Abstract
The placental vasculature is critical for nutrient, gas, and waste exchange between the maternal and fetal systems. Its development depends on the proper expression and interaction of angiogenesis and associated growth factors. Heme oxygenase (HMOX), the enzyme for heme degradation, plays a role in angiogenesis and is highly expressed in the placenta. To evaluate the role of maternal HMOX1, the inducible HMOX isozyme, on placental vasculature formation, mice with a partial deficiency in Hmox1 (Hmox1+/−) were used. Three-dimensional images of placental vasculatures as well as spiral arteries from Hmox1+/+ or Hmox1+/− placentas were created by vascular corrosion casting technique and imaged by micro-computerized tomography (microCT). The structures and morphologies of fetomaternal interfaces were observed by histological staining and the ultrastructure of uterine natural killer (uNK) cells, a major regulator in spiral artery remodeling, was analyzed by transmission electron microscopy. A group of growth factors and angiogenic factors from the decidua/mesometrial lymphoid aggregate of pregnancy (MLAp) as well as labyrinth regions were quantified using an angiogenesis PCR array kit and compared between Hmox1+/+ or Hmox1+/− placentas. In conclusion, a partial deficiency of maternal Hmox1 resulted in the malformation of fetomaternal interface, insufficiency of spiral artery remodeling, and alteration of uNK cell differentiation and maturation. These changes were independent of the fetal genotype, but relied on the maternal HMOX1 level, which determined the balance of expression levels of pro- and antiangiogenic factors in the decidua/MLAp region. These results implied that Hmox1 polymorphisms among the human population might contribute to some unexplained cases of pregnancy disorders, such as fetal growth retardation and preeclampsia.
Keywords: angiogenesis, heme oxygenase 1, intrauterine growth restriction (IUGR), placenta, spiral artery remodeling, uterine natural killer (uNK) cell
Maternal HMOX1 is essential for the formation of the fetomaternal interface, remodeling of the uterine spiral arteries, and differentiation and maturation of uNK cells; its effects are primarily mediated through the regulation of a group of pro- and anti-angiogenesis factors in the decidua/MLAp region.
INTRODUCTION
The placenta is a well-organized vascular network derived from both the maternal and fetal vasculature systems to form the fetomaternal interface. On the maternal side, uterine spiral arteries (SAs) undergo remodeling and enlargement to provide larger blood volume, but slower blood flow, into the maternal blood sinus to optimize gas and nutrient exchange. On the fetal side, respiratory gases and waste are transported via the umbilical cord and enter through branched vessels, finally ending at the fetal capillary space. The process is very fine-tuned, dependent on a balance between angiogenesis/vasculogenesis and the crosstalk between different cell types and multiple factors. Disruption of this balance can result in many pregnancy disorders, such as preeclampsia, spontaneous abortions, intrauterine growth restriction (IUGR), and premature births.
Heme oxygenase (HMOX) is the rate-limiting enzyme in the heme degradation pathway. The importance of HMOX1, the inducible isoform of HMOX, in maintaining a healthy pregnancy was revealed from the first and the only reported human case of HMOX1 deficiency in Japan. The parents were heterozygous carriers for a different mutant allele, and their boy had both alleles encoding a truncated HMOX1. He suffered from marked growth retardation and developmental delay, and eventually died. The mother, although healthy, had experienced two intrauterine fetal deaths [1]. In other reports, a reduction in placental HMOX1 expression was associated with recurrent miscarriages, spontaneous abortions, and preeclampsia [2, 3]. In addition to the existence of mutant alleles in the human population, Denschlag et al. [4] reported that there are different lengths of (GT) repeats in the human HMOX1 promoter regulatory region. These polymorphisms of HMOX1 are associated with idiopathic recurrent miscarriages.
Accumulating evidence has indicated that HMOX1 is involved in the maintenance and establishment of the vascular bed [5]. On the one hand, the antioxidative property of HMOX1 protects vessels from oxidative injury. Prominent intravascular hemolysis and endothelial cell injury were found in the HMOX1-deficient child [1]. In addition, an increased susceptibility to lipid-induced oxidative cell injury in vascular endothelial and smooth muscle cells was detected in the Hmox1 knockout (KO) mouse [6]. Conversely, the upregulation of Hmox1 and its metabolite, carbon monoxide (CO), can stimulate angiogenesis/vasculogenesis through the increased synthesis of proangiogenic factors, such as vascular endothelial growth factor (VEGF), monocyte chemotactic protein 1 (MCP-1; CCL2), transforming growth factor (TGF) β, and interleukin (IL) 8, and through the decreased production of antiangiogenic mediators, such as soluble Flt-1 (sFlt-1), soluble endoglin (sEng), and CXCL10 [7, 8].
Although a reduced Hmox1 expression arising from maternal interindividual variations has been associated with complications in pregnancy, the mechanism(s) by which this occurs is far from understood. Using a Hmox-1 KO mouse model, we have previously shown that wild-type (WT) crossbreedings yield about nine (called wWT) pups/litter, whereas Hmox1 heterozygous (Het, Hmox1+/−) breedings result in about five pups/litter (including WT [called hWT] and Het [called hHet] pups) because of intrauterine deaths of Hmox1−/− KO embryos [9]. Both embryos and placentas in Het pregnancies were growth restricted (73% to 88% of wWT) compared to WT pregnancies [9]. However, no significant difference in birth weight was observed between hWT and hHet pups, suggesting that the maternal genotype, and not the fetal genotype, determines fetal growth restriction [9].
