Abstract
Yokukansan (YKS) has been used in Japan as a remedy for neurosis, insomnia, and children with night crying. In a previous study, we reported that YKS controls scratching behavior and inhibits the development of atopic dermatitis (AD)-like lesions in NC/Nga mice. In this study, we investigated the effects of YKS on the development of AD-like lesions in socially isolated NC/Nga mice compared with the effects of fexofenadine and elucidated the mechanism of the ameliorating effect of YKS on the skin lesions. Ten-week-old male NC/Nga mice were divided into three groups (n = 5/group): the conventional control, the YKS-treated, and the fexofenadine-treated groups, and were kept isolated under conventional conditions for 6 weeks. Measurements were made of dermatitis scores and transepidermal water loss (TEWL), scratching and grooming behaviors. Immunohistochemistry and mRNA levels were also evaluated. We performed similar experiments under specific pathogen free (SPF) conditions that served as a SPF control. YKS and fexofenadine inhibited the aggravation of skin lesions and decreased TEWL, but only YKS decreased the numbers of scratching and pathologic grooming behaviors. Immunohistochemistry and RT-PCR revealed that N-methyl-d-aspartate (NMDA) receptor expression was increased in the skin of conventional control mice and was decreased in YKS-treated mice. Glutamate transporter-1 (GLT-1) mRNA levels were decreased in the skin of conventional control mice and were increased in YKS-treated mice. The results indicate that YKS ameliorates AD-like skin lesions in NC/Nga mice through a mechanism distinct from that of fexofenadine. Furthermore, the effects of YKS are suggested to be mediated via glutamate signaling in the skin lesions.
Keywords: Yokukansan, Atopic dermatitis, NC/Nga mice, Scratching behavior, NMDA receptor, GLT-1
Introduction
Atopic dermatitis (AD) is a chronic relapsing eczematous skin disease characterized by pruritus and inflammation with cutaneous physiological dysfunction. Long-lasting itching is an intolerable sensation for patients with severe AD. Psychosocial stresses such as human relationships, pressures of work, worries about career, and anxieties for independence affect the skin condition of patients with AD. Such patients tend to have habitual scratching behavior called ‘‘addictive scratching’’ or ‘‘scratch dependence’’; this behavior worsens their eruptions gradually and forms a vicious cycle called the ‘itch-scratch cycle’. Therefore, treatments are necessary for both the physical and the mental conditions of the patients [1, 2]. We term these adverse conditions of AD as “behavioral and psychological symptoms of atopic dermatitis (BPSA)”, and it has become an issue of public concern. Effective anti-pruritic and anti-BPSA drugs are needed by intractable AD patients.
NC/Nga mice have been regarded as an excellent model for AD since they develop AD-like skin lesions spontaneously under conventional conditions, but not under specific pathogen free (SPF) conditions [3]. Before starting the present study, we assessed the effects of the environment and stress on the skin of NC/Nga mice by comparing the mice bred in groups with those that were kept isolated under conventional conditions and under SPF conditions, respectively. It is well known that individually housed mice undergo strong social stress because mice are social animals that live in groups in the natural environment. Measurements were made of dermatitis scores, transepidermal water loss (TEWL), scratching behaviors as well as grooming behaviors. Under SPF conditions, dermatitis was not observed either in the group housed or the individually housed animals. In that study, we used data derived from the isolated mice kept under SPF conditions as a SPF control, which had no skin lesions. The results showed that isolated NC/Nga mice kept under conventional conditions manifested the most severe dermatitis with increased scratching behaviors and pathological grooming behaviors (data not shown). We concluded that socially isolated NC/Nga mice kept under conventional conditions exhibit skin lesions similar to those of human AD patients with BPSA under stressful conditions.
Previously, our group [4] reported that Paroxetine, a selective serotonin re-uptake inhibitor (SSRI), inhibits the development of AD-like lesions in NC/Nga mice. We expected that Paroxetine might be an alternative or complementary therapeutic option for the treatment of AD. Previous studies have indicated that centrally acting therapeutic drugs are useful for anti-pruritic therapy.
Yokukansan (YKS) is a traditional Japanese medicine called Kampo medicine. In Japan, Kampo medicines are often used for AD patients who do not respond to existing drugs or want to avoid using topical steroid ointment. Although Kampo medicines have long been used for many AD patients, there is no sufficient evidence about their efficacy so far. The reason is that Kampo medicines are usually prescribed individually for patients according to their symptoms, called “shou”, and therefore it is difficult to perform large-scale clinical studies using Kampo medicines. Recently, however, several studies have been performed using Kampo medicines and evidence-based data are now available. For example, Kobayashi et al. [5] reported that Hoshu-ekki-to is effective and safe for AD patients using multicenter, double-blind, randomized, placebo-controlled studies. Gao et al. [6] reported that Juzen-taiho-to, Hochu-ekki-to, Shofu-san and Oren-gedoku-to might correct the Th1/Th2 balance skewed to Th2, and thus inhibit dermatitis in NC/Nga mice. With this background, we examined the efficacy of YKS in the treatment of skin lesions in NC/Nga mice to obtain basic information regarding the treatment of AD in humans.
