Abstract
Objectives
FRNK, the C-terminal domain of focal adhesion kinase (FAK), is a tyrosine-phosphorylated, vascular smooth muscle cell (VSMC)-specific inhibitor of cell migration. FRNK inhibits both FAK and PYK2 in cultured VSMCs, and both kinases may be involved in VSMC invasion during vascular remodeling.
Methods and Results
Adenoviral-mediated gene transfer of GFP-tagged, wildtype (wt) FRNK into balloon-injured rat carotid arteries confirmed that FRNK overexpression inhibited both FAK and PYK2 phosphorylation and downstream signaling in vivo. To identify which kinase was involved in regulating VSMC invasion, adenoviral-mediated expression of specific shRNAs were used to “knock down” FAK vs. PYK2 in cultured VSMCs, but only FAK shRNA was effective in reducing VSMC invasion. The role of FRNK tyrosine phosphorylation was then examined using adenoviruses expressing nonphosphorylatable (Y168F-, Y232F-, and Y168,232F-) GFP-FRNK mutants. wtFRNK and all FRNK mutants localized to FAs, but only Y168 phosphorylation was required for FRNK to inhibit invasion. Preventing Y168 phosphorylation also increased FRNK-paxillin interaction, as determined by co-immunoprecipitation, total internal reflection fluorescence (TIRF)-microscopy, and fluorescence recovery after photobleaching (FRAP). Furthermore, wtFRNK competed with FAK for binding to p130Cas (a critically important regulator of cell migration), and prevented its phosphorylation. However, Y168F-FRNK was unable to bind p130Cas.
Conclusion
We propose a 3-stage mechanism for FRNK inhibition – FA targeting, Y168 phosphorylation, and competition with FAK for p130Cas binding and phosphorylation, which are all required for FRNK to inhibit VSMC invasion.
Keywords: focal adhesion kinase, PYK2, paxillin, vascular remodeling
INTRODUCTION
Vascular remodeling requires a complex interaction between growth factor receptors, extracellular matrix components, and integrins. Key proteins are involved in integrating extracellular signals and promoting the intracellular signal transduction required for vascular remodeling. One of these proteins is focal adhesion kinase (FAK), which is activated by growth factor receptors and integrin clustering, and which is critical for the assembly of a signaling complex within focal adhesions (FAs) that is required for cell migration and other aspects of the remodeling process.1
In addition to FAK, FAK-Related Non-Kinase (FRNK) is also a product of the PTK2 gene, but is autonomously expressed under control of an alternative, intronic promoter.2 FRNK is comprised of the noncatalytic, C-terminal region of FAK containing the focal adhesion targeting (FAT) sequence, and proline-rich domains important for adaptor protein binding. FRNK is selectively expressed in VSMCs, with very high levels found in large arterioles, and after arterial injury.3
Our laboratory has demonstrated that FRNK inhibits both FAK- and PYK2-dependent signaling in cultured VSMCs.4 We also showed that FRNK undergoes tyrosine phosphorylation at Y168 and Y232 after carotid artery injury in vivo, and in response to angiotensin II (AngII) in vitro.5,6 These phosphorylation sites are equivalent to the Y861 and Y925 phosphorylation sites within the C-terminal region of FAK.
The mechanisms responsible for FRNK inhibition of FAK and PYK2 signaling in VSMC invasion are uncertain. FAK localization to FAs is required for its activation, and its displacement from these sites results in decreased FAK activation.7 Since FRNK contains the identical FAT domain as FAK, we and others have proposed that FRNK inhibits FAK-dependent signaling by competitively displacing FAK from FAs.8,9 However, another possibility is that FRNK inhibits FAK signaling by acting as a sink for FAK binding proteins.10 One candidate binding partner is p130Cas, which is a critical regulator of cell migration.11 p130Cas binds to the first of two proline-rich domains (residues 711–717; APPKPSR) in the C-terminal region of FAK,12,13 but its binding appears to be regulated by FAK phosphorylation at Y861.14 Our evidence that FRNK can undergo tyrosine phosphorylation independently of FAK suggests that other factors in addition to FRNK targeting are important for its inhibitory function. These factors may also be responsible for the phenotypic differences observed between FAK-null and FRNK-overexpressing cells.9,15–17
Our recent observations indicate that FRNK inhibition of FAK autophosphorylation at Y397 is not related to FRNK’s ability to inhibit cell migration, so long as FA targeting is preserved. For instance, FRNK mutated at Y168 (Y168F-FRNK) retains its ability to target to focal adhesions and to inhibit FAK autophosphorylation, but fails to inhibit VSMC spreading and migration.5 One possibility is that FRNK’s inhibitory effect depends on its ability to inhibit the autophosphorylation of PYK2, rather than FAK. PYK2 is the other member of the FAK-family of nonreceptor protein tyrosine kinases. PYK2 is highly expressed in VSMCs, and shares both overlapping as well as distinct functions in integrin-dependent signaling. We now demonstrate that in addition to FAK, PYK2 is also up-regulated in balloon-injured rat carotid arteries, and overexpression of FRNK by adenoviral-mediated gene transfer immediately after injury reduces FAK, PYK2 and paxillin phosphorylation in vivo. To determine which kinase is involved in regulating cell invasion, shRNAs specific for each kinase were then used to “knock down” FAK vs. PYK2 in cultured VSMCs. We also examined the role of FRNK tyrosine phosphorylation on paxillin and p130Cas binding. Data are presented to indicate that competition for p130Cas binding to FAK, and subsequent inhibition of p130Cas phosphorylation mediates the inhibitory effect of FRNK on cell invasion.
METHODS
Experimental animals
Loyola University Medical Center’s Institutional Animal Care and Use Committee approved all procedures involving animals, which were handled in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. Please see the Online Supplemental Data Section (available at http://atvb.ahajournals.org) for a detailed description of the materials and methods used for these studies.
