Summary
All-trans-retinoic acid (atRA) serves essential functions during embryogenesis and throughout post-natal vertebrate life. Insufficient or excess atRA causes teratogenic and/or toxic effects in the developing embryo: interference with atRA biosynthesis or signaling likely underlies some forms of cancer. Many symptoms of vitamin A (atRA precursor) deficiency and/or toxicity overlap with those of another pleiotropic agent—ethanol. These overlapping symptoms have prompted research to understand whether interference with atRA biosynthesis and/or action may explain (in part) pathology associated with excess ethanol consumption. Ethanol affects many aspects of retinoid metabolism and mechanisms of action site-specifically, but no robust data support inhibition of vitamin A metabolism, resulting in decreased atRA in vivo during normal vitamin A nutriture. Actually, ethanol either has no effect on or increases atRA at select sites. Despite this realization, insight into whether interactions between ethanol and retinoids represent cause vs. effect requires additional research.
Keywords: retinol dehydrogenase, ethanol, fetal alcohol spectrum disorder, retinoic acid, vitamin A
INTRODUCTION
Two low molecular weight organic chemicals have enormous impact on human health: ethanol and retinol (vitamin A). All vertebrates require retinol, acting via its metabolite atRA, to reproduce, grow and remain healthy. Epithelial differentiation, nervous system development and function, immune system function, embryogenesis, and fertility require atRA within a fairly narrow concentration range at specific sites during restricted temporal windows (1–5). Failure to meet or regulate these demands for atRA results in birth defects, other forms of vitamin A toxicity, and increased cancer risk (6, 7). Similarly, excess ethanol consumption causes numerous pathologies, including cancer, fetal alcohol spectrum disorder (FASD), and abnormalities in many of the same processes regulated by atRA (8–10). Given the enveloping functions of atRA, and the pervasive pathologies and mechanisms of ethanol action, overlap of affected sites and processes seems inevitable (11).
Despite the unavoidable overlap of ethanol and retinoid effects, and the well-known ability of ethanol to interfere with the metabolism and function of multiple nutrients, a commonly held notion postulates that competitive inhibition of retinol’s conversion into atRA contributes to the pathology of ethanol (12–15). This hypothesis requires participation of alcohol dehydrogenase(s) (Adh) in retinol metabolism under physiological conditions, and decreased tissue atRA during normal vitamin A nutriture upon ethanol exposure. Neither seems to be the case. Rather, ethanol either has no effect on or increases tissue atRA, depending on the site (16, 17). Ethanol affects multiple enzymes, binding proteins, and receptors important to retinoid homeostasis and atRA biosynthesis and function (11, 18, 19). Thus, ethanol effects on atRA and retinoid function are difficult to predict by examining a single component of retinoid metabolism or signaling. The complexity of maintaining retinoid homeostasis, the interactions of various participants, and compensatory reactions, all impart tissue-specificity to ethanol’s effects on atRA concentrations. No determination has been made whether ethanol-induced changes in atRA contribute causally to ethanol pathology and/or FASD. A recent study of mouse limb development illustrates the complexity of the situation (20). An RAR pan-antagonist duplicated limb defects morphologically similar to those caused by ethanol, and atRA dosing prevented ethanol’s effects, but gene expression assessments revealed unexplained differences between ethanol and the RAR pan-antagonist.
RETINOID HOMEOSTASIS AND MECHANISMS OF ACTION
A brief review of retinoid biosynthesis and function will frame the context of ethanol actions (21). Liver stores the majority of retinol as retinyl esters (RE) and releases retinol bound with the serum retinol binding-protein (RBP) into circulation (Figure 1). RBP interacts with the plasma membrane receptor Stra6, which mediates transfer of retinol into cells (22). The cellular retinol binding-protein (Crbp1) and lecithin:retinol acyltransferase (LRAT) couple retinol uptake to RE formation. Accretion of RE in liver and other tissues promotes storage in lipid droplets and re-use of Crbp1. In contrast to the function of holo-Crpb1 (retinol bound to Crbp1) as chaperone for RE formation, apo-Crbp1 stimulates RE hydrolysis and inhibits LRAT. The ratio holo-Crbp1/apo-Crbp1 apparently signals retinol status and directs retinol flux into and out of storage as RE, while maintaining steady-state levels of holo-Crbp1 to support atRA biosynthesis.
Figure 1.
