Abstract
Estradiol has both negative and positive feedback actions upon gonadotropin-releasing hormone (GnRH) release; the latter actions trigger the preovulatory GnRH surge. Although neurobiological mechanisms of the transitions between feedback modes are becoming better understood, the roles of voltage-gated potassium currents, major contributors to neuronal excitability, are unknown. Estradiol alters two components of potassium currents in these cells: a transient current, IA, and a sustained current, IK. Kisspeptin is a potential mediator between estradiol and GnRH neurons and can act directly on GnRH neurons. We examined how estradiol, time of day, and kisspeptin interact to regulate these conductances in a mouse model exhibiting daily switches between estradiol negative (morning) and positive feedback (evening). Whole-cell voltage clamp recordings were made from GnRH neurons in brain slices from ovariectomized (OVX) mice and from OVX mice treated with estradiol (OVX+E). There were no diurnal changes in either IA or IK in GnRH neurons from OVX mice. In contrast, in GnRH neurons from OVX+E mice, IA and IK were greater during the morning when GnRH neuron activity is low and smaller in the evening when GnRH neuron activity is high. Estradiol increased IA in the morning and decreased it in the evening, relative to that in cells from OVX mice. Exogenously applied kisspeptin reduced IA regardless of time of day or estradiol status. Estradiol, interacting with time of day, and kisspeptin both depolarized IA activation. These findings extend our understanding of both the neurobiological mechanisms of estradiol negative vs. positive regulation of GnRH neurons and of kisspeptin action on these cells.
Keywords: diurnal, estradiol, estradiol receptor, feedback, GnRH receptor, gonadotropin-releasing hormone, ion channels
Estradiol, kisspeptin, and time of day interact to modulate potassium conductances in GnRH neurons, revealing novel aspects of the neurobiology of negative vs. positive feedback.
INTRODUCTION
Gonadotropin-releasing hormone (GNRH1 [GnRH]) neurons are the primary central neuronal cells controlling fertility. Their function is regulated, in part, by steroid feedback. Estradiol controls GnRH neuron activity and hormone release in both negative and positive modes, with the positive feedback action causing neurobiological changes that lead to a preovulatory surge of GnRH release. This surge is critical for initiating the luteinizing hormone (LH) surge that triggers ovulation [1]. Both modes of feedback are revealed on a diurnal basis in ovariectomized (OVX) female mice treated for 2–4 days with estradiol (E) implants (OVX+E), providing a constant physiological level of this steroid [2]. OVX+E mice exhibit low levels of serum LH and GnRH neuronal activity in the morning that are typical of estradiol negative feedback, and high LH and GnRH neuronal activity levels typical of estradiol positive feedback and the surge in the afternoon, peaking near lights out [2]. This mechanism provides a model for studying the switch in estradiol feedback mode without changing steroid hormone levels.
GnRH neurons express a variety of channels that are potential targets of estradiol feedback regulation [3–5]. Voltage-gated potassium channels are critical contributors to cellular physiology. These channels play major roles in setting a cell's resting membrane potential and in determining neuronal excitability and the rate of action potential firing and, thus, hormone release [6]. Activation of potassium channels under physiological conditions generates an outward, hyperpolarizing current that typically inhibits neuronal activity. Estradiol regulates potassium channels in other hypothalamic, neuroendocrine, and pituitary cells [7–9]. In previous studies of GnRH neurons from female mice, two main components of voltage-gated potassium currents, a rapidly inactivating transient A-type current (IA) and a noninactivating delayed rectifier (IK) current, were identified [4]. Both of these currents were suppressed by estradiol. At the time those studies were conducted, however, the daily surge was not appreciated in the OVX+E mouse, and hence, these important currents have not been studied in a model in which the timing of estradiol negative and positive feedback is well defined. Previous work in the daily surge model indicates that both intrinsic conductances of [10] and synaptic inputs to [11, 12] GnRH neurons change with time of day, suggesting both of these mechanisms can contribute to the switch in estradiol feedback action. In contrast, some other intrinsic properties [13] are not altered by time of day, suggesting their primary controller is estradiol alone, without input from additional diurnal signals. How potassium conductances are altered by the interaction of estradiol and time of day is unknown.
Many studies indicate that most of the estradiol feedback regulation of GnRH neurons likely occurs via estradiol receptor alpha (ERα; Esr1)-expressing afferents [11, 12, 14–16], although changes due to direct effects via ERβ (Esr2) expression cannot be ruled out [13]. Kisspeptin (encoded by the Kiss1 gene) is a major excitatory neuromodulator of GnRH neurons, and expression of kisspeptin mRNA levels is modulated by estradiol [17–22], although direct measures of whether estradiol alters kisspeptin release and/or kisspeptin neuron activity are lacking. GnRH neurons express the kisspeptin Kissr1 receptor and are strongly excited by its activation [17–19, 21, 23, 24]. Recent studies indicate kisspeptin action can involve changes in potassium and/or nonselective ionic conductances in GnRH neurons [18, 19, 21, 25], but effects on specific subtypes of potassium currents and the mechanisms by which estradiol and time of day alter kisspeptin action are less well understood.
Here we examined how potassium conductances of GnRH neurons are modulated during estradiol negative and positive feedback regulation of GnRH neurons, whether kisspeptin alters these conductances, and if the actions of kisspeptin are dependent on estradiol or time of day.
MATERIALS AND METHODS
Animals
Adult female mice that express green fluorescent protein (GFP) under the control of the GnRH promoter were used in all experiments [26]. Mice were maintained under a 14L:10D photoperiod, with Harlan 2916 chow (Harlan, Indianapolis, IN) and water available ad libitum. Female mice, age 42–125 days were OVX and either left with no further treatment or treated with estradiol implants (OVX+E) that produce circulating physiological levels of this steroid [2]. Experiments were performed 2–4 days after surgery in the mornings and in the evenings. Previous work demonstrates this estradiol treatment regimen induces negative and positive feedback effects on LH levels and GnRH neuronal activity during the mornings and evenings, respectively [2]. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Virginia.