RESULTS
FRNK overexpression in balloon-injured rat carotid artery reduces FAK and PYK2 autophosphorylation and downstream signaling
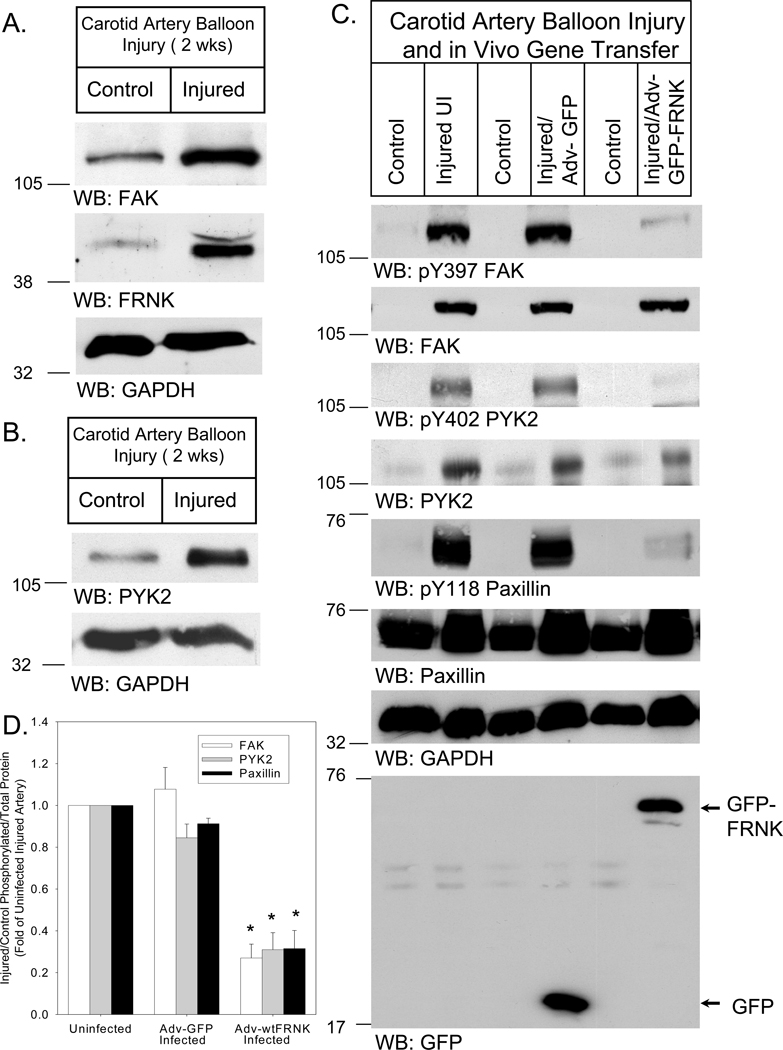
In initial experiments, we made use of a carotid artery balloon injury model5 to assess endogenous FAK, FRNK and PYK2 expression, and to determine if adenovirally-mediated gene transfer of GFP-wtFRNK inhibited FAK and PYK2 activation and downstream signaling in vivo. Western blot analysis demonstrated that, in addition to FAK and endogenous FRNK, PYK2 was markedly increased in rat carotid arteries 2wks after balloon injury (FAK=3.5±0.9-fold; FRNK=8.9±2.8-fold; PYK2=6.0±2.2-fold; n=3 animals; Figure 1). Increased FAK and PYK2 expression was also noted 1wk after injury, which was accompanied by increased FAK-Y397 and PYK2-Y402 phosphorylation, along with increased paxillin expression and Y118 phosphorylation. Gene transfer of a control Adv (Adv-GFP) immediately after balloon injury had no effect on FAK, PYK2 or paxillin phosphorylation or expression levels relative to injured, uninfected arteries 1wk later. However, Adv-GFP-wtFRNK gene transfer substantially reduced FAK, PYK2 and paxillin phosphorylation (n=4 animals), indicating that FRNK overexpression early in the course of balloon injury can inhibit both FAK and PYK2 activation and downstream signaling in vivo.
Figure 1. FRNK overexpression in balloon-injured rat carotid artery reduces FAK and PYK2 autophosphorylation and downstream signaling.
Representative Western blots of FAK (A) FRNK (A) and PYK2 (B) expression in balloon-injured and contralateral control carotid arteries 2wks after arterial injury. Quantitative analysis of expression levels is indicated in the text for n=3 animals. (C) Repesentative Western blots of FAK, PYK2 and paxillin phosphorylation and expression in contralateral (Control) and 1wk-injured, uninfected (UI) arteries, and in injured arteries subjected to Adv gene transfer with ~1010 pfu of Adv-GFP or Adv-GFP-wtFRNK. (D) Quantitative analysis of FAK-Y397, PYK2-Y402, and paxillin-Y118 phosphorylation in injured-uninfected, injured-Adv-GFP-infected, and injured-Adv-GFP-wtFRNK-infected arteries (relative to uninjured-uninfected control arteries) from n=4 rats. *P<0.05 vs. Uninfected-injured.
FRNK inhibits VSMC FAK and PYK2 phosphorylation and cell invasion
Confirming our previous studies,4,5 FRNK overexpression in cultured VSMCs also inhibited basal and AngII-induced FAK-Y397 autophosphorylation, and FAK phosphorylation at Y861 and Y925 (Supplemental Figure I). FRNK was also basally phosphorylated at Y168 and Y232 (the equivalent C-terminal phosphorylation sites on FAK), and FRNK phosphorylation at both sites increased further in response to AngII. However, FRNK’s inhibitory activity was not specific for FAK, as FRNK reduced basal and AngII-induced PYK2-Y402 phosphorylation. Finally, FRNK overexpression was associated with a marked inhibition of VSMC invasion, as assessed in a 3D-Boyden chamber assay.
FRNK-dependent inhibition of VSMC invasion is not dependent on PYK2
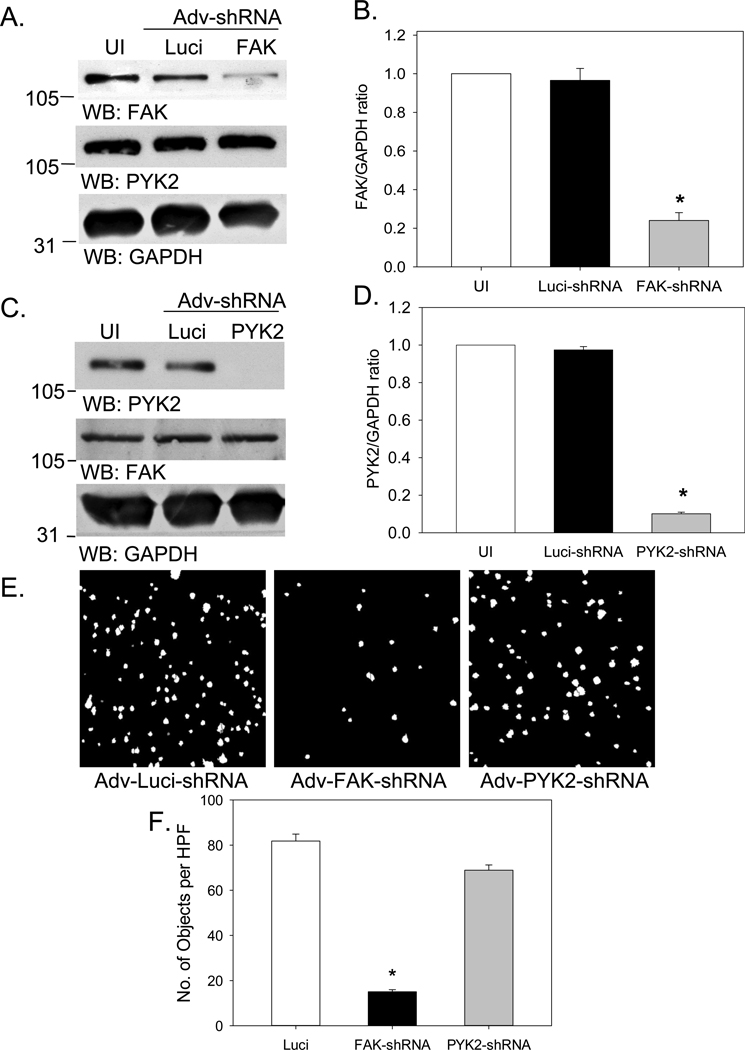
To ascertain whether FRNK’s inhibitory effect on cell invasion was dependent upon FAK or PYK2, we generated adenoviral (Adv) vectors that express shRNAs specific for each kinase. As seen in Figure 2, each pair of shRNA vectors successfully “knocked down” FAK or PYK2, without significantly affecting the expression of the other kinase. However, only FAK shRNAs reduced VSMC invasion in the 3D-Boyden chamber assay, indicating that FRNK-mediated inhibition of cell invasion was predominantly dependent on its inhibitory activity against FAK rather than PYK2.
