Abstract
Background
Semisynthetic collagen matrices are promising duraplasty grafts with low risk of cerebrospinal fluid (CSF) fistulas, good tissue integration and minor foreign body reaction. The present study investigates the efficacy and biocompatibility of a novel semisynthetic bilayered collagen matrix (BCM, B. Braun Aesculap) as dural onlay graft for duraplasty.
Methods
Thirty-four pigs underwent osteoclastic trepanation, excision of the dura, and placement of a cortical defect, followed by duraplasty using BCM, Suturable DuraGen™ (Integra Neuroscience), or periosteum. CSF tightness and intraoperative handling of the grafts were evaluated. Pigs were sacrificed after 1 and 6 months for histological analysis.
Findings
BCM and DuraGen™ showed superior handling than periosteum with a trend for better adhesion to dura and CSF tightness for BCM. Periosteum, which was sutured unlike the synthetic grafts, had the highest intraoperative CSF tightness. Duraplasty time with periosteum was significantly higher (14.4 ± 2.7 min) compared with BCM (2.8 ± 0.8 min) or DuraGen™ (3.0 ± 0.5 min). Tissue integration by fibroblast infiltration was observed after 1 month for all devices. More adhesions between graft and cortex were observed with DuraGen™ compared with BCM and periosteum. No relevant adhesions between leptomeninges and BCM were observed and all devices showed comparable lymphocytic reaction of the brain. All devices were completely integrated after 6 months. BCM and DuraGen™ showed a trend for an enhanced lymphocytic reaction of the brain parenchyma compared with periosteum. Implant rejection was not observed.
Conclusion
Semisythetic collagen matrices are an attractive alternative in duraplasty due to their easy handling, lower surgical time, and high biocompatibility.
Keywords: Collagen matrix, Dural substitute, Dural defect repair, Duraplasty, Cerebrospinal fluid leak, BCM, DuraGen
Introduction
Closure of dural defects is a necessity after neurosurgical procedures to prevent cerebrospinal fluid (CSF) leakage and to reduce the risk of perioperative infections [2, 12, 16, 18]. In several surgical settings primary closure is technically impossible, e.g. due to coagulation-induced shrinkage or retraction of dura, surgical excision of dura (resection of meningiomas), or dural injury after trauma and therefore reconstruction of the dural defect using a substitute is required. Reconstruction with endogenous material is most common. However, harvesting of periosteum or fascia lata may require extended surgical approach, additional incisions and time intensive suturing.
Numerous dura substitutes are currently commercially available. Among these dura substitutes, onlay grafts of semisynthetic collagen matrices appear promising, since they are thought to provide a matrix for ingrowth and subsequent replacement by endogenous connective tissue, while continuously presenting a mechanical barrier [7, 11]. Previous studies using DuraGen™ (Integra Neuroscience) showed that dura onlay grafts may be superior to other synthetic devices for duraplasty since they (1) do not require labour-intensive suturing, (2) allow dura reconstruction with sufficient tightness to avoid perioperative CSF fistulas effectively, and (3) cause no major reaction of the surrounding tissue [4, 11, 16, 18].
A novel semisynthetic, two-component collagen patch (bilayered collagen matrix, referred to as BCM, B. Braun Aesculap) has been designed as a suturable dural onlay graft. BCM consists of a non-cross-linked collagen sponge layer (manufactured from bovine split hide) and a watertight non-cross-linked collagen membrane based on lyophilized bovine pericardium (Lyoplant™, B. Braun Aesculap). Safety and efficacy of Lyoplant™ as a water tight dural substitute in a clinical trial has been reported [9]. Lyoplant™, like other collagen-based dural substitutes, has to be sutured to the dura. In contrast, BCM adheres to the dura without sutures due to its fine pore structure of the collagen sponge allowing capillary forces to act between the graft and the wet tissue, holding the graft in place. In addition, the pericardium layer within BCM provides a durable mechanical barrier and a watertight closure of the defect, while still allowing suturing.
The aim of the present study was to investigate the safety and efficacy of BCM in a porcine model. Sutured autologous periosteum and DuraGen™ used as onlay graft served as control materials. Periosteum sutured into the dural defect is used for duraplasty most frequently. DuraGen™ is one of the most widely used and best studied semisynthetic grafts for dural repair in clinical use.
In contrast to previous animal studies for dural repair [1, 5, 7, 8, 10, 13–15, 17–19], in the present study, a dural defect and a cortical lesion were created to study the adhesion of devices to the dura, their subsequent tissue integration, and potential adhesion and scar formation with the underlying lesioned cortex. Properties of the dural substitutes regarding intraoperative handling and workability, initial watertightness of the dural reconstruction during surgery, biocompatabiliy, tissue integration and formation of adhesion to the intact or lesioned cortex were analysed at 1 and 6 months after the surgery.
Materials and methods
Large animal model
All procedures were performed according to the animal care and use guidelines of the University of Pécs, Hungary under protocol number 1301-7/1999 approved by the Ethics Committee of the University of Pécs. Female Duroc pigs were used. Pigs were allowed to adapt for at least a week prior to surgery and were given pig feed and water ad libidum until the day of surgery. Pigs were monitored daily for general and implantation site related adverse events.
