Abstract
Noroviruses (NoVs) are second only to rotaviruses (RVs) as causative agents of acute gastroenteritis (AGE) in children. The proportional role of NoVs is likely to increase after control of RV by vaccination. We investigated NoVs in children seen in Tampere University Hospital either treated as outpatients or hospitalized because of AGE before universal RV vaccination was implemented in Finland. This prospective study was conducted from September 2006 to August 2008. A total of 1,128 children <15 years of age with symptoms of AGE were enrolled either in the hospital clinic or in a ward, and stool samples for NoV studies were obtained from 759 children. NoVs were found in 196 (26%) cases. In the first year, NoVs were found in 116 (34%) out of 341, and in the second year, in 80 (19%) out of 418 cases. RVs were found respectively in 128 (38%) and 260 (62%) cases in these two seasons. Both RV and NoV were present in 24 cases. NoV genotype GII.4 predominated with a 96% share of the NoV cases in the first season and an 80% share in the second season. Other NoV genotypes seen infrequently were GII.7, GIIb, GI.6, GII.1, GII.2, and GIIc. The median clinical severity of NoV AGE was 14 compared to 16 for RV AGE on a 20-point scale. Conclusion: NoVs were nearly as common as RVs as causative agents of severe AGE in children seen in hospital. After implementing universal RV vaccination, the importance of NoVs will still increase further.
Keywords: Acute gastroenteritis, Child, Human calicivirus, Norovirus, Rotavirus
Introduction
Noroviruses (NoVs) are major causative agents of acute gastroenteritis (AGE) in outbreaks in children and adults [1, 9–11]. In children, NoVs are also a common cause of seasonal AGE, as described in Finland in a study in 1993–1995 [15, 16] and later elsewhere [4, 9, 10, 24]. In hospital-based studies of seasonal AGE in children, the incidence of NoVs has been lower than that of RVs [9, 14, 15, 17, 26, 27], but particularly in community-based studies, NoVs have been the second most common, or sometimes even the most common causative agents of AGE in children [3, 5, 7, 10, 16–18]. Considering both frequency and severity, NoVs are the second most important cause of viral AGE in children [9, 16, 17, 26], and their importance will be further underscored following universal RV vaccination.
NoVs belong to human caliciviruses (HuCVs), which are divided into NoVs and sapoviruses (SaVs). Seasonal NoV AGE in children is most often caused by genogroup GII NoVs, and both GI and GII are seen in outbreaks. Since the mid 1990s, genotype GII.4 has emerged and become the predominant NoV type in outbreaks [23]. The emergence of GII.4 has also resulted in an overall increase of NoV outbreaks [14]. GII.4 is also the dominant strain detected in seasonal NoV AGE [23], which may have led to an increase of NoV AGE in children in general. The reason may be the greater virulence of GII.4 compared to other NoV genotypes [13, 28].
In this study, we investigated the occurrence and types of NoVs in children seen in Tampere University Hospital either as outpatients or admitted to a ward because of AGE. We also assessed the severity of NoV AGE in comparison to RV AGE.
Methods
Clinical methods
This prospective study was conducted in Tampere University Hospital in September 2006–August 2008. The hospital is the pediatric referral center for the Pirkanmaa Hospital District, a mainly urban area with a child population of about 79,000. The study protocol was approved by the Ethics Committee of Pirkanmaa Hospital District.
All children ≤15 years of age treated in the emergency room (ER), or admitted to the hospital ward for AGE, or caught nosocomial AGE while hospitalized for another reason in Tampere University Hospital, were eligible for enrolment. The diagnosis of AGE was made by a doctor in the hospital. The AGE was considered to be nosocomial if the symptoms began at least 24 h after hospitalization. Prior to enrolment, a parent or legal guardian was informed about the study and signed an informed consent form.
If the child had more than one ER visit or hospitalization during the study period, we deemed the AGE symptoms to belong to the same episode if there were fewer than seven symptom-free intervening days. Otherwise the visits were considered to represent two separate episodes.