Figure 2. FRNK inhibition of VSMC invasion is not dependent on PYK2.
RASMCs were maintained in serum-free medium (uninfected, or UI), or were infected with Adv-Luci-shRNA Adv-FAK-shRNAs, or Adv-PYK2-shRNAs (300moi, 96h). (A) For FAK-shRNAs, equal amounts of cell protein (50µg) were subjected to Western blotting, and blots were probed with total FAK, total PYK2 and GAPDH antibodies. (B) Quantitative analysis of total FAK relative to GAPDH. Data are means±SEM, n=4 experiments. (C) For PYK2-shRNAs, equal amounts of cell protein (50µg) were subjected to Western blotting, and blots were probed with total PYK2, total FAK, and GAPDH antibodies. (D) Quantitative analysis of total PYK2 relative to GAPDH. Data are means±SEM, n=4 experiments. The position of molecular weight markers is indicated to the left of each blot. (E–F) RASMCs were infected with Adv-Luci-shRNA, Adv-FAK-shRNAs or Adv-PYK2-shRNAs (300moi, 96h). Equal numbers of Adv-infected cells were suspended in serum-free medium, and placed in the upper chamber of Matrigel-coated Boyden chambers. AngII (100nM) was placed in the lower chamber. Cells were allowed to migrate for 2h, and cell invasion was quantified as in Figure 2. Data are means±SEM, n=4 experiments. *P<0.05 vs. UI or Luci-shRNA, where appropriate.
To further explore the role of FAK vs. PYK2 in VSMC invasion, we compared the invasive potential of VSMCs expressing GFP-wtFRNK with cells expressing GFP-wtCRNK, the C-terminal domain of PYK2. CRNK is a naturally occuring inhibitor of PYK2, which when overexpressed in HEK293 cells, blocked PYK2 but not FAK autophosphorylation.18 wtCRNK also inhibited basal, endothelin-1, and H2O2-induced PYK2 activation in neonatal rat cardiomyocytes.19 As seen in Supplemental Figure II, both wtFRNK and wtCRNK inhibited VSMC invasion in the 3D-Boyden chamber assay, but FRNK was significantly more effective, further indicating that FRNK’s inhibition of cell invasion was predominantly dependent on its inhibitory activity against FAK.
To explore the requirement for FAK phosphorylation on VSMC invasion, we made use of two different pharmacological agents that differentially inhibit FAK tyrosine phosphorylation.6,20 As seen in Supplemental Figure III, PF573,228, a FAK-specific kinase inhibitor20 reduced FAK autophosphorylation at Y397, and also indirectly prevented Src-dependent FAK phosphorylation at Y861 and Y925.6 In contrast, PP2, a Src-specific kinase inhibitor, reduced FAK phosphorylation at Y861 and Y925, but had no significant effect on basal or AngII-induced FAK autophosphorylation at Y397. However, both agents significantly inhibited VSMC invasion, indicating that FRNK’s ability to inhibit FAK autophosphorylation at Y397 was not the responsible mechanism.
FRNK tyrosine phosphorylation at Y168 is necessary for FRNK inhibition of VSMC invasion
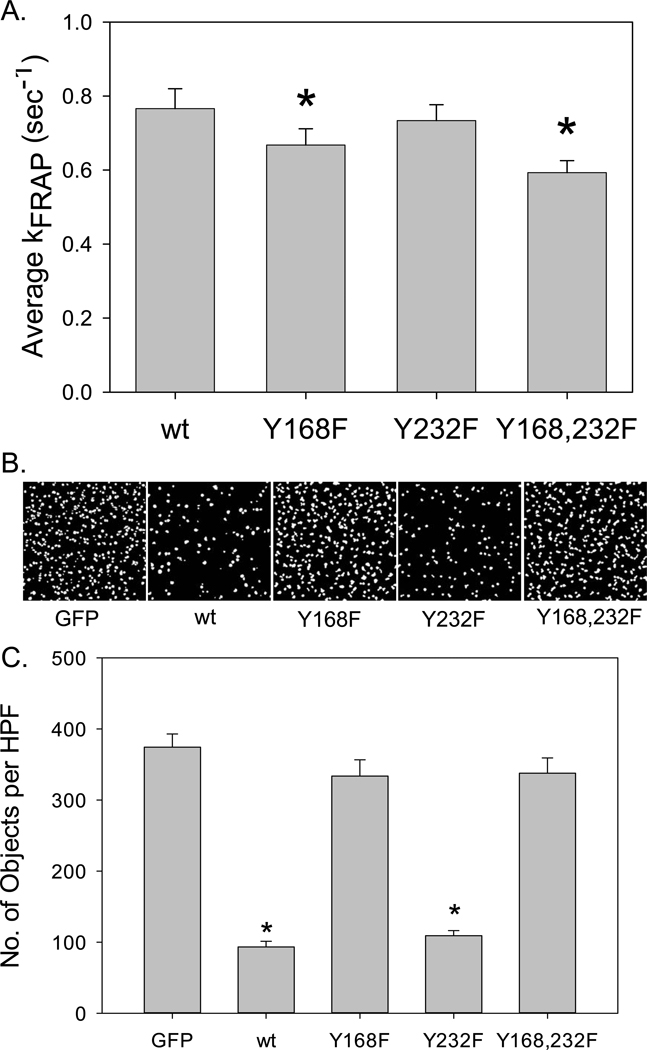
Since FRNK overexpression, PP2 and PF573,228 all blocked FAK tyrosine phosphorylation at Y861 and Y925, we next examined whether the same tyrosine phosphorylation sites on FRNK (i.e., Y168 and Y232) are required for FRNK inhibition of VSMC invasion. We generated replication-deficient Adv expressing GFP-tagged wtFRNK, Y168F-FRNK, Y232F-FRNK and Y168,232F-FRNK. Their effects on FAK phosphorylation, as well as FRNK targeting, binding kinetics, and inhibition of cell invasion were examined. As seen in Supplemental Figure IV, wtFRNK and the 3 FRNK phosphorylation mutants all displayed identical FA distribution patterns as observed by TIRF-microscopy of living cells. FA targeting of wtFRNK and the 3 FRNK mutants also reduced basal and AngII-induced FAK phosphorylation at Y397, Y861, and Y925, and markedly reduced downstream phosphorylation of paxillin and ERK1/2. Taken together, these results indicate that FRNK tyrosine phosphorylation is not required for its efficient FA targeting and inhibition of certain aspects of FAK-dependent signaling.
A critical requirement for FRNK-mediated inhibition of VSMC migration is its binding affinity to paxillin and other FA proteins.6 Therefore, we used TIRF-microscopy and FRAP analysis to examine the kinetics of wt and mutant FRNK binding to FAs. Surprisingly, we observed a small but statistically significant reduction in kFRAP for the Y168F and Y168,232F mutants (Figure 3 and Supplemental Figure V), indicating that their binding affinity was increased by rendering these sites nonphosphorylatable. These data were further analyzed by 2-way ANOVA, which indicated that mutation of the Y168 site was highly significant (P=0.007), whereas mutation of the Y232 site had no significant effect (P=0.224) on kFRAP. Also, there was no statistically significant interaction between mutation of the Y168 and Y232 sites (P=0.631). That is, the effect of the Y168 mutation was unaffected by the mutational status of Y232. Finally, the significant reduction in kFRAP was associated with a substantial loss of the inhibitory effect of the Y168F and Y168,232F mutants on VSMC invasion.