Brain Slice Preparation
All chemicals were obtained from Sigma Chemical Company (St. Louis, MO), unless noted otherwise. Brain slices were prepared as previously described [27, 28]. Mice were euthanized by decapitation either from 0800 to 0930 h (negative feedback groups) or from 1330 to 1500 h (positive feedback groups). Briefly, all solutions were bubbled with a 95% O2-5% CO2 mixture throughout the experiments and for at least 15 min before exposure to the tissue. The brain was rapidly removed and placed in an ice-cold, high-sucrose saline solution containing 250 mM sucrose, 3.5 mM KCl, 26 mM NaHCO3, 10 mM glucose, 1.25 mM Na2HPO4, 1.2 mM MgSO4, and 2.5 mM MgCl2. A Vibratome 3000 (Technical Products International, Inc., St. Louis, MO) was used to cut 300-μm coronal brain slices. Slices were incubated for 30 min at 30°C–32°C in a solution of 50% high-sucrose saline and 50% artificial cerebrospinal fluid (ACSF) containing 135 mM NaCl, 26 mM NaHCO3, 3.5 mM KCl, 10 mM glucose, 1.3 mM Na2HPO4, 1.2 mM MgSO4, and 2.5 mM CaCl2, pH 7.4, and were then transferred to a solution of 100% ACSF at room temperature and kept there for at least 30 min and no more than 4 h before recording. Negative feedback recordings were made from 1000 to 1330 h; positive feedback recordings were made from 1500 to 1900 h.
Electrophysiological Recordings
Brain slices were placed in a recording chamber superfused continuously with oxygenated ACSF solution and kept at 29°C–31°C with an inline heating unit (Warner Instruments, Hamden, CT). Brain tissue and cells were visualized with an Olympus BX50WI model upright fluorescence microscope with infrared differential interference contrast (Olympus, Central Valley, PA). GnRH-GFP neurons were identified by brief illumination at 470 nm to visualize the GFP signal. Borosilicate glass capillaries (1.65-mm OD × 1.12-mm ID; World Precision Instruments, Inc., Sarasota, FL) were pulled by using a Flaming/Brown P-97 unit (Sutter Instrument, Novato, CA) to make recording pipettes, which had a tip resistance of 2–4 MΩ. Pipettes were placed in contact with a GnRH neuron by using an MP-225 micromanipulator (Sutter Instruments).
Whole-Cell Voltage Clamp
To monitor recording quality, input resistance (Rin), series resistance (Rs), and membrane capacitance (Cm) were continually measured from the averaged membrane response to 10 5-mV hyperpolarizing voltage steps. Only recordings with an Rin >500 MΩ, a stable Rs <20 MΩ, and a stable Cm were used for analysis. Voltage commands were generated, and currents were obtained using an EPC-8 amplifier running PatchMaster software (HEKA, Mahone Bay, Nova Scotia, Canada). Pipettes were filled with a solution containing 120 mM potassium gluconate, 20 mM KCl, 10 mM HEPES, 5 mM ethylene glycol tetraacetic acid, 4.0 mM MgATP, 0.4 mM NaGTP, and 1.0 mM CaCl2, pH 7.3, 290 mOsm.
To isolate potassium currents, we superfused slices with ACSF supplemented with 0.5 μM tetrodotoxin and 200 μM CdCl2 to block voltage-gated sodium and calcium currents, respectively. Synaptic currents mediated by activation of ionotropic gamma aminobutyric acid (GABAA) or glutamatergic receptors were blocked with a combination of 100 μM picrotoxin, 20 μM APV [d-(–)-2-amino-5-phosphonovaleric acid] and 20 μM CNQX (6-cyano-7-nitroquinoxaline-2,3-dione). Cells were recorded in the whole-cell configuration and held at −60 mV in voltage clamp mode. After a 5-min stabilization period, specific voltage-clamp protocols with slight modifications, described previously [4], were used to record two types of voltage-gated potassium currents: the rapid IA type and the slower, delayed IK type. Because we included CdCl2 in the bath solution, calcium-activated K channels were not activated by this protocol; similarly, inwardly rectified K channels would not be activated because of different voltage dependence. To study inactivation, we hyperpolarized the cell membrane to −110 mV for 500 msec to remove inactivation, applied a 500-msec prepulse (−110 to −10 mV in 10-mV increments), and then measured the currents during a test pulse at −10 mV for 500 msec (Fig. 1A). To study activation, we hyperpolarized the membrane potential to −110 mV for 500 msec, applied a 500-msec prepulse of −110 mV or −40 mV, and then measured currents during test potentials (−90 mV to +40 mV, at 10-mV increments). All traces were leakage subtracted using a (P/-4) protocol to isolate voltage-gated potassium current from background (leakage) current [29]. Membrane potentials were corrected for a 10-mV liquid junction potential [30]. Only one cell was studied per slice and no more than four cells per animal.
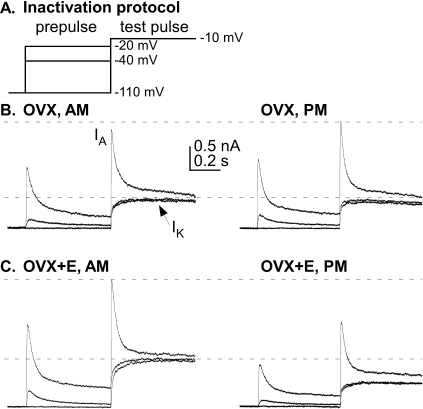
FIG. 1.
GnRH neurons from OVX+E mice but not from OVX mice exhibited a diurnal change in voltage-gated potassium currents. A) A voltage clamp protocol was used to identify voltage-gated potassium currents in GnRH neurons; for clarity, only three prepulse potentials (−110, −40, and −20 mV) are shown. Most of the 500 msec step to −100 mV for removal of inactivation from the A-type channel was omitted. Prepulses and test pulses shown were both 500 msec in duration. B) Representative traces of total potassium current illustrate two components: a fast transient current (IA) and a sustained current (IK) from GnRH neurons from OVX mice, recorded during negative feedback, morning hours (left), and during positive feedback, evening hours (right). The short duration current peak at the beginning of the −40 mV prepulse is the activation of IA, followed by its inactivation during the remainder of the prepulse, allowing measurement of isolated IK during the test pulse. C) Representative traces are shown from GnRH neurons from OVX+E mice recorded during morning hours (left) and evening hours (right).
Kisspeptin Effects on Potassium Currents
To test the effects of kisspeptin on voltage-gated potassium currents, we recorded cells before and during bath application of kisspeptin (10 nM; Phoenix Pharmaceuticals, Inc., Burlingame, CA). Specifically, to record total current (IA plus IK), the membrane was hyperpolarized for 500 msec at −110 mV to remove inactivation, then a 500-msec prepulse (−110 or −40 mV) was given, and the current was measured during a test pulse at −20 mV (500 msec). No differences were detected in kisspeptin action regardless of time of day or estradiol treatment, using this protocol. We therefore tested whether kisspeptin affects IA inactivation and IA or IK activation by using the voltage protocols described in the previous section (i.e., complete inactivation and activation protocols, with a test pulse at −10 mV) only in OVX+E cells in the morning.