Surgical procedure
Pigs (mean weight 16.06 kg; range 12–27 kg) were anaesthetized (125 mg of ketamine hydrochloride) after premedication with a cocktail of azaperonum (160 mg), ketamine (125 mg), diazepam (10 mg) and atropine sulphate (1 mg). Endotracheal intubation was followed by maintenance anaesthesia with 0.5% (v/v) halothane.
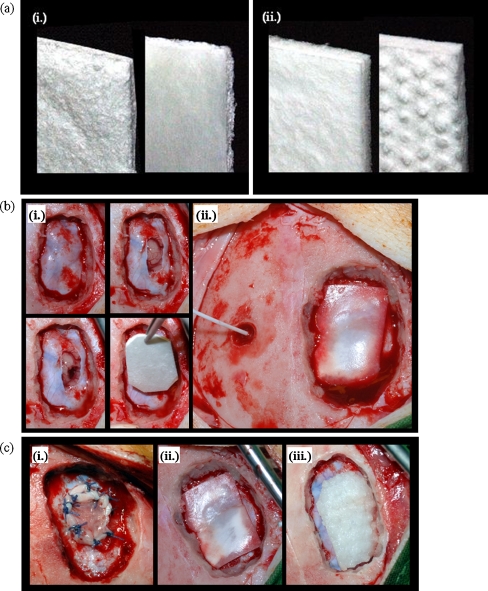
Animals were transferred to the prone position. After disinfection, a midline incision of the scalp was made and the periosteum was exposed. In animals selected for dural reconstruction with periosteum, a 2 × 2-cm sheet of periosteum was harvested and kept in a humidified chamber. A 4-mm drill hole was made in the right frontal region and an intracranial pressure probe (Codman ICP Monitoring System, Codman, Le Locle, Switzerland) was implanted into the right frontal white matter (Fig. 1). An osteoclastic craniotomy (2.5 × 2 cm) was performed in the left parietal region and a dural defect (1.5 × 1 cm) was created using surgical microscissors. A cortical defect (2–3 mm diameter) was made using mild suction (Fig. 1). Haemostasis was achieved where needed using Sangustop™ (B. Braun Aesculap, Tuttlingen, Germany). All foreign materials were removed before dural reconstruction. The galea was closed using Safil™ USP 2/0 (B. Braun Aesculap) and skin closure was done using Dafilon™ USP 2/0 (B. Braun Aesculap). Questionnaires evaluating workability, cutting behaviour, stiffness/flexibility, stability in a wet environment, watertightness and adhesion to dura were completed immediately after wound closure for each procedure. The following scoring was used: 1 = very good, 2 = good, 3 = acceptable, 4 = poor, 5 = not acceptable. Furthermore stickiness to instruments and gloves were evaluated as: 1 = without any problem, 2 = acceptable, 3 = not acceptable.
Fig. 1.
Surgical procedure. a Semisynthetic collagen matrices BCM (i) and DuraGen™ (ii) are shown. b illustrates the experimental approach: after creation of the osteoclastic defect, a dural defect and cortical lesion were created. After haemostasis, the dural defect was closed using an onlay graft (i). The intracranial pressure was monitored ((ii). c shows dural defects closed with periosteum (i), BCM (ii), and DuraGen™ (iii)
Autologous graft and dural substitutes
For dural reconstruction with endogenous periosteum, the periosteal sheet was sutured into the defect using Vicryl™ USP 4/0 (Ethicon, Norderstedt, Germany). For reconstruction using BCM (B. Braun Aesculap, Tuttlingen, Germany) or Suturable DuraGen™ (referred to as DuraGen™; Integra Neurosciences, Plainsboro, NJ, USA), appropriate patches of the implant were cut, hydrated with physiological saline and placed onto the dural defect with an overlap of 5 mm at the edges according to the manufacturers instructions (Fig. 1).
CSF tightness
To test graft adhesion and watertightness of the duraplasty, animals were positioned head down and a Valsalva manoeuvre was performed over 30 s. Intracranial pressure (ICP) was monitored continuously, and the ICP at which a CSF leak occurred was registered.
Specimens and histology
Animals were sacrificed at weeks 4 and 24 postoperatively following premedication as described above. Animals were anaesthesized and the left carotid artery was canulated and the right atrium was opened. Brain tissue was perfused with 500 ml physiological saline, followed by 500 ml neutral buffered 4% paraformaldehyde solution. The scalp was removed and the craniotomy site was exposed. Four weeks post-surgery, the osteoclastic defect showing varying degrees of ossification from the edges and a central soft tissue scar was identified in all animals. The defect was exposed by extending the craniotomy to 3 × 4 cm and histological specimens were taken as whole-tissue blocks containing brain parenchyma, meninges and duraplasty. Ossification closed the cranial defect entirely after 24 weeks in all animals. Craniotomy (3 × 4 cm) was performed with an oscillating osteotome (B. Braun Aesculap) and specimens were collected as whole-tissue blocks of the skull with adherent dura and duroplasty, meninges, and brain parenchyma. The specimens were fixed in 4% formaldehyde for at least 7 days. The fixed tissues were cut at the level of the parenchymal defect and embedded in paraffin; either in conjunction with overlaying dura or separately, depending on adhesions. In the group examined at 6 months after surgery brain parenchyma, dura and duragraft were partially removed from the mineralized craniotomy defect and embedded together with the cranial bone after decalcification using new decalc (Medite Medizintechnik, Burgdorf, Germany). Embedded tissues were sectioned (1-3 μm) and stained with haematoxylin and eosin for histology. Other sections were stained for connective tissue (elastica-van Gieson stain), basal membrane (silver reaction according to Gomori) and carbohydrates (periodic acid-Schiff stain).