YKS has been used as an anti-anxiety agent to treat neurosis, insomnia, and children with night crying. Clinically, it has been reported that YKS ameliorates excitement, anger, and hallucinations in behavioral and psychological symptoms of dementia (BPSD), and in patients with Alzheimer’s disease. BPSD treatment with YKS has been successful in clinical cases of dementia with Lewy bodies, Parkinsonian dementia, other forms of senile dementia, and also is successful in treating cases of neuropathic pain, schizophrenia, and restless legs syndrome [7–14]. Thus, YKS is a notable Kampo medicine in various fields.
In our previous study using NC/Nga mice, YKS-controlled scratching behaviors inhibited the development of AD-like lesions and showed a “preventive effect” as well as a “therapeutic effect” on dermatitis [15]. However, the mechanism by which YKS controls scratching behaviors and inhibits the development of AD-like lesions in NC/Nga mice remains unexplored. In the present study, we investigated the effects of YKS on the development of AD-like lesions in socially isolated NC/Nga mice and compared its effects with those of fexofenadine, a histamine H1-receptor antagonist.
Materials and methods
Animals
Since female NC/Nga mice have few skin lesions, male NC/Nga mice were used for this study. Male NC/Nga mice (10 weeks of age) were purchased from Japan SLC Inc. (Shizuoka, Japan) and were maintained under conventional conditions. The mice were divided into three groups: the control, the YKS-treated and the fexofenadine-treated groups (n = 5 per group). The mice were individually housed (under social isolation stress) throughout the experiment. We only used NC/Nga mice which had mild skin lesions for the experiments and mice with less severe or more severe lesions were excluded. The animal room was maintained at 24 ± 2°C, 55 ± 10% relative humidity, and a 12–12 h light–dark cycle. All mice were allowed access to water and food ad libitum. The individual cage size was 9(w) × 13(h) × 20(d) cm. All procedures performed on animals were approved by the Institutional Animal Care and Use Committee (IACUC) of Juntendo University, with the approval number of 220014.
Drugs
YKS, supplied by Tsumura & Co. (Tokyo, Japan), is a vacuum-concentrated extract of seven herbs in the following ratio: 4.0 g Atractylodes lancea rhizome, 4.0 g Hoelen, 3.0 g Cnidium rhizome, 3.0 g Japanese Angelica root, 2.0 g Bupleurum root, 1.5 g Glycyrrhiza root, and 3.0 g Uncaria thorn. Each plant material was authenticated by identification of external morphology and marker compounds of plant specimens according to the methods of the Japanese Pharmacopoeia and the Tsumura standard. The seven medicinal herbs were extracted with purified water at 95°C for 1 h, and the extraction solution was separated from the insoluble waste and was concentrated by removing water under reduced pressure. The spray-drying technique was used to produce a dried extract powder. Mice were given water containing YKS (Lot No.2060054010) at the dosage of 0.6% (1.0 g/kg). The control mice were given drug-free water ad libitum. Fexofenadine hydrochloride was supplied by LKT Laboratories, Inc. (Saint Paul, MN, USA) and dissolved at 0.03% (40 mg/kg), and was also provided to mice in water ad libitum.
Evaluation of the severity of dermatitis and observation of grooming behaviors
The severity of skin lesions was examined and scored at 0, 1, 3 and 6 weeks. Skin lesions on the dorsal skin were assessed according to the following four symptoms: erythema, edema, erosion and dryness, and the sum was considered as the individual score (0: no symptom, 1: mild, 2: moderate, 3: severe). The frequency of grooming behaviors was counted in a 10 min observation period. These assessments were performed by at least two investigators.
Measurement of scratching behaviors
Scratching behaviors of the hind paws were detected and evaluated using a Microact® (Neuroscience, Tokyo, Japan). Under ether anesthesia, a small Teflon-coated magnet (1 mm in diameter, 3 mm long) was implanted subcutaneously into the dorsal side of both hind paws of each mouse the day before the first recording of scratching behaviors. The magnet remained in situ throughout the entire experimental period. If the magnet was dislocated, it was implanted again. Each mouse with magnets was placed in the observation chamber (11 cm in diameter, 18 cm high) surrounded by a round coil. Movement of the hind paws with implanted magnets induced an electric current in the coil, which was amplified and recorded by the Microact® software. The number of scratching behaviors was measured for 24 h. In NC/Nga mice, long-lasting scratching behaviors (>1.5 s) are more important than short-lasting scratching behaviors (0.3–1.5 s) [16], so the number of long-lasting scratching behaviors was counted.
Measurement of transepidermal water loss on the dorsal skin
The hair on the dorsal skin of each mouse was shaved using an electric razor under ether anesthesia and TEWL was measured using a mobile Tewameter® MSC100/TM300 (Courage & Khazaka, Koeln, Germany) at 0, 1, 3 and 6 weeks. The values were recorded when stabilized approximately 10 s after the probe had been placed on the skin. TEWL was measured three times for each mouse and the mean values were obtained.
