Abstract
Prostaglandin E2 (PGE2) mediates many effects of the midcycle luteinizing hormone (LH) surge within the periovulatory follicle. Differential expression of the four PGE2 (EP) receptors may contribute to the specialized functions of each granulosa cell subpopulation. To determine if EP receptors are differentially expressed in granulosa cells, monkeys received gonadotropins to stimulate ovarian follicular development. Periovulatory events were initiated with human chorionic gonadotropin (hCG); granulosa cells and whole ovaries were collected before (0 h) and after (24–36 h) hCG to span the 40-h primate periovulatory interval. EP receptor mRNA and protein levels were quantified in granulosa cell subpopulations. Cumulus cells expressed higher levels of EP2 and EP3 mRNA compared with mural cells 36 h after hCG. Cumulus cell EP2 and EP3 protein levels also increased between 0 and 36 h after hCG. Overall, mural granulosa cells expressed low levels of EP1 protein at 0 h and higher levels 24–36 h after hCG. However, EP1 protein levels were higher in granulosa cells away from the follicle apex compared with apex cells 36 h after hCG. Higher levels of PAI-1 protein were measured in nonapex cells, consistent with a previous study showing EP1-stimulated PAI-1 protein expression in monkey granulosa cells. EP4 protein levels were low in all subpopulations. In summary, cumulus cells likely respond to PGE2 via EP2 and EP3, whereas PGE2 controls rupture of a specific region of the follicle via EP1. Therefore, differential expression of EP receptors may permit each granulosa cell subpopulation to generate a unique response to PGE2 during the process of ovulation.
Keywords: cumulus cells, granulosa cells, hormone receptors, ovulation, prostaglandins
Each of four PGE2 receptors is differentially expressed in subpopulations of granulosa cells, suggesting each subpopulation may generate a unique response to PGE2 during ovulation.
INTRODUCTION
Prostaglandin production by the primate ovarian follicle is required for successful ovulation. The ovulatory surge of luteinizing hormone (LH) stimulates the synthesis of prostaglandins by follicular granulosa cells, and prostaglandins reach peak concentrations in the follicle just prior to ovulation [1–4]. Prostaglandin E2 (PGE2) has been identified as the key prostaglandin in ovulatory processes. PGE2 acts within the follicle to regulate essential ovulatory processes, including cumulus expansion, follicle rupture, and oocyte release in a number of mammalian species, including primates [5].
PGE2 regulates cellular function through specific G protein-coupled receptors. PGE2 binds to each of four distinct receptors: PTGER1, PTGER2, PTGER3, and PTGER4 (referred to as EP1, EP2, EP3, and EP4, respectively) [6]. These four receptors couple to different G proteins and differentially regulate downstream signal transduction pathways [7].
EP receptor localization has been described in the granulosa cells of ovarian follicles. In the mouse follicle, EP2 and EP4 mRNA and proteins were detected in mural and cumulus granulosa cells, with enhanced expression following an ovulatory dose of gonadotropin [8, 9]. Similarly, EP2 and EP4 mRNA were expressed in the granulosa cells of the periovulatory follicle in the mare [10]. Granulosa-lutein cells from women undergoing treatment for infertility expressed EP1 and EP2 mRNA, whereas EP4 expression was reported to be low to nondetectable [11, 12]. This laboratory demonstrated that the mural granulosa cells of nonhuman primate follicles express all four EP mRNAs, as well as EP1, EP2, and EP3 proteins [13]. Most recently, we reported detection of EP2 and EP4 proteins in monkey cumulus cells [14].
Granulosa cells can be divided into subpopulations based on their location within the periovulatory follicle and their function in ovulation. Cumulus granulosa cells surround the oocyte and expand to permit oocyte release at ovulation, whereas mural granulosa cells line the follicle wall [15]. Mural granulosa cells may be further divided into subpopulations based on structural and functional characteristics. Basal mural granulosa cells are columnar cells located near the basement membrane; these cells contain large amounts of smooth endoplasmic reticulum and lipid droplets, suggesting specialization for efficient steroidogenesis. In contrast, antral mural granulosa cells are cuboidal or polygonal cells located near the follicle antrum and may produce lower levels of steroid hormones [16]. Mural granulosa cells located near the follicle apex (or apical region) are thought to participate in follicle rupture, distinguishing apex granulosa cells from mural granulosa cells lining the remainder of the follicle [17].
PGE2 regulates a diverse set of periovulatory events. Each of these events takes place within a different subpopulation of granulosa cells. For example, PGE2 stimulates production of matrix proteins by expanding cumulus cells [9, 18] while promoting expression of proteolytic enzymes and their inhibitors by mural granulosa cells [19]. Clearly, PGE2 triggers a different intracellular response in each subpopulation of granulosa cells. As a potential mechanism to explain this phenomenon, we hypothesized that each EP receptor is differentially expressed within these functional subpopulations of granulosa cells. Granulosa cell subpopulations were obtained from monkey follicles collected throughout the periovulatory period, and each granulosa cell subpopulation was assessed for EP receptor mRNA and protein expression. The results of these studies support the hypothesis that EP receptors are differentially expressed, both temporally and spatially, in distinct subpopulations of granulosa cells of the primate periovulatory follicle.
MATERIALS AND METHODS
Animals
Granulosa cells and whole ovaries were obtained from adult female cynomolgus macaques at Eastern Virginia Medical School (Norfolk, VA). All animal protocols and experiments were approved by the Eastern Virginia Medical School Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health's “Guide for the Care and Use of Laboratory Animals.” Animal husbandry and sample collections were performed as described previously [20]. Briefly, adult females with regular menstrual cycles were checked regularly for menstruation, and the first day of menstruation was designated Day 1 of the menstrual cycle. Blood samples were obtained following ketamine chemical restraint (5–10 mg/kg body weight) by femoral venipuncture. Blood samples were centrifuged for 10 min at 1600 × g, and serum was stored at −20°C. Aseptic surgeries were performed in a dedicated surgical suite under isoflurane anesthesia using midline laparotomy or laparoscopic procedures.