In this study, we evaluated the roles of HMOX1 in placentation, focusing primarily on vasculature development. We hypothesized that a maternal deficiency in HMOX1 is the determining factor that contributes to the impairment of fetomaternal interface through insufficient SA remodeling and modified expression of angiogenesis and the associated factors. In the murine model, the invasion of trophoblast cells into the decidua is normally very shallow. SA enlargement and remodeling are primarily mediated by fetal trophoblast cell invasion and uterine natural killer (uNK) cells, which play a major role in mouse placental development [10, 11]. These cells constitute 50–90% of the leukocytes in the decidua and regulate local cytokine production, growth factors, and adhesion/matrix proteins, which, in turn, are believed to mediate endovascular invasion and SA remodeling. Since insufficient dilation of the SAs was found in Het pregnancies, we speculated that a deficiency in HMOX1 might first affect uNK cells (CD16−CD56hi) in the decidua/mesometrial lymphoid aggregate of pregnancy (MLAp) region in the early stages of pregnancy. In a normal pregnancy, the uterine SAs are remodeled to vessels of low resistance and high capacitance to increase blood flow to the dynamically growing placenta. Its remodeling and enlargement occurs in the early stages of pregnancy. We have previously reported that SAs in the proximal decidual region in Het placentas were less dilated, and junction zones were significantly thinner than those of WT placentas by histological characterization [9]. Therefore, we speculated that a partial deficiency in HMOX1 results in a less efficient placental vascular adaptation. A Hmox1 Het mouse model used in this study mimics the human circumstance of HMOX1 polymorphism. This study will deepen our understanding of those unsolved cases of pregnancy disorders, especially IUGR.
MATERIALS AND METHODS
Animals
Mice were maintained under strict adherence to Stanford University institutional guidelines. FVB/N WT mice (6–8 wk old) were obtained from Charles River Laboratories. The FVB/HMOX1/KO mouse strain carries a targeted deletion of a large portion of Hmox1 gene and was generously provided by Dr. Phyllis A. Dennery (Philadelphia, PA). The original KO strain was established on a C57BL/6 background and obtained from Poss and Tonegawa [6]. To establish this strain on a predominant FVB/N background, C57BL/6 HMOX1/KO mice were backcrossed with FVB/N WT mice for at least six generations [12] and maintained for future breeding. Mice were mated at 6–10 wk of age and gestational ages were determined by vaginal plug day (Embryonic Day 0.5 [E0.5]).
Mouse Genotyping
Genotyping of embryos and offspring were performed by PCR as described previously [9]. Briefly, genomic DNA were isolated from fetal tissues or tail cuttings using the Tissue DNeasy Kit (Qiagen) and analyzed by PCR. For Hmox1+/+, Hmox1+/−, and Hmox1−/− genotyping, two sets of primers specially designed for the WT and mutants were used. The conditions for PCR were as follows: 95°C for 10 min for denaturing the genomic DNA, 94°C for 20 sec, 68°C for 30 sec, and 72°C for 40 sec, repeating 40 cycles. WT (510 bp) and mutant (390 bp) bands were analyzed using 1.8% (w/v) agarose gel electrophoresis.
Vascular Corrosion Casting
Vascular casts were prepared as described previously [13]. In brief, pregnant mice at ∼E16.5 were anesthetized with isoflurane and the abdominal region was surgically exposed. Heparin (0.05 ml at 100 IU/ml) was injected into the beating heart and saline was used to perfuse the vessels. To cast the uteroplacental vasculature, a catheter was placed in the descending thoracic aorta of the pregnant mice, and the MicroFil casting agent (Flow Tech, Inc.) was infused distally towards the uterus and placentas at a speed of 0.4 ml/min using an automatic infusion pump. After polymerization of the casting compound at 4°C, placentas were dissected and stored in 10% (v/v) formalin, and the associated embryos were collected for genotyping.
Micro-Computerized Tomography Scanning and Analysis
The cast placentas were staged and scanned in a benchtop micro-computerized tomography (microCT) scanner (Scanco μCT40, Scanco Medical AG) at 6-μm resolution using a 0.5-mm aluminum filter, five frames per view, 2000 views, 200-ms integration time, 55 kVp, and 144 μA. The DICOM files generated from the microCT scan were used to analyze the samples and to create rendered volumes of the specimens. The raw data files were oriented and cropped using AltaViewer (Numira Biosciences). The files were converted into a compatible file format amenable to Seg3D segmentation software (Scientific Computing and Imaging Institute, University of Utah). This software was used to create label maps associated with the region of interest. The volume rendering snapshots were saved using SCIRun (Scientific Computing and Imaging Institute) [14].
Histological Staining
Placentas were dissected and collected in Protocol* 10% (v/v) neutral buffered formalin (Fisher Scientific) for 24 h. The fixed placentas were then embedded in paraffin according to standard protocols. Six-millimeter-thick tissues were sectioned from paraffin-embedded blocks using a microtome. Following deparaffinization, sections were stained by hematoxylin and eosin (H&E; American MasterTech Scientific, Inc.) and placental structures were identified using light microscopy (Carl Zeiss Microimaging, Inc.).
Biotinylated Dolichos biflorus Agglutinin and Isolectin B4 Staining
After deparaffinization and rehydration, the paraffin-embedded sections were antigen retrieved using Citrate Plus solution (Biogenex) and treated by hydrogen peroxide. Biotinylated Dolichos biflorus agglutinin (DBA) or isolectin B4 (1:500 and 1:100 respectively; Vector Laboratories) was applied on the slide for 2 h. After rinsing with PBS, the slides were incubated with Vectastain RTU Elite ABC reagent (Vector Laboratories) for 30 min, and positive brown color was developed by incubation with 3,3′-diaminobenzidine (Vector Laboratories).
