Abstract
Introduction
Treatment regimens for childhood acute lymphoblastic leukemia (ALL) contain neurotoxic agents that may interfere with neuromuscular health. This study examined associations between neuromuscular impairments and physical function, and between neuromuscular impairments, and doses of vincristine and intrathecal methotrexate used to treat leukemia among survivors of childhood ALL.
Methods
ALL survivors 10+ years from diagnosis participated in neuromuscular performance testing. Treatment data were abstracted from medical records. Regression models were used to evaluate associations between treatment factors, neuromuscular impairments and physical performance.
Results
Among 415 survivors (median age 35 years; range 21–52), balance, mobility and six-minute walk (6MW) distances were 1.3 standard deviations below age- and sex-specific values in 15.4%, 3.6% and 46.5% of participants, respectively. Impairments included absent Achilles tendon reflexes (39.5%), active dorsiflexion range of motion (ROM) < 5 degrees (33.5%) and impaired knee extension strength (30.1%). In adjusted models (including cranial radiation), survivors treated with intrathecal methotrexate cumulative doses 215+ mg/m2 were 3.4 (95% CI 1.2–9.8) times more likely than survivors who received no intrathecal methotrexate, and those who received vincristine cumulative doses 39+ mg/m2 1.5 (95% CI 1.0–2.5) times more likely than those who received lower doses to have impaired ROM. Higher intrathecal methotrexate doses were associated with reduced knee extension strength and 6MW distances.
Conclusion
Neuromuscular impairments are prevalent in childhood ALL survivors and interfere with physical performance. Higher cumulative doses of vincristine and/or intrathecal methotrexate are associated with long-term neuromuscular impairments, which have implications on future function as these survivors age.
Keywords: Acute Lymphoblastic Leukemia, Survivor, Neuromuscular impairment, Function, Physical performance, Intrathecal methotrexate, Vincristine, Late effect
INTRODUCTION
The 5-year survival rate for children treated for acute lymphoblastic leukemia (ALL), even without cranial radiation, is now over 90%.1 Contemporary therapy has diminished relapse rates,2 second malignant neoplasms,3 and severe neurocognitive outcomes.4, 5 Nevertheless, current treatment regimens contain chemotherapeutic agents that have the potential to interfere with long-term neuromuscular health and physical function.6
Vincristine and methotrexate, chemotherapeutic agents integral to treatment for ALL, have been associated with acute peripheral neuropathy7 or polyradiculopathy.8 While previous reports have dismissed these toxicities as transient phenomena,9 recent findings indicate that many survivors have impaired neuromuscular function that persists after treatment has ended.9–14 Among these survivors, long-term dysfunction includes slowed motor nerve conduction velocities,10, 12–14 absent deep tendon reflexes,15 limited ankle range of motion11 and distal muscle weakness.13 Several small studies of survivors evaluated during childhood or adolescence indicate that these neuromuscular impairments are subtle in the early post-treatment period, and not always associated with significant limitations in physical performance.10, 12, 14, 16 Few studies have evaluated the impact of persistent neuromuscular impairment on physical performance among adult survivors of childhood ALL, many years from completion of initial cancer therapy.
Studies of the association between methotrexate and neuromuscular impairment have identified cumulative dose, the number of intrathecal accesses, and concomitant chemotherapy as predictors of an acute toxicity.6 This association has not been evaluated in very long-term ALL survivors. Investigations of the association between vincristine and neuromuscular toxicity have yielded inconsistent results,17,18 potentially because small sample sizes, and similar treatment protocols with homogeneous drug doses, limited the statistical power to detect differences.
The purpose of this study was to comprehensively assess the frequency of neuromuscular impairments and physical performance limitations, to determine the impact of specific neuromuscular impairments on physical performance, and to evaluate associations between vincristine and intrathecal methotrexate administration and loss of neuromuscular function in a cohort of adults who were treated for childhood ALL and who have survived at least ten years.
METHODS
Individuals included in these analyses were participants in the St. Jude Lifetime Cohort, a study designed to evaluate medical and psychosocial late effects of childhood cancer and its’ treatment.19 Potentially eligible participants (n=4024 all diagnoses, 1231 with ALL) were treated at St. Jude Children’s Research Hospital for childhood cancer between 1962 and 2001. All participants were 18 years of age or older and had survived at least 10 years since their original cancer diagnosis. Protocol enrollment started in November of 2007 and is ongoing. Potentially eligible participants were randomly assigned to blocks of 50 for recruitment. These analyses include survivors of childhood ALL who were assigned to one of the first 32 recruitment blocks and who have completed an initial medical follow-up visit and functional assessment between November 2007 and February 2011.