Quantitative real-time PCR
The dorsal skin of each mouse was excised using a surgical knife and was homogenized using a SK-100 (Tokken, Chiba, Japan), and total RNA was extracted using QIAzol (Qiagen, Hilden, Germany). cDNAs were synthesized using a high capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. Quantitative real-time PCR was done with Taqman universal PCR master mix, using an Applied Biosystems 7500 real-time PCR system (Applied Biosystems). Taqman probes and primers were purchased from Applied Biosystems. The expression of mRNA in each mouse was normalized to the expression of mouse β-actin mRNA as the endogenous control.
Histopathology
Formalin-fixed, paraffin-embedded skin samples from each mouse were sectioned and stained with hematoxylin and eosin. Skin samples were also stained with toluidine blue for identification of mast cells. Representative sections were observed at 10× magnification.
Immunohistochemical staining of paraffin sections
All mouse tissues were sliced as frozen sections at 5 μm thickness. Detection of mouse NMDA receptors was carried out using anti-NMDARζ1(C-20) goat polyclonal antibodies supplied by Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The goat polyclonal antibodies were raised against the peptide corresponding to the carboxy terminus of the human glutamate (NMDA) receptorζ1. That human sequence is identical with the sequences of mice and rats. Binding of the primary antibody was detected using the fluorescent anti-goat secondary antibody (Alexa Fluor & reg® 488 goat anti-rabbit IgG (H + L), Molecular Probes, Inc., Eugene, OR). Nuclei were stained with DAPI using ProLong® Gold anti-fade reagent (Molecular Probes, Inc.). Images were obtained using a confocal laser scanning microscope (FluoView FV1000 Confocal Microscope, Olympus Corporation, Tokyo, Japan).
Statistical analyses
All data are presented as means ± SE. Statistical comparison of real-time PCR was analyzed using the Wilcoxon test. For other results, statistical analysis was performed using one-way ANOVA followed by Bonferroni/Dunn test. P values of <0.05 are defined as statistically significant.
Results
Time courses of dermatitis scores, TEWL and the numbers of scratching behaviors and grooming behaviors
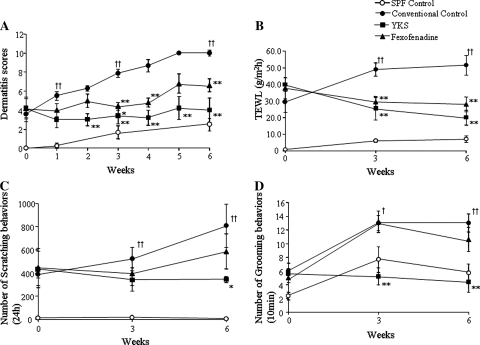
Dermatitis scores of the conventional control group were aggravated as time goes on. Both the YKS- and the fexofenadine-treated groups significantly inhibited the aggravation of skin lesions in NC/Nga mice from 3 weeks after the start of the experiments. The dermatitis score of the SPF control group was not more than a minor increase (Fig. 1a). The TEWL of the conventional control group was increased as time goes on. The YKS- and the fexofenadine-treated groups significantly inhibited the increase of TEWL compared with the conventional control mice from 3 weeks. The TEWL of the SPF group, which had no skin lesions, was not increased (Fig. 1b). Scratching behaviors of the conventional control and the fexofenadine-treated groups increased as time goes on. The YKS-treated group significantly decreased the scratching behaviors compared with the conventional control group after 6 weeks. The scratching behaviors of the SPF group was not increased (Fig. 1c). Grooming behaviors of the conventional control group and the fexofenadine-treated group were increased under social isolated conditions. The YKS-treated group significantly decreased the grooming behaviors compared to the conventional control mice from 3 weeks. The grooming behaviors of the SPF group increased under social isolated conditions (Fig. 1d).
Fig. 1.