Transmission Electron Microscopy
For transmission electron microscopy (TEM), the decidua/MLAp regions of placenta at E10.5 were dissected and fixed in 2.5% (v/v) glutaraldehyde and 2% (w/v) paraformaldehyde/0.1 M phosphate buffer (pH 7.4). Postfixation was performed in 1.5% (w/v) osmium tetroxide in the same buffer. After dehydration with a graded series of ethanol and infiltration by propylene oxide, specimens were embedded in epoxy resin (Polysciences Inc.). Ultrathin sections (90 nm thick) were prepared and analyzed by TEM (JEOL TEM1230).
Quantitative RT-PCR
After mice were euthanized, placentas were immediately dissected and stored in liquid nitrogen. Total RNA was extracted using the RNAeasy Mini Kit (Qiagen). Hmox1 and β-actin mRNA levels were quantified using the QuantiTect SYBR Green RT-PCR kit (Qiagen) as described previously [15, 16]. Amplification was performed using an Mx3005P QPCR System (Stratagene).
Angiogenesis PCR Array
After mice were euthanized, placental tissues were immediately dissected and stored in liquid nitrogen. Total RNA was extracted using the RNeasy Mini Kit (Qiagen). Complementary DNA was synthesized using a RT2 First Strand Kit (Qiagen). Real-time PCR was performed using RT2 Real-Time SYBR Green/ROX PCR Master Mix (Qiagen) on the Mx3005P QPCR system (Stratagene). For data analysis, the ΔΔCt method was used and fold changes were calculated as gene expression in Het compared to WT placentas (www.sabiosciences.com/pcrarraydataanalysis.php).
Statistical Analysis
For comparisons of experimental groups, analysis of variance was first performed for each set of experiments to determine statistically significant differences when P ≤ 0.05. To determine differences between individual experimental and control groups, a Dunnett test, which adjusts for multiple comparisons using the same control group, was used.
RESULTS
Impaired Vasculature in the Labyrinth Region
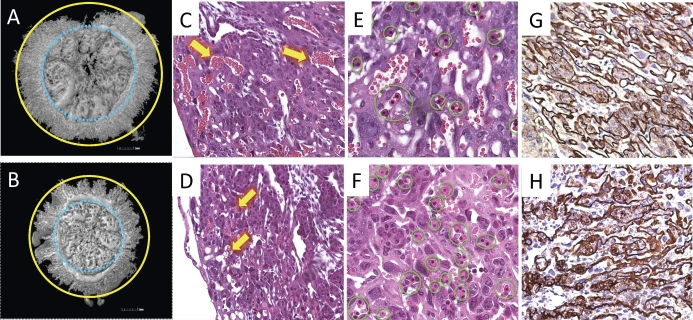
The labyrinth is the region where fetomaternal exchange occurs. To better understand the spatial organization of the placental vasculature in Hmox1-deficient pregnancies, we employed a vascular corrosion casting technique to visualize the maternal placental vasculature and then used microCT to produce detailed 3D images [13]. SAs, arterial canals, and sinusoid spaces of the labyrinth were identified, and a significant difference in the vasculature was detected between wWT and hHet placentas at E16.5. Compared to wWT placentas, total microvasculature vessel volumes in the labyrinth region were largely decreased in the hHet placenta (9.0 vs. 8.3 mm3, respectively; Fig. 1, A and B).
FIG. 1.
Impaired vascular structure in fetomaternal interface (labyrinth in hHet placentas). Upper panels (A, C, E, and G) are wWT placentas, and lower panels (B, D, F, and H) are hHet placentas. A and B) 3D reconstructions of cast placental vasculatures were prepared from placentas at E16.5 (n = 2) and shown from the fetal view. Significant reduction of vessel volumes and microvasculatures (the area between blue and yellow circles) are seen in the hHet labyrinth compared to the wWT. C and D) H&E staining of E12.5 labyrinths (original magnification ×200, n = 7 for each genotype) shows that maternal sinusoid spaces, indicated by yellow arrows, are significantly narrowed and distorted in the hHet placenta. E and F) More nucleated fetal red blood cells (circled in green) are present in the hHet placenta (original magnification ×400, n = 7 for each genotype). G and H) B4-lectin staining of fetal endothelial cells shows irregular fetal capillaries and base membranes in the hHet labyrinth (original magnification ×200). For each experiment, three placentas per mouse and three mice for each experiment were used.
This malformation of the hHet labyrinth was also observed by H&E staining. Compared to a similar region in the wWT labyrinth (Fig. 1C), the number of maternal sinusoid spaces in both hHet (Fig. 1D) and hWT (see Supplemental Fig. S1, available online at www.biolreprod.org) labyrinth was reduced, suggesting that this “hypo-” vasculature in Het pregnancies was independent of the fetal genotype. Even the few existing sinusoid spaces were very distorted with a loss of space. Although the change in the fetal capillary spaces was not obvious, we observed fewer maternal red blood cells (nonnucleated) and more fetal red blood cells (nucleated) (Fig. 1, E and F), indicating more fetal blood cells were entering into hHet placentas, possibly being compensatory.
To further characterize the fetomaternal interface, we stained the labyrinth region with B4 lectin, a marker of fetal endothelium and associated basement membranes. The fetal capillaries in hHet were disorganized, with their walls irregularly shaped and sometimes abnormally thickened (Fig. 1H), in contrast to the smooth, thin, and regularly appearing capillaries of wWT placentas (Fig. 1G). Similar defects were also detected in hWT placentas (see Supplemental Fig. S1).
