Abstract
Protein kinase C-θ (PKC-θ) translocates to the center of the immunological synapse, but the underlying mechanism and its importance in T cell activation are unknown. We found that the PKC-θ V3 domain is necessary and sufficient for IS localization mediated by Lck-dependent association with CD28. We identified a conserved proline-rich motif in V3 required for CD28 association and IS localization. CD28 association was essential for PKC-θ-mediated downstream signaling and TH2 and TH17, but not TH1, differentiation. Ectopic V3 expression sequestered PKC-θ from the IS and interfered with its functions. These results identify a unique mode of CD28 signaling, establish a molecular basis for the IS localization of PKC-θ, and implicate V3-based “decoys” as therapeutic modalities for T cell-mediated inflammatory diseases.
The induction of an immune response depends on effective communication between antigen-specific T cells and antigen presenting cells (APCs). When a T cell expressing a cognate T cell receptor (TCR) encounters an activated APC, both cells actively redistribute their receptors and ligands to the interface, creating a platform for effective signaling, known as the immunological synapse (IS). At steady state, the mature IS is composed of concentric rings with a central core (cSMAC) containing clusters of TCR and costimulatory molecules, and a peripheral ring (pSMAC) of adhesion molecules1. The engagement of these surface molecules triggers signaling cascades resulting in the recruitment of intracellular proteins, including kinases, adapter and cytoskeletal proteins to the IS2. One of the most prominent proteins to be recruited to the IS following antigen stimulation is protein kinase C-θ (PKC-θ), whose localization is limited to the cSMAC3,4.
PKC-θ is a member of the novel, Ca2+-independent PKC subfamily expressed predominantly in T cells, which plays important and non-redundant roles in T cell activation and survival (but not in T cell development)5–7, reflecting its unique ability to activate the transcription factors NF-κB, AP-1 and, more recently, also NFAT5,8–12. Studies using PKC-θ-deficient (Prkcq−/ −) mice have characterized the importance of PKC-θ in different disease models and have revealed that its requirement in different forms of immunity is quite selective and not absolute. Thus, TH2 responses against allergens or helminth infection13,14 and TH17-mediated autoimmune diseases15–17 require PKC-θ. In contrast, TH1 immune responses against intracellular pathogens such as Leishmania major13, as well as antiviral effector and memory cytotoxic T lymphocyte responses were relatively intact18–21. Consistent with these in vivo findings, Prkcq−/ − CD4+ T cells display impaired in vitro differentiation into the TH2 and TH17 lineages, while TH1 differentiation is only moderately reduced13–15. More recently, PKC-θ was found to be required for allograft rejection and graft vs. host (GvH) disease, but not for graft vs. leukemia (GvL) response in mice22.
Although the antigen-induced localization of PKC-θ to the IS and, more specifically, to the cSMAC is well established3,4, the molecular basis for this highly selective localization is not clear, nor is it known whether it is required for the signaling functions of PKC-θ in T cells. Here, we identified and characterized the hinge region of PKC-θ, known as the V3 domain, as playing a critical role in determining its IS/cSMAC localization via binding to CD28 and, consequently, dictating its signaling from the IS. Fine mapping further revealed an evolutionarily conserved proline-rich (PR) motif that is required for CD28 association, cSMAC localization and PKC-θ-mediated functions. Importantly, the isolated V3 domain of PKC-θ behaved as a decoy to block PKC-θ-dependent functions, including TH2- and TH17-, but not TH1-, differentiation and inflammation. These findings implicate a unique signaling mode of CD28, and establish the molecular basis for the specialized localization and function of PKC-θ in antigen-stimulated T cells.
RESULTS
PKC-θ IS localization and signaling requires the V3 domain
As a first step toward understanding the basis for unique antigen-induced PKC-θ localization to the IS/cSMAC, we compared the amino acid sequences of PKC-θ and its closest relative (62% identity and 75% homology) within the novel, Ca2+-independent PKC subfamily, i.e., PKC-δ (Supplementary Fig. 1). However, unlike PKC-θ, PKC-δ does not translocate to the IS upon interaction between T cells and APCs3. Sequence alignment revealed a significant divergence between the V3 (hinge) domains of these two isoforms (amino acids ~291–378 of human PKC-θ) (Supplementary Fig. 1), suggesting that this region might play a role in targeting PKC-θ to the IS. This region has not been previously implicated in regulation of PKC-θ localization or function, other than its general role as a flexible hinge that allows PKCs to undergo a conformational change from a resting state into an “open”, active conformation23.