Analysis
Peaks of IA and IK, average steady-state IK, IA inactivation, and IA and IK activation values were calculated using customized software written in Igor Pro (Wavemetrics, Lake Oswego, OR). To calculate inactivation of IA, IA was mathematically isolated using the voltage dependence of inactivation. Inactivation was complete at −40 mV (i.e., no fast transient current was present in current traces after the −40 mV prepulse). IA was isolated by subtracting the current after the −40 mV prepulse from that after more hyperpolarized prepulses. Peak current was normalized to the average of the values after prepulses to −90 and −70 mV. Current densities were obtained by dividing calculated peak values of the currents by capacitance of the cells from which the current was measured and were used in all comparisons. All data were transferred to Excel (Microsoft, Redmond, WA), InStat, or Prism software (Graph Pad Software, San Diego, CA) for statistical analysis. Potassium currents between groups were compared using two-way ANOVA with Student-Newman-Keuls post hoc tests. Kisspeptin effects on IA inactivation and IA and IK activation were compared using two-tailed paired t-tests. All data are presented as means ± SEM (unless noted otherwise), and significance was set at a P value of <0.05.
RESULTS
Diurnal Changes in Potassium Currents of GnRH Neurons Are Observed Only in Cells from Estradiol-Treated Mice
Changes in the response of the GnRH neuronal network to estradiol feedback is reproduced on a daily basis in OVX mice treated with constant-release estradiol implants [2]. To test the hypothesis that the diurnal shift between estradiol negative and positive feedback action is due in part to changes in voltage-gated potassium currents, we isolated and measured these currents using whole-cell voltage clamp recordings of GnRH neurons in brain slices from OVX and OVX+E mice at those times when estradiol-treated mice exhibited negative (morning) and positive (evening) feedback. Figure 1 shows the voltage protocol and representative raw current traces from all four groups; Figure 2 shows inactivation and summary data.
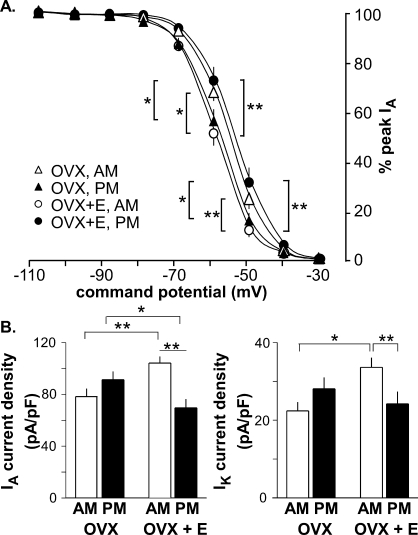
FIG. 2.
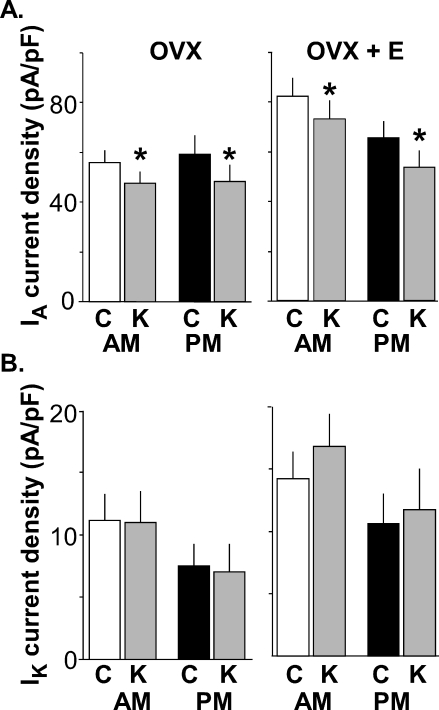
Estradiol alters inactivation of IA. All data are means ± SEM. A) IA inactivation (as normalized current) is shown as a function of command potential. B) Summary bar graphs show the effects of estradiol and time of day on subtracted IA current density following −100 mV prepulse (left) and IK current density following −40 mV prepulse (right) in GnRH neurons from OVX mice (morning, n = 11; evening, n = 11) and OVX+E mice (morning, n = 13; evening, n = 8) during morning hours (white bars) and evening hours (black bars). **P < 0.005; *P < 0.05. The test pulse was set at −10 mV. Significant differences in inactivation at −40 mV are observed between OVX evening and OVX+E evening and between OVX+E morning and OVX+E evening; these are not indicated in A for clarity (both, P < 0.05).
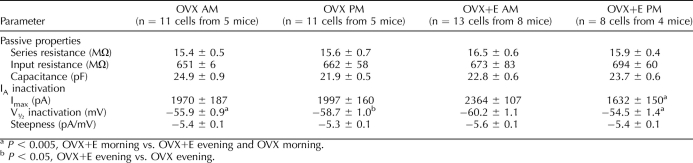
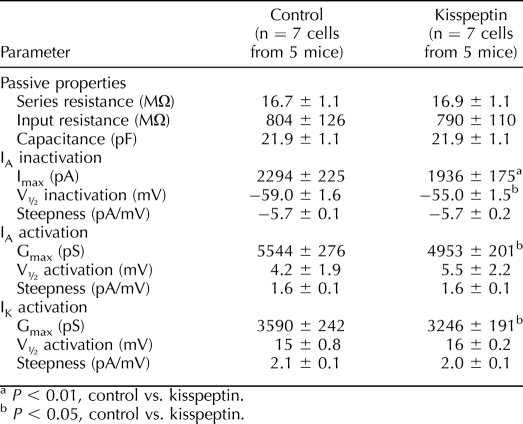
In GnRH neurons from OVX control mice, there were no diurnal changes in current density (amount of current normalized by the capacitance of the cell) of either IA or IK (Figs. 1B and 2B, and see Tables 1 and 2 for number of cells). Additionally, neither the voltage at which the IA level was half inactivated (V1/2 inact [Table 1]) nor the percentage of inactivation as a function of command potential was changed by time of day (Fig. 2A). In marked contrast, GnRH neurons from OVX+E mice showed diurnal changes in both IA and IK (Figs. 1C and 2B). Specifically, the current densities of both IA and IK were greater in GnRH neurons from OVX+E mice recorded in the morning during negative feedback than those recorded in the evening during positive feedback (OVX+E morning vs. OVX+E evening, P < 0.005 [Figs. 1C and 2B, left]). The V1/2 inact value during the morning was hyperpolarized relative to that in the evening in cells from OVX+E mice (Fig. 2A, Table 1, P < 0.005). Unlike inactivation, there were no differences in IA and IK activation characteristics (maximum current [Imax], V1/2 activation, and steepness) among groups (Table 2). Changes observed in current density and inactivation were likely due to alterations in channel properties because there were no differences in passive electrophysiological properties (Rin and Cm) or series resistance among groups (Table 1).
