant activity in sensitization to food proteins in murine models,17 regardless of site of exposure. Although we found that all sites could induce an allergic response given the appropriate environment (adjuvant), we observed exposure via the skin to be an especially potent route for the induction of IgE production with negligible protective IgA production. Our data show that exposure to food allergens through the skin is not inherently pro-allergenic, and argues against the site of exposure being the primary factor responsible for sensitization or tolerance. This is supported by studies in mice27, 28 and humans29 showing that desensitization can be achieved to food and aeroallergens by applying antigen to the skin.
Several studies have demonstrated that mice can be epicutaneously sensitized to a variety of antigens including OVA, hazelnut, and milk whey proteins via the epicutaneous route in the absence of exogenous adjuvant9–11. These models differed from ours in that they utilized occlusive dressings and/or prolonged exposure to the antigen. Prolonged duration of antigen exposure has been shown to result in adjuvant-independent immunity 18. It has also been shown that tape-stripping, that disrupts the barrier, is required for effective sensitization, and that this mechanical injury upregulates the cytokines IL-2118 and thymic stromal lymphopoietin (TSLP) 20. Alternatively, our use of TLR4-deficient C3H/HeJ mice eliminated adjuvant activity that could be provided by endotoxin contamination of standard antigen extracts 21, 22. In addition, adjuvant-independent sensitization may reflect inherent adjuvant properties of particular antigens. For example, they may mimic TLR signaling components (Der p 2) 23, or contain glycan structures that bind to pattern recognition receptors and cause maturation of DCs and downstream Th2 skewing (peanut) 24–26. This does not appear to be the case for milk allergens, at least in the context of TLR4-deficient mice.
Our model is a useful experimental tool for examining mechanisms of sensitization in mice and provides a robust model of allergic sensitization to food proteins. Although the depilatory cream used in our method could potentially cause changes to the skin barrier, no obvious irritation was noted and no clinical sensitization to ALA alone could be observed when it was applied to skin prepared in this method. Similarly cytokine production by T cells activated by OVA administered alone after hair removal was poor as compared to the robust cytokine response including IL-4 and IL-13 after administration of OVA together with CT. Thus our method of hair removal did not appear to be itself adjuvant-like, in the manner of tape stripping. Further studies to test the adjuvant-dependence of strong and weak food allergens need to be done to determine if the model could also be useful for predicting allergenicity of food antigens. CT was used as a model adjuvant. In humans, adjuvant activity may be inherent in the antigen or may be associated with states of inflammation such as that observed during eczema. Mast cell activation and local production of cytokines, such as TSLP or IL-21, may play an important role in sensitization to allergens by acting directly or indirectly on dermal DCs.19, 27–31 Adjuvant activity may also be provided by microbial stimuli such as that by skin-colonizing staphylococcal enterotoxin B (SEB). SEB has direct immunomodulatory effects on T cells and DCs.32–33 SEB-conditioned DCs drive naïve CD4 T-cells to differentiate into Th2 cells and SEB itself promotes the maturation of DCs.34