A controlled ovarian stimulation model, developed for the collection of multiple oocytes for in vitro fertilization, was used to obtain monkey granulosa cells [21]. Beginning within 3 days of initiation of menstruation, recombinant human follicle-stimulating hormone (r-FSH; 90 IU daily; Schering-Plough [now Merck & Co.], Whitehouse Station, NJ; and Serono Reproductive Biology Institute, Rockland, MA) was administered for 6–8 days, followed by daily administration of 90 IU of r-FSH plus 60 IU of recombinant human LH (Serono Biology Institute) for 2–3 days to stimulate the growth of multiple preovulatory follicles. The gonadotropin-releasing hormone antagonist ganirelix acetate (30 μg/kg per day, Merck and Co.) was also administered daily to prevent an endogenous ovulatory LH surge. Adequate follicular development was monitored by ultrasonography, and serum estradiol and progesterone levels were monitored using the Immulite 1000 immunoassay system (Siemens, Deerfield, IL) [22]. Follicular aspiration was performed before (0 h) and at 24 and 36 h after administration of 1000 IU of recombinant human chorionic gonadotropin (r-hCG; Serono). Follicles with a diameter of more than 4 mm were aspirated using a 22-gauge syringe, and aspirates were pooled to obtain granulosa cells and cumulus-oocyte complexes. Whole ovaries were also removed from additional monkeys undergoing controlled ovarian stimulation 0, 24, and 36 h after hCG administration. These times were selected to span the monkey periovulatory interval. Ovulation typically occurs about 40 h after the endogenous LH surge in natural menstrual cycles [23]. Consistent with our previous studies, ovulation was not observed at or before 36 h following hCG administration. Aspirates of multiple follicles from a single animal were pooled and considered a single entity in this study.
Cell and Tissue Preparation
To obtain granulosa cells and oocytes, aspirates were centrifuged at 300 × g. Pelleted cells were resuspended in media, and cumulus-oocyte complexes were mechanically removed. For some experiments, the complexes were fixed in 4% paraformaldehyde. For some experiments, cumulus cells were removed from oocytes with stripper tips (Mid-Atlantic Diagnostics, Mt. Laurel, NJ); pelleted cumulus cells were frozen in liquid nitrogen and stored at −80°C for isolation of total RNA. A mural granulosa cell-enriched population of the remaining cells was obtained by Percoll gradient centrifugation [24]. Mural granulosa cells were then frozen in liquid nitrogen and stored at −80°C for isolation of total RNA. Whole stimulated ovaries were bisected such that at least two periovulatory follicles greater than 4 mm in diameter were present on each piece. The ovarian pieces were covered with O.C.T. Compound (Sakura, Tokyo, Japan), frozen in liquid propane, and stored at −80°C prior to sectioning at 10 μm and mounting on glass slides (Fisher Scientific, Pittsburgh, PA).
Laser Capture Microscopy
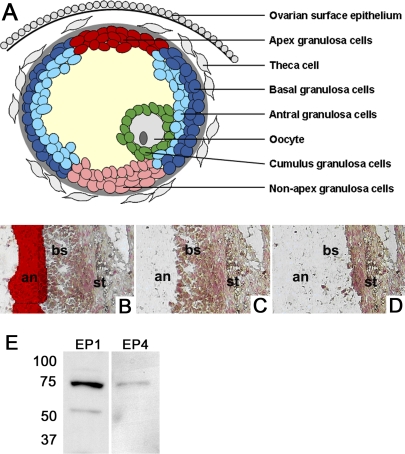
Monkey ovarian tissue sections were dehydrated with a series of ethanol gradients, distilled water, and xylene as described by the Arcturus HistoGene Frozen Section Staining Kit (Applied Biosystems, Carlsbad, CA), with the addition of a 2.5-min incubation with Nuclear Fast Red counterstain following distilled water incubations (Vector Laboratories). Subpopulations of granulosa cells were visualized and captured onto CapSure Macro laser capture microscopy (LCM) caps (Molecular Devices, Sunnyvale, CA) for isolation of RNA using an Arcturus AutoPix 100e LCM system (Arcturus, Mountain View, CA). Antral and basal mural subpopulations of granulosa cells were collected as illustrated in Figure 1. Areas of follicle wall containing a granulosa cell layer at least eight cells thick were used. Granulosa cells (at least four cell layers thick) nearest the antrum were captured as antral mural granulosa cells; granulosa cells (at least four cell layers thick) remaining were captured as basal mural granulosa cells. Similarly, the granulosa cells adjacent to the thinnest area of ovarian stroma were captured as apex mural granulosa cells, whereas granulosa cells opposite the apex cells were captured as nonapex mural granulosa cells.
FIG. 1.
Identification and assessment of granulosa cell subpopulations in periovulatory follicles. A) Granulosa cells were divided into subpopulations based on location within the periovulatory follicle: apex mural granulosa cells (red), nonapex mural granulosa cells (pink), basal mural granulosa cells (dark blue), antral mural granulosa cells (light blue), and cumulus granulosa cells (green). Other key follicle cell types (i.e., oocyte, theca cells, ovarian surface epithelial cells) are shown in gray. B–D) Laser capture microscopy was used to select antral mural granulosa cells (an; B, red highlight), selectively remove antral mural granulosa cells (C), and selectively remove basal mural granulosa cells (bs; D), leaving ovarian stroma (st) behind on a single tissue section. E) Granulosa cells obtained 30 h after hCG administration were subjected to Western blot analysis to confirm specificity of the EP4 receptor antibody. Similarly, homogenized kidney tissues were subjected to Western blot analysis to confirm EP1 receptor specificity. EP2 and EP3 receptor antibodies were previously assessed for specificity in monkey cells and tissues [13].
RNA Isolation, Amplification, and Real-Time PCR
Total RNA was isolated from granulosa cells using Trizol reagent according to the manufacturer's protocol (Invitrogen, Carlsbad, CA), with a modification of the addition of 25 μg/ml glycogen to the RNA precipitation step. For LCM samples, the caps were placed on a 0.5-ml microcentrifuge tube containing 200 μl of Trizol (Invitrogen) and inverted for 1 h, mixing every 30 min. About 4–10 caps were dissolved in one tube of Trizol. Cumulus and mural granulosa cells followed the same protocol, except 800 μl of Trizol was directly added to the pelleted granulosa cells.
Sample processing for RNA amplification was carried out using the first amplification cycle from the Small Sample Labeling Protocol Version VII [25]. This amplification was followed by reverse transcription of the T7-amplified RNA using M-MLV reverse transcriptase (Invitrogen). DNA was then purified using the Qiagen PCR purification kit (Valencia, CA).