Figure 3. FRNK tyrosine phosphorylation at Y168 is critical for FRNK inhibition of VSMC invasion.
RASMCs were infected (100moi, 24h) with Adv expressing GFP-tagged, wt, Y168F-, Y232F-, or Y168,232F-FRNK. (A) Average kFRAP values (sec−1) for peripheral focal adhesions of 20 cells from each of 4 different experiments (a total of 80 individual TIRF-FRAP recordings) were compared. Data are means±SEM; *P<0.05 vs. wtFRNK. (B–C) Equal numbers of Adv-infected cells were suspended in serum-free medium, and placed in the upper chamber of Matrigel-coated Boyden chambers. AngII (100nM) was placed in the lower chamber. Cells were allowed to migrate for 2h, and cell invasion was quantified as in Figure 2. (B) Representative object scoring maps from a single experiment. (C) Quantitative analysis of 20 object scoring maps per group from 3 separate experiments was compared. Data are means±SEM; *P<0.05 vs. GFP.
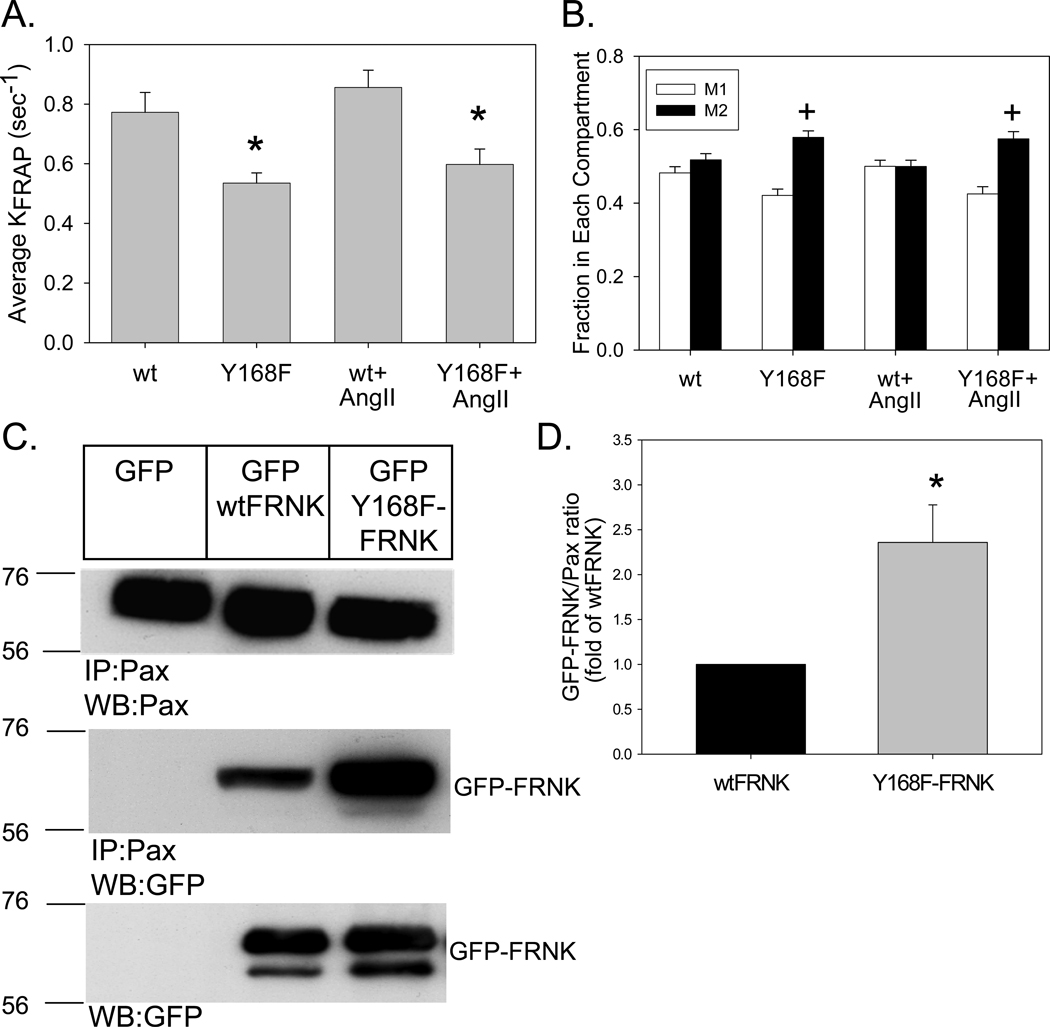
These initial studies were conducted using unstimulated cells which demonstrate somewhat lower levels of basal wtFRNK-Y168 and wtFRNK-Y232 phosphorylation (Supplemental Figure I). Since the invasion assays were conducted in the presence of AngII, we repeated the TIRF-FRAP analysis in unstimulated cells expressing wtFRNK or Y168F-FRNK, and in paired cells that were stimulated with AngII. As seen in Figure 4, AngII stimulation had no significant effect on the observed kFRAP for either wtFRNK or Y168F-FRNK (P=0.177; 2-way ANOVA). Only the presence of the Y168F mutation was significant (P<0.001) and there was no statistically significant interaction between Y168F and AngII (P=0.848).
Figure 4. Mutation of Y168 enhanced FA targeting and paxillin interaction.
RASMCs were infected (100moi, 24h) with Adv expressing GFP-tagged, wt or Y168F-FRNK. Paired cultures were stimulated with AngII (100nM, 15–45min). (A) Average kFRAP values (sec−1) for the 80 individual TIRF-FRAP recordings were compared. (B) 2-compartment analysis of wtFRNK and Y168F-FRNK binding kinetics. M1 and M2 represent the fraction of total fluorescence in the “fast” and “slow” compartments, respectively. (C–D) RASMCs were infected (100moi, 24h) with Adv-GFP, Adv-GFP-wtFRNK or Adv-GFP-Y168F-FRNK. (C) Cell extracts (500µg total protein) were co-immunoprecipitated with anti-paxillin mAb, and immunoblots were probed with anti-GFP mAb (to detect GFP-wtFRNK and GFP-Y168F-FRNK). The position of molecular weight markers is indicated to the left of each blot. (D) Quantitative analysis of GFP-wtFRNK and GFP-Y168F-FRNK co-immunoprecipitated with paxillin. Data are means±SEM; n=4 experiments. *P<0.05 vs. wtFRNK.
The 2-compartment analysis of wtFRNK and Y168F-FRNK binding kinetics also revealed that there was a significant increase in the percentage of Y168F-FRNK in the M2 or “slow” compartment, which was unaffected by AngII stimulation (Figure 4). These kinetic data were confirmed by co-immunoprecipitation analysis of paxillin, which revealed a substantial increase in the amount of Y168F-FRNK as compared to wtFRNK that was bound to paxillin under basal conditions. AngII stimulation had no further effect on steady-state paxillin binding to either wtFRNK or Y168F-FRNK (data not shown).