The effects of YKS and fexofenadine on dermatitis score (a), TEWL (b), the numbers of scratching behaviors (c) and grooming behaviors (d) for AD-like skin lesions in NC/Nga mice. a The skin lesions of the conventional control group were aggravated as time goes on. Both the YKS- and the fexofenadine-treated groups significantly inhibited the aggravation of skin lesions in NC/Nga mice from 3 weeks after the start of the experiments. The dermatitis score of the SPF control group was not more than a minor increase. b The TEWL of the conventional control group was increased as time goes on. The YKS- and the fexofenadine-treated groups significantly inhibited the increase of TEWL compared with the conventional control mice from 3 weeks. The TEWL of the SPF group, which had no skin lesions, was not increased. c Scratching behaviors of the conventional control and the fexofenadine-treated groups increased as time goes on. The YKS-treated group significantly decreased the scratching behaviors compared with the conventional control group after 6 weeks. The scratching behaviors of the SPF group were not increased. d Grooming behaviors of the conventional control group and also the fexofenadine-treated group were increased under social isolated conditions. The YKS-treated group significantly decreased the grooming behaviors compared with the conventional control mice from 3 weeks. The grooming behaviors of the SPF group increased under social isolated conditions. SPF control: unfilled circle, conventional control: filled circle, YKS: filled square, fexofenadine: filled triangle. Data are presented as means ± SE (n = 5–6). † p < 0.05, †† p < 0.01 vsersu SPF control, *p < 0.05, **p < 0.01 versus conventional control, one-way ANOVA followed by Bonferroni/Dunn test
Assessment of the number of infiltrating mast cells in the skin of NC/Nga mice
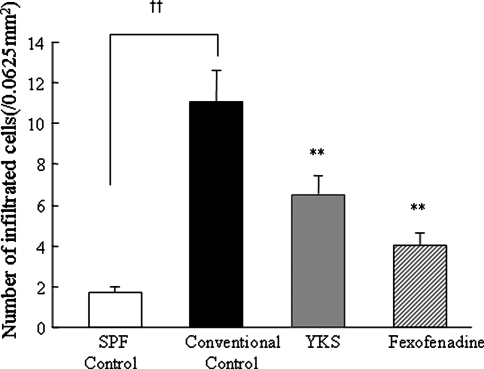
Using histological examination, the numbers of mast cells infiltrating the skin were enumerated. The numbers of infiltrating mast cells in 0.25 × 0.25 mm (0.0625 mm2) squares were counted using a microscope. The numbers of infiltrating mast cells in the YKS-treated or the fexofenadine-treated mice were significantly decreased compared with the conventional control mice (Fig. 2).
Fig. 2.
Effects of YKS and fexofenadine on the numbers of infiltrating mast cells in the skin of NC/Nga mice The numbers of mast cells in the skin of NC/Nga mice skin inside a 0.25 × 0.25 mm (0.0625 mm2) square were counted using a microscope. The numbers of mast cells of the conventional control group were significantly increased compared with the numbers of mast cells in the SPF control group. The numbers of mast cells of the YKS- and the fexofenadine-treated groups were significantly decreased compared with the numbers of mast cells in the conventional control group. Data are presented as means ± SE (n = 5–6). † p < 0.05, †† p < 0.01 versus SPF control, *p < 0.05, **p < 0.01 versus conventional control, one-way ANOVA followed by Bonferroni/Dunn test
Immunohistochemical staining of NMDA receptors in the skin of NC/Nga mice
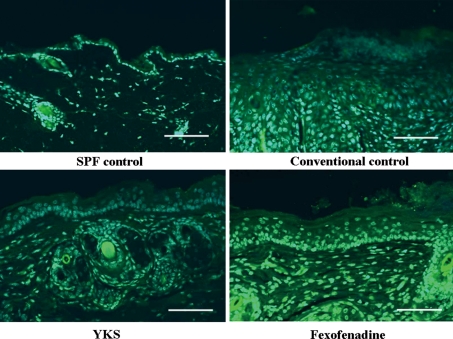
At the involved-skin lesion, expression of NMDA receptors were increased in the epidermis under the conventional control mice compared with the SPF control mice. After the treatment with YKS, the expression of NMDA receptors was significantly reduced in the conventional control mice, which was similar to that of SPF control mice (Fig. 3).
Fig. 3.
Immunohistochemical analysis of NMDA receptor expression in the skin of NC/Nga mice Sections were stained with a fluorescent anti-goat secondary antibody (Alexa Fluor® Anti-Goat IgG) (green). Nuclei were counterstained with 4′-6′-diamidino-2-phenylindole hydrochloride (DAPI) (blue). NMDA receptor expression in the conventional control was increased compared with the SPF control. NMDA receptor expression in the YKS group was decreased compared with the conventional control and the fexofenadine groups. The scale bar represents 100 μm
RT-PCR analyses of NMDA receptor, glutamate aspartate transporter, excitatory amino-acid carrier 1 and glutamate transporter-1 in the skin of NC/Nga mice
The level of NMDA receptor mRNA in the skin was significantly increased in the conventional control mice compared with the SPF control mice, and was significantly decreased in the YKS-treated mice compared with the conventional control mice. The level of glutamate transporter-1 (GLT-1) mRNA in the skin was decreased in the conventional control mice compared with the SPF control mice and the YKS-treated mice. The levels of glutamate aspartate transporter (GLAST) and excitatory amino-acid carrier 1 (EAAC1) mRNAs were not significantly different among the four groups (Fig. 4).
Fig. 4.