Real-time PCR amplification was performed for each sample using FastStart DNA Master SYBR Green I kit (Roche Diagnostics, Indianapolis, IN) following the manufacturer's instructions. Cynomolgus macaque-specific EP receptor and β-actin PCR primers were designed using LightCycler Probe Design software (Roche Diagnostics) as described previously [19]. Quantitative real-time PCR amplification of the LH/CG receptor (LHCGR) and CYP171A1 was performed using the following primers: GTTGATTCCCAAACCAAGG (LHCGR forward), GGCCACCACATTGAGA (LHCGR reverse), CCCATCTATTCGGTTCGT (CYP17A1 forward), and TGGACTGTCCGTTGTG (CYP17A1 reverse). Accession numbers for cynomolgus macaque LHCGR and CYP17A1 are HQ426149 and HQ426148, respectively. The LH receptor and CYP171A1 reactions used 4 μM Mg2+, 0.5 μM each primer, and an annealing temperature of 55°C. Levels of mRNA were assessed for each EP receptor (PTGER1-4), β-actin (ACTB), CYP17A1, and LHCGR by real-time PCR using a Roche LightCycler (Roche Diagnostics). All primers span an intron to prevent undetected amplification of genomic DNA, with the exception of EP4 (as described previously [13]). A standard curve was generated for each primer set over a five-log dilution series. All data are expressed as the ratio of mRNA of interest:β-actin mRNA for each sample. The β-actin mRNA levels in granulosa cells were not different before (0 h) and after hCG, and β-actin mRNA levels were proportional to total mRNA (data not shown).
Samples obtained from an ovary collected 24 h after hCG were excluded from the analysis of EP mRNA in apex and nonapex samples because CYP17A1 mRNA was detected in one sample of this pair, indicating contamination with theca cells of the ovarian stroma. LHCGR mRNA was low in cumulus and higher in mural cells obtained before (0 h) hCG (P < 0.05 by paired t-test), confirming proper selection of these granulosa cell subpopulations (data not shown). However, LHCGR mRNA was low in both cumulus and mural granulosa cells obtained after hCG administration, consistent with rapid desensitization of this receptor after exposure to an ovulatory stimulus [26].
Western Blot Detection of EP Receptors
Western blotting was performed as previously described [27] to confirm specificity of EP receptor antibodies (Fig. 1). Briefly, homogenized kidney tissue or granulosa cell lysate from cynomolgus macaques was loaded onto a 12% polyacrylamide Tris-HCl gel (Bio-Rad, Hercules, CA). Proteins were transferred to a polyvinylidene fluoride membrane (Imobilon; Millipore, Billerica, MA) and probed using antibodies against the EP1 and EP4 receptors (5 μg/ml; Cayman Chemical, Ann Arbor, MI). Membranes were incubated with anti-rabbit secondary antibody coupled to alkaline phosphatase (Applied Biosystems, Forest City, CA), and protein bands were visualized with Tropix CDP-Star according to the manufacturer's instructions (Applied Biosystems).
Immunofluorescent Detection of Proteins in Ovarian Sections
A methodology to perform semiquantitative analysis of protein levels following immunofluorescence staining was established. The specificity of each antibody was determined by Western blot analysis as described above or as reported in Markosyan et al. [13]. A titration was performed with each primary antibody in order to determine the optimal antibody concentration for detection of each protein by immunofluorescence. A region of the mural granulosa cell layer was selected from each digital image using free-form drawing tools included in MetaMorph (Universal Imaging Corp., Downingtown, PA). Fluorescence pixel area was determined and normalized to cell count for each selected region. Fluorescence (expressed as pixel area per cell) was plotted against antibody concentration. These data were used to generate a sigmoidal curve that plateaued, representing maximal EP detection at high concentrations of primary antibody. The lowest primary antibody concentration that resulted in maximal EP protein detection was used for assessment of EP receptor levels in granulosa cell subpopulations as described below. Using this approach, EP3 protein levels in mural granulosa cells of monkey follicles obtained before (0 h) and 36 h after hCG administration were determined to be 82 ± 33 and 155 ± 23 pixel areas per cell, respectively (n = 3 animals per treatment group), showing a 2-fold increase in EP3 protein in response to hCG treatment. This compares favorably with our previously published assessment of granulosa cell EP3 protein levels by Western blotting, which showed a 2.5-fold increase in EP3 protein following 36 h of hCG exposure [13]. This immunofluorescence approach was therefore used for semiquantitative analysis of EP receptor protein levels in subpopulations of granulosa cells.
Immunofluorescence detection of proteins in ovarian tissue sections was performed as previously described [19]. Ovarian tissue sections were fixed with buffered 10% formalin. Following citrate antigen retrieval (BioGenex Labs, San Ramon, CA), sections were blocked with 5% nonimmune goat serum (Vector Laboratories) in PBS containing 0.1% Triton (PBS-Triton). Sections were incubated overnight with one primary antibody (PTGER1 [1 μg/ml], PTGER2 [5 μg/ml], PTGER3 [2 μg/ml], or PTGER4 [3 μg/ml]; all from Cayman Chemical); PLAT (also known as tissue plasminogen activator [tPA]; 10 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA); and SERPINE1 (also known as plasminogen activator inhibitor-1 [PAI-1]; 10 μg/ml; EMD Biosciences, Gibbstown, NJ), followed by incubation with an Alexa Fluor 488-conjugated anti-mouse or anti-rabbit secondary antibody (1:500; Molecular Probes, Eugene, OR). After incubation with 1% Sudan Black in 70% methanol, the slides were coverslipped using Vectashield medium (Vector Laboratories). All images were obtained using an Olympus BX41 fluorescent microscope fitted with a DP70 digital camera and associated software (Olympus, Melville, NY). Preincubation of the primary antibody with an antibody-specific peptide or omission of primary antibody was used to determine background levels of fluorescence. Immunodetection was eliminated when primary antibodies were preincubated with a blocking peptide (Cayman Chemical) or primary antibody was omitted.
The expression of EP receptors in specific regions of the ovary was determined by analysis of fluorescence and cell counts using the MetaMorph system as described above, and all data are expressed as fluorescence pixel area per cell (Universal Imaging Corp.). Specific regions of the mural granulosa cell layer (apex, nonapex, antral, basal) were selected using the criteria for laser capture microscopy and were quantified by MetaMorph analysis as described above. Ovaries from three to five animals were analyzed, with two images analyzed for each EP receptor and each region at each time of hCG exposure.