In the gastrointestinal tract, CT feeding results in an increase in total CD11c+ DCs in the mesenteric lymph nodes, and maturation in the subset expressing CD103 15, which is a marker of cells draining from the lamina propria.35 In contrast to the findings in the mucosa, we did not observe any global increase in CD11c+ DCs in the skin draining lymph node nor did we see any changes in the sub-populations of DCs after cutaneous exposure. This is consistent with previous reports on the impact of CT on skin DCs and may reflect a lower basal migration rate in the skin compared to the gut.36 Detection of antigen-bearing DCs in the draining lymph node showed that they were a very small percentage of the total DC population, and therefore would not result in a detectable increase in the total DC number. We determined that the FITC-containing DCs driven to the skin draining lymph nodes by cholera toxin were langerinneg dermal DCs. DCs that captured antigen in vivo in the context of CT had enhanced Th2 stimulatory activity. This is consistent with a previous report showing that dermal DCs were the primary inducers of Th2 responses in the context of a Th2 adjuvant (papain), while Th1-promoting adjuvants (CpG, LPS) induced a slower migration of Langerhans cells that mediated a Th1 response.37 Langerin+ CD103+ dermal DCs have been shown to mediate EAE after immunization via the induction of Th1 and Th17 cells 38, indicating some level of functional specialization. Further studies are required to determine if adjuvant is driving an inherently Th2-skewing DC subset to the lymph node, or if adjuvant alters the phenotype of the Langerinneg dermal DC to generate a Th2-promoting cell. In addition, although we have shown that DCs are sufficient to prime a Th2 response to skin-derived antigens, we have not ruled out cooperation between DCs and innate sources of Th2 cytokines such as basophils. This cooperation has been shown to occur in response to the protease allergen papain.39
In conclusion, we have shown that the route of exposure influences the degree of sensitization induced. In addition, an adjuvant, CT, is required for sensitization to occur to the milk allergen ALA independent of route of exposure. We have shown that the skin is a potent and likely important physiologic route of sensitization whereby CT acts by causing an efflux of Langerinneg dermal DCs from the skin, resulting in a proliferation of T cells in the draining lymph node and ultimately an allergic response. Our data indicate that sensitization to foods may occur through intact skin with only small doses of Ag without occlusive dressings, prolonged exposures or the presence of inflammation when adjuvant is present.
Key Messages.
Allergic sensitization to milk proteins can be achieved by any physiologic route in the presence of exogenous adjuvant
Cutaneous sensitization to antigen induces maximal IgE production in the absence of IgA production
Cholera toxin promotes cutaneous sensitization by driving antigen-bearing langerin-negative dermal DCs into the draining lymph node
Supplementary Material
Acknowlegements
We thank Wei Wang, Paul Arnaboldi and Keren Rabinowitz for expert technical assistance.
This work was supported by NIH grants PO1 AI044236 and K12 HD052890
Abbreviations
- ALA
α-lactalbumin
- CT
cholera toxin
- DC
dendritic cell
- LPS
lipopolysaccharide
- MLN
mesenteric lymph node
- OVA
ovalbumin
- PP
Peyer’s patch
- SEB
staphylococcal enterotoxin B
- SLN
skin-draining lymph node
- TSLP
thymic stromal lymphopoietin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of seafood allergy in the United States determined by a random telephone survey. J Allergy Clin Immunol. 2004;114:159–165. doi: 10.1016/j.jaci.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 2.Baral VR, Hourihane JO. Food allergy in children. Postgrad Med J. 2005;81:693–701. doi: 10.1136/pgmj.2004.030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren E, et al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120:638–646. doi: 10.1016/j.jaci.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 4.Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. J Allergy Clin Immunol. 2003;112:1203–1207. doi: 10.1016/s0091-6749(03)02026-8. [DOI] [PubMed] [Google Scholar]
- 5.Lack G, Fox D, Northstone K, Golding J. Factors associated with the development of peanut allergy in childhood. N Engl J Med. 2003;348:977–985. doi: 10.1056/NEJMoa013536. [DOI] [PubMed] [Google Scholar]