Tyrosine phosphorylation at Y168 is critical for the binding of FRNK to p130Cas
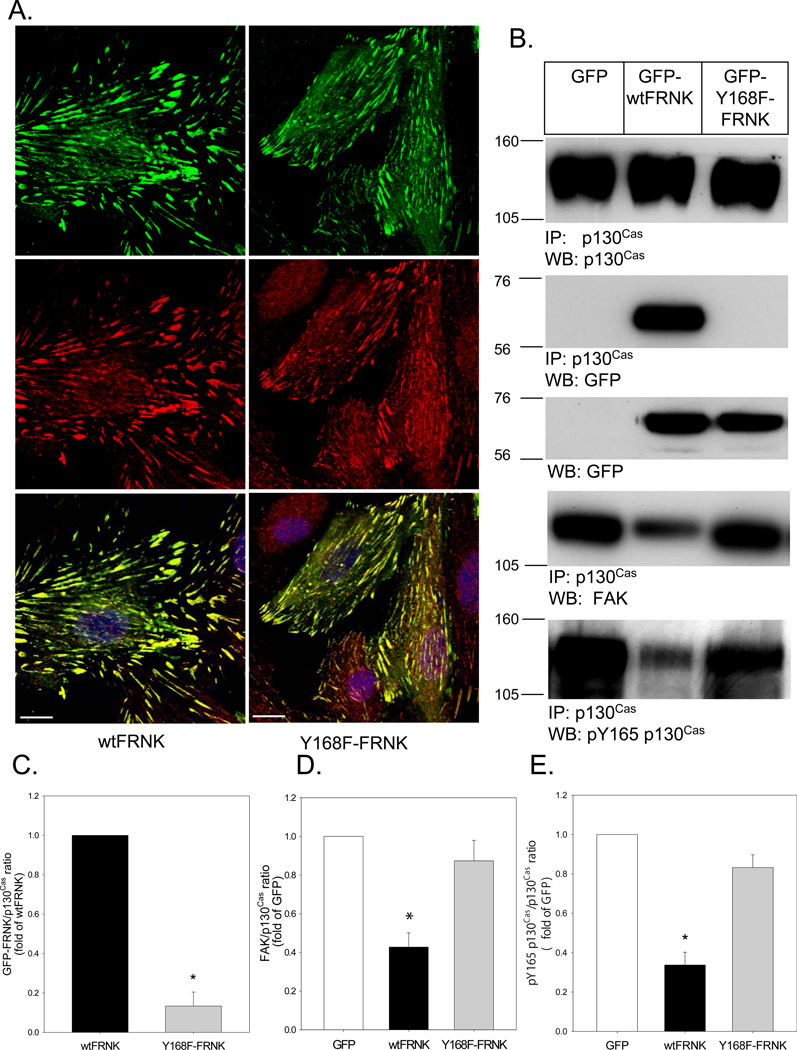
Like paxillin, p130Cas is a focal adhesion adaptor protein that binds to the first of two proline-rich regions in the C-terminal domain of FAK. Since FRNK contains the same proline-rich regions, we examined whether FRNK and p130Cas co-localize to VSMC focal adhesions. As seen in Figure 5A, GFP-tagged wtFRNK (green) was readily detected by confocal microscopy within linear structures at the cell-substratum interface of fully spread VSMCs. The identical structures also contained p130Cas (red). Co-localization was confirmed in the merged images (yellow). Interestingly, a similar co-localization pattern was observed for GFP-tagged Y168F-FRNK.
Figure 5. Tyrosine phosphorylation at Y168 is critical for the binding of FRNK to p130Cas.
(A) RASMCs were infected (100moi, 24h) with Adv-GFP-wtFRNK, or Adv-GFP-Y168F-FRNK (green). Cells were then fixed, permeabilized, counterstained with anti-p130Cas mAb (red), mounted with DAPI-containing mounting medium to detect cell nuclei (blue), and viewed by confocal microscopy. Co-localization of GFP fluorescence and p130Cas in these 1µm optical sections at the cell-substratum interface was represented in the merged image (yellow). The bar indicates 15µm. (B) RASMCs were infected (100moi, 24h) with Adv-GFP, Adv-GFP-wtFRNK or Adv-GFP-Y168F-FRNK. Cell extracts (1000µg total protein) were co-immunoprecipitated with anti-p130Cas mAb, and immunoblots were probed with anti-p130Cas, anti-GFP mAb (to detect GFP-wtFRNK and GFP-Y168F-FRNK), anti-N-terminal FAK mAb and pY165p130Cas. The position of molecular weight markers is indicated to the left of each blot. (C) Quantitative analysis of GFP-wtFRNK and GFP-Y168F-FRNK co-immunoprecipitated with p130Cas. (D) Quantitative analysis of FAK co-immunoprecipitated with p130Cas in the same pull-downs. (E) Quantitative analysis of pY165-p130Cas in the same pull-downs. Data are means±SEM; n=4 experiments. *P<0.05 vs. GFP control; +P<0.05 for wtFRNK vs. Y168-FRNK.
A substantial fraction of the p130Cas also co-localized with both wtFRNK and Y168F-FRNK at the leading edge of VSMCs during focal adhesion formation. As seen in Supplemental Figure VI-A, GFP-tagged wtFRNK and Y168F-FRNK were readily identified in linear structures at the cell-substratum interface of spreading VSMCs. Although most of the p130Cas was centrally located around the nucleus of spreading cells, it was also readily identified in peripheral focal adhesions, where it was found to co-localize with both wtFRNK and Y168F-FRNK. A similar distribution pattern was observed for paxillin, and wt and mutant FRNK (Supplemental Figure VI-B).
Interaction of p130Cas with FAK and FRNK
Co-immunoprecipitation was then used to examine the steady-state interaction of p130Cas with wtFRNK and Y168F-FRNK. As seen in a representative experiment (Figure 5B), equal amounts of endogenous p130Cas were immunoprecipitated in cells infected (100moi, 24h) with Adv-GFP, Adv-GFP-wtFRNK, or Adv-GFP-Y168F-FRNK. As predicted from its known structure, there was considerable steady-state interaction between p130Cas and wtFRNK. Surprisingly, this interaction was significantly reduced in cells expressing Y168F-FRNK. A quantitative analysis of wtFRNK vs. Y168F-FRNK binding in n=4 experiments is depicted in Figure 5C. Of note, direct analysis by Western blotting of cell extracts prior to co-immunoprecipitation revealed equal expression levels of GFP-tagged wtFRNK and Y168F-FRNK, indicating that the dramatic difference in p130Cas binding was not due to differences in the expression or stability of wt vs. mutant FRNK.
The same co-immunoprecipitates were then examined for the presence of endogenous FAK, which revealed evidence of competition for p130Cas binding between wtFRNK and FAK. A quantitative analysis of FAK/p130Cas binding from n=4 experiments is depicted in Figure 5D. As is evident from the figure, there was a significant reduction in p130Cas-FAK steady-state interaction in cells expressing wtFRNK. In contrast, there was no significant difference in FAK binding to p130Cas in cells expressing Y168F-FRNK.