Immunofluorescence Detection of EP Receptors in Cumulus-Oocyte Complexes
Cumulus-oocyte complexes were incubated for 30 min in 4% formaldehyde, washed in PBS, and stored at 4°C in PBS. Cumulus-oocyte complexes were blocked with 5% nonimmune goat serum (Vector Laboratories) in PBS-Triton. Complexes were incubated for 2 h in 2 μg/ml rabbit anti-human polyclonal primary antibody against one of the EP receptors. Cells were washed three times for 10 min each time in PBS-Triton and incubated with an Alexa Fluor 488-conjugated anti-rabbit secondary antibody (1:1000; Molecular Probes). Cells were washed three times for 10 min each time in PBS-Triton, then coverslipped using Vectashield medium with propidium iodine (Vector Laboratories). All images were obtained using a Zeiss LSM 510 confocal microscope (Thornwood, NY) and associated software at detector gain = 595 and pinhole size = 1. Z-stacks of 5-μm sections were generated, composed of 1024 × 1024 pixels, at a 40× magnification. Immunodetection was eliminated when primary antibodies were preincubated with a blocking peptide (Cayman Chemical) or primary antibody was omitted.
Measurement of EP receptor levels in cumulus cells was conducted by analysis of fluorescence and cell counts using the MetaMorph system as described above. For each cumulus complex, two different images from the Z-stack were analyzed. In each image, 5–10 cumulus cells were selected, and the intensity of fluorescence was determined. All data are expressed as mean intensity per cell. For each EP receptor, cumulus complexes from three animals per time point were analyzed.
Data Analysis
All data sets were assessed for heterogeneity of variance by Bartlett test; data were log10 transformed when Bartlett test yielded P < 0.05. Log-transformed data were subjected to Bartlett test to confirm that P > 0.05. The majority of data sets were log10 transformed before analysis; EP2 mRNA in antral and basal granulosa cells and all EP2 and EP3 protein data were not log10 transformed prior to further analysis. In all cases, untransformed data are presented. Protein and mRNA levels before and after hCG were compared by ANOVA, followed by a Duncan post hoc test. Comparisons between subpopulations were performed using a paired t-test. For comparisons between cumulus cells at 0 and 36 h after hCG, an unpaired t-test was used. All statistical tests were performed using StatPak version 4.12 software (Northwest Analytical, Portland, OR). For all tests, significance was assumed at P < 0.05. Data are expressed as mean ± SEM, n = 3–6 animals per group.
RESULTS
EP Receptor Expression in Cumulus and Mural Granulosa Cell Subpopulations
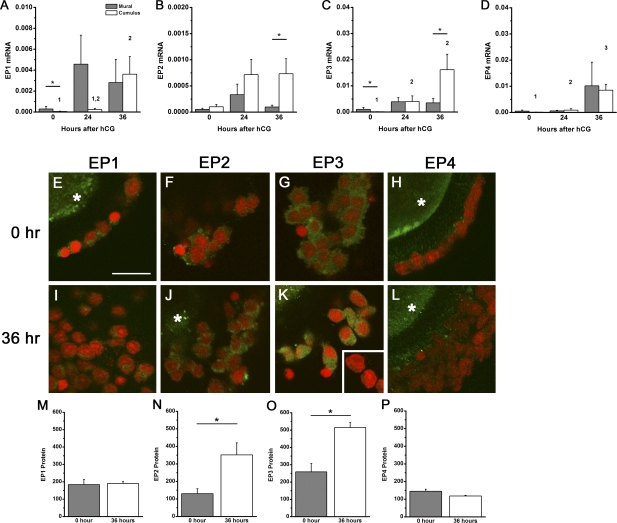
To determine whether EP receptors are differentially expressed in response to the ovulatory gonadotropin surge, cumulus and mural granulosa cells were obtained from periovulatory follicles at 0, 24, and 36 h after administration of an ovulatory dose of hCG. Variable expression levels of mRNA for all four EP receptors were detected in cumulus and mural granulosa cells throughout the periovulatory interval (Fig. 2). Cumulus granulosa cells demonstrate significantly higher levels of EP1, EP3, and EP4 mRNA expression at 36 h following hCG stimulation when compared with 0-h hCG. At 36 h after hCG, levels of EP2 and EP3 mRNA were higher in cumulus granulosa cells compared with mural granulosa cells. In contrast, EP1 and EP4 mRNA levels in cumulus cells were similar to mural granulosa cell levels 36 h after hCG. It is important to note that monkeys used in this study are not genetically identical or highly inbred. Thus, variation within the treatment groups reflects typical variations in natural populations as previously described [28, 29].
FIG. 2.
EP receptors in cumulus and mural granulosa cell subpopulations. EP1 (A), EP2 (B), EP3 (C), and EP4 (D) mRNAs were quantified within cumulus and mural granulosa subpopulations obtained before (0 h), 24 h after, and 36 h after hCG administration. Each EP mRNA was expressed relative to β-actin. Data are expressed as mean ± SEM, n = 3–6 monkeys per time point. Within each panel, each subpopulation was assessed by ANOVA, followed by Duncan; groups with no common superscripts are different (P < 0.05). Comparisons between subpopulations were performed using a paired t-test (*P < 0.05). E–L) Immunofluorescent detection (green) of EP1 (E and I), EP2 (F and J), EP3 (G and K), and EP4 (H and L) in cumulus granulosa cells obtained before (0 h; E–H) and 36 h after (I–L) hCG administration. Granulosa cell nuclei are red (propidium iodide staining). When visible, immunofluorescence reflecting presence of the oocyte in the image is indicated by an asterisk. Specific EP immunodetection in monkey oocytes was previously reported [14]. Absence of green fluorescence was confirmed when primary antibody was omitted (K, inset) or primary antibody was preabsorbed with the peptide used to generate the antibody (data not shown). All images are at the same magnification; bar in E = 20 μm. Protein levels for EP1 (M), EP2 (N), EP3 (O), and EP4 (P) are expressed as mean ± SEM (n = 3 animals per time point). Comparisons between time points were performed using an unpaired t-test (*P < 0.05).
Confocal laser scanning microscopy was used to detect EP receptor proteins in the cumulus granulosa cells of cumulus-oocyte complexes (Fig. 2). Immunodetection of EP2 and EP3 receptor proteins was low in cumulus cells obtained before (0 h) hCG and increased significantly in cumulus cells obtained 36 h after hCG administration. Cumulus cell levels of EP1 and EP4 receptor proteins did not change in response to hCG administration.