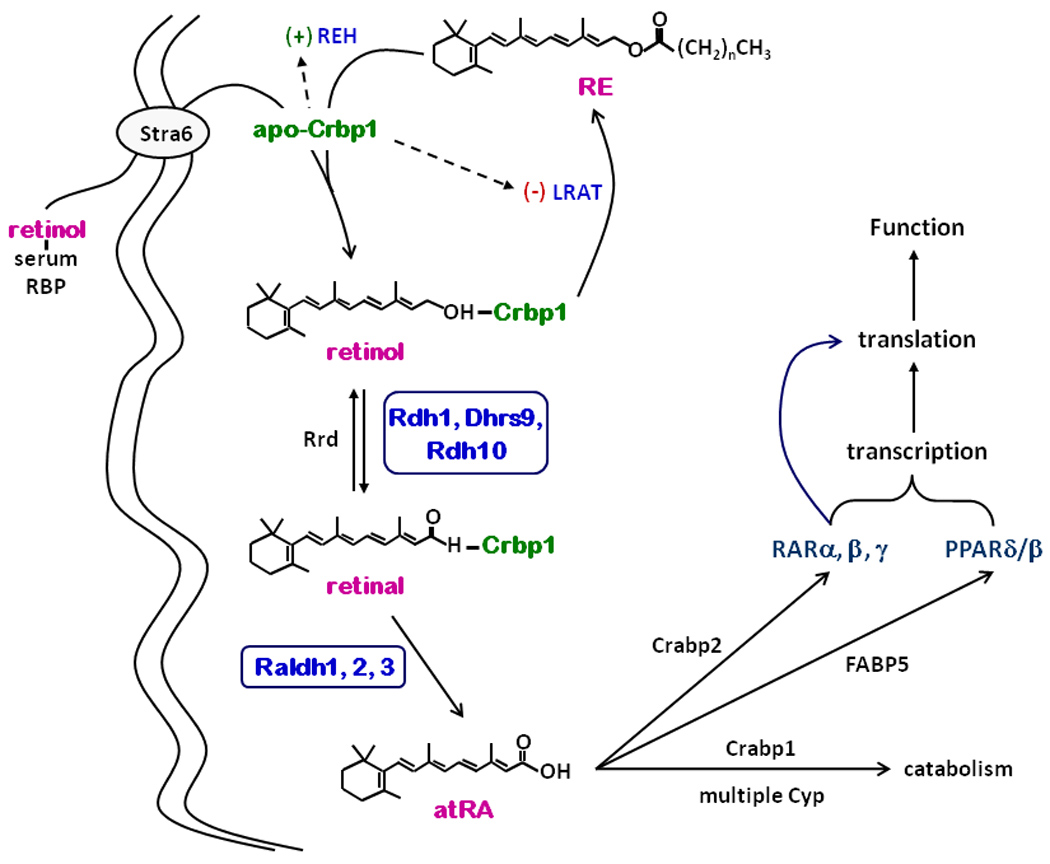
Retinoid homeostasis. Serum Rbp-transported retinol enters cells via Stra6-mediated uptake. Net uptake requires Crbp1 and/or LRAT. Incoming retinol undergoes LRAT-catalyzed esterification into RE. REH mobilizes RE to provide retinol for atRA biosynthesis. Three members of the short-chain dehydrogenase/reductase gene family catalyze the first and rate-limiting step of atRA biosynthesis: Rdh1, Dhrs9 and Rdh10. Retinal reductases, Rrd, reduce retinal into retinol. Three members of the Aldh gene family catalyze the second and irreversible step: Raldh1, 2, and 3 (aka Adlh1A1, 1A2, and 1A3). Three members of the FABP gene family determine intracellular disposition of atRA: Crabp1, Crabp2, and FABP5. atRA regulates translation and transcription via four nuclear receptors: RARα, β, and γ and PPARβ/δ. Genes regulated by atRA include Stra6, Crbp1, LRAT, Raldh, and Cyp.
The first of two dehydrogenations, conversion of retinol into retinal, limits the rate of atRA biosynthesis. Microsomes account for 80 to 94% of cellular retinal-generating capacity from holo-Crbp1, and microsomal rates exceed cytosolic rates by 5 to 20-fold (23). The microsomal retinol dehydrogenases (Rdh) that recognize retinol associated with Crbp1 have been identified as members of the short-chain dehydrogenase/reductase gene family. At least three Rdh seem physiologically important to atRA biosynthesis, based on biochemical, physiological and genetic evidence: Rdh1, Rdh10 and Dhrs9. Notably, ethanol does not function as a competitive inhibitor of Rdh, but rather stimulates activity in vitro. The next step involves a rapid (relative to retinol dehydrogenation) and irreversible dehydrogenation of retinal into atRA. At least three Raldh catalyze this step: Raldh1, 2, and 3 (Aldh1A1, 1A2 and 1A3).