Insufficient SA Remodeling and Structural Changes in the Decidua/MLAp Region
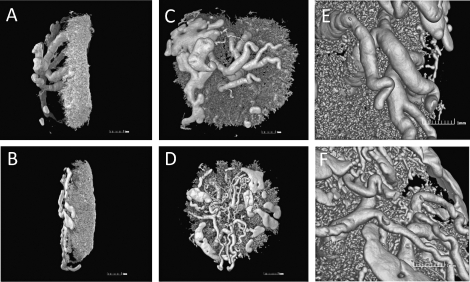
The 3D casting images further confirmed our findings and provided more detailed information. Compared to wWT placentas, hHet placentas appeared smaller and had thinner maternal vasculature regions (2.4 vs. 2.1 mm, respectively, Fig. 2, A and B). The affected region is where the SAs converged to form arterial canals to the placental bed [13], which includes the decidua, trophoblast giant cell region, and junction zone. Furthermore, the remodeled and enlarged SAs from hHet placentas also had significantly smaller diameters than those of wWT controls (0.30 ± 0.05 vs. 0.41 ± 0.04 mm, respectively, P < 0.001), but appeared to be highly branched (Fig. 2, C–F).
FIG. 2.
Reduced decidua/MLAp regions and insufficient SA remodeling in hHet placentas. Upper panels (A, C, and E) are 3D reconstructions of a casted wWT placenta, and lower panels (B, D, and F) are of an hHet placenta. A and B) Lateral view showing the thinner maternal vasculature regions in the hHet placenta. C and D) Maternal view reveals insufficient SA remodeling in the hHet placenta with smaller vessel diameters and more branching. E and F) Close-up view of the SA regions (n = 2).
Morphological Changes in uNK Cells
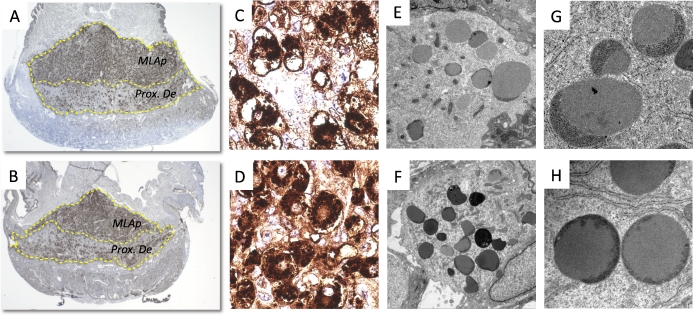
When we performed DBA staining of uNK cells at E10.5, we found that the majority of uNK cells reside in the MLAp region, with only a few in the proximal decidua (Fig. 3, A and B). Cells rarely infiltrated the junction zone or labyrinth. Both the proximal decidua and MLAp regions were significantly thinner in hHet placentas (Fig. 3, A and B). Under higher magnification, we could see significant morphological differences. wWT uNK cells were irregularly shaped, containing apoptotic nuclei and vacuolated cytoplasms. Granules were aggregated near the nuclei. Some cells did not have a clear boundary of cell membranes (Fig. 3C). In contrast, Het uNK cells were round, with DBA-positive granules heavily, but evenly, distributed in the cytoplasm, and with clear cell membranes and nuclear regions (Fig. 3D). The ultrastructure of uNK cells from the hWT placentas was very similar to that of an hHet placenta (see Supplemental Fig. S1).
FIG. 3.
Morphological changes in uNK cells. Upper panels (A, C, E, and G) are from wWT placentas, and lower panels (B, D, F, and H) are from hHet placentas. A and B) uNK cells stained by DBA lectin are located primarily in the MLAp region (MLAp) and some in proximal decidual region (Prox. De). Significantly thinner MLAp regions were observed in the hHet placentas. C and D) High-magnification areas (original magnification ×630) showing granules with DBA-positive staining. Morphological differences were observed between wWT and hHet placentas. E and F) TEM images showing increased granularity of hHet uNK cells, and their relatively smaller size compared to wWT placentas (original magnification ×4000). G and H) High-electron-density cap structures are seen in both wWT and hHet placentas, but the covered regions are different (original magnification ×30 000). For panels A–D, three placentas per mouse and three mice per experiment were used. For panels E–H, one placenta per mouse and three mice per experiment were used.
We then further characterized the ultrastructure of these cells by using TEM. WT uNK cell nuclei were found to have clumps of condensed chromatin, whereas the Het cells had euchromatin and conspicuous nucleoli. Compared to the WT, Het uNK cells had numerous granules, but were relatively smaller in size (Fig. 3, E and F). The granules in WT cells were observed to have regularly shaped cap structures and uniform electron densities (Fig. 3G), whereas the majority of those in Het cells had caps that covered three fourths of the total size of the granules (Fig. 3H). In addition, the endoplasmic reticula were seen frequently located very proximal to the granules in some Het cells (Fig. 3H).
Angiogenic Profiles in WT and Het Decidua/MLAp Regions
Since significant morphologic differences were found in uNK cells as well as insufficiencies in SA remodeling, we speculated that partial deficiency in maternal Hmox1 might affect the expression of angiogenic factors and their associated factors in the decidua/MLAp regions. To test our hypothesis, we used the Mouse Angiogenesis RT2 Profiler PCR Array to compare the expression of 84 genes that are involved in modulating the biological processes of angiogenesis between WT and Het pregnancies.