EP Receptor Expression in Apex and Nonapex Granulosa Cell Subpopulations
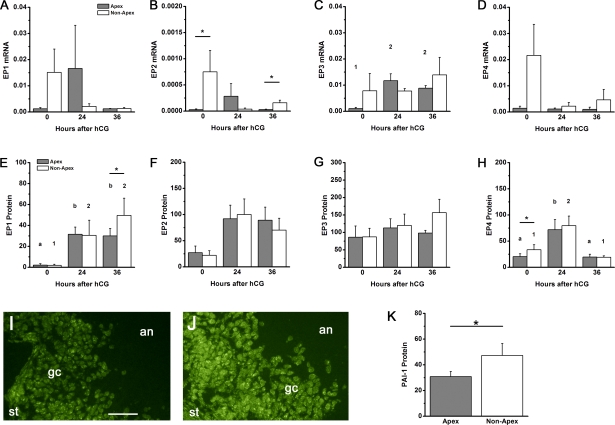
Follicle rupture is anticipated to occur where the follicle wall presents the thinnest barrier between the interior of the follicle and the exterior of the ovary. Mural granulosa cells in this area (the follicle apex) and granulosa cells opposite the apex (nonapex) were assessed for EP mRNA and proteins (Fig. 3, A–D). EP3 mRNA increased in response to hCG administration in apex, but not nonapex, mural granulosa cells; hCG-stimulated changes in EP1, EP2, and EP4 mRNAs were not observed. EP2 mRNA levels were significantly higher in apex granulosa cells compared with nonapex granulosa cells at 0 and 36 h after hCG administration. In contrast, differences in EP1, EP3, and EP4 receptor mRNA levels between apex and nonapex granulosa cells were never observed.
FIG. 3.
EP receptors and PAI-1 protein in apex and nonapex mural granulosa cell subpopulations. EP1 (A and E), EP2 (B and F), EP3 (C and G), and EP4 (D and H) mRNA (A–D) and protein (E–H) were quantified within granulosa subpopulations of ovaries obtained before (0 h), 24 h after, and 36 h after hCG administration. Each EP mRNA was expressed relative to β-actin. Each EP protein was expressed as fluorescence pixel area per cell. Data are expressed as mean ± SEM, n = 3–5 monkeys per time point. Within each panel, each subpopulation was assessed by ANOVA, followed by Duncan; groups with no common superscripts are different (P < 0.05). Comparisons between subpopulations were performed using a paired t-test (*P < 0.05). Representative immunodetection (green) of EP1 in apex (I) and nonapex (J) regions of ovarian follicles obtained 36 h after hCG administration. In each image, positions of ovarian stroma (st), mural granulosa cells (gc), and follicle antrum (an) are indicated. Both images are shown at the same magnification; bar in I = 50 μm. K) Protein levels for PAI-1 in apex and nonapex regions of the ovarian follicle are expressed as mean ± SEM (n = 4 animals). Comparisons between subpopulations were performed using a paired t-test (*P < 0.05).
EP protein levels did not follow the same patterns as levels of EP mRNA in these granulosa cell subpopulations (Fig. 3, E–H). EP1 protein levels were low before hCG and higher 24–36 h after hCG administration in both apex and nonapex mural granulosa cells. EP1 protein levels were not different between apex and nonapex cells at 0 and 24 h after hCG. However, EP1 protein levels were higher in nonapex compared with apex mural granulosa cells 36 h after hCG administration, or just before the expected time of ovulation. No differences between these subpopulations were identified for EP2 and EP3 protein levels at any time examined. In both apex and nonapex mural granulosa cells, EP4 protein levels were low before (0 h) hCG, higher 24 h after hCG, and low again 36 h after hCG administration. EP4 protein levels were lower in apex compared with nonapex mural granulosa cells before (0 h) hCG, whereas no differences in EP4 protein levels were noted between these subpopulations 24 and 36 h after hCG administration.
PAI-1 and tPA Expression in Apex and Nonapex Cells
Previous studies in this laboratory demonstrated that EP1 mediates PGE2-stimulated expression of PAI-1, which inhibits the proteolytic activity of tPA [19]. Mural granulosa cells in the apex and nonapex regions of the follicle were assessed for PAI-1 and tPA protein levels by semiquantitative immunofluorescence. Whereas tPA protein levels did not differ between apex and nonapex granulosa cell subpopulations (data not shown), PAI-1 protein levels were significantly higher in nonapex granulosa cells compared with granulosa cells located at the follicle apex (Fig. 3). Our current study shows a correlation between high EP1 protein and high PAI-1 protein in granulosa cells located away from the follicle apex.
EP Receptor Expression in Antral and Basal Granulosa Cell Subpopulations
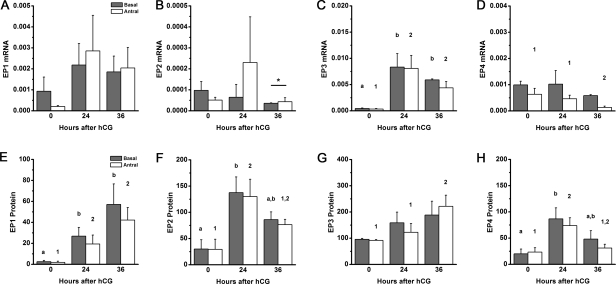
Mural granulosa cells near the follicle antrum (antral) and near the basement membrane (basal) were also assessed for EP mRNA and protein (Fig. 4). All EP receptor mRNAs were detected in both antral and basal mural granulosa cell subpopulations. EP1 and EP2 receptor mRNA levels were highly variable and did not change during the periovulatory period. EP2 mRNA levels were slightly, but significantly, higher in antral granulosa cells obtained 36 h after hCG compared with basal granulosa cells. EP3 mRNA was low before (0 h) hCG and increased in response to hCG to achieve high levels 24–36 h after hCG administration in both antral and basal granulosa cells. EP4 mRNA was highest before (0 h) hCG in both antral and basal granulosa cell subpopulations. Whereas EP4 mRNA levels remained unchanged in basal cells, EP4 mRNA levels in antral mural granulosa cells were high 0–24 h after hCG and declined to reach the lowest levels 36 h after hCG administration.
FIG. 4.