Once localized, FAK phosphorylates itself at a single tyrosine residue (Y397), which serves as a high-affinity binding site (pYAEI motif) for the SH2 domain of Src-family protein tyrosine kinases.21 Src also binds via its SH3 domain to the proline-rich region (RPLPSPP) of p130Cas,22 and Src then phosphorylates p130Cas at multiple sites within its substrate domain. Since p130Cas tyrosine phosphorylation is critical for downstream signaling required for cell migration,11 we examined whether the interaction of wtFRNK with p130Cas affected p130Cas phosphorylation at Y165. As seen in Figures 5B and 5E, the immunoprecipitated p130Cas was highly phosphorylated in control cells expressing GFP. wtFRNK expression markedly reduced p130Cas phosphorylation. However, p130Cas phosphorylation in cells expressing Y168F-FRNK was similar to control cells. Of note, the phosphorylation of p130Cas at Y165 was Src-dependent, as PP2 markedly suppressed basal- and AngII-stimulated phosphorylation at this site (Supplemental Figure VII-A). We interpret these results to indicate that the residual p130Cas binding to FAK within focal adhesions of cells expressing Y168F-FRNK was sufficient to maintain near normal levels of p130Cas phosphorylation by Src that is required for the initiation of cell migration.
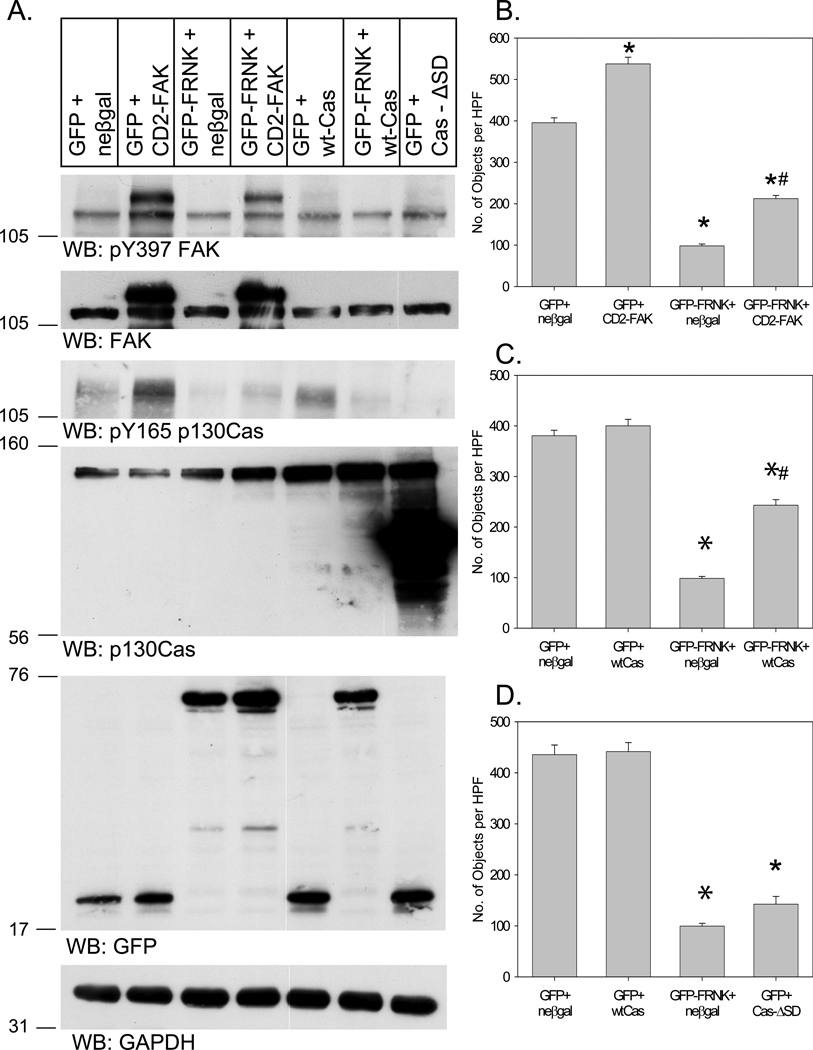
To further examine the relationship between FAK, FRNK and p130Cas, we attempted to “rescue” wtFRNK-induced inhibition of VSMC invasion by overexpressing a “constitutively active” mutant of FAK, known as CD2-FAK.23 Fusion of CD2 to the FAK N-terminus caused hyperphosphorylation of its Y397 site (Figure 6A), perhaps by membrane anchoring and unfolding its N-terminal autoinhibitory domain. As depicted in Figure 6B, this construct increased VSMC invasion in cells expressing GFP, and also partially rescued the FRNK-mediated inhibition of cell invasion. Similarly, wt-p130Cas overexpression also partially reversed the inhibitory effect of GFP-wtFRNK, but had no effect on VSMCs expressing GFP (Figure 6C). Finally, overexpression of a nonphosphorylatable mutant of p130Cas (i.e., Cas-ΔSD)24 reduced endogenous p130Cas phosphorylation (Figure 6A), and mimicked the inhibitory phenotype of wtFRNK overexpression (Figure 6D).
Figure 6. Rescue of wtFRNK inhibition of VSMC invasion by CD2-FAK and p130Cas.
RASMCs were infected (100moi, 24h) with either Adv-GFP or Adv-GFP-wtFRNK. To “rescue” the phenotype, cells were then infected with either Adv-CD2-FAK or Adv-wtCas (100moi, 24h). Adv-neßgal (100moi, 24h) was used to control for the nonspecific effects of Adv infection. To further analyze the importance of p130Cas phosphorylation, a nonphosphorylatable Cas mutant (Cas-ΔSD; 100moi, 24h) was also used. (A) Representative Western blots; (B–D) Quantitative analysis of cell invasion from n=4 experiments. *P<0.05 vs. GFP+neßgal; #P<0.05 vs. wtFRNK+neßgal.
DISCUSSION
FRNK is a naturally occurring, smooth-muscle specific protein that is markedly up-regulated in the vessel wall following vascular injury.3,5,16,25 In cultured VSMCs, FRNK overexpression has a number of effects, including inhibition of new protein synthesis,4 inhibition of cell proliferation and migration,5,6,16,26,27 and induction of serum- and TGF-β–stimulated smooth muscle marker gene expression.25 As FRNK can inhibit both FAK and PYK2, it is conceivable that some of FRNK’s inhibitory activity is mediated by inhibition of PYK2 rather than FAK. Indeed, both proteins have distinct as well as overlapping functions in cell signaling, including similar FAT sequences and proline-rich domains that are involved in protein-protein interactions. As we demonstrate in this report, the anti-invasive effect of FRNK is primarily due to its inhibitory effect on FAK, as FRNK overexpression, FAK knockdown, and PF-573228, a highly specific FAK kinase inhibitor, all showed similar results in our 3D-Boyden chamber invasion assay. Nevertheless, it is conceivable that other FRNK effects, including its regulation of both the ERK1/2 and the phosphatidylinositol 3-kinase/Akt pathways that are involved in AngII-induced VSMC protein synthesis, could be mediated by its inhibition of PYK2.28
Although FRNK is a potent inhibitor of VSMC migration in 2D and 3D culture, the mechanisms responsible for FRNK inhibition appear much more complicated that previously proposed. FRNK targeting to FAs is consistently required for FRNK inhibition of FAK-dependent signaling, including its effect on VSMC invasion.6 However, our data indicate that other factors, including FRNK tyrosine phosphorylation, are also necessary. In fact, rendering FRNK nonphosphorylatable at Y168 resulted in enhanced, rather than reduced FA targeting, as evident by a reduction in the dynamic exchange of FRNK between the cytoplasm and binding partners within VSMC FAs. Reduced exchange resulted in an increase in the steady-state interaction of FRNK with paxillin. Surprisingly however, enhanced FA targeting did not correlate with enhanced inhibitory activity with respect to cell invasion. Rather, the Y168F mutation abolished FRNK’s ability to inhibit cell invasion, yet the mutated FRNK still retained its ability to inhibit FAK tyrosine phosphorylation at multiple sites, paxillin phosphorylation, and downstream signaling to the ERK1/2 cascade.