RT-PCR analyses of GLAST, EAAC1, GLT-1 and NMDA receptor mRNA levels in the skin of NC/Nga mice RT-PCR analyses of total RNAs extracted from the skins of NC/Nga mice. NMDA receptor mRNA levels in the skin were significantly increased in the conventional control NC/Nga mice compared with the SPF control mice. YKS treatment significantly decreased the elevated NMDA receptor mRNA levels compared with the conventional control NC/Nga mice. GLT-1 mRNA levels in the skin were significantly decreased in the conventional NC/Nga mice control mice compared with the SPF control mice. YKS treatment significantly increased the GLT-1 mRNA levels compared with the conventional control mice. Data are presented as means ± SE (n = 5–6). † p < 0.05, †† p < 0.01 versus SPF control, *p < 0.05, **p < 0.01 versus conventional control, Wilcoxon’s test
Discussion
The results of the present study demonstrate that the traditional Japanese medicine, YKS, ameliorates the development of AD-like lesions and the increase in TEWL and also decreases the number of scratching behaviors and pathological grooming behaviors in socially isolated NC/Nga mice. It is also shown that NMDA receptors and GLT-1 in the skin are involved in the AD-like skin lesions of NC/Nga mice. YKS ameliorated the AD-like skin lesions as well as fexofenadine as shown in the present study. In our previous study, we demonstrated that YKS ameliorates the AD-like skin lesions in a dose-dependent manner in NC/Nga mice [15]. In the present study, we utilized a Microact® to count the scratching behaviors of the animals, which provides a more objective and accurate evaluation of scratching behaviors as compared with visual counting by the investigators. Long-lasting (>1.5 s) scratching behaviors were counted, so that scratching behaviors which are equivalent to human ‘‘addictive scratching’’ were evaluated in NC/Nga mice [16]. YKS significantly inhibited scratching behaviors compared with the conventional control mice and the fexofenadine-treated mice in which the numbers of scratching behaviors gradually increased. Although grooming behaviors are intrinsically essential in many animal species, anxiety and/or stress induce(s) the increase in involuntary grooming behaviors, which leads to inflammation, erosion, and/or ulceration in the skin. Such behaviors are also known to increase in mice that are models for obsessive compulsive disorder [17–19]. Obsessive–compulsive disorder is an anxiety disorder and is characterized by persistent intrusive thoughts (obsessions) and repetitive behaviors (compulsions). Our experimental results show that YKS reduces the numbers of aberrant grooming behaviors compared with the conventional control mice and the fexofenadine-treated mice, i.e., it was shown that YKS alleviates excessive anxiety and/or stress in NC/Nga mice. Our experimental results suggest that YKS acts on the central nervous system (CNS) inducing a tranquilizing effect.
Itching is one of the major diagnostic criteria of AD and is also one of the most troublesome symptoms in AD [1, 2]. The discomfort of itching causes anger and irritation in AD patients and in family members. The itching sensation disturbs sleep, work, school and social life in AD patients for a long period of time, and eventually their quality of life (QOL) will be debased. The effective control of itching will contribute to controlling the skin lesions and to improving the patients’ QOL. The purpose of our study was to suppress the intolerable itching sensations which are not readily cured.
YKS, a traditional Japanese medicine, has been used as a remedy for neurosis, insomnia, and children with night crying. Since Iwasaki et al. [7] reported that YKS is effective for treating BPSD in a randomized, observer-blind, controlled trial in 2005, many basic and clinical studies using YKS have been reported. Clinically, it has been reported that YKS ameliorates excitement, anger, and hallucinations in BPSD, and in patients with Alzheimer’s disease. Treatment of BPSD with YKS has been successful in clinical cases of dementia with Lewy bodies, Parkinsonian dementia and other forms of senile dementia, and also treatments of neuropathic pain, schizophrenia and restless legs syndrome with YKS have been successful [7–14]. Basic research has shown that YKS regulates both serotonin signaling and glutamate signaling. Serotonin is made from tryptophan and suppresses the excitement of neuronal cells. Serotonin receptors now number more than 14, one of them being the 5-HT1A receptor. YKS is a partial agonist of the 5-HT1A receptor, and down-regulates the 5-HT2A receptor. Terawaki et al. [20] stated that YKS is a partial agonist of 5-HT1A receptors. Egashira et al. [21] stated that YKS is down-regulated the 5-HT2A receptor. Kawana et al. [22] reported that 5-HT1A receptors agonist is effective for AD patients. This report suggests that YKS which has partial agonist effect of 5-HT1A receptors is effective for AD patients. Glutamate is a major excitatory neurotransmitter, and accounts for 70–80% of neurotransmitters in the CNS. Glutamate is related to cognition, memory, study, motion control and so forth. Excess glutamate itself has excitotoxicity and is related to several psychoses [23, 24]. Ikarashi et al. [25] reported that YKS inhibits glutamate-mediated excitotoxicity as one of its mechanisms of action. Kawakami et al. [23, 24] reported that YKS binds antagonistically to NMDA receptors and exerts a neuroprotective effect against glutamate-induced excitotoxicity, using cultured rat cortical astrocytes. Hiratsuka et al. [26] explained clearly that YKS inhibits neuronal death during ER stress by regulating the unfolded protein response. Accordingly, YKS controls extracellular glutamate concentrations by suppressing NMDA receptors and activating glutamate transport, and by suppressing glutamate-mediated excitotoxicity and ER stress, and ultimately inhibiting neuronal death in the CNS. Uchida et al. [27] reported that the effects of YKS might be mediated by inhibiting the activity of the dopaminergic system. Thus, YKS has beneficial effects on BPSD and on various psychoses [28, 29].