At enrollment, the parents were interviewed about the child’s AGE symptoms and treatment before the hospital visit, and their rotavirus vaccination status was ascertained. A questionnaire about the total duration of the symptoms after discharge was requested to be completed and returned after recovery. After discharge from the ER or the hospital ward, additional clinical information and possible laboratory test results of the AGE episode were collected from the medical records. A stool sample was collected while in the hospital. We failed to collect the sample if the child did not pass stools while in the hospital. If the parents did not return the questionnaire, the duration of symptoms at home remained unknown. Severity of the AGE episode was assessed using a 20-point score [19], in which ≥11 points is usually considered to be severe AGE. This score considers the following symptoms and features: fever, duration and intensity of diarrhea, duration and intensity of vomiting, degree of dehydration and treatment given, and need for hospitalization. If information on one or more of these items was missing, the case was excluded from the severity analysis. The statistical analyses were done using SPSS 15.0 with Mann–Whitney and Kruskal–Wallis tests.
Apart from the prospective surveillance, we also collected discharge information on all patients ≤15 years of age treated for AGE in Tampere University Hospital during the study period. For this purpose, all the ICD-10 diagnoses of groups A01–A09 were retrieved.
In the second follow-up season, an extensive waterborne AGE outbreak occurred in Nokia, a town close to Tampere. The outbreak was caused by massive contamination of drinking water by sewage water and caused extraordinary severe and mixed AGE [12, 21]. Because of the uncommon features associated with the AGE cases in this outbreak, we excluded the cases associated to this outbreak from this analysis; the cases have, however, been reported separately [19].
Laboratory methods
All stool samples were studied by reverse transcription (RT)-PCR for HuCVs and RVs. HuCVs were detected using a modified RT-PCR method introduced by Jiang and Farkas [2, 8]. These primers, localized in the RNA polymerase region, co-detect NoVs and SaVs: in this study, we describe the NoV findings. All PCR-positive amplicons were sequenced to confirm the PCR results and to determine the NoV genotype. Original genotypes for validation have been described earlier [2, 8]. We used NoV GI.1, GI.3, GI.4, GI.6, GII.1, GII.2, and GII.4 genotypes for PCR validation. In addition, we have later found other genotypes using this same PCR, such as GIIb, GII.7, GI.2, GII.9, GIIU, and GIId [6]. RV G types were determined by RT-PCR as described by Pang et al. [16] with the Taq polymerase replaced by GoTaq® polymerase (Promega, Madison, WI, USA). Both RT-PCR methods are highly sensitive detecting viruses and can be made from the smallest amount of sample as well as from diapers. Negative findings do not need confirming tests. If there was any uncertainty in the positive findings or sequencing, the tests were repeated.
The presence of RV antigen in stools was detected with ELISA using IDEIA® Rotavirus kit (Oxoid Ltd., UK) according to the manufacturer’s protocol. The ELISA test is not as sensitive as the PCR techniques and cannot be performed from diapers.
Results
A total of 1,723 patients ≤15 years of age presented with AGE in the Tampere University Hospital during the study period. The number of patients recruited was 1,193, of whom 65 were excluded because of association with the waterborne AGE outbreak in Nokia [20], and thus, 1,128 (65% of the total cases of AGE) were included in this study. Among these, there were 45 (4%) children, who were known to have received at least one dose of either of the RV vaccines. Stool samples were obtained from 759 children (67% of included and 44% of all eligible)—341 in the first AGE season (September 2006–August 2007) and 418 in the second season (September 2007–August 2008).