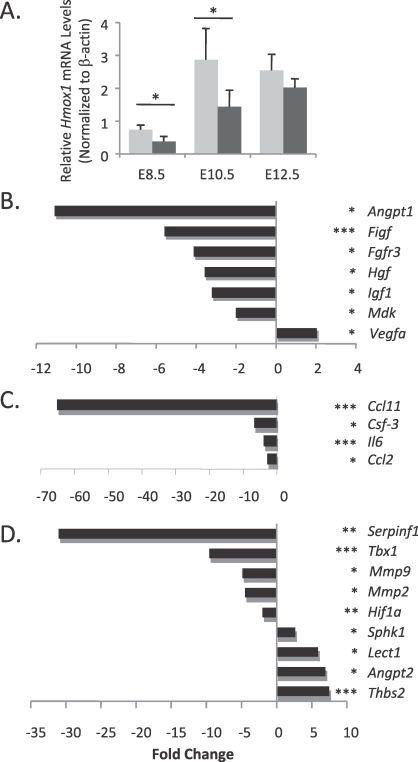
Decidua/MLAp regions were collected from placentas of WT and Het pregnancies. Hmox1 mRNA levels were found to be significantly lower in the Het decidua/MLAp region at different gestational ages (Fig. 4A). For the PCR array, samples used were from E10.5, the important gestational age when SA remodeling occurs and labyrinth vasculature starts to develop. This age corresponds to the period around the end of the first trimester and the beginning of the second trimester in the human pregnancy. Of the 84 genes we screened, 20 showed significant differences between WT and Het regions. The putative functions that are related to placenta vasculature development of each identified gene were also found by searching PubMed and links via the Array Analysis software.
FIG. 4.
Angiogenic profiles of decidua/MLAp regions. A) Hmox1 mRNA levels were detected in decidua/MLAp regions at different gestational ages and normalized by β-actin. Significant reductions in Hmox1 mRNA levels were seen in the hHet placentas at E8.5 and E10.5. *P < 0.05, n = 4. B–D) A mouse angiogenesis PCR array kit was used to detect changes in mRNA levels of angiogenic factors and their related genes in the decidua/MLAp regions of hHet placentas compared to wWT placentas. B) Growth factors and receptors. C) Cytokines and chemokines. D) Matrix proteins and transcriptional factors. P values are shown in parentheses following each gene name (*P < 0.05, **P < 0.01, or ***P < 0.005; n = 3).
In the growth factors and receptors group, six genes, including angiopoietin 1 (Angpt1), c-fos induced growth factor (Figf), fibroblast growth factor receptor 3 (Fgfr3), hepatocyte growth factor (Hgf), insulin-like growth factor 1 (Igf1), and midkine (Mdk), were significantly reduced (Fig. 4B). All six are proangiogenic factors. Among them, Angpt1 showed the largest reduction (−11.1-fold) in the Het regions.
In the cytokines and chemokines group, four genes were significantly decreased in Het tissues. They include: small chemokine ligand 11 (Ccl11, also called Eotaxin, −65-fold); chemokine ligand 2 (Ccl2, also called Mcp-1); colony-stimulating factor 3 (Csf3); and interleukin 6 (Il6) (Fig. 4C).
In the matrix proteins and transcriptional factors group, four out of five genes that were reduced were proangiogenic factors, which included matrix metallopeptidase 2 and 9 (Mmp2 and Mmp9), transcriptional factors T-box1 (Tbx1), and hypoxia-inducible factor 1A (Hif1a). On the other hand, three out of four upregulated genes were antiangiogenic factors, which are leukocyte cell-derived chemotaxin 1 (Lect1), angiopoietin 2 (Angpt2), and thrombospondin 2 (Thbs2) (Fig. 4D). However, we also found an increase in proangiogenic vascular endothelial growth factor A (Vegfa, +2-fold) and sphingosine kinase-1 (Sphk1, +3-fold), and a decrease in antiangiogenic serine peptidase inhibitor F1 (Serpinf1, −31-fold).
Angiogenic Profiles in WT and Het Labyrinth Regions
The majority of labyrinth cells, which include trophoblast cells, giant cells, endothelial cells, nucleated blood cells, and others, are of fetal origin. Since we have observed significant impairment of the fetoplacental interface in hWT and hHet placentas (Fig. 1), we investigated whether these defects originate from a partial fetal deficiency in Hmox1 or merely are downstream effects due to insufficient SA remodeling.
Labyrinth samples from wWT, hWT, and hHet placentas at E12.5 were collected and their angiogenic profiles characterized by using the Mouse Angiogenesis RT2 Profiler PCR Array. Interestingly, among the 84 genes screened, none showed statistically significant differences among wWT, hWT, and hHet labyrinth samples. However, we did find four genes that showed large increases. These four factors include coagulation factor II (F2, also called thrombin, +5.3-fold), fibroblast growth factor 1 (Fgf1, +4.0-fold), plasminogen (Plg, +12.7-fold), and Serpinf1 (+3.7-fold).
DISCUSSION
Pregnancy disorders, such as IUGR and preeclampsia, contribute significantly to preterm births as well as to perinatal morbidity and mortality. To date, there is no known prenatal treatment for these conditions [17, 18] and their pathological mechanisms are not well understood. It is believed that these disorders are rooted from placental defects, mediated by fetal defects, maternal factors, or both [19–22]. In our mouse model, we showed that a partial deficiency of maternal Hmox1 induces growth restriction in both the placenta and fetus and results in defects of the placental vasculature. These malformations are independent of the fetal genotype, but determined by the maternal Hmox1 expression level, which was also observed in the various mating combination experiments using WT and Het (data not shown). We propose that a partial deficiency of Hmox1 may modify the infiltration and differentiation of lymphocytes, affect their production of angiogenic and associated factors, cause insufficiency of SA remodeling, and subsequently result in defects in the fetomaternal interface.