The outcomes of interest for these analyses included clinical measures of neuromuscular impairment and physical performance limitations. Ankle tendon reflexes, touch and vibratory sensation, ankle range of motion and strength, quadriceps strength, balance, mobility and walking efficiency were evaluated. For ankle reflexes, the foot was gently dorsiflexed and the Achilles tendon tapped with a reflex hammer, while observing and palpating for a contraction of the gastrocnemius. If there was no response, the patient was asked to interlock and pull with flexed fingers to provide reinforcement.20 Reflexes were classified as absent only if there was no muscle contraction even with reinforcement. The plantar surface of the great toe was examined for protective touch sensation with a 5.07/10 gram Semmes Weinstein monofilament, and for vibration with a Bio-Thesiometer (Newbury, Ohio) at 120 cycles per second.21 Vibration was considered absent at thresholds greater than 0.616 microns.21 Active ankle dorsiflexion range-of-motion (ROM) was measured with a goniometer and considered impaired at values less than five degrees.22 While sitting with the hip and knee supported, lower extremity muscle strength was measured for isokinetic dorsiflexion, plantar flexion, and knee extension at 60 degrees per second (Biodex III Myometer, Shirley, New York). Peak torque from among five repetitions was used for analysis. Strength was considered impaired when the peak torque value for that motion was less than 1.3 standard deviations below height, weight, age and sex-specific mean values.23, 24
Balance, defined as the ability to maintain a standing position, was measured by having the participant complete the sensory organization test (SOT) on a computerized dynamic posturography system (Neurocom International, Clackamas, Oregon). Participants were asked to stand on dual force plates that were pitched up or down in an anterior-posterior direction to provoke ankle motion. A colored visual screen surrounded the participant on three sides. Visual and kinesthetic inputs were manipulated to create six different conditions, each of which included three 20 second trials. During testing, the device continuously recorded the participant’s center of pressure over the force plates. A difference score was computed from the normal range of anterior-posterior sway (12.5 degrees) and the maximum range of sway of the participant on each trial was averaged and expressed as a percentage. A higher score indicated less sway. Participants who scored below 70 percent on the SOT were classified as having a balance limitation.25, 26
Mobility was evaluated with the Timed Up and Go Test (TUG) and walking efficiency with the Six Minute Walk Test (6MW). For the TUG, participants were asked to rise from a sitting position in a chair, walk ten feet, turn and return to the sitting position. They were encouraged to complete this task as fast as possible. Two trials were attempted by each participant; the time taken to complete the second trial was recorded and used for analysis. Individuals with TUG times greater than 1.3 standard deviations from population based mean values were classified as having poor mobility.27 For the 6MW, participants were asked to walk as fast as possible along a corridor for 6 minutes. Customary walking aids (canes, walkers, crutches) were allowed, and the walking distance recorded in meters. Individuals who walked distances 1.3 or more standard deviations below sex-, age-, height-, and weight-, adjusted predicted distances were classified as having limited walking efficiency.28
Demographic and treatment information were abstracted from medical records. We considered sex as a dichotomous variable, age at diagnosis as a continuous variable, and cranial radiation and five chemotherapeutic agents as both dichotomous and continuous variables. Data distributions were examined to establish representative categories for treatment exposures, resulting in classifications of cranial radiation exposure as none or any; intrathecal methotrexate exposure as none, 47–214 mg/m2, or 215–694 mg/m2; and vincristine exposure as 3–38 mg/m2 or 39 or more mg/m2. Of note, glucocorticoids in prednisone equivalent doses, intravenous methotrexate, epipodophyllotoxins, and asparaginase were not associated with the outcomes, nor did they appreciably alter final models so they were not included in multivariable analyses.
Chronic conditions considered as confounders in the associations between neuromuscular impairments and balance, mobility and fitness included unresolved cardiac or pulmonary dysfunction, diabetes and peripheral vascular disease. Chronic conditions were confirmed with electrocardiography, electrocardiogram, pulmonary function testing, laboratory and clinical evaluation.
Descriptive statistics including frequencies, percentages, means, standard deviations, medians and ranges were calculated to characterize the demographic and treatment characteristics of the study population. Participants were compared to non-participants with Chi-squared statistics and paired t-tests. The percentages of survivors with impaired neuromuscular outcomes and physical performance limitations are also reported. The associations between demographic and treatment characteristics and the neuromuscular outcome variables were evaluated in multiple variable logistic regression models.29 Associations between reflexes, touch and vibratory sensation, ankle ROM and ankle strength and physical performance were evaluated in multiple variable regression models, using logistic regression29 when the outcome was dichotomous and general linear regression30 when the outcome was continuous. Models were adjusted for age, sex and prevalent cardiopulmonary conditions, diabetes and peripheral vascular disease. P-values < 0.05 were considered significant. SAS version 9.2 (Cary, North Carolina) was used for all analyses.
RESULTS
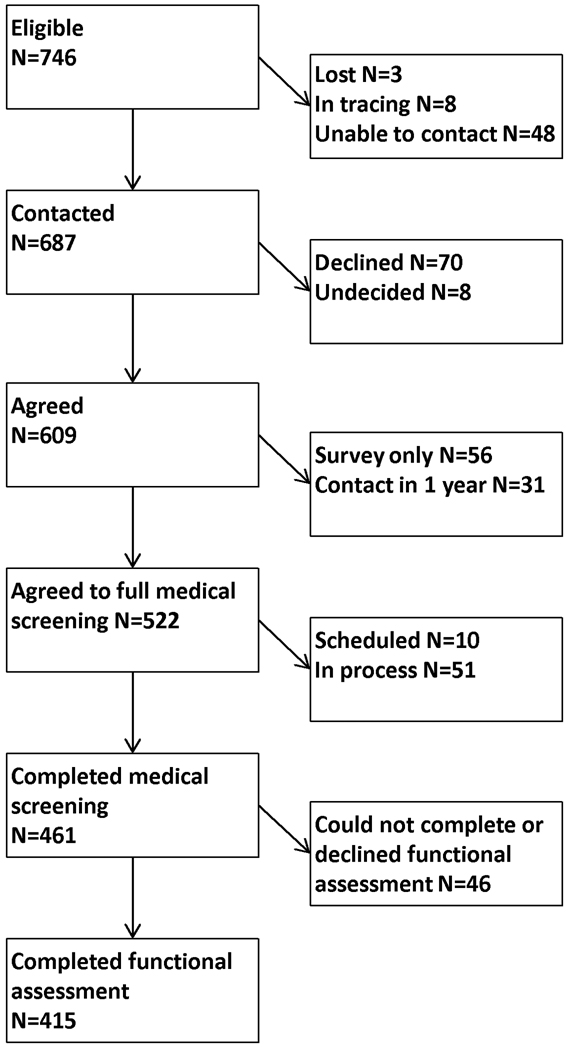
Among the 1559 members in the first 32 blocks of the St. Jude Life cohort, 746 had a previous diagnosis of ALL and were mailed a recruitment letter. Among these individuals, 687 have been contacted, 609 (88.6% of those contacted) agreed to undergo a risk-based medical evaluation, and 461 (67.1% of those contacted) have completed their assessments (Figure 1). Among the 461 participants who completed their medical evaluations, 46 (9.9%) did not complete the physical performance portion of the assessment: Thirteen had severe chronic cardiac or pulmonary problems, and seven had acute musculoskeletal injuries that precluded completion. Two individuals were paraplegic, three were too large for the equipment, six were unable to follow directions, and fifteen declined some or all of the testing procedures. Characteristics of the participants and non-participants are shown in Table 1. The median age of the study participants was 35.6 (range 21.9–52.3) years and the median time since diagnosis was 29.9 (13.7–46.5) years. Participants did not differ from non-participants by current age, age at diagnosis or time since diagnosis. Those who were unable to complete or who declined the functional assessment were more likely to be obese (65.2% vs. 46.5%), and had survived slightly longer than those who participated in the physical performance assessment (median 33; range 14–47 years vs. 29; range 13–46 years). Participants were more likely to be female (51.1% vs. 42.4%) and self-report their race as white (93.7% vs. 89.1%) than non-participants. The distribution of cranial radiation and chemotherapy doses were similar among participants and non-participants.