The cases of AGE positive for HuCV and RV are shown in Table 1. In the first season, 116 out of 341 (34%) and, in the second season, 80 out of 418 (19%) AGE cases were positive for NoV, and in both seasons combined 196 of all 759 (26%). Of the NoV-positive cases, 24 (12%) were mixed infections with RV. In the first season, 4 (1%) out of 341 stools and, in the second season, 8 out of 418 (2%) stools were positive for SaV; of these, 2 were mixed infections with RV. There were no cases with both NoV and SaV or more than one type of NoV in the stools at the same time. No child had more than one episode of NoV AGE during the follow-up. RVs were present in 128 (38%) AGE cases in the first, and 260 (62%) in the second follow-up season, of which 26 (7%) were mixed infections with a NoV or SaV. RV was found in seven specimens from the children who were known to have received RV vaccines; of these, three were vaccine-type viruses from recent vaccination.
Table 1.
Causative agents of acute gastroenteritis in children seen in Tampere University Hospital in September 2006–August 2008
| Virus | 1st season (%) | 2nd season (%) | Total (%) |
|---|---|---|---|
| Norovirus (NoV) | 105 (31) | 67 (16) | 172 (23) |
| Sapovirus (SaV) | 4 (<1) | 6 (1) | 10 (1) |
| Rotavirus (RV) | 117 (34) | 245 (59) | 362 (48) |
| Mixed NoV + RV | 11 (3) | 13 (3) | 24 (3) |
| Mixed SaV + RV | 0 (0) | 2 (<1) | 2 (<1) |
| Other/undefined | 104 (30) | 85 (20) | 189 (25) |
| Total | 341 (100) | 418 (100) | 759 (100) |
Genotype GII.4 was the predominant NoV genotype detected in both seasons. In the first season, 111 (96%) and, in the second season, 64 (80%) of the NoV-positive cases were of genotype GII.4 (Table 2).
Table 2.
Norovirus genotypes seen in acute gastroenteritis in children in two seasons (September 2006–August 2008) in Tampere University Hospital
| Genotype | 1st season (%) | 2nd season (%) | Total (%) |
|---|---|---|---|
| GII.4 | 111 (96) | 64 (80) | 175 (89) |
| GII.7 | 1 (<1) | 4 (5) | 5 (2) |
| GIIb | 0 (0) | 11 (14) | 11 (6) |
| GII.1 | 2 (2) | 0 (0) | 2 (1) |
| GIIc | 1 (<1) | 0 (0) | 1 (<1) |
| GI.6 | 1 (<1) | 0 (0) | 1 (<1) |
| GII.2 | 0 (0) | 1 (1) | 1 (<1) |
| Total | 116 (100) | 80 (100) | 196 (100) |
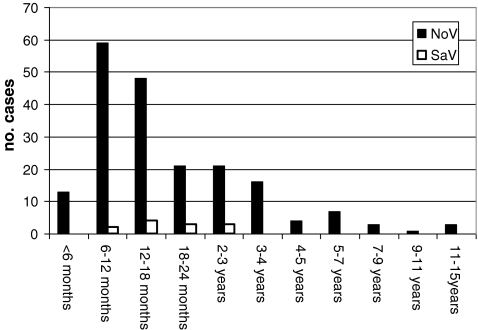
The age range of the children treated because of NoV AGE was from 19 days to 13 years 8 months. The median age was 15 months, and the peak incidence was between 6 and 18 months of age. Altogether, 72% of children were ≤24 months of age. In the children treated because of SaV AGE, the age ranged from 6 months to 2 years 10 months, the median being 22 months (Fig. 1).
Fig. 1.
Age distribution of children seen in Tampere University Hospital because of norovirus and sapovirus gastroenteritis in September 2006–August 2008
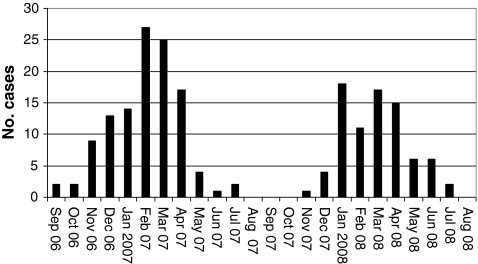
A clear seasonality was seen in NoV AGE in both years. The most active NoV period in the first season was from February to April 2007 and in the second season from January to April 2008 (Fig. 2). The SaV AGE cases were scattered with no obvious seasonality.