TABLE 1.
GnRH neuron passive properties and IA inactivation.
TABLE 2.
IA and IK activation in GnRH neurons.
During the time of negative feedback (morning), estradiol induced increases in current densities of both IA (P < 0.005) and IK (P < 0.05) compared to those in cells from OVX mice (Fig. 2B). During the time of positive feedback (evening), however, estradiol suppressed only IA current density (P < 0.05), without changing IK. Likewise, estradiol shifted the inactivation curve of IA relative to that in cells from OVX mice to more hyperpolarized values in the morning and more depolarized values in the evening (morning, P < 0.005; evening, P < 0.05 [Fig. 2A]). Together these data suggest that the presence of estradiol induces diurnal changes in aspects of voltage-gated potassium currents in GnRH neurons and that the direction of this alteration is consistent with observed changes in GnRH neuronal activity during negative and positive feedback. That is, increased voltage-gated potassium current was observed when GnRH neurons were more quiescent during negative feedback and vice versa [2].
Kisspeptin Reduces IA in GnRH Neurons and Its Actions Are Not Dependent on Estradiol or Time of Day
Previous work has indicated kisspeptin can change potassium currents in GnRH neurons, but specific subtypes were not investigated [21]. To determine which components of this current in GnRH neurons are altered by kisspeptin and also if kisspeptin action is dependent on estradiol and/or time of day, we made recordings of GnRH neurons from OVX and OVX+E mice during the morning (negative feedback) and the evening (positive feedback). Kisspeptin (10 nM) reduced IA current density in a similar manner in cells from all four groups of mice (representative traces are shown in Fig. 3; summary is shown in Fig. 4; P < 0.001 for all groups). Note that currents shown in Figures 3 and 4 were measured at a −20-mV test pulse and are thus smaller than those shown in Figures 1, 2, and 5, which were measured at −10 mV; this difference in test pulse would not affect comparisons made within each experiment or interpretation of results. Kisspeptin had no effect on IK in any group (representative traces are shown in Fig. 3; summary is shown in Fig. 4; P > 0.3). Transient currents such as IA are particularly sensitive to changes in recording quality over time. To control for this, we examined series resistance to ensure it was stable within recordings and among groups and found no differences (OVX+E morning control values were 15.2 ± 0.5 MΩ and 15.3 ± 0.5 MΩ for kisspeptin; OVX+E evening control values were 15.3 ± 0.9 MΩ, and 15.4 ± 0.9 MΩ for kisspeptin; OVX morning values for control were 14.6 ± 0.3 MΩ and 14.5 ± 0.3 MΩ for kisspeptin; and OVX evening control values were 15.2 ± 0.9 MΩ and 14.4 ± 0.9 MΩ for kisspeptin; P > 0.9). Furthermore, recordings of additional cells demonstrated that peak IA current was maintained at a steady level for the duration of time required to test the effect of kisspeptin. There was no change in IA or IK over time in these control cells (sham treatment included n = 4 cells from three mice; IA in control was 83.6 ± 8.2 pA/pF and 83.6 ± 9.2 pA/pF for sham; and Ik in control was 14.7 ± 1.7 pA/pF and 16.1 ± 3.1 pA/pF for sham; both, P > 0.7 [Fig. 3C]). These data show that the part of kisspeptin actions that increases GnRH neuronal activity involves reduction in IA and that this effect does not appear to be dependent on estradiol or time of day.
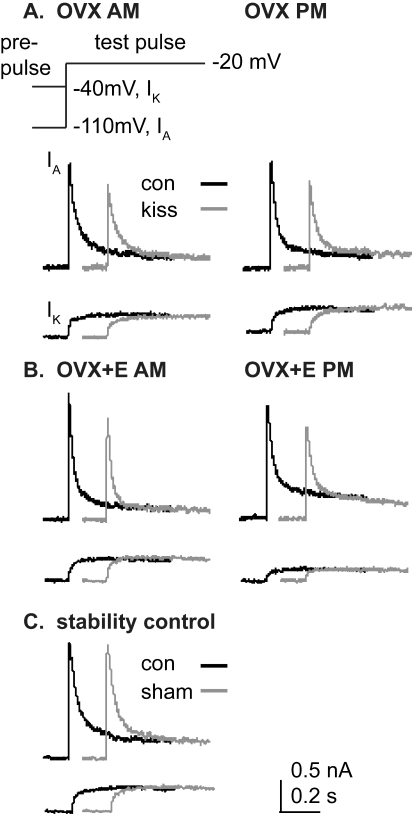
FIG. 3.
Kisspeptin reduces IA in GnRH neurons regardless of time of day or estradiol treatment but has no effect on IK. A and B) Top of part A shows the voltage protocol; only the final 200 msec of the 500-msec prepulse is shown for both the protocol and the data below. Representative traces of IA and IK were obtained during control conditions (black) and after kisspeptin treatment (gray) in GnRH neurons from an OVX (A) and OVX+E (B) mouse recorded during morning hours (left) and during evening hours (right). C) Representative traces of IA and IK from an untreated GnRH neuron from an OVX+E mouse recorded during morning illustrating recording stability in the absence of treatment are shown.
FIG. 4.
Summary bar graphs show effects of kisspeptin (gray bars) on IA and IK in GnRH neurons from OVX (n = 9, morning; n = 8, evening) and OVX+E (n = 9, morning; n = 8, evening) mice recorded in the morning (white bars) and evening (black bars). A and B) IA and IK current densities are shown, each at a test pulse of −20 mV. C, control; K, kisspeptin. All data are means ± SEM. *P < 0.001, control vs. kisspeptin.
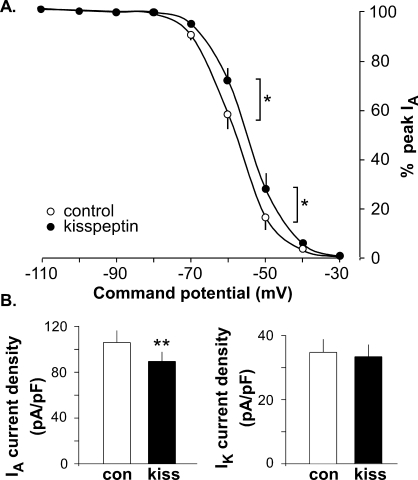
Kisspeptin Depolarizes IA Inactivation but Does Not Change Activation
The above-described results showed that kisspeptin reduced IA current density regardless of estradiol status or time of day. To further investigate the effects of kisspeptin, we examined whether it altered inactivation and/or activation of IA. Because there was no time of day or estradiol difference, these studies were conducted only in cells from OVX+E mice in the morning, before and after kisspeptin treatment. Kisspeptin significantly depolarized V1/2 inact (Table 3) and the inactivation curve of IA (Fig. 5) but had no effect on V1/2 act of either IA or IK (Table 3). As shown in Figures 3 and 4, kisspeptin significantly reduced IA current density and had no effect on IK current density. Kisspeptin also reduced the maximum conductance of both IA and IK (Table 3, −10-mV test pulse).