Figure 1.
Consort diagram
Table 1.
Characteristics of the study population
| Participants N=415 |
Participants unable to complete physical performance testing+ N=46 |
Non-participants N=285 |
||||||
|---|---|---|---|---|---|---|---|---|
| Median | Range | Median | Range | p-value* | Median | Range | p-value# | |
| Current age (years) | 35.6 | 21.9–52.3 | 36.6 | 27.8–55.2 | 0.18 | 36.0 | 21.8–52.0 | 0.96 |
| Age at diagnosis (years) | 4.8 | 0.2–18.8 | 4.1 | 1.6–17.2 | 0.17 | 4.4 | 0.1–21.0 | 0.42 |
| Time since diagnosis (years) | 29.9 | 13.7–46.5 | 33.0 | 14.3–47.3 | 0.02 | 30.3 | 13.7–47.6 | 0.54 |
| N | % | N | % | N | % | |||
| Sex | ||||||||
| Female | 212 | (51.1) | 30 | (65.2) | 0.07 | 121 | (42.5) | 0.02 |
| Male | 203 | (48.9) | 16 | (34.8) | 164 | (57.5) | ||
| Race | ||||||||
| White | 389 | (93.7) | 42 | (91.3) | 0.50 | 254 | (89.1) | 0.03 |
| Black | 23 | (5.5) | 4 | (8.7) | 28 | (9.8) | ||
| Other | 3 | (0.7) | 0 | (0.0) | 3 | (1.1) | ||
| Cranial radiation | ||||||||
| No | 110 | (26.5) | 7 | (15.2) | 0.10 | 74 | (26.0) | 0.81 |
| Yes | 305 | (73.5) | 39 | (84.8) | 211 | (74.0) | ||
| Vincristine | ||||||||
| 3–38 mg/m2 | 209 | (50.4) | 29 | (63.0) | 0.10 | 163 | (57.2) | 0.08 |
| 39–220 mg/m2 | 206 | (49.6) | 17 | (37.0) | 122 | (42.8) | ||
| Intrathecal Methotrexate | ||||||||
| None | 30 | (7.2) | 4 | (8.7) | 0.39 | 21 | (7.4) | 0.58 |
| 47–214 mg/m2 | 280 | (67.5) | 33 | (71.7) | 186 | (65.3) | ||
| 215–694 mg/m2 | 105 | (25.3) | 9 | (19.6) | 78 | (27.4) | ||
| Chronic conditions | ||||||||
| Arrhythmia | 26 | (6.3) | 4 | (8.7) | 0.53 | |||
| Heart valve disorder | 85 | (20.5) | 10 | (21.7) | 0.84 | |||
| Left ventricular dysfunction | 32 | (7.7) | 6 | (13.0) | 0.21 | |||
| Peripheral vascular disease | 27 | (6.5) | 5 | (10.9) | 0.27 | |||
| Reduced pulmonary function | 28 | (6.7) | 5 | (10.9) | 0.30 | |||
| Diabetes Mellitus | 24 | (5.8) | 6 | (13.0) | 0.06 | |||
| Obesity^ | 193 | (46.5) | 30 | (65.2) | 0.02 | |||
participated in St. Jude Lifetime Cohort Study, but unable to complete or declined performance testing
comparing those unable to complete functional assessment to participants
comparing those who did not participate to those who did participate
Body mass index 30 kilograms/meter squared or greater
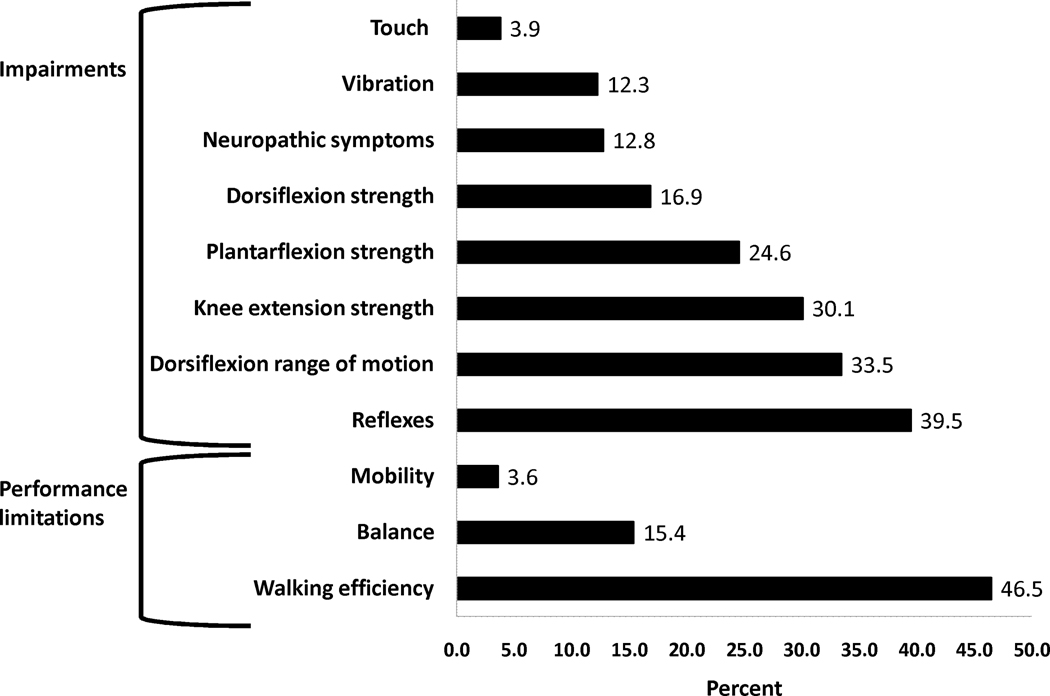
Figure 2 shows the percentages of impairments and physical performance limitations among the survivors. Over one-third of participants had absent Achilles’ deep tendon reflexes (39.5%), and/or active ankle dorsiflexion ROM less than five degrees (33.5%). Among the 138 survivors with impaired active ankle dorsiflexion ROM, 20 (4.8% of the cohort) had no passive ankle dorsiflexion ROM past neutral. Knee extension weakness was prevalent among 30.1% of the participants, ankle plantar flexion weakness among 24.6%, and in ankle dorsal flexion weakness among 16.9%. Neuropathic symptoms (numbness, tingling, difficulty buttoning or tying shoes) were reported by 12.8% of survivors. Vibration and protective touch sensation at the great toe were absent among 12.3% and 3.9% of survivors, respectively. The median number of impairments in this cohort was 1 (range 0–8). Nearly half (49.9%) of the cohort had two or more impairments, and slightly more than one-forth (27.0%) had three or more impairments.