The fetomaternal interface consists of endothelial cells from fetal capillaries, their associated basement membranes, and a trilaminar layer of trophoblast cells that directly lines the maternal sinusoid spaces. Any alterations of the exchange surface area, barrier thickness, and cell composition will affect placental transport capacity. The significant abnormal changes we observed in Het labyrinth was very similar to the characteristic pathological findings observed in the severe IUGR placenta, which displays a “persisting immaturity,” a deficiency of terminal villi, a reduction in villous trophoblasts, and a thickened placental exchange barrier. All these abnormalities affect the permeability of the placenta, causing placental insufficiency and resulting in IUGR. In our model, the fewer fetomaternal interface exchange units in the Het placenta are very likely to be the result of insufficient SA enlargement. It has been suggested that maternal blood flow is normally matched by the fetomaternal exchange area mediated via SA branching morphogenesis [23]. In addition, the suppression of angiogenic factors secreted in the decidua/MLAp region raises the possibility of a long-distance effect of these molecules on the fetoplacental vasculature.
Murine uNK cells, which represent the largest leukocyte population (∼70%) in the decidua/MLAp region, increase dramatically in number and size from Day 8 to Day 12 of pregnancy and decrease from Day 13 onward [10]. We observed the greatest difference in the morphologies and ultrastructures between WT and Het uNK cells at E10.5. According to the uNK four-subtype classification proposed by Paffaro et al. [24], uNK cells in Hmox1 WT or Het seemed to have dissimilar differentiation and maturation rates. These cells can either be recruited from peripheral blood or can develop from preexisting uterine progenitor cells in situ. Although it is known that neither a conceptus nor a trophoblast is required for uNK cell development [10, 11], the factors that contribute to uNK recruitment, differentiation, positioning, or activation are not quite clear. In our studies, the embryonic genotype (hWT or hHet) had no effect on uNK cell maturation, but the maternal genotype may, suggesting that uNK cell morphology and function are determined primarily by maternal factors. A partial deficiency in maternal Hmox1 could affect rates of proliferation and differentiation on peripheral NK cells or on the progenitors themselves. We have reported that HMOX1 plays a key role in limiting the proliferation and differentiation of hematopoietic progenitor cells during stress, most likely by reducing CO-dependent activation of the p38MAPK pathway and leading to low levels of CDKN1A in rapidly dividing cells [12]. However, a partial deficiency in Hmox1 may also change the local decidual environment and subsequently affect uNK cells or other infiltrating leukocytes, which constitute 40% of the total cell population in the decidua [11].
During the establishment of the uteroplacental circulation in Het pregnancies, the major genes that regulate proangiogenic factors are downregulated, while genes that control antiangiogenic factors are induced. This supports the hypothesis that a partial deficiency in maternal Hmox1 can change angiogenic profiles, and results in a suppression of angiogenesis. Six genes in the growth factors and receptors group were downregulated in the Het placenta. FGF and ANGPT are the two major angiogenic factors in the placental vasculature [25]. FGF1, along with FGF2, has been described in the placenta and implicated in normal physiological processes such as embryonic and fetal development, neovascularization, and nerve regeneration. [26]. Angpt1 acts to enlarge blood vessels without inducing sprouting [27], and this unique property may play a critical role during SA remodeling and enlargement. HGF is expressed abundantly in the human placenta and may play a role in promoting trophoblast growth and initiating neocapillary formation [17]. IGF1 is an important regulator gene of fetal growth, and circulating IGF1 concentrations are reduced in IUGR fetuses [28]. In addition, four genes from the cytokines and chemokines group were decreased. CCL11 is a chemotactic for eosinophils, whereas CCL2 is for monocytes, suggesting their roles in the attraction of lymphocytes and their subsequent infiltration into the decidua/MLAp region. IL6 is normally produced by the placenta during pregnancy, and studies have shown that preeclampsia is associated with decreased placental IL6 production [29]. MMP2 and MMP9, the two matrix proteins, were also down-modulated and have been suggested to play an important role in SA remodeling by destruction of the uterine arterial wall [30]. Most of these growth factors, cytokine/chemokines, and matrix factors were likely produced by infiltrated leukocytes, which include uNK cells and macrophages [20, 31, 32].
However, the increases of proangiogenic factors Vegfa and Sphk1, and the decrease of antiangiogenic Serpinf1, were not inconsistent with this trend. VEGFA is well known for its potent angiogenesis function in many tissues. Serpinf1, also called pigment epithelium-derived factor, has been described as a natural and potent angiogenesis inhibitor, which suppresses unwanted neovascularization [33]. Sphk1−/− female mice are infertile because of impaired decidualization with vessel defects [34]. It has been reported that hypoxia leads to the downregulation of Serpinf1 but the upregulation of Vegf mRNA. Generally, oxygen is an important mediator of cell proliferation, growth factor production, and cell invasion. Early in pregnancy (e.g., the first trimester in human pregnancy) the fetal environment is hypoxic, where the partial pressure of oxygen (pO2) in the intervillous space is around 18 mm Hg, compared to 61 mm Hg at the end of the third trimester [35]. It is not quite well understood if the Het placenta has a lower or higher oxygen level than that in WT pregnancies early in pregnancy. It needs to be further investigated if these three genes contribute to the increased number of SA vessels we observed in the casted Het placenta (Fig. 2, D and F).