TABLE 3.
Kisspeptin effects on potassium currents in GnRH neurons.
FIG. 5.
Kisspeptin depolarized IA inactivation in GnRH neurons from the OVX+E morning group. A) Voltage dependence of IA inactivation is shown as a function of command potential. B) Summary bar graphs show means ± SEM values of kisspeptin effect on IA (left) and IK (right) current density (n = 7). *P < 0.05; **P < 0.01.
DISCUSSION
Steroid milieu alters the firing activity of GnRH neurons and hormone release [2, 31–35]. Estradiol is of particular interest as it exerts both negative and positive feedback actions on GnRH neuron activity and LH secretion [35–42], with positive feedback being a critical aspect of the preovulatory neuroendocrine signal. Both of these actions can be studied in a reduced-variable setting by using an animal model that exhibits diurnal changes in GnRH neuron activity and LH levels subsequent to OVX plus physiological-level estradiol replacement [2]. We used this model to study the role of voltage-gated potassium current modulation in estradiol feedback regulation of GnRH neurons. Both the rapidly inactivating transient A-type current, IA, and the noninactivating delayed rectifier, IK, current are targets of feedback interactions between estradiol and time of day. In contrast, the ability of kisspeptin to alter potassium currents is limited to the transient subtype IA, and this action is not dependent upon time of day or estradiol.
Potassium channels are among the most important determinants of both a neuron's intrinsic excitability and its responsiveness to both inhibitory and excitatory synaptic inputs [43]. Modulation of the current that flows through these channels could thus have a major effect on action potential firing and subsequent hormone release from a cell. The present data suggest that an underlying mechanism for the switch between estradiol negative and positive feedback is the fact that estradiol interacts with a daily signal(s) to alter two components of voltage-gated potassium currents that are intrinsic to GnRH neurons, IA and IK. Specifically, during negative feedback, estradiol increased IA and IK. Because these currents tend to hyperpolarize the membrane potential, this finding is consistent with decreased excitability. This increase was relative to both that in cells from OVX mice at the same time of day and to that in cells from OVX+E mice during the time of positive feedback. IK of GnRH neurons from OVX mice was similar to that of GnRH neurons from OVX+E mice during the time of positive feedback, suggesting changes in this current are likely more involved in the suppression of GnRH neurons by estradiol during negative feedback than in their activation during positive feedback. These data support those from a previous study carried out before the diurnal aspects of estradiol feedback were appreciated in the mouse [5]. In that study, evening recordings made after a longer estradiol treatment revealed an inhibition of potassium currents by estradiol. By extending those studies to defined modes of estradiol feedback, the present studies reveal that the action of estradiol is not a simple unidirectional inhibition of these currents but rather that estradiol can enhance the same currents during other parts of the diurnal cycle and thus play a role in both the activation and suppression of GnRH neuron activity. An interesting question is whether these changes are restricted to GnRH neurons or whether they also occur in other cells. In this regard, our previous work indicated magnocellular neurons of the paraventricular nucleus did not exhibit changes in response to a slightly different regimen of estradiol feedback [4]. Further studies with the daily surge model are needed to more clearly address this question.
Increased outward potassium currents such as those observed during negative feedback would tend to hyperpolarize the cell's membrane potential and thus inhibit firing rate. Increased potassium currents within GnRH neurons would also reduce responsiveness to excitatory synaptic inputs. The low GnRH neuronal activity observed during negative feedback is thus likely the result, at least in part, of increased voltage-gated potassium current tone. Similarly, the estradiol-induced reduction in potassium currents during the time of positive feedback would tend to increase intrinsic excitability of GnRH neurons as well as their response to excitatory inputs. The lack of diurnal changes in potassium currents in GnRH neurons from OVX mice indicates these currents are altered as a result of interactions between estradiol and the circadian pacemaker. This observation is consistent with that from previous studies in this animal model in which no diurnal change in LH levels, GnRH neuron firing activity, fast synaptic transmission, or calcium currents were observed in GnRH neurons in cells from OVX mice in the absence of estradiol [10–12]. It is important to point out that other types of potassium currents, such as M-type and inward rectifiers, may also be correlated with changes in GnRH neuron activity in this model [44, 45].
The estradiol-induced changes in voltage-gated potassium currents in GnRH neurons that arose subsequent to estradiol/time of day interactions could be caused by either the direct action of estradiol on GnRH neurons through ERβ or indirect actions through ERα- or ERβ-expressing afferents that alter signaling and/or gene expression in GnRH neurons. With regard to the former, GnRH neurons express clock genes [46], and many tissues have been shown to be capable of maintaining aspects of circadian organization in the absence of the primary circadian pacemaker in the suprachiasmatic nuclei (SCN) [47]. Circadian changes in potassium channel function have been reported in neurons from Bulla gouldiana [48], and immortalized GnRH neurons appear to express an endogenous clock [49]. Considerable previous work has demonstrated a role for the SCN in estradiol positive feedback, suggesting this region may be dominant over clocks within individual GnRH neurons [50–52]. There are direct connections from the SCN to GnRH neurons via vasoactive intestinal polypeptide (VIP) and GABAergic neurons [53–55], as well as indirect connections via anterior ventral periventricular (AVPV) nuclei [56]. AVPV nuclei appear to serve as integrators of the effects of estradiol and time of day in the surge response [57].
One possible candidate for conveying the integrated signal from AVPV nuclei to GnRH neurons is the neuromodulator kisspeptin. AVPV kisspeptin neurons express both nuclear estrogen receptor isoforms, the expression of kisspeptin mRNA in this region is enhanced by estradiol, and these neurons express c-Fos, a surrogate marker of neuronal activation, during estradiol positive feedback [15, 22, 58, 59]. Recent studies have shown kisspeptin can excite GnRH neurons via reduction of potassium currents, but previously, only inwardly rectified potassium currents have been identified as specific targets for kisspeptin. Different subtypes of potassium channels are activated at different membrane potentials and thus can affect different aspects of cellular function. Here we show that kisspeptin decreases specifically the IA component of voltage-gated potassium currents. Because activation of IA occurs at more hyperpolarized potentials, this current can be activated near the interspike, or “resting,” membrane potential in GnRH neurons [4]. This characteristic makes IA particularly interesting as a modulator of GnRH neuron activity because changes near this membrane potential can have a marked effect on the ability of a cell to initiate action potential (spike) firing. IA increases the latency to action potential initiation following a depolarizing stimulus and can even block spike initiation; both of these actions would lower the neuronal firing rate [4]. The present observation that IA is inhibited by kisspeptin is consistent with that of previous work that demonstrated kisspeptin generates a depolarizing current in GnRH neurons held near the interspike potential (−60 mV) [21].