Figure 2.
Percentages of participants with neuromuscular impairments and physical performance limitations
The most common physical performance limitation was limited walking efficiency; 46.5% of participants achieved distances during the six-minute walk in the lowest tenth percentile when compared to age, gender, height and weight matched normative values.28 In fact, the average distance walked by participants in our study was similar to the predicted six-minute walk distance for persons of the same height, weight and gender distribution of individuals fifty years of age (569±109 meters ALL survivors vs. 566±58 meters predicted for 50 year olds).28 Impaired balance was demonstrated in 15.4% of survivors, and mean scores on the sensory organization test were slightly lower than those typically reported for persons 60–69 years old (76.0 vs. 77.6 percent).31 Condition specific balance scores among those with limited balance were global and lower than expected in each of the six conditions, deteriorating and becoming widely variable with each added balance challenge. Mean percentage of time spent within the 12.5 degree sway envelope were 92.4±4.7; 88.2±5.8; 84.3±13.3; 67.4±18.4; 36.0±20.2; and 33.9±21.6 for conditions one through six respectively Poor performance on the TUG, an indicator of significant mobility limitations, was present in only 3.6% of survivors. Over half of the cohort had at least one physical performance limitation (54.5%). However, only 9.2% had more than one physical performance limitation.
Table 2 shows the results of final multivariable models examining associations between sex, impaired active dorsiflexion ROM, knee extension strength, loss of protective sensation and performance on tests of balance, mobility, and walking efficiency. All models were adjusted for age. The models with the mobility and walking efficiency outcomes were additionally adjusted for height and weight. The walking efficiency outcome model was also adjusted for echocardiographic evidence of left ventricular dysfunction. Other predictors of interest (vibratory sensation, reflexes, dorsiflexion strength, plantar flexion strength, acknowledged neuropathic symptoms) and potential confounders (pulmonary conditions, diabetes, peripheral vascular disease) were not independently associated with the outcomes, nor did they influence the strength of the associations, so they were not included in final models.
Table 2.
Associations between neuromuscular impairments and physical performance limitations
| Balance limitation* | N | % limited | OR | 95% CI | Mean | SE | p-value |
|---|---|---|---|---|---|---|---|
| Total | 415 | (15.4) | |||||
| Sex | |||||||
| Female | 212 | (19.3) | 2.5 | 1.3–4.5 | 70.2 | 2.1 | 0.005 |
| Male | 203 | (11.3) | 1.0 | 74.4 | 2.0 | ||
| Impaired dorsiflexion range of motion | |||||||
| Yes | 139 | (19.4) | 1.3 | 0.7–2.3 | 72.0 | 2.0 | 0.61 |
| No | 276 | (13.4) | 1.0 | 72.7 | 2.0 | ||
| Impaired knee extension strength | |||||||
| Yes | 125 | (20.8) | 2.0 | 1.1–3.7 | 70.3 | 2.2 | 0.01 |
| No | 290 | (13.1) | 1.0 | 74.4 | 1.9 | ||
| Loss of protective sensation | |||||||
| Yes | 16 | (37.5) | 2.9 | 0.9–8.8 | 69.4 | 3.6 | 0.11 |
| No | 399 | (14.5) | 1.0 | 75.2 | 0.8 | ||
| Mobility limitation* | |||||||
| Total | 415 | (3.6) | |||||
| Sex | |||||||
| Female | 212 | (5.2) | 5.6 | 1.6–19.3 | 6.4 | 0.2 | <0.001 |
| Male | 203 | (2.0) | 1.0 | 5.5 | 0.2 | ||
| Impaired dorsiflexion range of motion | |||||||
| Yes | 139 | (6.5) | 2.2 | 0.7–6.8 | 6.1 | 0.2 | 0.03 |
| No | 276 | (2.2) | 1.0 | 5.8 | 0.2 | ||
| Impaired knee extension strength | |||||||
| Yes | 125 | (8.8) | 8.2 | 2.3–29.0 | 6.4 | 0.2 | <0.001 |
| No | 290 | (1.4) | 1.0 | 5.5 | 0.2 | ||
| Loss of protective sensation | |||||||
| Yes | 16 | (0.0) | NE | NE | 6.1 | 0.4 | 0.65 |
| No | 399 | (3.8) | 5.9 | 0.1 | |||
| Limited walking efficiency*# | |||||||
| Total | 415 | (46.5) | |||||
| Sex | |||||||
| Female | 212 | (50.0) | 1.8 | 0.9–3.4 | 541.1 | 13.2 | 0.003 |
| Male | 203 | (42.9) | 1.0 | 579.2 | 12.4 | ||
| Impaired dorsiflexion range of motion | |||||||
| Yes | 139 | (64.0) | 3.9 | 2.4–6.3 | 531.8 | 12.1 | <0.001 |
| No | 276 | (37.7) | 1.0 | 588.5 | 13.3 | ||
| Impaired knee extension strength | |||||||
| Yes | 125 | (52.0) | 2.3 | 1.4–4.1 | 530.1 | 13.2 | <0.001 |
| No | 290 | (44.1) | 1.0 | 590.2 | 11.5 | ||
| Loss of protective sensation | |||||||
| Yes | 16 | (37.5) | 0.6 | 0.2–1.9 | 573.4 | 21.8 | 0.24 |
| No | 399 | (46.8) | 1.0 | 546.9 | 5.0 | ||
Adjusted for age,
Adjusted for height and weight,
OR=Odds ratio, CI=Confidence Interval, SE=Standard Error
Females were 2.5 times (95% CI 1.3–4.5) more likely to have balance problems, and 5.6 times (95% CI 1.6–19.3) more likely to have limited mobility than were males. Individuals with impaired active dorsiflexion ROM were more likely than those without impaired active dorsiflexion ROM to have limited walking efficiency (OR 3.9; 95% CI 2.4–6.3). Survivors with impaired knee extension strength were 2.0 times (95% CI 1.1–3.7) times more likely to have poor balance, 8.2 times (95% CI 2.3–29.0) more likely to have limited mobility, and 2.3 times (95% CI 1.4–4.1) more likely to have limited walking efficiency than were those with adequate knee extension strength. The impact of impaired active dorsiflexion ROM and knee extension strength upon mobility and fitness was also reflected by mean comparisons of the time to complete the TUG and the distance covered during the 6MW.