- 6.Fox AT, Sasieni P, du Toit G, Syed H, Lack G. Household peanut consumption as a risk factor for the development of peanut allergy. J Allergy Clin Immunol. 2009;123:417–423. doi: 10.1016/j.jaci.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Beezhold DH, Sussman GL, Liss GM, Chang NS. Latex allergy can induce clinical reactions to specific foods. Clin Exp Allergy. 1996;26:416–422. [PubMed] [Google Scholar]
- 8.Roth-Walter F, Berin MC, Arnaboldi P, Escalante CR, Dahan S, Rauch J, et al. Pasteurization of milk proteins promotes allergic sensitization by enhancing uptake through Peyer's patches. Allergy. 2008;63:882–890. doi: 10.1111/j.1398-9995.2008.01673.x. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh KY, Tsai CC, Wu CH, Lin RH. Epicutaneous exposure to protein antigen and food allergy. Clin Exp Allergy. 2003;33:1067–1075. doi: 10.1046/j.1365-2222.2003.01724.x. [DOI] [PubMed] [Google Scholar]
- 10.Birmingham NP, Parvataneni S, Hassan HM, Harkema J, Samineni S, Navuluri L, et al. An adjuvant-free mouse model of tree nut allergy using hazelnut as a model tree nut. Int Arch Allergy Immunol. 2007;144:203–210. doi: 10.1159/000103993. [DOI] [PubMed] [Google Scholar]
- 11.Gonipeta B, Parvataneni S, Tempelman RJ, Gangur V. An adjuvant-free mouse model to evaluate the allergenicity of milk whey protein. J Dairy Sci. 2009;92:4738–4744. doi: 10.3168/jds.2008-1927. [DOI] [PubMed] [Google Scholar]
- 12.Chehade M, Mayer L. Oral tolerance and its relation to food hypersensitivities. J Allergy Clin Immunol. 2005;115:3–12. doi: 10.1016/j.jaci.2004.11.008. quiz 3. [DOI] [PubMed] [Google Scholar]
- 13.Allam JP, Wurtzen PA, Reinartz M, Winter J, Vrtala S, Chen KW, et al. Phl p 5 resorption in human oral mucosa leads to dose-dependent and time-dependent allergen binding by oral mucosal Langerhans cells, attenuates their maturation, and enhances their migratory and TGF-beta1 and IL-10-producing properties. J Allergy Clin Immunol. 2010;126:638–645. doi: 10.1016/j.jaci.2010.04.039. e1. [DOI] [PubMed] [Google Scholar]
- 14.Mascarell L, Lombardi V, Louise A, Saint-Lu N, Chabre H, Moussu H, et al. Oral dendritic cells mediate antigen-specific tolerance by stimulating TH1 and regulatory CD4+ T cells. J Allergy Clin Immunol. 2008;122:603–609. doi: 10.1016/j.jaci.2008.06.034. e5. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez D, Keller AC, Faquim-Mauro EL, de Macedo MS, Cunha FQ, Lefort J, et al. Bacterial lipopolysaccharide signaling through Toll-like receptor 4 suppresses asthma-like responses via nitiric oxide synthase 2 activity. J Immunol. 2003;171(2):1001–1008. doi: 10.4049/jimmunol.171.2.1001. [DOI] [PubMed] [Google Scholar]
- 16.Blazquez AB, Berin MC. Gastrointestinal dendritic cells promote Th2 skewing via OX40L. J Immunol. 2008;180:4441–4450. doi: 10.4049/jimmunol.180.7.4441. [DOI] [PubMed] [Google Scholar]
- 17.Berin MC, Shreffler WG. T(H)2 adjuvants: implications for food allergy. J Allergy Clin Immunol. 2008;121:1311–1320. doi: 10.1016/j.jaci.2008.04.023. quiz 21-2. [DOI] [PubMed] [Google Scholar]
- 18.Naito S, Maeyama J, Mizukami T, Takahashi M, Hamaguchi I, Yamaguchi K. Transcutaneous immunization by merely prolonging the duration of antigen presence on the skin of mice induces a potent antigen-specific antibody response even in the absence of an adjuvant. Vaccine. 2007;25:8762–8770. doi: 10.1016/j.vaccine.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 19.Jin H, Oyoshi MK, Le Y, Bianchi T, Koduru S, Mathias CB, et al. IL-21R is essential for epicutaneous sensitization and allergic skin inflammation in humans and mice. J Clin Invest. 2009;119:47–60. doi: 10.1172/JCI32310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oyoshi MK, Larson RP, Ziegler SF, Geha RS. Mechanical injury polarizes skin dendritic cells to elicit a T(H)2 response by inducing cutaneous thymic stromal lymphopoietin expression. J Allergy Clin Immunol. 2010;126:976–984. doi: 10.1016/j.jaci.2010.08.041. 84 e1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brix S, Kjaer TM, Barkholt V, Frokiaer H. Lipopolysaccharide contamination of beta-lactoglobulin affects the immune response against intraperitoneally and orally administered antigen. Int Arch Allergy Immunol. 2004;135:216–220. doi: 10.1159/000081306. [DOI] [PubMed] [Google Scholar]