EP receptors in basal and antral mural granulosa cell subpopulations. EP1 (A and E), EP2 (B and F), EP3 (C and G), and EP4 (D and H) mRNA (A–D) and protein (E–H) levels were quantified within granulosa subpopulations of ovaries obtained before (0 h), 24 h after, and 36 h after hCG administration. Each EP mRNA was expressed relative to β-actin. Each EP protein was expressed as fluorescence pixel area per cell. Data are expressed as mean ± SEM, n = 4–5 monkeys per time point. Within each panel, each subpopulation was assessed by ANOVA, followed by Duncan; groups with no common superscripts are different (P < 0.05). Comparisons between subpopulations were performed using a paired t-test (*P < 0.05).
Immunofluorescent detection of EP receptors in antral and basal mural granulosa cell subpopulations confirmed our previous report of EP detection in monkey mural granulosa cells (Fig. 4) [13]. EP1 protein levels were low before (0 h) hCG and higher 24–36 h after hCG administration. EP2 and EP4 protein levels were low before (0 h) hCG, peaked 24 h after hCG, and reached intermediated levels 36 h after hCG administration. EP3 protein was easily detected before (0 h) hCG, rose by 24 h, and peaked 36 h after hCG administration; this trend was statistically significant in antral, but not basal, mural granulosa cells. Importantly, significant differences in EP receptor levels were never detected between antral and basal mural granulosa cell subpopulations at any time examined.
DISCUSSION
This study was the first to examine in detail the expression of each of the four PGE2 receptors within distinct, functional subpopulations of granulosa cells in the periovulatory follicle. Numerous studies have demonstrated that the PGE2 signal is transduced by these four EP receptors [30]. Protein and mRNA for PGE2 receptors were expressed by monkey follicular granulosa cells, and expression of these EP receptors increased in response to the ovulatory gonadotropin surge [13]. Peak levels of EP1, EP2, and EP3 were observed late in the periovulatory interval, when elevated follicular PGE2 regulates events that lead to ovulation [1]. Cumulus and functionally distinct subpopulations of mural granulosa cells play different roles in the overall process of ovulation, so differential expression of EP receptors may permit these subpopulations to respond differently to ovulatory concentrations of PGE2.
Data from the present study demonstrate that cumulus granulosa cells of the monkey follicle express primarily EP2 and EP3 receptors. Increases in both EP2 and EP3 receptors were noted following exposure to an ovulatory dose of hCG, paralleling increasing EP3 mRNA and a trend toward increased EP2 mRNA. Whereas EP1 mRNA levels in cumulus granulosa cells were low prior to hCG and increased following hCG exposure, a similar pattern in EP1 protein levels was not observed. Differences in mRNA and protein levels are not uncommon [31] and have been previously reported for EP receptors [13]. Importantly, receptor protein levels are more likely than mRNA to correlate with function. Monkey cumulus cells contained low levels of EP1 and EP4 receptor proteins, both of which were not altered by hCG. These data contrast with findings in the mouse ovary, where primarily EP2 and EP4 receptors were expressed in cumulus granulosa cells before and after an ovulatory dose of gonadotropin [9, 11]. Inhibition of ovarian cyclooxygenase (COX) activity and reduced prostaglandin production in mice led to impaired cumulus expansion, including decreased hyaluronic acid synthesis and reduced expression of the hyaluronan cross-linking protein tumor necrosis factor-α-stimulated gene 6 (Tsg6; official symbol Tnfaip6), both of which are required to form the extracellular matrix surrounding expanded cumulus granulosa cells [32, 33]. Cumulus expansion fails in mice lacking EP2 receptors, demonstrating an essential role for PGE2 and EP2 receptors in this essential periovulatory process [34]. Both EP2 and EP4 receptors typically couple to G proteins to increase cAMP [30], so both of these receptors may respond to PGE2 with elevated cAMP within cumulus cells to stimulate cumulus expansion. Histological evidence indicates that PGE2 produced via PTGS2 (COX-2) is required for successful cumulus expansion in nonhuman primate follicles as well. Administration of COX inhibitors orally or directly into the periovulatory follicle led to oocytes trapped within luteinizing follicles, an indication that failure of cumulus expansion prevented release of the cumulus-oocyte complex into the follicle antrum [35, 36]. The present study demonstrates that monkey cumulus cells express primarily EP2 and EP3 receptors, both of which have been shown to increase cAMP levels in monkey granulosa cells in vitro [13]. Taken together, these data support the conclusion that EP2 and EP3 receptors mediate the PGE2 signal to promote cumulus expansion in primate periovulatory follicles.
Differential EP1 expression in apex and nonapex granulosa cells suggests a role for EP1 in the process of follicle rupture. Whereas EP1 receptors were detected in all mural granulosa cell subpopulations throughout the periovulatory interval, EP1 protein was present in apex granulosa cells at lower levels than were measured in granulosa cells not at the follicle apex 36 h after hCG, or just before the expected time of follicle rupture. Granulosa cells located at the apex of the follicle preferentially express matrix metalloproteinases and have been implicated in the control of LH-stimulated follicle rupture [37]. Previous studies in this laboratory showed that EP1 mediates PGE2-stimulated expression of PAI-1, which inhibits the proteolytic activity of tPA [19]. The present study shows higher levels of PAI-1 protein in the nonapex region of the follicle where EP1 protein is maximal. These findings suggest that low levels of EP1 may lead to reduced PAI-1 protein, and thereby permit tPA-driven proteolysis at the follicle apex. EP1 receptors typically couple to Gq to activate phospholipase C and regulate intracellular calcium [38, 39]. In human granulosa-lutein cells, PGE2 transiently raised intracellular calcium levels, and this increase was prevented by the EP1 antagonist AH6809 [11]. Similarly, an EP1 agonist increased intracellular calcium in monkey granulosa cells obtained 36 h after hCG; agonists selective for other EP receptors did not alter calcium levels in these cells [13]. Taken together, these data suggest that PGE2 regulates intracellular calcium in mural granulosa cells primarily, if not solely, through EP1 receptors. EP1 may respond to PGE2 to mediate differential expression of enzymes and inhibitors involved in proteolytic degradation, resulting in selection of the follicle apex in the region where EP1 expression is lowest.
Few differences were identified in EP mRNA and protein levels between antral and basal mural granulosa cell subpopulations. Administration of an ovulatory dose of hCG increased EP3 receptor levels in antral, but not basal, mural granulosa cells. These data suggest that EP3 receptor stimulation may preferentially regulate the function of antral mural granulosa cells. However, no specific periovulatory functions have been attributed to antral mural granulosa cells to date. In contrast, basal mural granulosa cells are specialized for production of steroid hormones in response to gonadotropin stimulation [16]. Consistent with this role, basal mural granulosa cells preferentially express the LH receptor [40] and steroidogenic enzymes [41]. Limited studies in mice suggest that PGE2 regulates progesterone production by granulosa cells [42], but inhibition of COX activity or replacement with PGE2 does not appear to alter steroidogenesis by primate periovulatory follicles [35, 36]. Future studies will require identification of additional PGE2-mediated periovulatory events occurring specifically in these subpopulations of mural granulosa cells to elucidate the role of each EP in the ovulatory process.