Fig. 2.
Seasonality of the noroviruses seen in acute gastroenteritis in children (September 2006–August 2008) in Tampere University Hospital
Of the AGE cases that had NoV as the only causative agent, 80 out of 172 (47%) were admitted to the hospital and 81 (47%) were treated as outpatients. The remaining 11 cases (6%) were nosocomially acquired. For comparison, of the 362 AGE cases with a RV as a single causative agent, 189 (52%) were admitted to the hospital, 161 (44%) were treated as outpatients, and 12 (3%) were nosocomial. Of the children admitted to the hospital because of AGE, NoV was the causative agent in 21% and RV in 50% of the cases.
Of the 369 cases from which the stool sample could not be obtained, 298 (81%) were treated as outpatients, 64 (17%) were admitted to a ward, and 7 (2%) were nosocomially acquired AGE. We enrolled 48 cases of nosocomially acquired AGE and obtained stool samples from 41 of these. In 11 (27%) of these, the causative agent was NoV, in 12 (29%) the causative agent was RV, and in 4 (10%) both NoV and RV were found. No SaVs were found in nosocomial AGE.
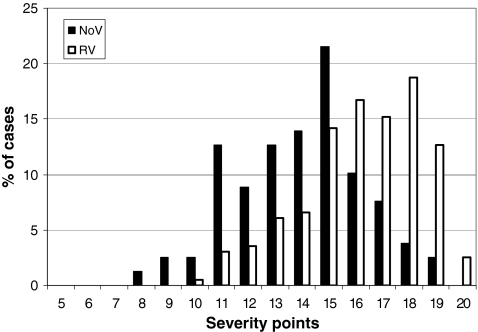
Clinical severity score could be calculated in 79 out of 171 (46%) of NoV-positive and RV-negative, and 196 out of 356 (55%) RV-positive and NoV-negative AGE cases. The severity scores of AGE cases caused by NoV ranged from 8 to 19, and cases caused by RV from 10 to 20 (Fig. 3). Median severity of AGE cases with a NoV as single causative agent was 14, and with a RV as a single causative agent, median was 16. The difference between the severities of AGE caused by NoV and RV was statistically significant (P < 0.01).
Fig. 3.
Severity of clinical symptoms of acute gastroenteritis caused either by norovirus (n = 79) or rotavirus (n = 196) (September 2006–August 2008). Severity is assessed with a 20-point scale in which ≥11 points is considered as severe
Discussion
This study confirmed both the prevalence and clinical importance of NoVs as the causative agent of seasonal AGE in children. At the hospital level, NoVs are, after RVs, the second most important causative agents of AGE in children admitted to a hospital or treated as outpatients. In some seasons, they may be as important as RVs, being as frequent as RV AGE, which was the situation in the first season, resulting in significantly severe cases of AGE. Overall, the 26% proportion of NoVs in the total material and the 21% proportion among children hospitalized with AGE appear higher than generally reported in the past. For example, in a review of published studies from 1990 to 2008, Patel et al. counted that the pooled proportion of NoVs was 11% [17]. We propose that the proportional increase may also reflect an absolute increase of NoV AGE in children, possibly due to greater virulence of the recently emerged genotype GII.4.
Of the 1,723 eligible children, 1,193 were recruited. The rest were lost mainly because of the ER being too busy to be able to recruit all the eligible cases. Sixty-five cases belonging to the AGE outbreak in Nokia were excluded from this analysis because of the extraordinary features of this outbreak leaving 1,128 cases in this study material. Furthermore, stool samples could be obtained only from 759 of the 1,128 enrolled children because the stools were collected only in the hospital. There were many children who spent only a few hours in the ER passing no stools during that time. A possibility of a bias in the AGE-causing agents resulting from these matters cannot be totally excluded, and the majority of stool samples are from the severe AGE cases. However, we could see no relation between the causative agent and passing stools in the ER. Even with these limitations, our study represents almost half (47%) of all the AGE cases seen in the hospital in children in the 2-year period.