- 23.Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457:585–588. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shreffler WG, Castro RR, Kucuk ZY, Charlop-Powers Z, Grishina G, Yoo S, et al. The major glycoprotein allergen from Arachis hypogaea, Ara h 1, is a ligand of dendritic cell-specific ICAM-grabbing nonintegrin and acts as a Th2 adjuvant in vitro. J Immunol. 2006;177:3677–3685. doi: 10.4049/jimmunol.177.6.3677. [DOI] [PubMed] [Google Scholar]
- 25.Pochard P, Vickery B, Berin MC, Grishin A, Sampson HA, Caplan M, et al. Targeting Toll-like receptors on dendritic cells modifies the T(H)2 response to peanut allergens in vitro. J Allergy Clin Immunol. 2010;126:92–97. doi: 10.1016/j.jaci.2010.04.003. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu SC, Chen CH, Tsai SH, Kawasaki H, Hung CH, Chu YT, et al. Functional interaction of common allergens and a C-type lectin receptor, dendritic cell-specific ICAM3-grabbing non-integrin (DC-SIGN), on human dendritic cells. J Biol Chem. 2010;285:7903–7910. doi: 10.1074/jbc.M109.058370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 28.Ebner S, Nguyen VA, Forstner M, Wang YH, Wolfram D, Liu YJ, et al. Thymic stromal lymphopoietin converts human epidermal Langerhans cells into antigen-presenting cells that induce proallergic T cells. J Allergy Clin Immunol. 2007;119:982–990. doi: 10.1016/j.jaci.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGowen AL, Hale LP, Shelburne CP, Abraham SN, Staats HF. The mast cell activator compound 48/80 is safe and effective when used as an adjuvant for intradermal immunization with Bacillus anthracis protective antigen. Vaccine. 2009;27:3544–3552. doi: 10.1016/j.vaccine.2009.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 32.Laouini D, Kawamoto S, Yalcindag A, Bryce P, Mizoguchi E, Oettgen H, et al. Epicutaneous sensitization with superantigen induces allergic skin inflammation. J Allergy Clin Immunol. 2003;112:981–987. doi: 10.1016/j.jaci.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Huvenne W, Callebaut I, Plantinga M, Vanoirbeek JA, Krysko O, Bullens DM, et al. Staphylococcus aureus enterotoxin B facilitates allergic sensitization in experimental asthma. Clin Exp Allergy. 2010;40:1079–1090. doi: 10.1111/j.1365-2222.2010.03464.x. [DOI] [PubMed] [Google Scholar]
- 34.Mandron M, Aries MF, Brehm RD, Tranter HS, Acharya KR, Charveron M, et al. Human dendritic cells conditioned with Staphylococcus aureus enterotoxin B promote TH2 cell polarization. J Allergy Clin Immunol. 2006;117:1141–1147. doi: 10.1016/j.jaci.2005.12.1360. [DOI] [PubMed] [Google Scholar]
- 35.Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, et al. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203:519–527. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anjuere F, George-Chandy A, Audant F, Rousseau D, Holmgren J, Czerkinsky C. Transcutaneous immunization with cholera toxin B subunit adjuvant suppresses IgE antibody responses via selective induction of Th1 immune responses. J Immunol. 2003;170:1586–1592. doi: 10.4049/jimmunol.170.3.1586. [DOI] [PubMed] [Google Scholar]
- 37.Sen D, Forrest L, Kepler TB, Parker I, Cahalan MD. Selective and site-specific mobilization of dermal dendritic cells and Langerhans cells by Th1- and Th2-polarizing adjuvants. Proc Natl Acad Sci U S A. 2010;107:8334–8339. doi: 10.1073/pnas.0912817107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.King IL, Kroenke MA, Segal BM. GM-CSF-dependent, CD103+ dermal dendritic cells play a critical role in Th effector cell differentiation after subcutaneous immunization. J Exp Med. 2010;207:953–961. doi: 10.1084/jem.20091844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang H, Cao W, Kasturi SP, Ravindran R, Nakaya HI, Kundu K, et al. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol. 2010;11:608–617. doi: 10.1038/ni.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.