Kisspeptin reduced IA in GnRH neurons regardless of estradiol status or time of day. One possible interpretation of these findings is that the response of GnRH neurons to kisspeptin is independent of time of day or estradiol milieu and that diurnal changes in endogenous kisspeptin release (low during negative feedback; high during positive feedback) help drive the estradiol-induced changes in IA in GnRH neurons. At present, direct measures of kisspeptin release or kisspeptin neuron activity as a function of defined estradiol feedback mode have not been published. Indirect evidence, however, is consistent with this postulate of diurnal changes in kisspeptin release. First, the ability of exogenous kisspeptin to increase GABAergic transmission to GnRH neurons during the time of negative feedback is occluded during positive feedback [60], suggesting increased endogenous release of kisspeptin during positive feedback. Note that the recording conditions for studying GABAergic transmission are different from those needed to isolate potassium currents, and the use of tetrodotoxin in the present study, while necessary to record potassium currents, would impair endogenous kisspeptin release. Second, as mentioned above, kisspeptin neurons express c-Fos during the LH surge [22, 61]. Third, during positive feedback, there is an increase in spontaneous GABAergic transmission to GnRH neurons [11]. AVPV kisspeptin neurons were recently shown to coexpress the synthetic enzyme for GABA, indicating that they are GABAergic [62]. Because GABAergic AVPV neurons project to GnRH neurons [63], kisspeptin is expressed by AVPV neurons, and kisspeptin-positive fibers make close appositions with GnRH neurons [64], it is possible that the increase in GABAergic transmission marks an increase in the corelease of kisspeptin. Fourth, excitatory actions of kisspeptin on GnRH neurons have also been shown to involve changes in nonselective cationic conductances, as well as suppression of GABAB receptor-mediated inhibition of GnRH neurons [19, 65]. These parameters might also be differentially affected by circadian rhythms and hormonal status.
Although estradiol and time of day interacted to alter both IA and IK in GnRH neurons, kisspeptin affected only the A-type potassium current. This suggests that either estradiol itself directly modulates IK of GnRH neurons through ERβ or that it acts through estradiol-sensitive afferents containing different neuromodulators/neurotransmitters. In this regard, estradiol affects GnRH neuronal activity via direct mechanisms [13, 66] and regulation of both GABAergic and glutamatergic transmission to GnRH neurons. Specifically, in vivo estradiol treatment reduces GABAergic and glutamatergic transmission during negative feedback and increases GABAergic transmission during positive feedback [11, 12]. The neuromodulator VIP is another candidate that potentially alters IK [67]; both estradiol and time of day affect the GnRH neuron response to this peptide. Of interest, VIP has been shown to reduce a similarly delayed rectifier potassium current in neurons from the suprachiasmatic nucleus [68].
Whether estradiol and neuromodulators act directly on GnRH neurons or indirectly via afferents, or both, to change potassium currents in these cells, there are several cellular biological mechanisms that may be deployed within the GnRH neuron to bring about the observed changes in potassium currents. Expression of either pore-forming or accessory channel subunits could be altered [7, 69]. The phosphorylation state of these proteins could be changed [70]; in this regard, previous studies in GnRH neurons indicated in vivo estradiol effects that reduced potassium conductances could be reversed in vitro with a broad-spectrum kinase inhibitor [4]. Alterations in channel trafficking to the membrane may occur [71]. Voltage-gated potassium currents are also sensitive to lipid environment [72], which might be altered by kisspeptin signaling via changes in phospholipids [73, 74]. These mechanisms are not mutually exclusive and may act in different combinations to bring about the observed changes.
The present findings extend our understanding of the neurobiological actions of estradiol negative and positive feedback actions on GnRH neurons by demonstrating that voltage-gated potassium currents intrinsic to GnRH neurons are modulated by estradiol in a manner that is dependent on time of day and are also targets of kisspeptin action. This opens the possibility for future studies to further elucidate the relationship between estradiol and kisspeptin in regulating GnRH neurons, as well as to determine signaling pathways involved in the alterations of voltage-gated potassium channels in GnRH neurons. Together with previous work, these data indicate that estradiol acts through multiple direct and trans-synaptic mechanisms to modulate GnRH neuronal activity and that its effects on GnRH output are likely via an integration of these mechanisms.
ACKNOWLEDGMENTS
We thank Debra Fisher and Laura Burger for excellent technical assistance and Catherine Christian, Laura Burger, Garrett Gaskins, and Elizabeth Wagenmaker for editorial comments.
Footnotes
Supported by National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development grant R01 HD41469. Portions of this work were presented at the 39th Annual Meeting of the Society for Neuroscience, October 17–21, 2009, Chicago, Illinois, and at the 92nd Annual Meeting of the Endocrine Society, June 19–22, 2010, San Diego, California.