As described in this study, granulosa cells of the periovulatory follicle can be clearly divided into subpopulations, with different locations within the follicle, histological appearances, and periovulatory functions. Previous studies have made substantial progress to assess the role of PGE2 in ovulatory events using mice lacking expression of individual EP receptors. Some of these mice failed to ovulate, others showed no reproductive dysfunction, and still other EP knockout mice died before adult reproductive function could be assessed [43]. Gene deletion technologies have clear strengths and weaknesses in the assessment of EP receptor function in the process of ovulation. Using an alternative approach, this was the first study to demonstrate that EP receptors can be preferentially localized to specific subpopulations of granulosa cells. EP2 and/or EP3 are likely the key receptors mediating the ability of PGE2 to stimulate cumulus expansion. Low levels of EP1, coupled with low PAI-1, were a characteristic of the follicle apex, suggesting a role for this PGE2 receptor in defining the site of follicle rupture. Modulation of EP receptor levels, but not absence of any individual EP receptor, defined each subpopulation and may regulate response to periovulatory concentrations of PGE2. The ability to stimulate or inhibit certain periovulatory events with EP receptor-selective agonists or antagonists may lead to the development of novel contraceptives or methods to promote fertility in infertile patients.
ACKNOWLEDGMENTS
The authors would like to acknowledge the dedicated work of Ms. Kim Hester in animal training and animal handling. Recombinant human FSH and Ganirelix were generously provided by Schering-Plough [now Merck & Co.], Whitehouse Station, NJ. Serono Reproductive Biology Institute, Rockland, MA, generously provided recombinant human LH.
Footnotes
Supported by grant funding from the National Institutes of Health/National Institute of Child Health and Human Development (HD054691) and Virginia's Commonwealth Health Research Board.
REFERENCES
- Duffy DM, Stouffer RL. The ovulatory gonadotrophin surge stimulates cyclooxygenase expression and prostaglandin production by the monkey follicle. Mol Hum Reprod 2001; 7: 731 739. [DOI] [PubMed] [Google Scholar]
- Sirois J. Induction of prostaglandin endoperoxide synthase-2 by human chorionic gonadotropin in bovine preovulatory follicles in vivo. Endocrinology 1994; 135: 841 848. [DOI] [PubMed] [Google Scholar]
- Sirois J, Dore M. The late induction of prostaglandin G/H synthase-2 in equine preovulatory follicles supports its role as a determinant of the ovulatory process. Endocrinology 1997; 138: 4427 4434. [DOI] [PubMed] [Google Scholar]
- Wong WY, Richards JS. Evidence for two antigenically distinct molecular weight variants of prostaglandin H synthase in the rat ovary. Mol Endocrinol 1991; 5: 1269 1279. [DOI] [PubMed] [Google Scholar]
- Murdoch WJ, Hansen TR, McPherson LA. A review–role of eicosanoids in vertebrate ovulation. Prostaglandins 1993; 46: 85 115. [DOI] [PubMed] [Google Scholar]
- Coleman RA, Smith WL, Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev 1994; 46: 205 229. [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev 1999; 79: 1193 1226. [DOI] [PubMed] [Google Scholar]
- Segi E, Haraguchi K, Sugimoto Y, Tsuji M, Tsunekawa H, Tamba S, Tsuboi K, Tanaka S, Ichikawa A. Expression of messenger RNA for prostaglandin E receptor subtypes EP4/EP2 and cyclooxygenase isozymes in mouse periovulatory follicles and oviducts during superovulation. Biol Reprod 2003; 68: 804 811. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Morrow JD, Wang H, Dey SK. Cyclooxygenase-2-derived prostaglandin E(2) directs oocyte maturation by differentially influencing multiple signaling pathways. J Biol Chem 2006; 281: 37117 37129. [DOI] [PubMed] [Google Scholar]
- Sayasith K, Bouchard N, Dore M, Sirois J. Gonadotropin-dependent regulation of the prostaglandin E2 receptor in equine preovulatory follicles during the ovulatory process in mares. Mol Reprod Dev 2009; 76: 191 201. [DOI] [PubMed] [Google Scholar]
- Harris TE, Squires PE, Michael AE, Bernal AL, Abayasekara DR. Human granulosa-lutein cells express functional EP1 and EP2 prostaglandin receptors. Biochem Biophys Res Commun 2001; 285: 1089 1094. [DOI] [PubMed] [Google Scholar]
- Narko K, Saukkonen K, Ketola I, Butzow R, Heikinheimo M, Ristimaki A. Regulated expression of prostaglandin E(2) receptors EP2 and EP4 in human ovarian granulosa-luteal cells. J Clin Endocrinol Metab 2001; 86: 1765 1768. [DOI] [PubMed] [Google Scholar]
- Markosyan N, Dozier BL, Lattanzio FA, Duffy DM. Primate granulosa cell response via prostaglandin E2 receptors increases late in the periovulatory interval. Biol Reprod 2006; 75: 868 876. [DOI] [PubMed] [Google Scholar]
- Duffy DM, McGinnis LK, Vandevoort CA, Christenson LK. Mammalian oocytes are targets for prostaglandin E2 (PGE2) action. Reprod Biol Endocrinol 2010; 8: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsafriri A, Reich R. Molecular aspects of mammalian ovulation. Exp Clin Endocrinol Diabetes 1999; 107: 1 11. [DOI] [PubMed] [Google Scholar]
- Lipner H. Mechanism of mammalian ovulation. : Knobil EA, Neill JD. (eds.), The Physiology of Reproduction, vol. 1. New York: Raven Press Ltd.; 1988: 477 488. [Google Scholar]
- Tadakuma H, Okamura H, Kitaoka M, Iyama K, Usuku G. Association of immunolocalization of matrix metalloproteinase 1 with ovulation in hCG-treated rabbit ovary. J Reprod Fertil 1993; 98: 503 508. [DOI] [PubMed] [Google Scholar]