The stained slides were analyzed for (1) adhesions between the device and the leptomeninges or the brain tissue, (2) cellular reactions in the device, (3) cellular reactions in the brain tissue, especially in the brain lesion and (4) the integration of the device. The following scoring scale was used for evaluations:
Histological adhesion score for meningeal structures (a) and cortex (b): 0 = no adhesions detectable, 1 = adhesions between device and (a) leptomeninges or (b) cortex, 2 = adhesions with fibroblast reaction and fibrosis in (a) leptomeninges or (b) cortex.
Macrosopical adhesion score (6 months time point only); quantification of the adherence between device and cortex: 0 = no adherence, 1 = lose adherence, 2 = considerable adherence.
Cellular reaction in the device: 0 = no cellular infiltrates (i.e. lymphocytes), 1 = sparse lymphocytic infiltrates, 2 = considerable lymphocytic infiltrates, 3 = foreign body reaction/suture granuloma.
Cellular reaction in the cortical lesion below the device: 0 = no cellular infiltrates (i.e. lymphocytes), 1 = sparse lymphocytic infiltrates, 2 = considerable lymphocytic infiltrates, 3 = fibroblastic reaction in the brain tissue.
Integration of the device: 0 = no integration, 1 = ingress of fibroblasts into the device, 2 = only parts of the device remaining detectable, 3 = complete integration.
Statistical analysis
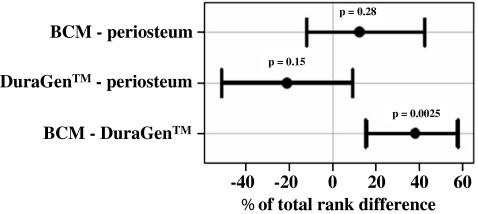
A composite primary endpoint analysis was performed (cf. Fig. 5). The variable used for the analysis of this study was derived by establishing a rank order of the experimental animals using a hierarchical composite outcome score. Statistical evaluation was done using standard non-parametric Wilcoxon statistics on the rankings. This approach is an extension of the methods described by Follmann et al. [6] and by Bjorling et al. [3], respectively. For the secondary endpoints, namely dura closure time and CSF tightness, p values were computed using unpaired Wilcoxon tests with a 95% confidence interval. A p value <0.05 was considered statistically significant.
Fig. 5.
Statistical evaluation of the composite primary endpoint. Paired difference of the composite score and 95% confidence intervals between treatments from unpaired exact Wilcoxon test. Bars not overlapping the vertical line at t = 0 indicate significant effects
Results
Animals were assigned to three groups: duraplasty with periosteum (n = 11), DuraGen™ (n = 10) and BCM (n = 13).
Intraoperative handling of dural substitutes and water tight dura reconstruction
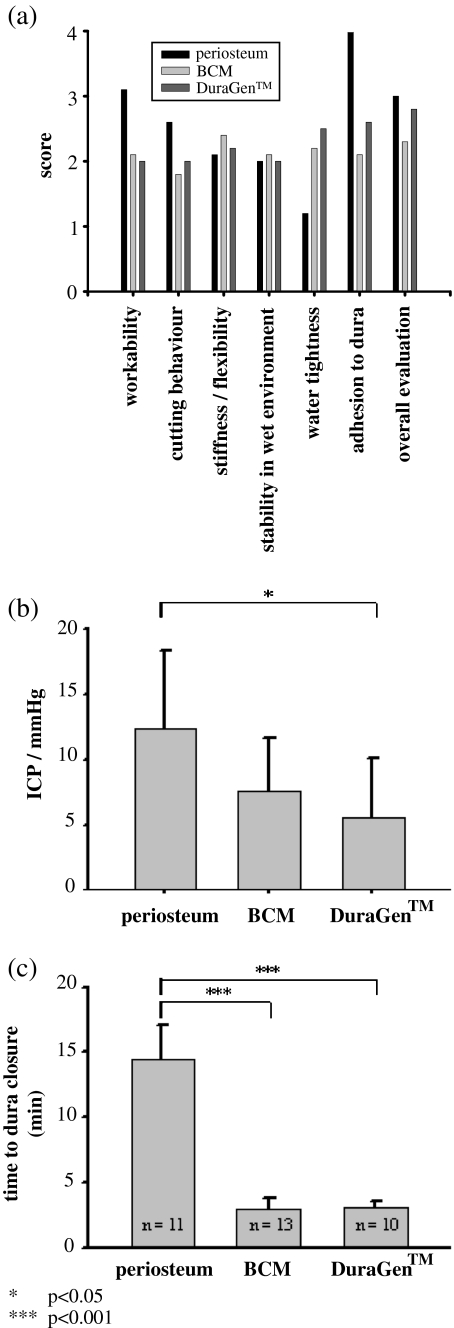
Table 1 and Fig. 2 summarize the results of the questionnaires completed after wound closure to evaluate device handling. Results for both onlay grafts were similar, with a trend for better adhesion to dura and higher CSF tightness for BCM. Workability of the onlay grafts was better compared with periosteum. However, periosteum yielded higher watertightness. This is supported by the CSF tightness measurements, which revealed CSF tightness at higher ICPs for duraplasty with periosteum compared with BCM (12.3 ± 6.1 vs 7.7 ± 4.0 mmHg, p = 0.098) and significantly higher compared with DuraGen™ (5.5 ± 4.6 mmHg, p = 0.02). No significant differences between the two semisynthetic products were found (p = 0.34). The time required for duraplasty with periosteum was significantly higher (14.4 ± 2.7 min) compared with BCM (2.8 ± 0.8 min) or DuraGen™ (3.0 ± 0.5 min).