REFERENCES
- Christian CA, Moenter SM. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr Rev 2010; 31: 544 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Mobley JL, Moenter SM. Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci U S A 2005; 102: 15682 15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Ronnekleiv OK, Ibrahim N, Lagrange AH, Wagner EJ. Estrogen modulation of K(+) channel activity in hypothalamic neurons involved in the control of the reproductive axis. Steroids 2002; 67: 447 456. [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Moenter SM. Estradiol feedback alters potassium currents and firing properties of gonadotropin-releasing hormone neurons. Mol Endocrinol 2002; 16: 2255 2265. [DOI] [PubMed] [Google Scholar]
- Moenter SM. Identified GnRH neuron electrophysiology: a decade of study. Brain Res 2010; 1364: 10 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ion channels of excitable membranes. Sunderland, MA: Sinauer Associates, Inc.; 2001. [Google Scholar]
- Roepke TA, Malaya A, Bosch MA, Kelly MJ, Ronnekleiv OK. Estrogen regulation of genes important for K+ channel signaling in the arcuate nucleus. Endocrinology 2007; 148: 4937 4951. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Chen C, Clarke IJ. Estrogen transiently increases delayed rectifier, voltage-dependent potassium currents in ovine gonadotropes. Neuroendocrinology 1999; 69: 254 260. [DOI] [PubMed] [Google Scholar]
- Kow LM, Devidze N, Pataky S, Shibuya I, Pfaff DW. Acute estradiol application increases inward and decreases outward whole-cell currents of neurons in rat hypothalamic ventromedial nucleus. Brain Res 2006; 1116: 1 11. [DOI] [PubMed] [Google Scholar]
- Sun J, Chu Z, Moenter SM. The diurnal in vivo and rapid in vitro effects of estradiol on voltage-gated calcium channels (VGCCs) in gonadotropin-releasing hormone (GnRH) neurons. J Neurosci 2010; 30: 3912 3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Moenter SM. Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. J Neurosci 2007; 27: 1913 1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Pielecka-Fortuna J, Moenter SM. Estradiol suppresses glutamatergic transmission to gonadotropin-releasing hormone neurons during negative feedback. Biol Reprod 2009; 80: 1128 1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Z, Andrade J, Shupnik MA, Moenter SM. Differential regulation of gonadotropin-releasing hormone neuron activity and membrane properties by acutely applied estradiol: dependence on dose and estrogen receptor subtype. J Neurosci 2008; 29: 5616 5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 2005; 146: 2976 2984. [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 2005; 146: 3686 3692. [DOI] [PubMed] [Google Scholar]
- Christian CA, Glidewell-Kenney C, Jameson JL, Moenter SM. Classical estrogen receptor alpha signaling mediates negative and positive feedback on gonadotropin-releasing hormone neuron firing. Endocrinology 2008; 149: 5328 5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 2005; 25: 11349 11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumalska I, Wu M, Morozova E, Liu R, van den Pol A, Alreja M. Excitatory effects of the puberty-initiating peptide kisspeptin and group I metabotropic glutamate receptor agonists differentiate two distinct subpopulations of gonadotropin-releasing hormone neurons. J Neurosci 2008; 28: 8003 8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Roepke TA, Kelly MJ, Rønnekleiv OK. Kisspeptin depolarizes gonadotropin–releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci 2008; 28: 4423 4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci 2006; 26: 6687 6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology 2008; 149: 1979 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J. d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci 2008; 28: 8691 8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A. et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A 2005; 102: 1761 1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE, de Tassigny X, Doran J, Colledge WH. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology 2010; 151: 312 321. [DOI] [PubMed] [Google Scholar]
- Liu X, Lee K, Herbison AE. Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology 2008; 149: 4605 4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology 2000; 141: 412 419. [DOI] [PubMed] [Google Scholar]
- Nunemaker CS, DeFazio RA, Moenter SM. Estradiol-sensitive afferents modulate long-term episodic firing patterns of GnRH neurons. Endocrinology 2002; 143: 2284 2292. [DOI] [PubMed] [Google Scholar]
- Chu Z, Moenter SM. Endogenous activation of metabotropic glutamate receptors modulates GABAergic transmission to gonadotropin-releasing hormone neurons and alters their firing rate: a possible local feedback circuit. J Neurosci 2005; 25: 5740 5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F, Armstrong CM. Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol 1977; 70: 549 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry PH. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Methods 1994; 51: 107 116. [DOI] [PubMed] [Google Scholar]
- Pielecka J, Moenter SM. Effect of steroid milieu on gonadotropin-releasing hormone-1 neuron firing pattern and luteinizing hormone levels in male mice. Biol Reprod 2006; 74: 931 937. [DOI] [PubMed] [Google Scholar]
- Pielecka J, Quaynor SD, Moenter SM. Androgens increase gonadotropin-releasing hormone neuron firing activity in females and interfere with progesterone negative feedback. Endocrinology 2006; 147: 1474 1479. [DOI] [PubMed] [Google Scholar]
- Levine JE, Norman RL, Gliessman PM, Oyama TT, Bangsberg DR, Spies HG. In vivo gonadotropin-releasing hormone release and serum luteinizing hormone measurements in ovariectomized, estrogen-treated rhesus macaques. Endocrinology 1985; 117: 711 721. [DOI] [PubMed] [Google Scholar]
- Clarke IJ. Variable patterns of gonadotropin-releasing hormone secretion during the estrogen-induced luteinizing hormone surge in ovariectomized ewes. Endocrinology 1993; 133: 1624 1632. [DOI] [PubMed] [Google Scholar]
- Moenter SM, Brand RC, Karsch FJ. Dynamics of gonadotropin-releasing hormone (GnRH) secretion during the GnRH surge: insights into the mechanism of GnRH surge induction. Endocrinology 1992; 130: 2978 2984. [DOI] [PubMed] [Google Scholar]
- Levine JE, Ramirez VD. In vivo release of luteinizing hormone-releasing hormone estimated with push-pull cannulae from the mediobasal hypothalami of ovariectomized, steroid-primed rats. Endocrinology 1980; 107: 1782 1790. [DOI] [PubMed] [Google Scholar]
- Caraty A, Locatelli A, Martin GB. Biphasic response in the secretion of gonadotrophin-releasing hormone in ovariectomized ewes injected with oestradiol. J Endocrinol 1989; 123: 375 382. [DOI] [PubMed] [Google Scholar]
- Karsch FJ, Cummins JT, Thomas GB, Clarke IJ. Steroid feedback inhibition of pulsatile secretion of gonadotropin-releasing hormone in the ewe. Biol Reprod 1987; 36: 1207 1218. [DOI] [PubMed] [Google Scholar]
- Moenter SM, Caraty A, Karsch FJ. The estradiol-induced surge of gonadotropin-releasing hormone in the ewe. Endocrinology 1990; 127: 1375 1384. [DOI] [PubMed] [Google Scholar]
- Moenter SM, Caraty A, Locatelli A, Karsch FJ. Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: existence of a preovulatory GnRH surge. Endocrinology 1991; 129: 1175 1182. [DOI] [PubMed] [Google Scholar]