Our current observations now explain the significance of FRNK Y168 phosphorylation, and reveal its importance in regulating the competition of FRNK and FAK for p130Cas binding. Unlike paxillin which binds to the FAT domain of FAK and FRNK and regulates targeting,29,30 p130Cas binds via its SH3 domain to the first of two proline-rich regions (APPKPSR) in both FAK and FRNK.12 SH3-domain-mediated binding of p130Cas to FAK is critically important in promoting cell migration through the coordinated activation of Rac at membrane extensions.31 Importantly, Lim et al.14 showed that p130Cas binding to FAK was influenced by the phosphorylation state of FAK at Y861. We now clearly demonstrate that p130Cas binding to FRNK is highly dependent on the phosphorylation state of FRNK at Y168. When overexpressed, FRNK successfully competed for p130Cas binding as an essential mechanism for its inhibitory activity. However, FRNK overexpression also prevented the downstream phosphorylation of p130Cas, which is necessary for the initiation of cell migration.11 Therefore, we propose a 3-stage mechanism for FRNK inhibition – FA targeting, Y168 phosphorylation, and competition with FAK for p130Cas binding and phosphorylation, which are all required for FRNK to inhibit VSMC invasion. In this scenario, FRNK undergoes phosphorylation at Y168, targets to FAs, and competes with the FAK/Src complex for p130Cas. Because FRNK has no kinase activity or SH2 binding site for Src, it cannot phosphorylate p130Cas and initiate migration. This scenario is schematically depicted in Supplemental Figure VII-B.
Nevertheless, the relationship between FRNK-Y168/FAK-Y861 phosphorylation and p130Cas binding remains unknown. X-ray crystallographic analysis of the FAT domain has revealed a highly compact, 4 α-helix bundle that interacts via 2 hydophobic patches with the LD2 and LD4 domains of paxillin.32 However, Campbell and colleagues33,34 have suggested by solution-phase NMR and molecular dynamics simulations that conformational flexibility within the FAT domain promotes an open conformation of Helix-1. This unfolding of the hinge region of the FAT domain would disrupt paxillin binding, and facilitate the Src-dependent phosphorylation of FAK at Y925, thereby creating a recognition site for binding of the adaptor protein Grb2. Our recent data6 support this model, as reducing the affinity of FRNK for paxillin (by introduction of a hydrophilic Ser in place of a hydrophobic Leu at position 341) increased the phosphorylation of FRNK at Y232, which is equivalent to the Y925 site on FAK. These results also suggest that a similar mechanism may be operative in regulating p130Cas binding to FRNK and FAK. Src-dependent phosphorylation at Y168/Y861 may induce a more open conformation in the intervening sequences between the kinase and FAT domains, exposing the proline-rich domain, and thereby allowing p130Cas interaction with both FRNK and FAK. Interestingly, we observed that rendering FRNK nonphosphorylatable at Y168 not only reduced p130Cas-FRNK interaction, but also increased FRNK’s interaction with paxillin. Thus, the Y168F mutation must also have favored the closed conformation of the FAT domain and reduced its dynamic exchange with paxillin in FAs.
There may be potential therapeutic value in pharmacologically manipulating FAK phosphorylation during vasculogenesis and neointima formation. However, the structural similarities between FAK and FRNK require caution in manipulating FAK-dependent signaling in VSMCs, as FAK and FRNK phosphorylation may occur by similar mechanisms, but result in opposing effects on cell spreading, migration and invasion.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Mr. Daniel Blackwell for assistance with TIRF-microscopy and FRAP analysis. These studies were supported in part by NIH 2PO1 HL062426, NIH 1F32 HL096143, and a grant from the Dr. Ralph and Marian Falk Medical Research Trust. Y.E.K. was also an American Heart Association Postdoctoral Fellow and S.J.E. was an American Heart Association Predoctoral Fellow during the time these studies were performed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: none
REFERENCES
- 1.Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Schaller MD, Borgman CA, Parsons JT. Autonomous expression of a noncatalytic domain of the focal adhesion- associated protein tyrosine kinase pp125FAK. Mol Cell Biol. 1993;13:785–791. doi: 10.1128/mcb.13.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayasaka H, Simon K, Hershey ED, Masumoto KH, Parsons JT. FRNK, the autonomously expressed C-terminal region of focal adhesion kinase, is uniquely regulated in vascular smooth muscle: analysis of expression in transgenic mice. J Cell Biochem. 2005;95:1248–1263. doi: 10.1002/jcb.20501. [DOI] [PubMed] [Google Scholar]
- 4.Govindarajan G, Eble DM, Lucchesi PA, Samarel AM. Focal adhesion kinase is involved in angiotensin II-mediated protein synthesis in cultured vascular smooth muscle cells. Circ Res. 2000;87:710–716. doi: 10.1161/01.res.87.8.710. [DOI] [PubMed] [Google Scholar]
- 5.Koshman YE, Engman SJ, Kim T, Iyengar R, Henderson KK, Samarel AM. Role of FRNK tyrosine phosphorylation in vascular smooth muscle spreading and migration. Cardiovasc Res. 2010;85:571–581. doi: 10.1093/cvr/cvp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koshman YE, Kim T, Chu M, Engman SJ, Iyengar R, Robia SL, Samarel AM. FRNK inhibition of focal adhesion kinase-dependent signaling and migration in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2010;30:2226–2233. doi: 10.1161/ATVBAHA.110.212761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen Y, Schaller MD. Focal adhesion targeting: the critical determinant of FAK regulation and substrate phosphorylation. Mol Biol Cell. 1999;10:2507–2518. doi: 10.1091/mbc.10.8.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson A, Parsons T. A mechanism for regulation of the adhesion-associated proteintyrosine kinase pp125FAK. Nature. 1996;380:538–540. doi: 10.1038/380538a0. [DOI] [PubMed] [Google Scholar]
- 9.Heidkamp MC, Bayer AL, Kalina JA, Eble DM, Samarel AM. GFP-FRNK disrupts focal adhesions and induces anoikis in neonatal rat ventricular myocytes. Circ Res. 2002;90:1282–1289. doi: 10.1161/01.res.0000023201.41774.ea. [DOI] [PubMed] [Google Scholar]
- 10.Neff L, Zeisel M, Druet V, Takeda K, Klein JP, Sibilia J, Wachsmann D. ERK 1/2- and JNKs-dependent synthesis of interleukins 6 and 8 by fibroblast-like synoviocytes stimulated with protein I/II, a modulin from oral streptococci, requires focal adhesion kinase. J Biol Chem. 2003;278:27721–27728. doi: 10.1074/jbc.M212065200. [DOI] [PubMed] [Google Scholar]