Table 3 shows the association between treatment and impaired active dorsiflexion ROM, knee extension strength, and protective sensation, and between treatment exposure and limited balance, mobility and walking efficiency. After adjusting for cranial radiation, sex, age at diagnosis and current age, both vincristine and intrathecal methotrexate doses were associated with impaired active dorsiflexion ROM. Survivors who received total vincristine doses of 39 mg/m2 or more were 1.5 (95% CI 1.0–2.5) times more likely than those who received dose less than 39 mg/m2 to have impaired active dorsiflexion ROM. Survivors who received intrathecal methotrexate doses from 215–694 mg/m2 3.4 times (95% CI 1.2–9.8), more likely than those who did not receive any intrathecal methotrexate to have impaired active dorsiflexion ROM. Intrathecal methotrexate doses were also associated with limited walking distance (OR 4.0, 95% CI 1.5–10.7 for 47–214 mg/m2 vs. no intrathecal methotrexate; and OR 5.8, 95% CI 2.2–15.4 for 215–694 mg/m2 vs. no intrathecal methotrexate), and with reduced knee extension strength (OR 3.7, 95% CI 1.2–11.2 for 47–214 mg/m2 vs. no intrathecal methotrexate; and OR 4.1, 95% CI 1.3–13.2 for 215–694 mg/m2 vs. no intrathecal methotrexate).
Table 3.
Associations between treatment and neuromuscular impairments or performance limitations
| Impaired dorsiflexion range of motion (N=139) |
Loss of protective sensation (N=16) |
Impaired knee extension strength (N=125) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Row % | OR* | 95% CI | Row % | OR* | 95% CI | Row % | OR* | 95% CI | |
| Cranial radiation | ||||||||||
| No | 110 | (22.7) | 1.0 | (0.9) | 1.0 | (19.1) | 1.0 | |||
| Yes | 305 | (37.4) | 1.6 | 0.9–2.9 | (4.9) | 4.6 | 0.5–10.8 | (34.1) | 1.5 | 0.8–2.9 |
| Vincristine | ||||||||||
| 3–38 mg/m2 | 209 | (30.1) | 1.0 | (2.9) | 1.0 | (32.1) | 1.0 | |||
| 39–220 mg/m2 | 206 | (36.9) | 1.5 | 1.0–2.5 | (4.9) | 1.7 | 0.5–5.6 | (28.2) | 1.2 | 0.7–2.1 |
| Intrathecal Methotrexate | ||||||||||
| None | 30 | (30.0) | 1.0 | (10.0) | 1.0 | (23.3) | 1.0 | |||
| 47–214 mg/m2 | 280 | (30.0) | 2.0 | 0.8–5.5 | (2.9) | 0.4 | 0.1–2.7 | (31.1) | 3.7 | 1.2–11.2 |
| 215–694 mg/m2 | 105 | (43.8) | 3.4 | 1.2–9.8 | (4.8) | 1.0 | 0.1–7.7 | (29.5) | 4.1 | 1.3–13.2# |
|
Balance limitation (N=64) |
Mobility limitation (N=15) |
Limited walking efficiency (N=193) |
||||||||
| N | Row % | OR* | 95% CI | Row % | OR* | 95% CI | Row % | OR* | 95% CI | |
| Cranial radiation | ||||||||||
| No | 110 | (11.8) | 1.0 | (0.9) | 1.0 | (50.9) | 1.0 | |||
| Yes | 305 | (16.7) | 0.9 | 0.4–2.0 | (4.6) | 2.5 | 0.3–22.1 | (44.9) | 0.8 | .5–1.2 |
| Vincristine | ||||||||||
| 3–38 mg/m2 | 209 | (16.8) | 1.0 | (5.7) | 1.0 | (43.1) | 1.0 | |||
| 39–220 mg/m2 | 206 | (14.0) | 1.1 | 0.6–2.0 | (1.5) | 0.3 | 0.1–1.2 | (50.0) | 1.3 | 0.9–2.1 |
| Intrathecal Methotrexate | ||||||||||
| None | 30 | (16.7) | 1.0 | (0.0) | NE | (20.0) | 1.0 | |||
| 47–214 mg/m2 | 280 | (15.7) | 1.6 | 0.5–5.5 | (4.3) | (44.6) | 4.0 | 1.5–10.7 | ||
| 215–694 mg/m2 | 105 | (14.3) | 2.3 | 0.6–8.3 | (2.9) | (59.6) | 5.8 | 2.2–15.4# | ||
Adjusted for sex, age and age at diagnosis,
N= Number. OR=Odds Ratio, CI=Confidence Interval, mg/m2= milligrams per meter squared
p-value for trend < 0.001
DISCUSSION
This study indicates that less than half of adult survivors of childhood ALL experience long-term lower-extremity neuromuscular impairments. However, when these neuromuscular impairments are present, they adversely impact physical performance. In addition, models adjusted for age, sex and cranial radiation indicate an association between intrathecal methotrexate doses and persistent problems with ankle ROM, knee extension strength, and, ultimately, performance on the six-minute walk test. Cranial radiation was not associated with these problems.
Limited ankle ROM and quadriceps muscle weakness are particularly concerning in a cohort of adults in their mid-thirties. In other populations, loss of normal ankle ROM limits ability to maintain upright standing during daily tasks that require reaching;32 is associated with early foot ulceration,33 and predicts reduced functional abilities and physical activity levels.34 Knee extension weakness is associated with the development of osteoarthritis,35 reduced walking speed,36 and even with early mortality.37 Newman et al,37 in the health, aging and body composition cohort study, reported hazard ratios for death of 1.51 (95% CI 1.28–1.79) among men and of 1.65 (95% CI 1.19–2.30) among women for each 38 Newton meters decrease in quadriceps strength.