Binding proteins of the fatty acid binding-protein gene family sequester atRA: Crabp1, Crabp2 and FABP5 (24). Crabp2 transfers atRA to RAR, whereas FABP5 transfers atRA to PPARδ/β, and Crabp1 (so far) only seems to mediate atRA catabolism (25, 26). The receptors regulate transcription and RARα, also regulates translation (27–29). Thus, selective atRA delivery, mediated by binding proteins, provides a mechanism to induce dissimilar atRA actions.
atRA regulates retinol and its own concentrations. Simultaneous induction of Stra6, Crbp1, and LRAT by atRA insures coupled retinol uptake and esterification with “excess” retinol directed into RE. atRA also induces Cyp to stimulate its catabolism (30). Finally, interactions between Rdh and Raldh provide another level of maintaining atRA concentrations. Despite Dhrs9 function as an Rdh, knocking down Dhrs9 in primary astrocytes increases atRA production by increasing Raldh1 mRNA expression and activity (5).
ETHANOL DISRUPTS RETINOID HOMEOSTASIS AND SIGNALLING
The effects of acute and chronic ethanol on vitamin A homeostasis have been documented since the early 1980’s and have been reviewed in detail (11, 14, 19, 31, 32). Ethanol affects multiple sites of retinoid metabolism and function outlined in Figure 1. Briefly, ethanol ingestion: 1) causes massive depletion of liver RE and alters the tissue distribution of retinol; 2) enhances liver-mediated retinol and atRA catabolism; 3) induces Crpb1 mRNA in the embryo and postnatal brain; 4) alters RAR mRNA expression in testis and brain (33, 34); 5) increases Crabp1 expression in the mouse embryo (35); 6) increases hepatic concentrations of the carotenoid cleavage enzyme, CMO1, which generates retinal (36). Generally, mechanisms of these effects have not been established, but likely reflect pleiotropic effects of ethanol on cellular processes, including gene regulation.
For the most part, distinct enzymes catalyze retinoid vs. ethanol metabolism, suggesting an indirect impact of ethanol on retinoid metabolism. Raldh1 (Aldh1a1) may represent an exception. Raldh1 (Aldh1A1) has a K0.5 value ~150 µM for acetaldehyde and 0.8 µM for the Crbp1-retinal complex, providing potential for interaction between the two substrates (37). Blood acetaldehyde levels in rat reach <40 µM after a 5 g/kg dose of ethanol, however, whereas tissue retinal concentrations vary between 0.05 and 0.2 µM (38). These values, along with the Raldh1 kinetic constants, suggest limited possibility of competitive interaction. Furthermore, Raldh1 does not catabolize the major portion of acetaldehyde. Nevertheless, Raldh1 polymorphisms have been implicated in alcoholism and alcohol sensitivity (39). Cyp2E1 may provide another exception. This enzyme has not been implicated in atRA metabolism under physiological conditions, but higher concentrations of chlormethiazole, an inhibitor of Cyp2E1 mRNA expression, modestly preserves RE levels in livers of rats fed ethanol for 1 month, likely through sparing retinol and atRA (40).
ETHANOL IMPACT ON RA
An impact of ethanol on the concentrations of atRA likely would have a large impact on processes governed by vitamin A. Yet, until recently no analytically robust test has reported an effect of ethanol on serum and tissue atRA concentrations during normal vitamin A nutriture. The recent application of LC/MS/MS assays has allowed direct quantification of ethanol’s effects on serum and tissue atRA (41). LC/MS data showed that acute dosing of ethanol (2 × 5 g/kg, 2 hr apart) to postnatal day 4 rat pups caused cerebellar atRA levels to increase ~5-fold 2 hr after the second dose (16). A second study quantified atRA in a variety of mouse tissues and brain areas after both acute and chronic ethanol exposure (17). Only the highest single ethanol dose (3.5 g/kg) affected atRA, and the affect was an increase in hippocampus, testis, and liver, and no change in serum or kidney. Chronic exposure (6.5% ethanol diet, one month) increased atRA in testis, serum, hippocampus and cortex, and had no impact on atRA in the other tissues and brain areas assayed (Table 1). Additionally, exposure of pregnant dams to ethanol starting on e13 increased atRA in proportion to dam BAC% in the hippocampus and cortex of e19 embryos (Table 2). These data illustrate several points: 1) short-term effects of ethanol on atRA do not predict longer term effects; 2) in no instance did ethanol decrease serum or tissue atRA; 3) effects were tissue specific; 4) serum atRA levels did not predict tissue atRA after chronic ethanol exposure; 5) ethanol ingestion by dams increases embryo atRA at select sites in the developing brain. The last point suggests a connection between FASD and the impact of ethanol on atRA, because even modest increases in atRA cause toxicity, especially in the embryo (42).