Furthermore, it has become clear that glutamate signaling also functions in non -neuronal tissues and occurs in sites as diverse as bone, pancreas, and skin [30, 31]. It was reported by Langerstrom et al. that vesicular glutamate transporters (VGLUT2) regulate the chronic itch sensation in mice [32]. Intriguingly, Skerry and Genever [31] reported that keratinocytes, dermal fibroblasts, melanocytes and Merkel cells also express glutamate transporters and NMDA receptors. They also reported that NMDA receptors are expressed on keratinocytes, and GLAST and GLT-1 are expressed by fibroblasts. Our experimental results indicate that YKS might have effects on NMDA receptors and GLT-1, i.e., YKS might act on keratinocytes and/or fibroblasts. In addition, Fuziwara et al. and others [9, 33–36] reported that glutamate plays an important role as a signal in cutaneous barrier homeostasis and in epidermal hyperplasia induced by barrier disruption. We postulate that YKS might affect glutamate transports and NMDA receptors in the skin, and thus might ameliorate homeostasis of the skin. YKS activates glutamate transport and reduces free glutamate among neurons. Thus, we examined the effects of YKS on dermatitis in a mouse model for AD, NC/Nga mice. In addition, when AD-like lesions in NC/Nga mice worsen, the glutamate concentration in nerve terminals is expected to be high due to the inhibition of GLT-1 activation. YKS might activate GLT-1, and the extracellular concentration of glutamate would be normalized as a result. It is expected that YKS would suppress glutamate-mediated cytotoxicity, and would normalize keratinocytes and ameliorate AD-like lesions in NC/Nga mice.
Our previous study showed that YKS controls scratching behaviors and inhibits the development of AD-like lesions in isolated NC/Nga mice. In the present study, we compared the efficacy of YKS and fexofenadine using the same experimental system. Both YKS and fexofenadine inhibit the aggravation of AD-like symptoms in socially isolated NC/Nga mice with respect to TEWL and dermatitis scores. However, YKS decreases the scratching and grooming behaviors in socially isolated NC/Nga mice. Thus, we speculate that YKS inhibits the aggravation of AD-like skin lesions in isolated NC/Nga mice due to mechanisms different from fexofenadine. According to the immunohistological study and RT-PCR for NMDA receptors and GLT-1, YKS has a tendency to decrease the mRNA levels of NMDA receptors and to increase the mRNA levels of GLT-1. Thus, YKS is an excellent cell excitement modulator due to its regulation of intracellular glutamate concentrations in the skin.
Several studies analyzed the function of NMDA receptors in the skin. NMDA receptors reside on keratinocytes and are known to be involved in regulating their proliferation and differentiation and in skin barrier repair [30, 31, 33, 34]. The role of GLT-1 which resides on keratinocytes and fibroblasts in the skin is not well understood. From literature on the function of NMDA receptor in the skin, when aggravating AD-like lesions occur in NC/Nga mice, the NMDA receptors may be activated and stimulate the keratinocytes, so that skin barrier repair is delayed. YKS inhibits NMDA receptor activity, thus skin barrier repair might be normalized. Further, YKS ameliorates the development of AD-like lesions in NC/Nga mice.
In conclusion, the present study suggests that YKS ameliorates the development of AD-like skin lesions and scratching behaviors in NC/Nga mice due to a mechanism different from fexofenadine. YKS suppresses the activity of NMDA receptors and enhances the activity of GLT-1 in the skin. We expect that YKS inhibits NMDA receptors and activates GLT-1 by adjusting the extracellular concentration of glutamate in the skin of NC/Nga mice. Further study is necessary to characterize the mechanism(s) of glutamate signaling and the relationship between the itch sensation and glutamate signaling function in the skin of NC/Nga mice. The authors plan to investigate the role of glutamate in the induction of itch sensation in future experiments. This study showed that YKS was effective for treating AD-like skin lesions with NC/Nga mice, and might be a useful therapeutic strategy for AD patients with BPSA under stressful conditions. In addition, we disclosed the glutamate signaling pathway involved in the AD-like skin lesions with NC/Nga mice.