In our array results, except for the increase of VEGFA, we did not see any changes in the other major players in placental angiogenesis and vasculogenesis, such as placenta growth factor (Plgf) [36], Vegfb, Vegfc, Flt1, Tgfb1, Tgfb2, Tgfb3, and their receptors [7, 25]. This observation is actually in agreement with the clinical findings that there are no differences in VEGF and PlGF expression in severe IUGR placentas [35]. In preeclampsia, the importance of the VEGF family appears in late pregnancy (such as Flt1), whereas our assay was performed in early gestational samples. However, besides the VEGF family, other molecules/pathways and their crosstalk are more than likely to be actively involved. More importantly, it may be the maintenance of the delicate balance between angiogenic inducers and inhibitors that is most critical in the process of normal placental vascular formation rather than the simple up- or downregulation of just one pathway.
In our previous study, we showed that in the hHet labyrinth the levels of mRNA of Hmox2, the constitutive isoform of Hmox, showed no change, whereas both mRNA of NOS2 and NOS3 were significantly increased. [9] The Nos/NO system shares many similarities with the HMOX1/CO system; expression of NOS2 and NOS3 was found significantly reduced in the trophoblast cells of placentas in IUGR infants. Local NO can interact with angiogenic factors to coordinate placental angiogenesis and blood flow; therefore, an increase in NOS2 and NOS3 might be a compensation for a deficiency in Hmox1 so that overall angiogenesis can be maintained.
Clinically, other than chromosomal abnormalities and viral infections, most IUGR births are attributed to an inadequate substrate supply to the fetus. This may be because of maternal malnutrition [37] or because of placental insufficiency. Maternal microvascular diseases, such as hypertension, diabetes, systemic lupus erythematosus, and the antiphospholipid syndrome, may impair placental development and function, but these are thought to account for less than half the cases of placental insufficiency [17]. In most cases of IUGR associated with placental insufficiency, the underlying causes remain unknown, despite clinically relevant placental pathology [17]. Although results derived from animal studies cannot be completely extrapolated to humans, our model of partial HO-1 deficiency may afford insights into the mechanism by which interindividual variations of some maternal genes can cause placental insufficiency, and in turn result in pregnancy disorders, such as IUGR and preeclampsia.
Supplementary Material
ACKNOWLEDGMENTS
We thank Mr. Brad Johnston of Numira Bioscience, Inc., and Dr. Timothy C. Doyle and Dr. Hedi Razavi for their help with microCT image reconstructions and data analyses. We also thank Mrs. Pauline Chu and Mrs. Madeleine Huey for their help with histological staining and Mr. John J. Perrino for performing the transmission electron microscopy. Finally, we thank Dr. Nihar R. Nayak and Dr. Qiujun Fan for thoughtful discussion.
Footnotes
Supported by National Institutes of Health grant HD050351, the Hess Research Fund, and the Mary L. Johnson Research Fund.
REFERENCES
- Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest 1999; 103: 129 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge SA, Smith GN. HO in pregnancy. Free Radic Biol Med 2005; 38: 979 988. [DOI] [PubMed] [Google Scholar]
- Lyall F, Myatt L. The role of the placenta in pre-eclampsia—a workshop report. Placenta 2002; 23 (suppl A): S142 S145. [DOI] [PubMed] [Google Scholar]
- Denschlag D, Marculescu R, Unfried G, Hefler LA, Exner M, Hashemi A, Riener EK, Keck C, Tempfer CB, Wagner O. The size of a microsatellite polymorphism of the haem oxygenase 1 gene is associated with idiopathic recurrent miscarriage. Mol Hum Reprod 2004; 10: 211 214. [DOI] [PubMed] [Google Scholar]
- Loboda A, Jazwa A, Grochot-Przeczek A, Rutkowski AJ, Cisowski J, Agarwal A, Jozkowicz A, Dulak J. Heme oxygenase-1 and the vascular bed: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 2008; 10: 1767 1812. [DOI] [PubMed] [Google Scholar]
- Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci U S A 1997; 94: 10925 10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechend R, Luft FC. Angiogenesis factors and preeclampsia. Nat Med 2008; 14: 1187 1188. [DOI] [PubMed] [Google Scholar]
- Cudmore M, Ahmad S, Al-Ani B, Fujisawa T, Coxall H, Chudasama K, Devey LR, Wigmore SJ, Abbas A, Hewett PW, Ahmed A. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation 2007; 115: 1789 1797. [DOI] [PubMed] [Google Scholar]
- Zhao H, Wong RJ, Kalish FS, Nayak NR, Stevenson DK. Effect of heme oxygenase-1 deficiency on placental development. Placenta 2009; 30: 861 868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilinski MJ, Thorne JG, Oh MJ, Leonard S, Murrant C, Tayade C, Croy BA. Uterine NK cells in murine pregnancy. Reprod Biomed Online 2008; 16: 218 226. [DOI] [PubMed] [Google Scholar]
- Goldman-Wohl D, Yagel S. NK cells and pre-eclampsia. Reprod Biomed Online 2008; 16: 227 231. [DOI] [PubMed] [Google Scholar]