NoV AGE showed seasonality with activity peaking in wintertime and only little NoV activity in the summer months. This confirms that NoVs are not involved only in outbreaks, but are also important causative agents in seasonal epidemic AGE. Like RVs, NoVs have winter seasonality, but in this study the seasons were distinct, with NoVs occurring earlier than RVs.
In the first follow-up year, from September 2006 to August 2007, RV activity was unusually low and associated with several RV G types. By contrast, RV activity in the second year from September 2007 to August 2008 was high and associated with the most common RV G-type G1P [8]. In the circumstances of low RV activity in the first season, the NoVs caused as many AGE hospitalizations as RVs, but when RV activity was high, the proportion of NoV AGE cases seen in the hospital was lower. Furthermore, the “virulent” NoV genotype GII.4 predominated particularly in the first season with a 96% share. It is tempting to speculate that simultaneous occurrence of the high NoV activity with the virulent GII.4 genotype and the low RV activity with multiple G types may be more than a coincidence. In any case, while RV G1P [8] re-emerged as the dominant RV type in the second season of follow-up, the NoV activity decreased, the proportion of GII.4 became less, and other NoV types appeared.
In general, NoV GII.4 has been the most common NoV strain detected in children, as well as in adults, this century. GII.4 activity varies from one season to another [22, 23, 28]. Such variation may be associated with mutations in the antigenic epitopes or other parts of the NoV genome [25, 28].
SaVs were not the point of primary interest in this study and are not discussed at length. SaV findings were single cases scattered throughout the follow-up: 12 cases were seen, of which 2 (17%) were mixed AGE cases with RV.
We supposedly did not obtain information about all the nosocomially acquired AGE in the hospital in the study period. If the child is hospitalized for another reason and gets AGE symptoms, he/she is usually discharged as soon as possible, and unfortunately, often no ICD-10 diagnosis of AGE is recorded. Nosocomial AGE is not discussed in-depth here because its real incidence is probably higher than what is seen in this study.
We used the 20-point severity scale to assess the severity of those AGE episodes which were not associated with the Nokia AGE outbreak of November–December 2007. The severity of NoV cases seen in the hospital was somewhat lower than that of RV cases (Fig. 3). However, the median severities of the AGE cases caused either by NoV, RV, or both were over 11, which is considered severe. If we assess the severity of AGE using only the hospitalization rate among the children, NoV AGE cases seem almost as severe as RV cases: of the NoV AGE cases, 47% were hospitalized and 47% were treated as outpatients; of the RV AGE cases, 54% were hospitalized and 42% were treated as outpatients. The number of non-GII.4 types seen was small and did not permit statistical comparison of clinical severity between GII.4 and other NoV genotypes. Median severity of the AGE caused by GII.4 NoV was 14 (n = 71) with a range from 8 to 19.
Presumably, the overall high severity of the AGE cases in our study was reflected in the hospital-based study design and in the fact that we did not obtain stool samples for analysis from many of the cases not needing hospitalization. However, the NoV AGE cases seen in this study were unexpectedly severe, even in a hospital setting. This may be a sign of the proposed greater severity of genotype GII.4 NoVs compared to the other genotypes. The omission of such cases may influence the assessment of mean clinical severity of AGE associated with NoV or other gastroenteritis viruses.
There are only a few prospective follow-up studies on both endemic circulating NoV genotypes and severity of endemic NoV AGE in children. The results of this survey also serve as a baseline to subsequent studies of AGE in children to be conducted after the introduction of universal RV vaccination in Finland in September 2009. It is expected that the proportional role of NoV will increase along with a decrease in RVs. It will be of particular interest to see if the absolute numbers of NoV AGE also increase in the future.
Acknowledgments
We thank the children and their parents for participating in this study. We also thank the study personnel, the staff of the virology laboratory, and the nursing personnel in Tampere University Hospital. Special thanks to Marjo Salonen for her outstanding work as the study nurse.