Table 1.
atRA concentrations (pmol/g) in mouse tissues after 1-month feeding a liquid diet ± ethanol.
| Diet | liver | serum | testis | kidney | Hp | Cx | Ob | Cb | St | Th |
|---|---|---|---|---|---|---|---|---|---|---|
| control | 5.6 ± 0.4 | 2.2 ± 0.4 | 8.3 ± 0.4 | 3 ± 0.3 | 23 ± 3.6 | 13. 9 ± 1.5 | 77 ± 21 | 55 ± 4 | 78 ± 33 | 81 ± 6 |
| ethanol | 6.2 ± 0.9 | 24 ± 2* | 14 ± 0.8* | 3.3 ± 0.2 | 511 ± 253* | 37.5 ± 10* | 79 ± 13 | 52 ± 7 | 90 ± 33 | 60 ± 10 |
Data are means ± SE. Mice were fed liquid diets. The “ethanol” diet contained 6.5% ethanol. atRA concentrations were quantified by LC/MS/MS.
Abbreviations: Hp, hippocampus; Cx, cortex; Ob, olfactory bulb; Cb, cerebellum; St, striatum; Th, Thalamus.
P values for serum, testis, hippocampus and cortex were <0.0001, <0.0002, ~0.02 and <0.05, respectively.
Table 2.
Effects of dam blood alcohol on mouse embryo atRA (pmol/g) concentrations.
| control | L1 | L2 | L3 | L4 | |
|---|---|---|---|---|---|
| BAC% | 0 | 0.01 | 0.025 | 0.1 | 0.13 |
| hippocampus | 20 ± 1 | 30 ± 2 | 47 ± 3 | 81± 4 | 422 ± 8 |
| cortex | 13 ± 3 | 29 ± 3 | 112 ± 3 | 210 ± 4 | 650 ± 11 |
Data are means ± SE. Five individual dams were exposed to a control liquid diet or a liquid diet with 6.5 % ethanol from e13 through e19.
MECHANISMS OF ETHANOL EFFECTS ON RA
The results of the two studies detailed above may seem enigmatic: increased atRA; tissue-specific impact of ethanol on atRA; differences in acute vs. chronic exposure. Considering these effects in context of retinoid homeostasis, as outlined in Figure 1, however, produces some insight.
The liver reacts to ethanol exposure by mobilizing RE, which increases hepatic retinol and provides additional substrate to drive atRA biosynthesis (Figure 2). With time, ethanol induces expression of two genes in liver that encode enzymes involved in atRA biosynthesis, Dhrs9 (~3-fold) and Raldh3 (~5-fold) (17). But chronic increases in atRA and ethanol induce atRA catabolism, such that the elimination t½ of atRA decreases nearly 9-fold. The result is a short-term increase in liver atRA, with atRA catabolism eventually offsetting the increase in retinol concentrations and Dhrs9 and Raldh3 induction. In contrast, the hippocampus reacts to both acute and chronic ethanol exposure with increases in atRA, driven in the short term by increased retinol and maintained in the longer term by increased expression of Stra6 and Crbp1, insuring increased retinol uptake. Also, Raldh1 activity increases, which contributes to increased atRA, even though the amount of Raldh1 protein decreases. Thus, understanding the impact of ethanol on atRA requires longer-term study, quantification of atRA, assessment of multiple contributors to atRA homeostasis, and assessments of activity (not just protein expression assays).
Figure 2.
Impact of acute and chronic ethanol exposure on atRA in liver and hippocampus.