In this cohort of 30 to 40 year old survivors, balance testing and six-minute walk values corresponded to scores typically seen in much older adults. Poor performance on the sensory organization test is associated with increased risk for falls in elderly populations.38 Because long-term ALL survivors are at increased risk for low bone mineral density at an early age,39 fall-related fractures and their associated morbidities are of heightened concern. Also, because six minute walk distances are highly correlated with cardiac and pulmonary function and with mortality in other populations,40, 41 our findings suggest that ALL survivors, who have known risk factors for cardiovascular morbidity and mortality,42 need regular follow-up to monitor their cardiorespiratory health.
We found signs and symptoms of sensory and motor deficits among ALL survivors in this cohort. These included absent reflexes, diminished detection of vibratory sensation, and limited ankle ROM. Cumulative vincristine doses greater than 38 mg/m2 were associated with ROM impairment. Past research has documented acute axonal peripheral neuropathy among children during,17 just after completion,10 and up to ten years after treatment for ALL.13 This distal polyneuropathy, usually attributed to vincristine administration,17 can be initially painful, affecting both sensory and motor functions. Reinders-Messelink et al.7 reported mild deficits in vibratory perception, reduced amplitude of action potentials in median, ulnar and fibular sensory nerves, and mild muscle weakness in 11 children after receiving 8 doses of vincristine at 1.5 mg per dose for remission induction and intensification therapy. Wright et al.11 reported 10 degree differences in ankle dorsiflexion ROM when they compared ALL survivors at least one year off therapy to age and gender matched normal controls, and Ramchandren et al.14 reported abnormalities in both sensory and motor nerve conduction studies in a group of 37 ALL survivors at an average of 7.4 years post treatment. Our study of a large adult cohort of childhood ALL survivors, at an average of 28 years from their original cancer diagnosis, indicates that these problems are present and negatively impact neuromuscular function.
To our knowledge, our study is the first to document an association between dose of intrathecal methotrexate administration and neuromuscular impairment in long-term adult survivors of childhood ALL. Several case studies and small clinical series provide some evidence that involvement may also include more proximal neural structures, with these deficits potentially related to administration of intrathecal methotrexate. Anderson et al43 reported acute polyradiculopathy in a three-year old girl following intrathecal chemotherapy during the maintenance phase of therapy for ALL. She was treated with intravenous immunoglobulin. Electromyographic findings five months after symptom onset were consistent with denervation and early reinervation. Koh et al.44 reported progressive paraparesis after intrathecal methotrexate administration for CNS prophylaxis in three children with ALL. Gadolinium enhancements of anterior lumbosacral spinal nerve roots were evident acutely. Motor function returned to baseline after steroid administration in two of the three children. Harila-Saari et al.10 evaluated motor evoked potentials (MEPs) in 32 children upon completing treatment for ALL and found prolonged latencies in both upper and lower extremity axons when responses were compared to an age, gender and height matched control group. These authors12 also employed somatosensory evoked potentials (SEP) to evaluate central and peripheral axonal integrity in 31 childhood ALL survivors who were at least two years off therapy. When compared to age and gender matched controls, median nerve stimulation in ALL survivors demonstrated prolonged SEP latencies at the level of the brachial plexus and spinal cord, as well as prolonged latencies at the level of spinal cord and cortex with tibial nerve stimulation at the knee. Lehtinen et al.13 showed that peripheral neuropathy persisted up to 5 years off-therapy as measured by MEPs in 27 children treated for ALL. Among our cohort of adult survivors, motor impairment was more common than sensory loss. Additionally, intrathecal methotrexate was associated, in a dose response fashion, with poor motor outcomes, perhaps indicating a lasting effect of this treatment on ventral nerve roots.
Our findings also indicate that these lasting neuromuscular impairments are associated with eventual limitations in balance, mobility and walking efficiency. This is in contrast to another recently published study that evaluated this association among 37 child (ages 8–18) ALL survivors.14 These young survivors were, on average, 7.4 years from diagnosis. While nearly all had evidence of motor neuropathy involving both upper and lower extremities, only one child scored below expected values on the test of motor proficiency. These authors concluded that objective deficits in nerve conduction and persistent signs and symptoms of peripheral neuropathy were not associated with limited physical performance in their young population. These differential findings may be because the physical performance measures we used were very different, or because younger survivors are better able to compensate for neuromuscular deficits than were our older group of survivors. We speculate that long term ROM losses and weakness may initiate a cycle of disuse and or physical inactivity. Early neuromuscular deficits, when combined with the increased body weight that can accompany aging, may stress skeletal and joint structures. Additional loss of strength and motion may eventually progress to such a level that they interfere with abilities to maintain balance, move from sit to stand, and walk at speeds required for efficient community mobility.
Female sex was associated with both balance and mobility limitations in our ALL survivor cohort. These findings are consistent with a study among 234 older adults (mean age 76±5 years) by Wolfson et al.45 who reported that women demonstrated greater sway than men when presented with balance challenges during the sensory organization test, and with a study by Vereeck et al.,46 who evaluated both balance and mobility in a cohort of 318 healthy volunteers (180 females, mean age 48.9±18.7 years). These authors reported that females were less likely than males to be able to stand for 30 seconds with their feet in a tandem position with their eyes closed, to stand on one leg for 10 seconds with their eyes opened, and to stand on foam for 30 seconds with their eyes closed. Veereck et al,46 also reported that women, on average, took longer than men to complete the TUG, and that the effect of age on performance during this task was greater among women than among men. The reasons for these differences are likely multifactorial. They may be biomechanical, or perhaps related to experience. Women are not as strong as men, even when body size is taken into account, and they may be less likely to participate in activities that require the use of complex balance strategies, limiting their opportunities to develop optimal responses to postural perturbation.
The results of this study should not be interpreted without taking into account potential study limitations. First of all, although our testing included validated measures of neuromuscular function to document impairment, we did not use quantitative nerve conduction velocity testing or evoked potentials to evaluate peripheral nerve integrity in our study participants. It is possible that our outcomes reflect only the effects of long-term disuse rather than persistent nervous system damage in this population. Bidirectional effects or complex interactions among neuromuscular impairments are likely to contribute to limitations in performance in this population. Secondly, because we did not complete performance based testing on these individuals during treatment, we do not know whether or not those who experienced poor long-term outcomes were those who had neuromuscular impairment acutely. Finally, our survivors were at least ten years from their original diagnosis. It is possible that our findings may not directly apply to children treated with contemporary regimens for ALL. Nevertheless, we remain concerned that our findings are pertinent to patients treated with current treatment protocols featuring intensive vincristine and intrathecal methotrexate at doses similar to those received by the participants in our study cohort.