Conflict of interests
The writers do not have any conflict of interest concerning this study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Atmar RL, Estes MK. The epidemiologic and clinical importance of norovirus infection. Gastroenterol Clin North Am. 2006;35:275–290. doi: 10.1016/j.gtc.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Farkas T, Zhong WM, Jing Y, et al. Genetic diversity among sapoviruses. Arch Virol. 2004;149:1309–1323. doi: 10.1007/s00705-004-0296-9. [DOI] [PubMed] [Google Scholar]
- 3.Ferreira MSR, Victoria M, Caravalho-Costa FA, et al. Surveillance of norovirus infection in the state of Rio de Janeiro, Brazil 2005–2008. J Med Virol. 2010;82:1442–1448. doi: 10.1002/jmv.21831. [DOI] [PubMed] [Google Scholar]
- 4.Froggatt PC, Barry Vipond I, Ashley CR, et al. Surveillance of norovirus infection in a study of sporadic childhood gastroenteritis in South West England and South Wales, during one winter season (1999–2000) J Med Virol. 2004;72:307–311. doi: 10.1002/jmv.10569. [DOI] [PubMed] [Google Scholar]
- 5.Harada S, Okada M, Yahiro S, et al. Surveillance of pathogens in outpatients with gastroenteritis and characterization of sapovirus strains between 2002 and 2007 in Kumamoto Prefecture. Japan J Med Virol. 2009;81:1117–1127. doi: 10.1002/jmv.21454. [DOI] [PubMed] [Google Scholar]
- 6.Huhti L, Szakal ED, Puustinen L, et al. Norovirus GII-4 causes a more severe gastroenteritis than other noroviruses in young children. J Infect Dis. 2011 doi: 10.1093/infdis/jir039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iturriza Gomara M, Simpson R, Perault AM, et al. Structured surveillance of infantile gastroenteritis in East Anglia, UK: incidence of infection with common viral gastroenteric pathogens. Epidemiol Infect. 2008;136:23–33. doi: 10.1017/S0950268807008059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang X, Huang PW, Zhong WM, et al. Design and evaluation of a primer pair that detects both Norwalk- and Sapporo-like caliciviruses by RT-PCR. J Virol Methods. 1999;83:145–154. doi: 10.1016/S0166-0934(99)00114-7. [DOI] [PubMed] [Google Scholar]
- 9.Junquera CG, de Barada CS, Mialdea OG, et al. Prevalence and clinical characteristics of norovirus gastroenteritis among hospitalized children in Spain. Pediatr Infect Dis J. 2009;28:604–607. doi: 10.1097/INF.0b013e318197c3ca. [DOI] [PubMed] [Google Scholar]
- 10.Koopmans M. Progress in understanding norovirus epidemiology. Curr Opin Infect Dis. 2008;21:544–552. doi: 10.1097/QCO.0b013e3283108965. [DOI] [PubMed] [Google Scholar]
- 11.Kroneman A, Verhoef L, Harris J, et al. Analysis of integrated virological and epidemiological reports of norovirus outbreaks collected within the Foodborne Viruses in Europe network from 1 July 2001 to 30 June 2006. J Clin Microbiol. 2008;46:2959–2965. doi: 10.1128/JCM.00499-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laine J, Huovinen E, Virtanen MJ et al (2011) An extensive gastroenteritis outbreak after drinking-water contamination by sewage effluent, Finland. Epidemiol Infect 139:1–9 [DOI] [PubMed]
- 13.Lopman B, Vennema H, Kohli E, et al. Increase in viral gastroenteritis outbreaks in Europe and epidemic spread of new norovirus variant. Lancet. 2004;363:682–688. doi: 10.1016/S0140-6736(04)15641-9. [DOI] [PubMed] [Google Scholar]
- 14.Nakagomi T, Correia JB, Nakagomi O, et al. Norovirus infection among children with acute gastroenteritis in Recife, Brazil: disease severity is comparable to rotavirus gastroenteritis. Arch Virol. 2008;153:957–960. doi: 10.1007/s00705-008-0060-7. [DOI] [PubMed] [Google Scholar]