ADH1 CONTRIBUTES TO RETINOL TOXICITY
Ethanol effects have been attributed to reduced tissue atRA via inhibition of Adh. This conclusion originated from reports that the same human liver and testis enzymes catalyze oxidation of ethanol and retinol in vitro, and low ethanol concentrations competitively inhibit retinol oxidation (43, 44). This conclusion has persisted as a result of indirect estimation of atRA in vivo, rather than quantification with validated analytical assays, and by assessing ethanol’s impact on the metabolism of pharmacological amounts of retinol dosed in a bolus (13, 15, 45, 46). Indirect estimation relies on a RARβ response element driving a Lacz reporter. This approach has multiple design and practical limitations, does not generate “real time” data, and has not been validated as an assay for atRA. Siegenthaler et al. compared reporter data to LC/MS generated data and concluded that the former was “not useful” to assess atRA in the developing cortex (47). Additionally, it is not clear how the reporter could distinguish interference with atRA biosynthesis from cumulative ethanol effects on the multiple processes required to produce a blue tint.
Participation of Adh in retinol metabolism under physiological conditions has not been verified and seems unlikely. Adh do not recognize the physiological form of retinol, i.e. holo-Crbp. Unbound retinol occurs in low nM concentrations in vivo, and is more likely to associate with membranes than occur solvated in the aqueous medium. Adh recognition of high concentrations of unbound retinol in vitro does not inform that Adh function with physiological concentrations of intracellular retinol. Consistent with this, studies of the various Adh-isozyme-null mice have not reported pathology, retinoid metabolism changes (including in atRA), or gene expression changes related to atRA deficiency. The mechanisms responsible for the limited phenotypes of Adh-isozyme-null mice remain unclear. In contrast, physiological participation of Rdh1 and Rdh10 in atRA biosynthesis has been verified by direct quantification of retinoids, pathology, gene expression changes, and/or mechanistic studies.
Decreased serum atRA after ethanol dosing or in Adh1-null mice has been observed after administration of toxic retinol doses (50 to 100 mg/kg) (46). A dose of 50 mg/kg delivers ~300-fold more than the recommended daily intake of retinol and drives serum atRA ~1600-fold higher than the steady-state value of ~2 pmol/ml in wild-type mice. Although this high serum atRA concentration decreases ~87% after ethanol dosing and in the Adh1-null mouse, the atRA concentrations in both remain ~200-fold higher than normal. These data indicate that Adh1 contributes to converting massive doses of retinol into toxic amounts of atRA, but do not address physiological function for Adh in atRA biosynthesis.
A recent study of ethanol’s effects on Xenopus embryogenesis showed that manipulating Adh did not affect expression of atRA-responsive genes, in contrast to manipulating Raldh2 (48). Although these data illustrate lack of participation of Adh in atRA generation, it is not certain how they relate to the complex process of FASD during mammalian embryogenesis. Conversion of retinal into atRA involves at least three Raldh, and Raldh2 has not been shown to metabolize acetaldehyde in vivo, or to react to ethanol dosing in mammals.
CONCLUDING REMARKS
Increasing insight into the physiological aspects of retinoid metabolism, along with analytically robust atRA assays, have improved ability to examine the impact of ethanol on atRA concentrations. Generating data that reflect realistically the vitamin A/ethanol interaction entails quantification of atRA in tissues, after chronic ethanol dosing to vertebrates, maintained via normal vitamin A nutriture. Understanding mechanism involves evaluating multiple enzymes, binding proteins, transport proteins and receptors. The complexity of retinoid homeostasis and compensatory reactions does not allow extrapolation from ethanol effects on a single gene or protein (especially in vitro or in non-vertebrate models) to tissue atRA status.
Abnormal concentrations of atRA could contribute to ethanol’s toxicity, including FASD. Modest excesses of atRA can produce teratogenic CNS effects and emerging insight indicates that atRA affects learning and cognitive abilities, in addition to nervous system development and neuron specification. To address this issue, future research must distinguish ethanol’s pathology caused by changes in atRA from the pathology of ethanol changing vitamin A homeostasis as a secondary effect. Cause and effect have not been established: the distinction has not been made between retinoid-dependent processes responding to vs. contributing to the pathology of ethanol.
ACKNOWLEDGMENT
The results discussed were supported in part by NIAAA grant AA017927. I thank Charles Krois for critically reading this manuscript.