Acknowledgments
Conflict of interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- AD
Atopic dermatitis
- GLT-1
Glutamate transporter 1
- NMDA receptor
N-methyl-d-aspartate receptor
- SPF
Specific pathogen free
- TEWL
Transepidermal water loss
- YKS
Yokukansan
References
- 1.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh) 1980;92(Suppl):44–47. [Google Scholar]
- 2.Saeki H, Furue M, Furukawa F, et al. Guidelines for management of atopic dermatitis. J Dermatol. 2009;36(10):563–577. doi: 10.1111/j.1346-8138.2009.00706.x. [DOI] [PubMed] [Google Scholar]
- 3.Suto H, Matsuda H, Mitsuishi K, et al. NC/Nga mice: a mouse model for atopic dermatitis. Int Arch Allergy Immunol. 1999;120(suppl 1):70–75. doi: 10.1159/000053599. [DOI] [PubMed] [Google Scholar]
- 4.Jiang J, Kuhara T, Ueki R, et al. Inhibitory effects of paroxetine on the development of atopic dermatitis-like lesions in NC/Nga mice. J Dermatol Sci. 2007;47:244–247. doi: 10.1016/j.jdermsci.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi H, Ishii M, Takeuchi S, et al. Efficacy and safety of a traditional herbal medicine, hochu-ekki-to in the long-term management of kikyo (delicate constitution) patients with atopic dermatitis: a 6-month, multicenter, double-blind, randomized, placebo-controlled study. Evid Based Complement Alternat Med. 2008;7(3):8367–8373. doi: 10.1093/ecam/nen003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao XK, Fuseda K, Shibata T, et al. Kampo medicines for mite antigen-induced allergic dermatitis in NC/Nga mice. Evid Based Complement Alternat Med. 2005;2(2):191–199. doi: 10.1093/ecam/neh077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwasaki K, Satoh-Nakagawa T, Maruyama M, et al. A randomized, observer-blind, controlled trial of the traditional Chinese medicine Yi-Gan San for improvement of behavioral and psychological symptoms and activities of daily living in dementia patients. J Clin Psychiatry. 2005;66(2):248–252. doi: 10.4088/JCP.v66n0214. [DOI] [PubMed] [Google Scholar]
- 8.Iwasaki K, Maruyama M, Tomita N, et al. Effects of the traditional Chinese herbal medicine Ti-Gan San for cholinesterase inhibitor-resistant visual neuropsychiatric symptoms in patients with dementia with Lewy bodies. J Clin Psychiatry. 2005;66(12):1612–1613. doi: 10.4088/JCP.v66n1219a. [DOI] [PubMed] [Google Scholar]
- 9.Kawanabe T, Yoritaka A, Shimura H, et al. Successful treatment with Yokukansan for behavioral and psychological symptoms of Parkinsonian dementia. Prog Neuro Psychopharmacol Biol Psychiatry. 2010;34(2):284–287. doi: 10.1016/j.pnpbp.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Miyaoka T, Furuya M, Yasuda H, et al. Yi-Gan san as adjunctive therapy for treatment-resistant schizophrenia: open-label study. Clin Neurophamacol. 2009;32(1):6–9. doi: 10.1097/WNF.0b013e31817e08c3. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura Y, Tajima K, Kawagoe I, et al. Efficacy of traditional herbal medicine Yokukansan on patients with neuropathic pain. Masui. 2009;58(10):1248–1255. [PubMed] [Google Scholar]
- 12.Okahara K, Ishida Y, Hayashi Y, et al. Effects of Yokukansan on behavioral and psychological symptoms of dementia in regular treatment for Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(3):532–536. doi: 10.1016/j.pnpbp.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Satoh T, Takahashi T, Iwasaki K, et al. Traditional Chinese medicine on four patients with Hunchington’s disease. Mov Disord. 2009;24(3):453–455. doi: 10.1002/mds.22447. [DOI] [PubMed] [Google Scholar]
- 14.Shinno H, Yamanaka M, Ishikawa I, et al. Successful treatment of restless legs syndrome with herbal prescription Yokukansan. Prog Neuro Psychopharmacol Biol Psychiatry. 2010;34(1):252–253. doi: 10.1016/j.pnpbp.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Jiang J, Yamaguchi T, Funakushi N, et al. Oral administration of Yokukansan inhibits the development of atopic dermatitis-like lesions in isolated NC/Nga mice. J Dermatol Sci. 2009;56(1):37–42. doi: 10.1016/j.jdermsci.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Takaoka A, Arai I, Sugimoto M, et al. Involvement of IL-31 on scratching behavior on NC/Nga mice with atopic-like dermatitis. Exp Dermatol. 2006;15:161–167. doi: 10.1111/j.1600-0625.2006.00405.x. [DOI] [PubMed] [Google Scholar]
- 17.Feusner J, Hembacher E, Phillips KA. The mouse who couldn’t stop washing: pathologic grooming in animals and humans. CNS Spectr. 2009;14(9):503–513. doi: 10.1017/s1092852900023567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupts MA, Gupta AK. Psychiatric and psychological co-morbidity in patients with dermatologic disorders. Am J Clin Dermatol. 2003;4(12):833–842. doi: 10.2165/00128071-200304120-00003. [DOI] [PubMed] [Google Scholar]