Table 1.
Intraoperative handling of graft and implants: the parameters evaluated after the surgical procedures. Scores of 1 (very good), 2 (good), 3 (acceptable), 4 (poor), 5 (not acceptable) were given for each category by two surgeons immediately following the implantation. Scores are detailed in “Materials and methods”
| Autologous periosteum | BCM | DuraGen™ | |
|---|---|---|---|
| n = 11 | n = 13 | n = 10 | |
| Mean scorea (range) | Mean scorea (range) | Mean scorea (range) | |
| Workability | 3.1 (3–4) | 2.1 (2–3) | 2.0 |
| Cutting behavior | 2.6 (2–3) | 1.8 (1–2) | 2.0 |
| Flexibility | 2.1 (2–3) | 2.4 (1–4) | 2.2 (2–3) |
| Handling in wet environment | 2.0 | 2.1 (2–3) | 2.0 |
| Watertightness | 1.2 (1–4) | 2.2 (1–4) | 2.5 (1–5) |
| Adhesion to dura | - | 2.1 (1–3) | 2.6 (1–4) |
| Overall evaluation | 3.0 (2–4) | 2.3 (2–4) | 2.8 (2–5) |
Fig. 2.
Intraoperative handling, CSF tightness, time for duraplasty. a Scores evaluated after surgical procedures (see “Materials and methods”). b CSF tightness was similar between the devices with best CSF tightness of periosteum. c Time required for closure of the dura mater was significantly lower with BCM and DuraGen™ compared with periosteum
Postoperative course and adverse events
In the periosteum group, one animal died intraoperatively from anaesthesiological complications post duraplasty and two animals died on days 4 and 10, but autopsy revealed no implantation-site-related cause of death. In the DuraGen™ group, one, two and one animal died on postoperative days 11, 12 and 25, respectively. Subgaleal abscesses were found in animals that died on days 11 and 12 during autopsy; for the other animal, no abnormalities at the implantation site were found. All other animals experienced a regular gain of size and weight at 4 weeks (mean of 11.8 kg; range 16–41 kg) and 24 weeks (mean of 69.6 kg; range 79–91 kg) and showed no signs of systemic or local incompatibility. Clinically apparent meningitis was not observed.
Macroscopic observations
At 4 weeks post-implantion
Animals from periosteum (n = 4), DuraGen™ (n = 3), and BCM (n = 8) groups were sacrificed at 4 weeks postoperatively. The osteoclastic defects with beginning osseous consolidation were visible in all groups and showed no differences between groups. Subcutaneous abscesses were found in periosteum and BCM groups (n = 1 in each), an abscess in the osteoclastic defect was observed in the BCM group (n = 1). CSF fistulas or seromas or signs of intracranial infection were not detected in any of the groups.
At 24 weeks post-implantation
Animals from periosteum (n = 4), DuraGen™ (n = 3) and BCM (n = 5) groups were sacrificed at 24 weeks postoperatively. At this time point, reossification had closed the defects in the former osteoclastic craniotomy area in all animals. CSF fistulas, abscesses, infections, seromas or signs of intracranial infection were not evident in any of the animals. In the BCM group, one of five specimens (20%) showed no adhesion, while soft or considerable adhesion of device to the cortex was detected in one of five (20%) and three of five (60%), respectively. In the DuraGen™ group, two of three specimens (66.6%) showed no adhesion, while one of three (33.3%) showed soft adhesion between device and the cortex. In the periosteum group, considerable adherence (75%) and soft adhesion (25%) between the graft and the cortex was observed (Table 3, Fig. 3).
Table 3.