Abbreviations
- Adh
alcohol dehydrogenase
- atRA
all-trans-retinoic acid
- Crabp
cellular RA binding protein
- Crbp
cellular retinol binding-protein
- FASD
fetal alcohol spectrum disorder
- LRAT
lecithin:retinol acyltransferase
- RAR
retinoic acid receptor(s)
- RBP
serum retinol binding protein
- Rdh
retinol dehydrogenase
- REH
retinyl ester hydrolase
REFERENCES
- 1.Mora JR, von Andrian UH. Role of retinoic acid in the imprinting of gut-homing IgA-secreting cells. Semin Immunol. 2009;21:28–35. doi: 10.1016/j.smim.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noy N. Between death and survival: retinoic acid in regulation of apoptosis. Annu Rev Nutr. 2010;30:201–217. doi: 10.1146/annurev.nutr.28.061807.155509. [DOI] [PubMed] [Google Scholar]
- 3.Hogarth CA, Griswold MD. The key role of vitamin A in spermatogenesis. J Clin Invest. 2010;120:956–962. doi: 10.1172/JCI41303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gudas LJ, Wagner JA. Retinoids regulate stem cell differentiation. J Cell Physiol. 2011;226:322–330. doi: 10.1002/jcp.22417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheng N, Xie Z, Wang C, Bai G, Zhang K, Zhu Q, Song J, Guillemot F, Chen YG, Lin A, Jing N. Retinoic acid regulates bone morphogenic protein signal duration by promoting the degradation of phosphorylated Smad1. Proc Natl Acad Sci U S A. 2010;107:18886–18891. doi: 10.1073/pnas.1009244107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fields AL, Soprano DR, Soprano KJ. Retinoids in biological control and cancer. J Cell Biochem. 2007;102:886–898. doi: 10.1002/jcb.21530. [DOI] [PubMed] [Google Scholar]
- 7.Lee GS, Liao X, Shimizu H, Collins MD. Genetic and pathologic aspects of retinoic acid-induced limb malformations in the mouse. Birth Defects Res A Clin Mol Teratol. 2010;88:863–882. doi: 10.1002/bdra.20712. [DOI] [PubMed] [Google Scholar]
- 8.Smith SM. Alcohol-induced cell death in the embryo. Alcohol Health Res World. 1997;21:287–297. [PMC free article] [PubMed] [Google Scholar]
- 9.Druesne-Pecollo N, Tehard B, Mallet Y, Gerber M, Norat T, Hercberg S, Latino-Martel P. Alcohol and genetic polymorphisms: effect on risk of alcohol-related cancer. Lancet Oncol. 2009;10:173–180. doi: 10.1016/S1470-2045(09)70019-1. [DOI] [PubMed] [Google Scholar]
- 10.Hellemans KG, Sliwowska JH, Verma P, Weinberg J. Prenatal alcohol exposure: fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci Biobehav Rev. 2010;34:791–807. doi: 10.1016/j.neubiorev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zachman RD, Grummer MA. The interaction of ethanol and vitamin A as a potential mechanism for the pathogenesis of Fetal Alcohol syndrome. Alcohol Clin Exp Res. 1998;22:1544–1556. [PubMed] [Google Scholar]
- 12.French SW. Nutrition in the pathogenesis of alcoholic liver disease. Alcohol Alcohol. 1993;28:97–109. [PubMed] [Google Scholar]
- 13.Deltour L, Ang HL, Duester G. Ethanol inhibition of retinoic acid synthesis as a potential mechanism for fetal alcohol syndrome. FASEB J. 1996;10:1050–1057. [PubMed] [Google Scholar]
- 14.Wang XD. Chronic alcohol intake interferes with retinoid metabolism and signaling. Nutr Rev. 1999;57:51–59. doi: 10.1111/j.1753-4887.1999.tb01778.x. [DOI] [PubMed] [Google Scholar]
- 15.Parlesak A, Menzl I, Feuchter A, Bode JC, Bode C. Inhibition of retinol oxidation by ethanol in the rat liver and colon. Gut. 2000;47:825–831. doi: 10.1136/gut.47.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCaffery P, Koul O, Smith D, Napoli JL, Chen N, Ullman MD. Ethanol increases retinoic acid production in cerebellar astrocytes and in cerebellum. Brain Res Dev Brain Res. 2004;153:233–241. doi: 10.1016/j.devbrainres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Kane MA, Folias AE, Wang C, Napoli JL. Ethanol elevates physiological all-trans-retinoic acid levels in select loci through altering retinoid metabolism in multiple loci: a potential mechanism of ethanol toxicity. FASEB J. 2010;24:823–832. doi: 10.1096/fj.09-141572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leo MA, Lieber CS. Alcohol, vitamin A, and beta-carotene: adverse interactions, including hepatotoxicity and carcinogenicity. Am J Clin Nutr. 1999;69:1071–1085. doi: 10.1093/ajcn/69.6.1071. [DOI] [PubMed] [Google Scholar]