- 19.Welch JM, Lu J, Rodriguiz RM, et al. Cortico-striatal synaptic defects and OCD-like behaviors in SAPAP3 mutant mice. Nature. 2007;448(7156):894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terawaki K, Ikarashi Y, Sekiguchi K, et al. Partial agonistic effect of yokukansan on human recombinant serotonin 1A receptors expressed in the membranes of Chinese hamster ovary cells. J Ethnopharmacol. 2010;127(2):306–312. doi: 10.1016/j.jep.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Egashira N, Iwasaki K, Ishibashi A, et al. Repeated administration of Yokukansan inhibits DOI-induced head-twitch response and decreases expression of 5-hydroxytryptamine (5-HT) 2A receptors in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1516–1520. doi: 10.1016/j.pnpbp.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Kawana S, Kato Y, Omi T. Efficacy of 5-HT1a receptor agonist in atopic dermatitis. Clin Exp Dermatol. 2010;35:835–840. doi: 10.1111/j.1365-2230.2009.03771.x. [DOI] [PubMed] [Google Scholar]
- 23.Kawakami Z, Kanno H, Ueki T, et al. Neuroprotective effects of Yokukansan, a traditional Japanese medicine, on glutamate-mediated excitotoxity in cultured cells. Neuroscience. 2009;159(4):1397–1407. doi: 10.1016/j.neuroscience.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Kawakami Z, Ikarashi Y, Kase Y. Glycyrrhizin and its metabolite 18 beta-glycyrrhetinic acid in glycyrrhiza, a constituent herb of yokukansan ameliorate thiamine deficiency-induces dysfunction of glutamate transport in cultured rat cortical astrocytes. Eur J Pharmacol. 2010;626(2–3):154–158. doi: 10.1016/j.ejphar.2009.09.046. [DOI] [PubMed] [Google Scholar]
- 25.Ikarashi Y, Iizuka S, Imamura S, et al. Effects of yokukansan, a traditional Japanese medicine, on memory disturbance and behavioral and psychological symptoms of dementia in thiamine-deficient rats. Biol Pharm Bull. 2009;32(10):1701–1709. doi: 10.1248/bpb.32.1701. [DOI] [PubMed] [Google Scholar]
- 26.Hiratsuka T, Matsuzaki S, Miyata S, et al. Yokukansan inhibits neuronal death during ER stress by regulating the unfolded protein response. PLoS One. 2010;5(10):e13280. doi: 10.1371/journal.pone.0013280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uchida N, Egashira N, Iwasaki K, et al. Yokukansan inhibits social isolation-induced aggression and methamphetamine-induced hyperlocomotion in rodents. Biol Pharm Bull. 2009;32(3):372–375. doi: 10.1248/bpb.32.372. [DOI] [PubMed] [Google Scholar]
- 28.Doo AR, Kim SN, Park JY, et al. Neuroprotective effects of an herbal medicine, Yi-Gan San on MPP+/MPTP-induced cytotoxicity in vitro and in vivo. J Ethnopharmacol. 2010;131(2):433–442. doi: 10.1016/j.jep.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Karlsson RM, Tanaka K, Heilig M, et al. Loss of glial glutamate and aspartate transporter (excitatory amino acid transporter 1) causes locomotor hyperactivity and exaggerated responses to psychotomimetics: rescue by haloperidol and metabotropic glutamate 2/3 agonist. Biol Psychiatry. 2008;64(9):810–814. doi: 10.1016/j.biopsych.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Genever P, Maxfield S, Kennovin G, et al. Evidence for a novel glutamate-mediated signaling pathway in keratinocytes. J Invest Dermatol. 1999;112:337–342. doi: 10.1046/j.1523-1747.1999.00509.x. [DOI] [PubMed] [Google Scholar]
- 31.Skerry T, Genever P. Glutamate signaling in non-neuronal tissues. Trends Pharmacol Sci. 2001;22(4):174–181. doi: 10.1016/S0165-6147(00)01642-4. [DOI] [PubMed] [Google Scholar]
- 32.Lagerström MC, Rogoz K, Abrahamsen B, et al. VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron. 2010;68(3):529–542. doi: 10.1016/j.neuron.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fischer M, Glanz D, William T, et al. N-methyl-d-aspartate receptors influence the intracellular calcium concentration of keratinocytes. Exp Dermatol. 2004;13(8):512–519. doi: 10.1111/j.0906-6705.2004.00200.x. [DOI] [PubMed] [Google Scholar]
- 34.Fuziwara S, Inoue K, Denda M. NMDA-type glutamate receptor is associated with cutaneous barrier homeostasis. J Invest Dermatol. 2002;120(6):1023–1029. doi: 10.1046/j.1523-1747.2003.12238.x. [DOI] [PubMed] [Google Scholar]
- 35.Morhenn VB, Murakami M, O’Grady T, et al. Characterization of the expression and function of N-methyl-d-aspartate receptor in keratinocytes. Exp Dermatol. 2004;13(8):505–511. doi: 10.1111/j.0906-6705.2004.00186.x. [DOI] [PubMed] [Google Scholar]
- 36.Nahm WK, Philpot BD, Adams MM, et al. Significance of N-methyl-d-aspartate (NMDA) receptor-mediated signaling in human keratinocytes. J Cell Physiol. 2004;200(2):309–317. doi: 10.1002/jcp.20010. [DOI] [PubMed] [Google Scholar]