Macroscopic findings: the macroscopic adhesion scores collected from the specimens obtained after 6 months
| Adhesion of graft to CNS tissue | Autologous graft | DuraGen™ | BCM |
|---|---|---|---|
| mean ± SDM | mean ± SDM | mean ± SDM | |
| 6 months | 1.75 ± 0.50 | 0.33 ± 0.58 | 1.4 ± 0.89 |
Fig. 3.
Histological and macroscopic analysis at 6 months postoperatively. Skull was carefully separated from the specimens before embedding. During this procedure the adhesion was scored (compare results section)
Histological analysis of implant integration
At 4 weeks post-implantion
In the BCM group, seven of eight specimens (87.5%) showed no adhesions of the graft to the leptomeninges, whereas the leptomeninges were adherent to the device in one of eight (12.5%). Cortical lesion was adherent to the graft in six of eight specimens (75%), while one of eight (12.5%) showed no adhesion, and scar formation was observed in one of eight (12.5%). Lymphocytic infiltration of the graft was absent in two of eight (25%) and isolated lymphocytic infiltration present in three of eight (37.5%). In two of eight (25%) there was massive lymphocytic infiltration, and a granuloma was observed in one of eight (12.5%), most likely due to intraoperative contamination. Isolated lymphocytic infiltration of the cortex were observed in five of eight specimens (62.5%), but it was massive in two of eight (25%) of specimens. One of eight (12.5%) specimens showed ingrowth of connective tissue into the cortex. Advanced integration of the graft into the dura with little acellular collagen remaining was found in seven of eight (87.5%) specimens, but one of eight (12.5%) showed a complete integration (Table 2, Fig. 4).
Table 2.
Histological evaluation: the scores collected from the specimens obtained after 1 month
| Autologous graft | DuraGen™ | BCM | |
|---|---|---|---|
| mean ± SDM | mean ± SDM | mean ± SDM | |
| Adhesion of graft to leptomeningeal surface | |||
| 1 month | 0.25 ± 0.50 | 0.00 ± 0.00 | 0.25 ± 0.46 |
| Adhesion of graft to cortical defect | |||
| 1 month | 1.00 ± 0.82 | 2.00 ± 0.00 | 1.00 ± 0.53 |
| Cellular reactions in the device | |||
| 1 month | 3.00 ± 0.00a | 2.00 ± 1.00 | 1.25 ± 1.04 |
| Cellular reactions in the brain tissue | |||
| 1 month | 1.25 ± 0.50 | 1.67 ± 1.15 | 1.50 ± 0.76 |
| 6 months | 0.00 ± 0.00 | 1.67 ± 1.15 | 2.00 ± 1.15 |
| Integration of device | |||
| 1 month | 3.00 ± 0.00 | 3.00 ± 0.00 | 2.13 ± 0.35 |
aForeign body reaction to suture material in all cases
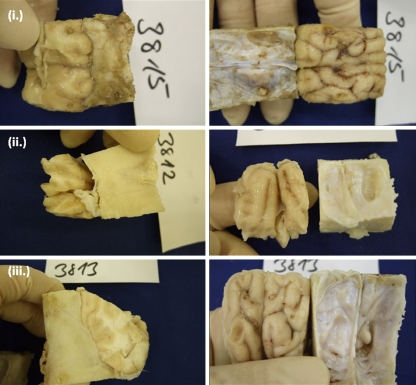
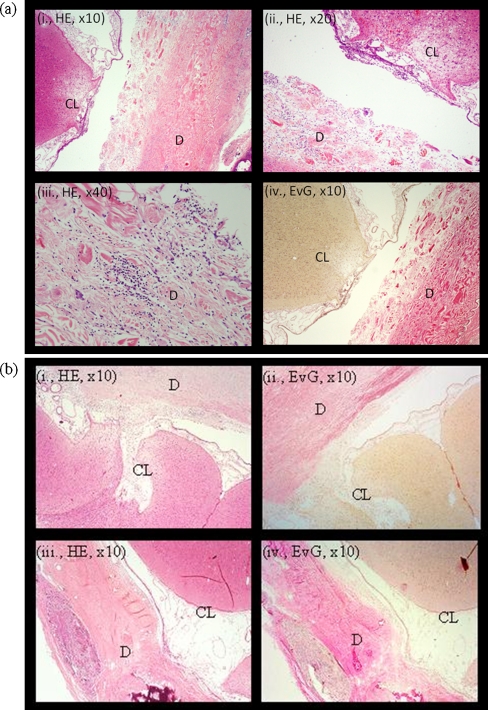
Fig. 4.
Histological analysis at 1 month postoperatively. a Histology of an animal randomized to duraplasty with BCM. Neither the cortical lesion (CL) nor the leptomeninges were markedly adherent to the device (D), which showed isolated lymphocytic infiltration. b Duraplasty with DuraGen™ (i, ii) or periosteum (iii, iv)
In the DuraGen™ group, no adhesions between leptomeninges and the graft were detected. However, scar formation between the graft and the lesioned cortex was observed in all three animals. In one of three specimens (33.3%) isolated lymphocytic infiltration of the graft was observed, in one of three (33.3%) it was massive. One specimen showed suture granuloma (inaccurately placed subcutaneous suture). In two of three (66.7%), isolated lymphocytic infiltration of the cortex was observed; one of three (33.3%) showed ingrowth of connective tissue. All specimens showed nearly complete integration of the device into the dura (Table 2, Fig. 4).