- 19.Wang XD. Alcohol, vitamin A, and cancer. Alcohol. 2005;35:251–258. doi: 10.1016/j.alcohol.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Johnson CS, Zucker RM, Hunter ES, 3rd, Sulik KK. Perturbation of retinoic acid (RA)-mediated limb development suggests a role for diminished RA signaling in the teratogenesis of ethanol. Birth Defects Res A Clin Mol Teratol. 2007;79:631–641. doi: 10.1002/bdra.20385. [DOI] [PubMed] [Google Scholar]
- 21.Napoli JL. Interactions of retinoid binding proteins and enzymes in retinoid metabolism. Biochim Biophys Acta. 1999;1440:139–162. doi: 10.1016/s1388-1981(99)00117-1. [DOI] [PubMed] [Google Scholar]
- 22.Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science. 2007;315:820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- 23.Boerman MH, Napoli JL. Cellular retinol-binding protein-supported retinoic acid synthesis. Relative roles of microsomes and cytosol. J Biol Chem. 1996;271:5610–5616. doi: 10.1074/jbc.271.10.5610. [DOI] [PubMed] [Google Scholar]
- 24.Noy N. Retinoid-binding proteins: mediators of retinoid action. Biochem J. 2000;348(Pt 3):481–495. [PMC free article] [PubMed] [Google Scholar]
- 25.Noy N. Ligand specificity of nuclear hormone receptors: sifting through promiscuity. Biochemistry. 2007;46:13461–13467. doi: 10.1021/bi7018699. [DOI] [PubMed] [Google Scholar]
- 26.Dong D, Ruuska SE, Levinthal DJ, Noy N. Distinct roles for cellular retinoic acid-binding proteins I and II in regulating signaling by retinoic acid. J Biol Chem. 1999;274:23695–23698. doi: 10.1074/jbc.274.34.23695. [DOI] [PubMed] [Google Scholar]
- 27.Chen N, Onisko B, Napoli JL. The nuclear transcription factor RARalpha associates with neuronal RNA granules and suppresses translation. J Biol Chem. 2008;283:20841–20847. doi: 10.1074/jbc.M802314200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laserna EJ, Valero ML, Sanz L, del Pino MM, Calvete JJ, Barettino D. Proteomic analysis of phosphorylated nuclear proteins underscores novel roles for rapid actions of retinoic acid in the regulation of mRNA splicing and translation. Mol Endocrinol. 2009;23:1799–1814. doi: 10.1210/me.2009-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sidell N, Feng Y, Hao L, Wu J, Yu J, Kane MA, Napoli JL, Taylor RN. Retinoic acid is a cofactor for translational regulation of vascular endothelial growth factor in human endometrial stromal cells. Mol Endocrinol. 2010;24:148–160. doi: 10.1210/me.2009-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thatcher JE, Isoherranen N. The role of CYP26 enzymes in retinoic acid clearance. Expert Opin Drug Metab Toxicol. 2009;5:875–886. doi: 10.1517/17425250903032681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lieber CS. Alcohol, liver, and nutrition. J Am Coll Nutr. 1991;10:602–632. doi: 10.1080/07315724.1991.10718182. [DOI] [PubMed] [Google Scholar]
- 32.Crabb DW, Pinairs J, Hasanadka R, Fang M, Leo MA, Lieber CS, Tsukamoto H, Motomura K, Miyahara T, Ohata M, Bosron W, Sanghani S, Kedishvili N, Shiraishi H, Yokoyama H, Miyagi M, Ishii H, Bergheim I, Menzl I, Parlesak A, Bode C. Alcohol and retinoids. Alcohol Clin Exp Res. 2001;25:207S–217S. doi: 10.1097/00000374-200105051-00034. [DOI] [PubMed] [Google Scholar]
- 33.Li H, Kim KH. Effects of ethanol on embryonic and neonatal rat testes in organ cultures. J Androl. 2003;24:653–660. doi: 10.1002/j.1939-4640.2003.tb02723.x. [DOI] [PubMed] [Google Scholar]