- 11.Meenderink LM, Ryzhova LM, Donato DM, Gochberg DF, Kaverina I, Hanks SK. P130Cas Src-binding and substrate domains have distinct roles in sustaining focal adhesion disassembly and promoting cell migration. PLoS One. 2010;5:e13412. doi: 10.1371/journal.pone.0013412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polte TR, Hanks SK. Interaction between focal adhesion kinase and Crk-associated tyrosine kinase substrate p130Cas. Proc Natl Acad Sci USA. 1995;92:10678–10682. doi: 10.1073/pnas.92.23.10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harte MT, Hildebrand JD, Burnham MR, Bouton AH, Parsons JT. p130Cas, a substrate associated with v-Src and v-Crk, localizes to focal adhesions and binds to focal adhesion kinase. J Biol Chem. 1996;271:13649–13655. doi: 10.1074/jbc.271.23.13649. [DOI] [PubMed] [Google Scholar]
- 14.Lim Y, Han I, Jeon J, Park H, Bahk YY, Oh ES. Phosphorylation of focal adhesion kinase at tyrosine 861 is crucial for Ras transformation of fibroblasts. J Biol Chem. 2004;279:29060–29065. doi: 10.1074/jbc.M401183200. [DOI] [PubMed] [Google Scholar]
- 15.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 16.Taylor JM, Mack CP, Nolan K, Regan CP, Owens GK, Parsons JT. Selective expression of an endogenous inhibitor of FAK regulates proliferation and migration of vascular smooth muscle cells. Mol Cell Biol. 2001;21:1565–1572. doi: 10.1128/MCB.21.5.1565-1572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu LH, Yang X, Bradham CA, Brenner DA, Baldwin AS, Jr, Craven RJ, Cance WG. The focal adhesion kinase suppresses transformation-associated, anchorage-independent apoptosis in human breast cancer cells. Involvement of death receptor-related signaling pathways. J Biol Chem. 2000;275:30597–30604. doi: 10.1074/jbc.M910027199. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Dy RC, Cance WG, Graves LM, Earp HS. Interactions between two cytoskeleton-associated tyrosine kinases: calcium-dependent tyrosine kinase and focal adhesion tyrosine kinase. J Biol Chem. 1999;274:8917–8924. doi: 10.1074/jbc.274.13.8917. [DOI] [PubMed] [Google Scholar]
- 19.Hart DL, Heidkamp MC, Iyengar R, Vijayan K, Szotek EL, Barakat JA, Leya M, Henze M, Scrogin K, Henderson KK, Samarel AM. CRNK gene transfer improves function and reverses the myosin heavy chain isoenzyme switch during post-myocardial infarction left ventricular remodeling. J Mol Cell Cardiol. 2008;45:93–105. doi: 10.1016/j.yjmcc.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slack-Davis JK, Martin KH, Tilghman RW, Iwanicki M, Ung EJ, Autry C, Luzzio MJ, Cooper B, Kath JC, Roberts WG, Parsons JT. Cellular characterization of a novel focal adhesion kinase inhibitor. J Biol Chem. 2007;282:14845–14852. doi: 10.1074/jbc.M606695200. [DOI] [PubMed] [Google Scholar]
- 21.Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamoto T, Sakai R, Ozawa K, Yazaki Y, Hirai H. Direct binding of C-terminal region of p130Cas to SH2 and SH3 domains of Src kinase. J Biol Chem. 1996;271:8959–8965. doi: 10.1074/jbc.271.15.8959. [DOI] [PubMed] [Google Scholar]
- 23.Chan PY, Kanner SB, Whitney G, Aruffo A. A transmembrane-anchored chimeric focal adhesion kinase is constitutively activated and phosphorylated at tyrosine residues identical to pp125FAK. J Biol Chem. 1994;269:20567–20574. [PubMed] [Google Scholar]
- 24.Kovacic-Milivojevic B, Roediger F, Almeida EA, Damsky CH, Gardner DG, Ilic D. Focal adhesion kinase and p130Cas mediate both sarcomeric organization and activation of genes associated with cardiac myocyte hypertrophy. Mol Biol Cell. 2001;12:2290–2307. doi: 10.1091/mbc.12.8.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sayers RL, Sundberg-Smith LJ, Rojas M, Hayasaka H, Parsons JT, Mack CP, Taylor JM. FRNK expression promotes smooth muscle cell maturation during vascular development and after vascular injury. Arterioscler Thromb Vasc Biol. 2008;28:2115–2122. doi: 10.1161/ATVBAHA.108.175455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hauck CR, Hsia DA, Schlaepfer DD. Focal adhesion kinase facilitates platelet-derived growth factor-BB-stimulated ERK2 activation required for chemotaxis migration of vascular smooth muscle cells. J Biol Chem. 2000;275:41092–41099. doi: 10.1074/jbc.M005450200. [DOI] [PubMed] [Google Scholar]
- 27.Brewster LP, Ucusian AA, Brey EM, Liwanag M, Samarel AM, Greisler HP. FRNK overexpression limits the depth and frequency of vascular smooth muscle cell invasion in a three-dimensional fibrin matrix. J Cell Physiol. 2010;225:562–568. doi: 10.1002/jcp.22239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rocic P, Lucchesi PA. Down-regulation by antisense oligonucleotides establishes a role for the proline-rich tyrosine kinase PYK2 in angiotensin ii-induced signaling in vascular smooth muscle. J Biol Chem. 2001;276:21902–21906. doi: 10.1074/jbc.M101684200. [DOI] [PubMed] [Google Scholar]
- 29.Hildebrand JD, Schaller MD, Parsons JT. Paxillin, a tyrosine phosphorylated focal adhesion-associated protein binds to the carboxyl terminal domain of focal adhesion kinase. Mol Biol Cell. 1995;6:637–647. doi: 10.1091/mbc.6.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheswohl DM, Harrell JR, Rajfur Z, Gao G, Campbell SL, Schaller MD. Multiple paxillin binding sites regulate FAK function. J Mol Signal. 2008;3:1. doi: 10.1186/1750-2187-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chodniewicz D, Klemke RL. Regulation of integrin-mediated cellular responses through assembly of a CAS/Crk scaffold. Biochim Biophys Acta. 2004;1692:63–76. doi: 10.1016/j.bbamcr.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi I, Vuori K, Liddington RC. The focal adhesion targeting (FAT) region of focal adhesion kinase is a four-helix bundle that binds paxillin. Nat Struct Biol. 2002;9:101–106. doi: 10.1038/nsb755. [DOI] [PubMed] [Google Scholar]
- 33.Dixon RD, Chen Y, Ding F, Khare SD, Prutzman KC, Schaller MD, Campbell SL, Dokholyan NV. New insights into FAK signaling and localization based on detection of a FAT domain folding intermediate. Structure. 2004;12:2161–2171. doi: 10.1016/j.str.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 34.Prutzman KC, Gao G, King ML, Iyer VV, Mueller GA, Schaller MD, Campbell SL. The focal adhesion targeting domain of focal adhesion kinase contains a hinge region that modulates tyrosine 926 phosphorylation. Structure. 2004;12:881–891. doi: 10.1016/j.str.2004.02.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.