CONCLUSION
Long term adult survivors of childhood ALL in their mid-thirties are at risk for neuromuscular impairments and physical performance limitations that are typically not seen in otherwise normal adults until at least their 6th decade of life. These late effects were associated with increased cumulative dose of intrathecal methotrexate, and to a lesser extent, with vincristine exposure. These findings are novel and important as these treatments continue to be administered to children treated on contemporary clinical protocols. The neuromuscular impairments, principally limited ROM and muscle weakness, may be amenable to simple interventions like stretching and strengthening so that long-term functional limitations are ameliorated.
Acknowledgments
Research Support: This work was supported in part by the Cancer Center Support (CORE) grant CA 21765 from the National Cancer Institute and by the American Lebanese Syrian Associated Charities (ALSAC).
REFERENCES
- 1.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krishnan S, Wade R, Moorman AV, et al. Temporal changes in the incidence and pattern of central nervous system relapses in children with acute lymphoblastic leukaemia treated on four consecutive Medical Research Council trials, 1985–2001. Leukemia. 2010;24:450–459. doi: 10.1038/leu.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaatsch P, Reinisch I, Spix C, et al. Case-control study on the therapy of childhood cancer and the occurrence of second malignant neoplasms in Germany. Cancer Causes Control. 2009;20:965–980. doi: 10.1007/s10552-009-9315-1. [DOI] [PubMed] [Google Scholar]
- 4.Kadan-Lottick NS, Brouwers P, Breiger D, et al. A comparison of neurocognitive functioning in children previously randomized to dexamethasone or prednisone in the treatment of childhood acute lymphoblastic leukemia. Blood. 2009;114:1746–1752. doi: 10.1182/blood-2008-12-186502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kadan-Lottick NS, Brouwers P, Breiger D, et al. Comparison of neurocognitive functioning in children previously randomly assigned to intrathecal methotrexate compared with triple intrathecal therapy for the treatment of childhood acute lymphoblastic leukemia. J Clin Oncol. 2009;27:5986–5992. doi: 10.1200/JCO.2009.23.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sioka C, Kyritsis AP. Central and peripheral nervous system toxicity of common chemotherapeutic agents. Cancer Chemother Pharmacol. 2009;63:761–767. doi: 10.1007/s00280-008-0876-6. [DOI] [PubMed] [Google Scholar]
- 7.Reinders-Messelink HA, Van Weerden TW, Fock JM, et al. Mild axonal neuropathy of children during treatment for acute lymphoblastic leukaemia. Eur J Paediatr Neurol. 2000;4:225–233. doi: 10.1053/ejpn.1999.0310. [DOI] [PubMed] [Google Scholar]
- 8.Kumar N, Sandroni P, Steensma DP, Luthra HS, Habermann TM. Polyradiculopathy due to methotrexate-induced ebv-associated lymphoproliferative disorder. Neurology. 2008;71:1644–1645. doi: 10.1212/01.wnl.0000334757.16882.37. [DOI] [PubMed] [Google Scholar]
- 9.Legha SS. Vincristine neurotoxicity. Pathophysiology and management. Med Toxicol. 1986;1:421–427. doi: 10.1007/BF03259853. [DOI] [PubMed] [Google Scholar]
- 10.Harila-Saari AH, Huuskonen UE, Tolonen U, Vainionpaa LK, Lanning BM. Motor nervous pathway function is impaired after treatment of childhood acute lymphoblastic leukemia: a study with motor evoked potentials. Med Pediatr Oncol. 2001;36:345–351. doi: 10.1002/mpo.1084. [DOI] [PubMed] [Google Scholar]
- 11.Wright MJ, Halton JM, Barr RD. Limitation of ankle range of motion in survivors of acute lymphoblastic leukemia: a cross-sectional study. Med Pediatr Oncol. 1999;32:279–282. doi: 10.1002/(sici)1096-911x(199904)32:4<279::aid-mpo7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 12.Harila-Saari AH, Vainionpaa LK, Kovala TT, Tolonen EU, Lanning BM. Nerve lesions after therapy for childhood acute lymphoblastic leukemia. Cancer. 1998;82:200–207. doi: 10.1002/(sici)1097-0142(19980101)82:1<200::aid-cncr25>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 13.Lehtinen SS, Huuskonen UE, Harila-Saari AH, Tolonen U, Vainionpaa LK, Lanning BM. Motor nervous system impairment persists in long-term survivors of childhood acute lymphoblastic leukemia. Cancer. 2002;94:2466–2473. doi: 10.1002/cncr.10503. [DOI] [PubMed] [Google Scholar]
- 14.Ramchandren S, Leonard M, Mody RJ, et al. Peripheral neuropathy in survivors of childhood acute lymphoblastic leukemia. J Peripher Nerv Syst. 2009;14:184–189. doi: 10.1111/j.1529-8027.2009.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilchrist LS, Tanner L, Hooke MC. Measuring chemotherapy-induced peripheral neuropathy in children: development of the Ped-mTNS and pilot study results. Rehabilitation Oncology. 2009;27:3–15. [Google Scholar]
- 16.Hartman A, van den Bos C, Stijnen T, Pieters R. Decrease in peripheral muscle strength and ankle dorsiflexion as long-term side effects of treatment for childhood cancer. Pediatr Blood Cancer. 2008;50:833–837. doi: 10.1002/pbc.21325. [DOI] [PubMed] [Google Scholar]
- 17.Vainionpaa L. Clinical neurological findings of children with acute lymphoblastic leukaemia at diagnosis and during treatment. Eur J Pediatr. 1993;152:115–119. doi: 10.1007/BF02072486. [DOI] [PubMed] [Google Scholar]
- 18.Hartman A, van den Bos C, Stijnen T, Pieters R. Decrease in motor performance in children with cancer is independent of the cumulative dose of vincristine. Cancer. 2006;106:1395–1401. doi: 10.1002/cncr.21706. [DOI] [PubMed] [Google Scholar]
- 19.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: Study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort Study. Pediatric Blood & Cancer. 2011;56:825–836. doi: 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall GL, Little JW. Deep tendon reflexes: a study of quantitative methods. J Spinal Cord Med. 2002;25:94–99. doi: 10.1080/10790268.2002.11753608. [DOI] [PubMed] [Google Scholar]
- 21.Halar EM, Hammond MC, LaCava EC, Camann C, Ward J. Sensory perception threshold measurement: an evaluation of semiobjective testing devices. Arch Phys Med Rehabil. 1987;68:499–507. [PubMed] [Google Scholar]
- 22.Boone DC, Azen SP, Lin CM, Spence C, Baron C, Lee L. Reliability of goniometric measurements. Phys Ther. 1978;58:1355–1390. doi: 10.1093/ptj/58.11.1355. [DOI] [PubMed] [Google Scholar]
- 23.Holmback AM, Porter MM, Downham D, Lexell J. Reliability of isokinetic ankle dorsiflexor strength measurements in healthy young men and women. Scand J Rehabil Med. 1999;31:229–239. [PubMed] [Google Scholar]
- 24.Biodex. Isokinetic testing and data interpretation normative database. [accessed February 28, 2011];Biodex. Available from: http://www.biodex.com/rehab/system4/resources/data_int/normative.pdf.