- Ochsner SA, Day AJ, Rugg MS, Breyer RM, Gomer RH, Richards JS. Disrupted function of tumor necrosis factor-alpha-stimulated gene 6 blocks cumulus cell-oocyte complex expansion. Endocrinology 2003; 144: 4376 4384. [DOI] [PubMed] [Google Scholar]
- Markosyan N, Duffy DM. Prostaglandin E2 acts via multiple receptors to regulate plasminogen-dependent proteolysis in the primate periovulatory follicle. Endocrinology 2009; 150: 435 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seachord CL, VandeVoort CA, Duffy DM. Adipose differentiation-related protein: a gonadotropin- and prostaglandin-regulated protein in primate periovulatory follicles. Biol Reprod 2005; 72: 1305 1314. [DOI] [PubMed] [Google Scholar]
- Stouffer RL, Zelinski-Wooten MB. Overriding follicle selection in controlled ovarian stimulation protocols: quality vs quantity. Reprod Biol Endocrinol 2004; 2: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandevoort CA, Baughman WL, Stouffer RL. Comparison of different regimens of human gonadotropins for superovulation of rhesus monkeys: ovulatory response and subsequent luteal function. J In Vitro Fert Embryo Transf 1989; 6: 85 91. [DOI] [PubMed] [Google Scholar]
- Weick RF, Dierschke DJ, Karsch FJ, Butler WR, Hotchkiss J, Knobil E. Periovulatory time courses of circulating gonadotropic and ovarian hormones in the rhesus monkey. Endocrinology 1973; 93: 1140 1147. [DOI] [PubMed] [Google Scholar]
- Chaffin CL, Stouffer RL, Duffy DM. Gonadotropin and steroid regulation of steroid receptor and aryl hydrocarbon receptor messenger ribonucleic acid in macaque granulosa cells during the periovulatory interval. Endocrinology 1999; 140: 4753 4760. [DOI] [PubMed] [Google Scholar]
- Small Sample Labeling Protocol vII, University of Kansas Medical Center, Kansas City, Kansas. 2004. World Wide Web (URL: http://www2.kumc.edu/siddrc/microarray/protocols.html) (December 10, 2010).
- Menon KM, Munshi UM, Clouser CL, Nair AK. Regulation of luteinizing hormone/human chorionic gonadotropin receptor expression: a perspective. Biol Reprod 2004; 70: 861 866. [DOI] [PubMed] [Google Scholar]
- Duffy DM, Dozier BL, Seachord CL. Prostaglandin dehydrogenase and prostaglandin levels in periovulatory follicles: implications for control of primate ovulation by prostaglandin E2. J Clin Endocrinol Metab 2005; 90: 1021 1027. [DOI] [PubMed] [Google Scholar]
- Disotell TR, Tosi AJ. The monkey's perspective. Genome Biol 2007; 8: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER, Remington KA, Strausberg RL, Venter JC, Wilson RK, Batzer MA, Bustamante CD. et al. Evolutionary and biomedical insights from the rhesus macaque genome. Science 2007; 316: 222 234. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem 2007; 282: 11613 11617. [DOI] [PubMed] [Google Scholar]
- Greenbaum D, Colangelo C, Williams K, Gerstein M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol 2003; 4: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani S, Elvin JA, Yan C, DeMayo J, DeMayo FJ, Horton HF, Byrne MC, Matzuk MM. Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol Endocrinol 2002; 16: 1154 1167. [DOI] [PubMed] [Google Scholar]
- Ochsner SA, Day AJ, Rugg MS, Breyer RM, Gomer RH, Richards JS. Disrupted function of tumor necrosis factor-alpha-stimulated gene 6 blocks cumulus cell-oocyte complex expansion. Endocrinology 2003; 144: 4376 4684. [DOI] [PubMed] [Google Scholar]
- Hizaki H, Segi E, Sugimoto Y, Hirose M, Saji T, Ushikubi F, Matsuoka T, Noda Y, Tanaka T, Yoshida N, Narumiya S, Ichikawa A. Abortive expansion of the cumulus and impaired fertility in mice lacking the prostaglandin E receptor subtype EP(2). Proc Natl Acad Sci U S A 1999; 96: 10501 10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy DM, Stouffer RL. Follicular administration of a cyclooxygenase inhibitor can prevent oocyte release without alteration of normal luteal function in rhesus monkeys. Hum Reprod 2002; 17: 2825 2831. [DOI] [PubMed] [Google Scholar]
- Hester KE, Harper MJ, Duffy DM. Oral administration of the cyclooxygenase-2 (COX-2) inhibitor meloxicam blocks ovulation in non-human primates when administered to simulate emergency contraception. Hum Reprod 2010; 25: 360 367. [DOI] [PubMed] [Google Scholar]
- Tadakuma H, Okamura H, Kitaoka M, Iyama K, Usuku G. Association of immunolocalization of matrix metalloproteinase 1 with ovulation in hCG-treated rabbit ovary. J Reprod Fertil 1993; 98: 503 508. [DOI] [PubMed] [Google Scholar]
- Tang CH, Yang RS, Fu WM. Prostaglandin E2 stimulates fibronectin expression through EP1 receptor, phospholipase C, protein kinase Calpha, and c-Src pathway in primary cultured rat osteoblasts. J Biol Chem 2005; 280: 22907 22916. [DOI] [PubMed] [Google Scholar]
- Watabe A, Sugimoto Y, Honda A, Irie A, Namba T, Negishi M, Ito S, Narumiya S, Ichikawa A. Cloning and expression of cDNA for a mouse EP1 subtype of prostaglandin E receptor. J Biol Chem 1993; 268: 20175 20178. [PubMed] [Google Scholar]
- Amsterdam A, Koch Y, Lieberman ME, Lindner HR. Distribution of binding sites for human chorionic gonadotropin in the preovulatory follicle of the rat. J Cell Biol 1975; 67: 894 900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner KJ, Macpherson S, Millar MR, McNeilly AS, Williams K, Cranfield M, Groome NP, Sharpe RM, Fraser HM, Saunders PTK. Development and validation of a new monoclonal antibody to mammalian aromatase. J Endocrinol 2002; 172: 21 30. [DOI] [PubMed] [Google Scholar]
- Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM. Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol Endocrinol 1999; 13: 1035 1048. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Narumiya S. Function of prostanoid receptors: studies on knockout mice. Prostaglandins Other Lipid Mediat 2002; 68–69: 557 573. [DOI] [PubMed] [Google Scholar]