- 34.Boucheron C, Alfos S, Enderlin V, Husson M, Pallet V, Jaffard R, Higueret P. Age-related effects of ethanol consumption on triiodothyronine and retinoic acid nuclear receptors, neurogranin and neuromodulin expression levels in mouse brain. Neurobiol Agin. 2006;27:1326–1334. doi: 10.1016/j.neurobiolaging.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 35.Bi J, Hu X, Zhou FC, Wei LN. Upregulation of cellular retinoic acid-binding protein I expression by ethanol. Dev Growth Differ. 2001;43:553–561. doi: 10.1046/j.1440-169x.2001.00591.x. [DOI] [PubMed] [Google Scholar]
- 36.Luvizotto RA, Nascimento AF, Veeramachaneni S, Liu C, Wang XD. Chronic alcohol intake upregulates hepatic expression of carotenoid cleavage enzymes and PPAR in rats. J Nutr. 2010;140:1808–1814. doi: 10.3945/jn.110.123398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Penzes P, Wang X, Napoli JL. Enzymatic characteristics of retinal dehydrogenase type I expressed in Escherichia coli. Biochim Biophys Acta. 1997;1342:175–181. doi: 10.1016/s0167-4838(97)00102-7. [DOI] [PubMed] [Google Scholar]
- 38.Nishiguchi M, Kinoshita H, Mostofa J, Taniguchi T, Ouchi H, Minami T, Hatake K, Utsumi T, Motomura H, Hishida S. Different blood acetaldehyde concentration following ethanol administration in a newly developed high alcohol preference and low alcohol preference rat model system. Alcohol Alcohol. 2002;37:9–12. doi: 10.1093/alcalc/37.1.9. [DOI] [PubMed] [Google Scholar]
- 39.Vasiliou V, Pappa A, Estey T. Role of human aldehyde dehydrogenases in endobiotic and xenobiotic metabolism. Drug Metab Rev. 2004;36:279–299. doi: 10.1081/dmr-120034001. [DOI] [PubMed] [Google Scholar]
- 40.Liu C, Chung J, Seitz HK, Russell RM, Wang XD. Chlormethiazole treatment prevents reduced hepatic vitamin A levels in ethanol-fed rats. Alcohol Clin Exp Res. 2002;26:1703–1709. doi: 10.1097/01.ALC.0000037135.09289.69. [DOI] [PubMed] [Google Scholar]
- 41.Kane MA, Napoli JL. Quantification of endogenous retinoids. Methods Mol Biol. 2010;652:1–54. doi: 10.1007/978-1-60327-325-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tzimas G, Collins MD, Burgin H, Hummler H, Nau H. Embryotoxic doses of vitamin A to rabbits result in low plasma but high embryonic concentrations of all-trans-retinoic acid: risk of vitamin A exposure in humans. J Nutr. 1996;126:2159–2171. doi: 10.1093/jn/126.9.2159. [DOI] [PubMed] [Google Scholar]
- 43.Mezey E, Holt PR. The inhibitory effect of ethanol on retinol oxidation by human liver and cattle retina. Exp Mol Pathol. 1971;15:148–156. doi: 10.1016/0014-4800(71)90095-5. [DOI] [PubMed] [Google Scholar]
- 44.Van Thiel DH, Gavaler J, Lester R. Ethanol inhibition of vitamin A metabolism in the testes: possible mechanism for sterility in alcoholics. Science. 1974;186:941–942. doi: 10.1126/science.186.4167.941. [DOI] [PubMed] [Google Scholar]
- 45.Khalighi M, Brzezinski MR, Chen H, Juchau MR. Inhibition of human prenatal biosynthesis of all-trans-retinoic acid by ethanol, ethanol metabolites, and products of lipid peroxidation reactions: a possible role for CYP2E1. Biochem Pharmacol. 1999;57:811–821. doi: 10.1016/s0006-2952(98)00362-1. [DOI] [PubMed] [Google Scholar]
- 46.Molotkov A, Duester G. Retinol/ethanol drug interaction during acute alcohol intoxication in mice involves inhibition of retinol metabolism to retinoic acid by alcohol dehydrogenase. J Biol Chem. 2002;277:22553–22557. doi: 10.1074/jbc.M201603200. [DOI] [PubMed] [Google Scholar]
- 47.Siegenthaler JA, Ashique AM, Zarbalis K, Patterson KP, Hecht JH, Kane MA, Folias AE, Choe Y, May SR, Kume T, Napoli JL, Peterson AS, Pleasure SJ. Retinoic acid from the meninges regulates cortical neuron generation. Cell. 2009;139:597–609. doi: 10.1016/j.cell.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kot-Leibovich H, Fainsod A. Ethanol induces embryonic malformations by competing for retinaldehyde dehydrogenase activity during vertebrate gastrulation. Dis Model Mech. 2009;2:295–305. doi: 10.1242/dmm.001420. [DOI] [PMC free article] [PubMed] [Google Scholar]