In the periosteum group, no adhesions between device and leptomeninges were visible in three of four specimens (75%); the leptomeninges were adherent to the device in one of four (25%). In two of four (50%), the cortical lesion was adherent to the device; in one of four, (25%) scar formation was observed; in one of four (25%), there was no adhesion between graft and cortex. As expected, suture granulomas were observed in all samples. In three of four (75%), isolated lymphocytic infiltration of the cortex was found. In one of four (25%), a massive lymphocytic infiltration of the cortex was found. All samples showed a complete integration into the dura (Table 2, Fig. 4).
At 24 weeks post-implantion
All cranial defects were completely mineralized. Histologically, the implants were not distinguishable in any case due to their complete integration into the scar tissue; therefore only the lymphocytic reaction of the brain parenchyma was analysed histologically. Lymphocytic infiltration was isolated in two of four specimens (50%) and ingrowth of connective tissue was observed in two of four (50%) in BCM group. In one specimen of BCM, the cortical lesion could not be clearly identified; this specimen was therefore not evaluated histologically. In the DuraGen™ group, isolated lymphocytic infiltration was found in two of three specimens (66.6%), ingrowth of connective tissue was found in one of three specimens (33.3%). Significant lymphocytic reaction was not detected in the periosteum group (Table 3).
Stastistical evaluation of the primary endpoints
A rank order was defined using a composite endpoint constructed from the histological assessment, survival, tight pressure, dura closure time, and handling characteristics. The ranks were analysed by unpaired Wilcoxon tests, and non-parametric 95% confidence intervals were computed. On a rank-range scale of 0–100, BCM was superior to DuraGen™ by 37.9 points with a 95% confidence interval of the difference of 15.2–57.6 (p = 0.0025), whereas compared with periosteum no significant difference was computed (p = 0.28). The 95%confidence interval of the difference was −12 to 42. Finally, no significant difference was found between DuraGen™ and periosteum, indicated by a 95% confidence interval of the difference −52 to 9 (Fig. 5).
Discussion
The present study investigates efficacy and safety of duraplasty with a novel bilayered semisynthetic collagen matrix (BCM, B.Braun Aesculap). In particular, duraplasty with BCM, DuraGen™ (Integra Neurosciences), and periosteum was compared.
A dura substitute should be easy to handle, not require to extend the surgical intervention, allow reconstruction with high watertightness, be biologically inert, resistant to disintegration while fully integrating into host dura, induce no adhesion between cortex and the dura or dura substitute, induce no adverse local or systemic reaction (immunological, toxic, prion infection) with high biocompatibility. Semisynthetic collagen matrices derived from animal sources meet these conditions [2, 5, 11, 12, 16, 18].
Our results show both DuraGen™ and BCM to be superior than periosteum in handling and time required for duraplasty, while sutured periosteum was better in providing intraoperative watertightness. Postoperative infections and deaths were observed in the periosteum and DuraGen™ groups but not in the BCM group. Four weeks post-implantation subcutaneous abscesses were found in one animal each from the periosteum and the BCM group. One animal of the BCM group showed an abscess within the osteoclastic defect. Interestingly, infection was limited only to the extracranial space and did not traverse the device. None of the animals showed intracranial infection. Due to the small number of animals in our study, extra- and intracranial infections cannot be correlated with the type of device.
Clinical studies using semisynthetic collagen matrix DuraGen™ for duraplasty showed no increased risk of infection [11, 12, 16, 18]. It is therefore likely that the infections observed in the present study may be related to the difficulty of maintaining an aseptic environment of the surgical wounds in our porcine model. Future studies will be needed to show whether the bilayered BCM with a mechanically durable percardial layer is a better barrier against bacterial infections.
No CSF fistulas were detected, either clinically or during specimen collection. Taken together, these obervations indicate that both semisynthetic collagen matrices (DuraGen™, BCM) provide sufficient barrier, despite lower intraoperative CSF tightness compared with sutured periosteum. These results are supported by clinical studies reporting 0% in 79 patients [12] and 0.4% in 439 patients [16] of CSF fistulas after duraplasty with DuraGen™. Furthermore, Barth and co-workers [2] demonstrated that, in 53 patients with supratentorial craniotomies who received an adaptive dura closure only, the risk of dura closure-related complications was not significantly higher compared with patients receiving watertight duraplasty. This shows that watertight suturing of the onlay grafts provides no additional advantage if the graft was placed on the dura with sufficient overlap, but could be helpful in cases where the remaining dural tissue does not allow sufficient overlap of graft and dura or where tension on the dura or the graft may prevent safe graft positioning.
For clinical use, caution is necessary when onlay grafts are used in patients with hydrocephalus or for the closure of infratentorial or basal defects. In this case, the lower CSF tightness observed with the onlay grafts may play a more significant role. Whether the risk of CSF fistulas in high-risk patients can be avoided by using suturing of the grafts, which is possible with BCM and DuraGen™, remains to be determined.