- Cao YA, Wagers AJ, Karsunky H, Zhao H, Reeves R, Wong RJ, Stevenson DK, Weissman IL, Contag CH. Heme oxygenase-1 deficiency leads to disrupted response to acute stress in stem cells and progenitors. Blood 2008; 112: 4494 4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson SL, Lu Y, Whiteley KJ, Holmyard D, Hemberger M, Pfarrer C, Cross JC. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev Biol 2002; 250: 358 373. [DOI] [PubMed] [Google Scholar]
- Vasquez SX, Gao F, Su F, Grijalva V, Pope J, Martin B, Stinstra J, Masner M, Shah N, Weinstein DM, Farias-Eisner R, Reddy ST. Optimization of MicroCT imaging and blood vessel diameter quantitation of preclinical specimen vasculature with radiopaque polymer injection medium. PLoS ONE 2011; 6: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Wong RJ, Doyle TC, Nayak N, Vreman HJ, Contag CH, Stevenson DK. Regulation of maternal and fetal hemodynamics by heme oxygenase in mice. Biol Reprod 2008; 78: 744 751. [DOI] [PubMed] [Google Scholar]
- Zhao H, Wong RJ, Nguyen X, Kalish F, Mizobuchi M, Vreman HJ, Stevenson DK, Contag CH. Expression and regulation of heme oxygenase isozymes in the developing mouse cortex. Pediatr Res 2006; 60: 518 523. [DOI] [PubMed] [Google Scholar]
- Somerset DA, Afford SC, Strain AJ, Kilby MD. Fetal growth restriction and hepatocyte growth factor. Arch Dis Child Fetal Neonatal Ed 1997; 77: F244 F248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiser D, Nguyen X, Wong R, Seidman D, Stevenson D, Quan S, Abraham N, Dennery PA. Heme oxygenase-1 modulates fetal growth in the rat. Lab Invest 2002; 82: 687 692. [DOI] [PubMed] [Google Scholar]
- Cross JC. The genetics of pre-eclampsia: a feto-placental or maternal problem? Clin Genet 2003; 64: 96 103. [DOI] [PubMed] [Google Scholar]
- Dokras A, Hoffmann DS, Eastvold JS, Kienzle MF, Gruman LM, Kirby PA, Weiss RM, Davisson RL. Severe feto-placental abnormalities precede the onset of hypertension and proteinuria in a mouse model of preeclampsia. Biol Reprod 2006; 75: 899 907. [DOI] [PubMed] [Google Scholar]
- Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod 2003; 69: 1 7. [DOI] [PubMed] [Google Scholar]
- Zenclussen AC, Fest S, Joachim R, Klapp BF, Arck PC. Introducing a mouse model for pre-eclampsia: adoptive transfer of activated Th1 cells leads to pre-eclampsia-like symptoms exclusively in pregnant mice. Eur J Immunol 2004; 34: 377 387. [DOI] [PubMed] [Google Scholar]
- Cross JC, Nakano H, Natale DR, Simmons DG, Watson ED. Branching morphogenesis during development of placental villi. Differentiation 2006; 74: 393 401. [DOI] [PubMed] [Google Scholar]
- Paffaro VA, Jr, Bizinotto MC, Joazeiro PP, Yamada AT. Subset classification of mouse uterine natural killer cells by DBA lectin reactivity. Placenta 2003; 24: 479 488. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Redmer DA. Angiogenesis in the placenta. Biol Reprod 2001; 64: 1033 1040. [DOI] [PubMed] [Google Scholar]
- Bottcher RT, Niehrs C. Fibroblast growth factor signaling during early vertebrate development. Endocr Rev 2005; 26: 63 77. [DOI] [PubMed] [Google Scholar]
- Schiessl B, Innes BA, Bulmer JN, Otun HA, Chadwick TJ, Robson SC, Lash GE. Localization of angiogenic growth factors and their receptors in the human placental bed throughout normal human pregnancy. Placenta 2009; 30: 79 87. [DOI] [PubMed] [Google Scholar]
- Randhawa RS. The insulin-like growth factor system and fetal growth restrictionn. Pediatr Endocrinol Rev 2008; 6: 235 240. [PubMed] [Google Scholar]
- Kauma SW, Wang Y, Walsh SW. Preeclampsia is associated with decreased placental interleukin-6 production. J Soc Gynecol Investig 1995; 2: 614 617. [DOI] [PubMed] [Google Scholar]
- Verlohren S, Geusens N, Morton J, Verhaegen I, Hering L, Herse F, Dudenhausen JW, Muller DN, Luft FC, Cartwright JE, Davidge ST, Pijnenborg R. et al. Inhibition of trophoblast-induced spiral artery remodeling reduces placental perfusion in rat pregnancy. Hypertension 2010; 56: 304 310. [DOI] [PubMed] [Google Scholar]
- Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V. et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 2006; 12: 1065 1074. [DOI] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science 2005; 308: 1592 1594. [DOI] [PubMed] [Google Scholar]
- Gao G, Li Y, Zhang D, Gee S, Crosson C, Ma J. Unbalanced expression of VEGF and PEDF in ischemia-induced retinal neovascularization. FEBS Lett 2001; 489: 270 276. [DOI] [PubMed] [Google Scholar]
- Mizugishi K, Li C, Olivera A, Bielawski J, Bielawska A, Deng CX, Proia RL. Maternal disturbance in activated sphingolipid metabolism causes pregnancy loss in mice. J Clin Invest 2007; 117: 2993 3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A, Kilby MD. Hypoxia or hyperoxia in placental insufficiency? Lancet 1997; 350: 826 827. [DOI] [PubMed] [Google Scholar]
- Tayade C, Hilchie D, He H, Fang Y, Moons L, Carmeliet P, Foster RA, Croy BA. Genetic deletion of placenta growth factor in mice alters uterine NK cells. J Immunol 2007; 178: 4267 4275. [DOI] [PubMed] [Google Scholar]
- Belkacemi L, Nelson DM, Desai M, Ross MG. Maternal undernutrition influences placental-fetal development. Biol Reprod 2010; 83: 325 331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.