- Evans NP, Dahl GE, Padmanabhan V, Thrun LA, Karsch FJ. Estradiol requirements for induction and maintenance of the gonadotropin-releasing hormone surge: implications for neuroendocrine processing of the estradiol signal. Endocrinology 1997; 138: 5408 5414. [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 2006; 52: 271 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D, Hoffman DA, Magee JC, Poolos NP, Watanabe S, Colbert CM, Migliore M. Dendritic potassium channels in hippocampal pyramidal neurons. J Physiol 2000; 525: 75 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Levine JE, Ronnekleiv OK, Kelly MJ. Gonadotropin-releasing hormone neurons express K(ATP) channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J Neurosci 2007; 27: 10153 10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Roepke TA, Zhang C, Ronnekleiv OK, Kelly MJ. Gonadotropin-releasing hormone (GnRH) activates the M-current in GnRH neurons: an autoregulatory negative feedback mechanism? Endocrinology 2008; 149: 2459 2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell PE, White RS, Mellon PL. Circadian gene expression regulates pulsatile gonadotropin-releasing hormone (GnRH) secretory patterns in the hypothalamic GnRH-secreting GT1-7 cell line. J Neurosci 2003; 23: 11202 11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science 2000; 288: 682 685. [DOI] [PubMed] [Google Scholar]
- Michel S, Geusz ME, Zaritsky JJ, Block GD. Circadian rhythm in membrane conductance expressed in isolated neurons. Science 1993; 259: 2399 2241. [DOI] [PubMed] [Google Scholar]
- Chappell PE, Goodall CP, Tonsfeldt KJ, White RS, Bredeweg E, Latham KL. Modulation of gonadotrophin-releasing hormone secretion by an endogenous circadian clock. J Neuroendocrinol 2009; 21: 339 345. [DOI] [PubMed] [Google Scholar]
- Brown-Grant K, Raisman G. Abnormalities in reproductive function associated with the destruction of the suprachiasmatic nuclei in female rats. Proc R Soc Lond B Biol Sci 1977; 198: 279 296. [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E, Bridson WE, Goy RW. Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology 1980; 31: 147 157. [DOI] [PubMed] [Google Scholar]
- Miller BH, Olson SL, Turek FW, Levine JE, Horton TH, Takahashi JS. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol 2004; 14: 1367 1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath TL, Cela V, van der Beek EM. Gender-specific apposition between vasoactive intestinal peptide-containing axons and gonadotrophin-releasing hormone-producing neurons in the rat. Brain Res 1998; 795: 277 281. [DOI] [PubMed] [Google Scholar]
- Van der Beek EM, Horvath TL, Wiegant VM, Van den Hurk R, Buijs RM. Evidence for a direct neuronal pathway from the suprachiasmatic nucleus to the gonadotropin-releasing hormone system: combined tracing and light and electron microscopic immunocytochemical studies. J Comp Neurol 1997; 384: 569 579. [DOI] [PubMed] [Google Scholar]
- Strecker GJ, Wuarin JP, Dudek FE. GABAA-mediated local synaptic pathways connect neurons in the rat suprachiasmatic nucleus. J Neurophysiol 1997; 78: 2217 2220. [DOI] [PubMed] [Google Scholar]
- Watson RE, Langub MC, Engle MG, Maley BE. Estrogen-receptive neurons in the anteroventral periventricular nucleus are synaptic targets of the suprachaismatic nucleus to the gonadotropin-releasing hormone system: combined tracing and light and electron microscopic immunocytochemical studies. Brain Res 1995; 689: 254 264. [DOI] [PubMed] [Google Scholar]
- de la Iglesia HO, Blaustein JD, Bittman EL. The suprachiasmatic area in the female hamster projects to neurons containing estrogen receptors and GnRH. Neuroreport 1995; 6: 1715 1722. [DOI] [PubMed] [Google Scholar]
- Dungan HM, Gottsch ML, Zeng H, Gragerov A, Bergmann JE, Vassilatis DK, Clifton DK, Steiner RA. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci 2007; 27: 12088 12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology 2009; 150: 3664 3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielecka-Fortuna J, Moenter SM. Kisspeptin increases gamma-aminobutyric acidergic and glutamatergic transmission directly to gonadotropin-releasing hormone neurons in an estradiol-dependent manner. Endocrinology 2010; 151: 291 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman GE, Le WW, Franceschini I, Caraty A, Advis JP. Expression of Fos and in vivo median eminence release of LHRH identifies an active role for preoptic area kisspeptin neurons in synchronized surges of LH and LHRH in the ewe. Endocrinology 2011; 152: 214 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J, Jr, Atkin S, Bookout AL, Rovinsky S, Frazao R, Lee CE, Gautron L, Zigman JM, Elias CF. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience 2011; 173: 37 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottem EN, Godwin JG, Krishnan S, Petersen SL. Dual-phenotype GABA/glutamate neurons in adult preoptic area: sexual dimorphism and function. J Neuorsci 2004; 24: 8097 8105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus: sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 2006; 147: 5817 5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Rønnekleiv OK, Kelly MJ. Gamma-aminobutyric acid B receptor mediated inhibition of gonadotropin-releasing hormone neurons is suppressed by kisspeptin-G protein-coupled receptor 54 signaling. Endocrinology 2009; 150: 2388 2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe H, Keen KL, Terasawa E. Rapid action of estrogens on intracellular calcium oscillations in primate luteinizing hormone-releasing hormone-1 neurons. Endocrinology 2008; 149: 1155 1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Moenter SM. Vasoactive intestinal polypeptide can excite gonadotropin-releasing hormone neurons in a manner dependent on estradiol and gated by time of day. Endocrinology 2008; 149: 3130 3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakhotin P, Harmar AJ, Verkhratsky A, Piggins H. VIP receptors control excitability of suprachiasmatic nuclei neurones. Pflugers Arch 2006; 452: 7 15. [DOI] [PubMed] [Google Scholar]
- Malyala A, Kelly MJ, Ronnekleiv OK. Estrogen modulation of hypothalamic neurons: activation of multiple signaling pathways and gene expression changes. Steroids 2005; 70: 397 406. [DOI] [PubMed] [Google Scholar]
- Park KS, Yang JW, Seikel E, Trimmer JS. Potassium channel phosphorylation in excitable cells: providing dynamic functional variability to a diverse family of ion channels. Physiology 2008; 23: 49 57. [DOI] [PubMed] [Google Scholar]
- Muchekehu RW, Harvey BJ. Estradiol rapidly induces the translocation and activation of the intermediate conductance calcium activated potassium channel in human eccrine sweat gland cells. Steroids 2009; 74: 212 217. [DOI] [PubMed] [Google Scholar]
- Oliver D, Lien C-C, Soom M, Baukrowitz T, Jonas P, Fakler B. Functional conversion between A-type and delayed rectifer K+ channels by membrane lipids. Science 2004; 304: 265 270. [DOI] [PubMed] [Google Scholar]
- Castano JP, Martinez-Fuentes AJ, Gutierrez-Pascual E, Vaudry H, Tena-Sempere M, Malagon MM. Intracellular signaling pathways activated by kisspeptins through GPR54: do multiple signals underlie function diversity? Peptides 2009; 30: 10 15. [DOI] [PubMed] [Google Scholar]
- Constantin S, Caligioni CS, Stojilkovic S, Wray S. Kisspeptin-10 Facilitates a Plasma Membrane-Driven Calcium Oscillator in Gonadotropin-Releasing Hormone-1 Neurons. Endocrinology 2009; 150: 1400 1412. [DOI] [PMC free article] [PubMed] [Google Scholar]