Histological analysis showed tissue integration by fibroblast invasion into all devices after 4 weeks. Implant rejection was not observed in any group. Both DuraGen™ and BCM showed remaining structures of the implant; most notably of the compact layer of BCM derived from the pericardium facing the subcutaneous tissue. Periosteum was integrated and was identifiable only at overlapping edges of the implant and dural defect as a slightly different connective tissue structure of the connective tissue layers. Although lymphocytic reaction of the implants was lowest in the periosteum group, foreign body reaction to suture material was observed consistently. Lymphocytic infiltration tended to be lower in BCM compared with DuraGen™. Cortical reaction at the lesion site showed a fibrous reaction with DuraGen™ (one of three cases) and with BCM (one of eight cases). There was no relevant adhesion to the leptomeninges, while adhesion to the lesioned cortex tended to be higher with DuraGen™ compared with BCM and periosteum. However, macroscopic graft adhesion to the cerebral tissue after 6 months post-implantation was lower with DuraGen™ compared with periosteum and BCM. Implant rejection was not observed in any group 6 months postoperatively. Histological analysis of the grafts and graft adhesions to leptomeninges and cortex was impossible due to their complete integration and mineralization. Lymphocytic infiltration of the brain in the area of the cortical lesion tended to be higher with DuraGen™ and BCM compared with periosteum (no significant lymphocytic infiltration).
To the best of our knowledge, previous animal studies [7, 8, 17, 19] did not investigate adhesions between semisynthetic collagen onlay grafts and lesioned cortex. Adhesions between graft and cortex are of significance in clinical use, since they might act as an epileptic focus. In the present study, we observed no seizures in animals implanted with semisynthetic grafts. Furthermore, adhesions are of clinical relevance in the case of reoperation. In this case a strong adhesion between dura mater or dural substitute and cortex may complicate the surgical procedure and increase the risk of neurological trauma. The present study provides no definitive answer to the question of whether separation of the brain and the dura mater in surgery is complicated by the use of semisynthetic collagen onlay grafts. A relatively high rate of fibrous reactions within the brain tissue at the lesioned site with both onlay grafts (BCM, DuraGen™) could be associated with the specifics of wound healing in our model of growing pigs, since according to our experience and a histopathological study [11] such changes are rarely observed in humans. After 6 months, all cranial defects were completely mineralized with ossification extending far into the soft tissue scar, which, in our opinion, is rarely observed in humans and may be associated with our animal model.
Results of this study demonstrate high biocompatibility for all dural substitutes, except for mild lymphocytic infiltration of the brain parenchyma associated with the onlay grafts, severe local or systemic toxicity, immunological reactions were not observed clinically or histologically. However, clinical relevance of slightly enhanced lymphocytic infiltration of brain parenchyma observed with onlay grafts after 6 months remains to be determined.
In conclusion, the dura substitutes BCM and DuraGen™ (semisynthetic collagen matrices of bovine origin) appear to be a promising alternative to duraplasty with endogenous periosteum (Fig. 5), which is consistent with other studies [11, 12, 16, 18]. Clinical studies are needed to show whether BCM provides an alternative in clinical practice and whether because of its bilayered structure provides a stronger mechanical barrier which could be advantageous in complex reconstructions.
Acknowledgements
We would like to acknowledge the operating room staff, surgical and anaesthesiological team and the staff of the animal care facility of the Department of Surgical Research and Techniques, Medical School at the University of Pécs, Hungary whose expertise has greatly facilitated this work. We thank Dr. Dieter Menne (Menne Biomed Consulting, Tuebingen, Germany) for the statistical analysis of our data. This study has been carried out in cooperation with and sponsored by B. Braun Aesculap AG, Tuttlingen, Germany.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
Comment
This extensive animal model testing two artificial dural materials against homograft in pigs, seeks to clarify if an ideal dural graft exists that does not require the extensive dissection required for harvesting of homograft material.
It would appear that one well-tried artificial substitute is quite acceptable but there may be very slight advantages in a newer material. Homograft has also very satisfactory properties.