- 15.Pang XL, Honma S, Nakata S, Vesikari T. Human caliciviruses in acute gastroenteritis of young children in the community. J Infect Dis. 2000;181(2):S288–S294. doi: 10.1086/315590. [DOI] [PubMed] [Google Scholar]
- 16.Pang XL, Joensuu J, Vesikari T. Human calicivirus-associated sporadic gastroenteritis in Finnish children less than 2 years of age followed prospectively during a rotavirus vaccine trial. Pediatr Infect Dis J. 1999;18:420–426. doi: 10.1097/00006454-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Patel MM, Widdowson MA, Glass RI, et al. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis. 2008;14:1224–1231. doi: 10.3201/eid1405.071426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips G, Tam CC, Conti S, et al. Community incidence of norovirus-associated infectious intestinal disease in England: improved estimates using viral load for norovirus diagnosis. Am J Epidemiol. 2010;171:1014–1022. doi: 10.1093/aje/kwq021. [DOI] [PubMed] [Google Scholar]
- 19.Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis. 1990;22:259–267. doi: 10.3109/00365549009027046. [DOI] [PubMed] [Google Scholar]
- 20.Räsänen S, Lappalainen S, Halkosalo A, et al. Rotavirus gastroenteritis in Finnish children in 2006–2008, at the introduction of rotavirus vaccination. Scand J Infect Dis. 2011;43:58–63. doi: 10.3109/00365548.2010.508462. [DOI] [PubMed] [Google Scholar]
- 21.Räsänen S, Lappalainen S, Kaikkonen S, et al. Mixed viral infections causing acute gastroenteritis in children in a waterborne outbreak. Epidemiol Infect. 2010;138:1227–1234. doi: 10.1017/S0950268809991671. [DOI] [PubMed] [Google Scholar]
- 22.Siebenga JJ, Vennema H, Duizer E, Koopmans MP. Gastroenteritis caused by norovirus GGII.4, the Netherlands, 1994–2005. Emerg Infect Dis. 2007;13:144–146. doi: 10.3201/eid1301.060800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siebenga JJ, Vennema H, Zheng DP, et al. Norovirus illness is a global problem: emergence and spread of norovirus GII.4 variants, 2001–2007. J Infect Dis. 2009;200:802–812. doi: 10.1086/605127. [DOI] [PubMed] [Google Scholar]
- 24.Simpson R, Aliyu S, Iturriza-Gomara M, et al. Infantile viral gastroenteritis: on the way to closing the diagnostic gap. J Med Virol. 2003;70:258–262. doi: 10.1002/jmv.10386. [DOI] [PubMed] [Google Scholar]
- 25.Tan M, Fang P, Chachiyo T, et al. Noroviral P particle: structure, function and applications in virus–host interaction. Virology. 2008;382:115–123. doi: 10.1016/j.virol.2008.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tran A, Talmud D, Lejeune B, et al. Prevalence of rotavirus, adenovirus, norovirus, and astrovirus infections and coinfections among hospitalized children in Northern France. J Clin Microbiol. 2010;48:1943–1946. doi: 10.1128/JCM.02181-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Victoria M, Carvalho-Costa FA, Heinemann MB, et al. Prevalence and molecular epidemiology of noroviruses in hospitalized children with acute gastroenteritis in Rio de Janeiro, Brazil, 2004. Pediatr Infect Dis J. 2007;26:602–606. doi: 10.1097/INF.0b013e3180618bea. [DOI] [PubMed] [Google Scholar]
- 28.Zheng DP, Widdowson MA, Glass RI, Vinje J. Molecular epidemiology of genogroup II-genotype 4 noroviruses in the United States between 1994 and 2006. J Clin Microbiol. 2010;48:168–177. doi: 10.1128/JCM.01622-09. [DOI] [PMC free article] [PubMed] [Google Scholar]