- 25.Cohen H, Heaton LG, Congdon SL, Jenkins HA. Changes in sensory organization test scores with age. Age Ageing. 1996;25:39–44. doi: 10.1093/ageing/25.1.39. [DOI] [PubMed] [Google Scholar]
- 26.NeuroCom International Inc. SOT Norms. Clackamas, OR: Neurocom International Inc; 2007. [Google Scholar]
- 27.Isles RC, Choy NL, Steer M, Nitz JC. Normal values of balance tests in women aged 20–80. J Am Geriatr Soc. 2004;52:1367–1372. doi: 10.1111/j.1532-5415.2004.52370.x. [DOI] [PubMed] [Google Scholar]
- 28.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 29.Allison PD. Logistic Regression Using the SAS System. Cary, NC: SAS Institute; 1999. [Google Scholar]
- 30.Kleinbaum D, Kupper L, Muller K. Applied regression analysis. 2nd ed. Boston, MA: PWS-Kent Publishing Co; 1988. [Google Scholar]
- 31.Nashner LM. Computerized Dynamic Posturography. In: Jacobson GP, Newman CW, Kartush JM, editors. Handbook of Balance Function Testing. Clifton Park: Thompson Delmar Learning; 1997. pp. 280–307. [Google Scholar]
- 32.Mecagni C, Smith JP, Roberts KE, O'Sullivan SB. Balance and Ankle Range of Motion in Community-Dwelling Women Aged 64 to 87 Years: A Correlational Study. Physical Therapy. 2000;80:1004–1011. [PubMed] [Google Scholar]
- 33.Fernando DJ, Masson EA, Veves A, Boulton AJ. Relationship of limited joint mobility to abnormal foot pressures and diabetic foot ulceration. Diabetes Care. 1991;14:8–11. doi: 10.2337/diacare.14.1.8. [DOI] [PubMed] [Google Scholar]
- 34.Barr EL, Browning C, Lord SR, Menz HB, Kendig H. Foot and leg problems are important determinants of functional status in community dwelling older people. Disabil Rehabil. 2005;27:917–923. doi: 10.1080/09638280500030506. [DOI] [PubMed] [Google Scholar]
- 35.Slemenda C, Brandt KD, Heilman DK, et al. Quadriceps weakness and osteoarthritis of the knee. Ann Intern Med. 1997;127:97–104. doi: 10.7326/0003-4819-127-2-199707150-00001. [DOI] [PubMed] [Google Scholar]
- 36.Ostchega Y, Dillon CF, Lindle R, Carroll M, Hurley BF. Isokinetic leg muscle strength in older americans and its relationship to a standardized walk test: data from the national health and nutrition examination survey 1999–2000. J Am Geriatr Soc. 2004;52:977–982. doi: 10.1111/j.1532-5415.2004.52268.x. [DOI] [PubMed] [Google Scholar]
- 37.Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 38.Girardi M, Konrad HR, Amin M, Hughes LF. Predicting fall risks in an elderly population: computer dynamic posturography versus electronystagmography test results. Laryngoscope. 2001;111:1528–1532. doi: 10.1097/00005537-200109000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Thomas IH, Donohue JE, Ness KK, Dengel DR, Baker KS, Gurney JG. Bone mineral density in young adult survivors of acute lymphoblastic leukemia. Cancer. 2008;113:3248–3256. doi: 10.1002/cncr.23912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rasekaba T, Lee AL, Naughton MT, Williams TJ, Holland AE. The six-minute walk test: a useful metric for the cardiopulmonary patient. Intern Med J. 2009;39:495–501. doi: 10.1111/j.1445-5994.2008.01880.x. [DOI] [PubMed] [Google Scholar]
- 41.Wise RA, Brown CD. Minimal clinically important differences in the six-minute walk test and the incremental shuttle walking test. Copd. 2005;2:125–129. doi: 10.1081/copd-200050527. [DOI] [PubMed] [Google Scholar]
- 42.Ness KK, Armenian SH, Kadan-Lottick N, Gurney JG. Adverse effects of treatment in childhood acute lymphoblastic leukemia: general overview and implications for long-term cardiac health. Expert Rev Hematol. 2011;4:185–197. doi: 10.1586/ehm.11.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson SC, Baquis GD, Jackson A, Monteleone P, Kirkwood JR. Ventral polyradiculopathy with pediatric acute lymphocytic leukemia. Muscle Nerve. 2002;25:106–110. doi: 10.1002/mus.1219. [DOI] [PubMed] [Google Scholar]
- 44.Koh S, Nelson MD, Jr, Kovanlikaya A, Chen LS. Anterior lumbosacral radiculopathy after intrathecal methotrexate treatment. Pediatr Neurol. 1999;21:576–578. doi: 10.1016/s0887-8994(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 45.Wolfson L, Whipple R, Derby CA, Amerman P, Nashner L. Gender differences in the balance of healthy elderly as demonstrated by dynamic posturography. J Gerontol. 1994;49:M160–M167. doi: 10.1093/geronj/49.4.m160. [DOI] [PubMed] [Google Scholar]
- 46.Vereeck L, Wuyts F, Truijen S, Van de Heyning P. Clinical assessment of balance: normative data, and gender and age effects. Int J Audiol. 2008;47:67–75. doi: 10.1080/14992020701689688. [DOI] [PubMed] [Google Scholar]