Michael Powell
London, UK
References
- 1.Barbolt TA, Odin M, Léger M, Kangas L, Hoiste J, Liu SH. Biocompatibility evaluation of dura mater substitutes in an animal model. Neurol Res. 2001;23:813–820. doi: 10.1179/016164101101199405. [DOI] [PubMed] [Google Scholar]
- 2.Barth M, Tuettenberg J, Thomé C, Weiss C, Vajkoczy P, Schmiedek P. Watertight dural closure: is it necessary? A prospective randomized trial in patients with supratentorial craniotomies. Neurosurgery. 2008;63:352–358. doi: 10.1227/01.NEU.0000310696.52302.99. [DOI] [PubMed] [Google Scholar]
- 3.Bjorling LE, Hodges JS. Rule-based ranking schemes for antiretroviral trial. Stat Med. 1997;16:1175–1191. doi: 10.1002/(SICI)1097-0258(19970530)16:10<1175::AID-SIM522>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 4.Danish SF, Samdani A, Hanna A, Storm P, Sutton L. Experience with acellular human dura and bovine collagen matrix for duraplasty after posterior fossa decompression for Chiari malformations. J Neurosurg. 2006;104:16–20. doi: 10.3171/ped.2006.104.1.16. [DOI] [PubMed] [Google Scholar]
- 5.Filippi R, Derdilopoulos A, Heimann A, Krummenauer F, Perneczky A, Kempski O. Tightness of duraplasty in rabbits: a comparative study. Neurosurgery. 2000;46:1470–1476. doi: 10.1097/00006123-200006000-00032. [DOI] [PubMed] [Google Scholar]
- 6.Follmann D, Wittes J, Cuttler JA. The use of subjectives rankings in clinical trials with an application to cardiovascular disease. Stat Med. 1992;11:427–438. doi: 10.1002/sim.4780110402. [DOI] [PubMed] [Google Scholar]
- 7.Knopp U, Christmann F, Reusche E, Sepehrnia A. A new collagen biomatrix of equine origin versus a cadaveric dura graft for the repair of dural defects—a comparative animal experimental study. Acta Neurochir (Wien) 2005;147:877–887. doi: 10.1007/s00701-005-0552-0. [DOI] [PubMed] [Google Scholar]
- 8.Laquerriere A, Yun J, Tiollier J, Hemet J, Tadie M. Experimental evaluation of bilayered human collagen as a dural substitute. J Neurosurg. 1993;78:487–491. doi: 10.3171/jns.1993.78.3.0487. [DOI] [PubMed] [Google Scholar]
- 9.Laun A, Tonn JC, Jerusalem C. Comparative study of lyophilized human dura mater and lyophilized bovine pericardium as dural substitutes in neurosurgery. Acta Neurochir (Wien) 1990;107:16–21. doi: 10.1007/BF01402607. [DOI] [PubMed] [Google Scholar]
- 10.Maher CO, Anderson RE, McClelland RL, Link MJ. Evaluation of a novel propylene oxide-treated collagen material as a dural substitute. J Neurosurg. 2003;99:1070–1076. doi: 10.3171/jns.2003.99.6.1070. [DOI] [PubMed] [Google Scholar]
- 11.Narotam PK, van Dellen JR, Bhoola KD. A clinicopathological study of collagen sponge as a dural graft in neurosurgery. J Neurosurg. 1995;82:406–412. doi: 10.3171/jns.1995.82.3.0406. [DOI] [PubMed] [Google Scholar]
- 12.Narotam PK, Reddy K, Fewer D, Qiao F, Nathoo N. Collagen matrix duraplasty for cranial and spinal surgery: a clinical and imaging study. J Neurosurg. 2007;106:45–51. doi: 10.3171/jns.2007.106.1.45. [DOI] [PubMed] [Google Scholar]
- 13.Palm SJ, Kirsch WM, Zhu YH, Peckham N, Kihara S, Anton R, Anton T, Balzer K, Eickmann T. Dural closure with nonpenetrating clips prevents meningoneural adhesions: an experimental study in dogs. Neurosurgery. 1999;45:875–881. doi: 10.1097/00006123-199910000-00029. [DOI] [PubMed] [Google Scholar]
- 14.Preul MC, Bichard WD, Spetzler RF. Toward optimal tissue sealants for neurosurgery: use of a novel hydrogel sealant in a canine durotomy repair model. Neurosurgery. 2003;53:1189–1198. doi: 10.1227/01.NEU.0000089481.87226.F7. [DOI] [PubMed] [Google Scholar]
- 15.Preul MC, Campbell PK, Bichard WD, Spetzler RF. Application of a hydrogel sealant improves watertight closures of duraplasty onlay grafts in a canine craniotomy model. J Neurosurg. 2007;107:642–650. doi: 10.3171/JNS-07/09/0642. [DOI] [PubMed] [Google Scholar]
- 16.Sade B, Oya S, Lee JH (2011) Non-watertight dural reconstruction in meningioma surgery: results in 439 consecutive patients and a review of the literature. J Neurosurg 114(3):714–718 [DOI] [PubMed]
- 17.San-Galli F, Darrouzet V, Rivel J, Baquey C, Ducassou D, Guérin J. Experimental evaluation of a collagen-coated vicryl mesh as a dural substitute. Neurosurgery. 1992;30:396–401. doi: 10.1227/00006123-199203000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Stendel R, Danne M, Fiss I, Klein I, Schilling A, Hammersen S, Pietilae T, Jänisch W, Hopfenmüller W. Efficacy and safety of a collagen matrix for cranial and spinal dural reconstruction using different fixation techniques. J Neurosurg. 2008;109:215–221. doi: 10.3171/JNS/2008/109/8/0215. [DOI] [PubMed] [Google Scholar]
- 19.Zerris VA, James KS, Roberts JB, Bell E, Heilman CB. Repair of the dura mater with processed collagen devices. J Biomed Mater Res B Appl Biomater. 2007;83:580–588. doi: 10.1002/jbm.b.30831. [DOI] [PubMed] [Google Scholar]