To determine if the V3 domain is required for the IS localization of PKC-θ, we constructed a V3 deletion mutant (PKC-θ-ΔV3). When retrovirally transduced into Prkcq−/ − TCR-transgenic (Tg) ovalbumin (Ova)-specific OT-II CD4+ T cells, wild-type PKC-θ localized in the center of IS, i.e., the cSMAC, following stimulation with Ova peptide-pulsed APCs (Fig. 1a,b), as evident from its central localization relative to that of talin, a known pSMAC marker4. In contrast, PKC-θ-ΔV3 did not translocate to the IS and instead remained largely cytosolic. Since V3 domain deletion could cause a gross conformational change, we next generated an exchange mutant, in which the native V3 domain of PKC-θ was replaced with the corresponding domain of PKC-δ (PKC-θ+δV3). Similar to PKC-θ-ΔV3, this mutant also failed to translocate to the IS/cSMAC (Fig. 1a,b). The peripheral IS localization of talin in T cells expressing both mutants indicates that the organization of a mature IS was not grossly impaired in the absence of wild-type PKC-θ. These data suggest that the unique V3 domain of PKC-θ is required for its selective IS/cSMAC localization.
Figure 1.
Requirement of PKC-θ-V3 for IS-cSMAC localization and signaling. (a) Immunofluorescence imaging of Prkcq−/ − OT-II Tg CD4+ T cells infected with retrovirus expressing GFP-tagged wild-type (WT) PKC-θ, PKC-θ-ΔV3 or PKC-θ+δV3 (green) and mixed (1:1 ratio) with CMAC (blue)-labeled APC, pre-incubated with or without Ova peptide. Fixed conjugates were stained with anti-talin plus a secondary Alexa 647-coupled antibody (red). (b) Quantitation of PKC-θ IS-cSMAC localization analyzed in ~40 T-APC conjugates as described in a. Only conjugates that had reorganized their talin and that had detectable PKC-θ were analyzed. ** p < .05. (c) Normalized luciferase (Luc) activity in MCC-specific T hybridoma cells cotransfected with empty vector or the indicated Xpress-tagged PKC-θ vectors, together with CD28-response element (RE/AP)-Luc reporter and a β-Gal reporter. Cells were incubated with I-Ek- and B7-1-expressing DCEK fibroblasts in the absence or presence of MCC peptide for 6 h. Transfected PKC-θ expression revealed by anti-Xpress immunoblotting is shown at the bottom. ** p < .05. Data are from three experiments. (d–e) CD69 (d) or CD25 and PKC-θ. (e) expression in GFP+ CD4+ T cells sorted from Rag1−/ − mice reconstituted with Prkcq−/ − BM cells transduced with empty vector, WT PKC-θ, or PKC-θ+δV3 and left unstimulated or stimulated overnight with anti-CD3 plus anti-CD28 mAbs. Data are representative of two experiments. (f–g) IL-2 production (f) and cell proliferation (g) in GFP+ CD4+ T cells isolated as in d and stimulated or not with anti-CD3 plus anti-CD28 mAbs for 48 h. Data are from two experiments.
The quality of T cell activation appears to correlate with the clustering of PKC-θ in the IS4,24. However, direct evidence that the IS/cSMAC localization of PKC-θ is essential for its downstream functions is missing. Therefore, we investigated whether loss or replacement of the PKC-θ V3 domain impairs the activation of key transcription factors that are essential for productive T cell activation and are known targets of PKC-θ5,8–12, i.e. NF-κB, AP-1 and NFAT (Fig. 1c). Stimulation of empty vector-transfected cells with peptide-APCs resulted consistently in minimal reporter gene stimulation (see also Figs. 3d, 4c, 6c), most likely reflecting the relatively weak stimulus provided by peptide-APC stimulation as compared to the more standard use of saturating anti-CD3-CD28 antibody concentrations. T cells transfected with wild-type PKC-θ showed a significant increase in the basal activities of a CD28 response element (RE/AP; Fig. 1c) and NFAT (Supplementary Fig. 2), which was further increased by peptide-APC stimulation. However, the activation of these reporter genes was completely abrogated in T cells transfected with PKC-θ-ΔV3 or PKC-θ+δV3 (Fig. 1c and Supplementary Fig. 2).
Figure 3.
A PR motif in the V3 domain of PKCθ determines its IS localization and interacts with CD28. (a) PKCθ localization in CD4+ T cells from OT-II Tg mice infected with retrovirus expressing GFP-tagged WT PKCθ, PKCδ with an inserted PR motif (PKCδ+θPR), or WT PKCδ (green). Cells were stimulated, and conjugates were fixed, stained and analyzed as in Fig. 1a. (b) Quantitative analysis of the results shown in (a) was performed as in Fig. 1a. ** p < .05. (c) PKC-CD28 association in Prkcq−/ − CD4+ T cells infected with retrovirus expressing WT PKCθ, PKCδ+θPR, or WT PKCδ. Cells were harvested, restimulated with CD3+CD28 mAbs for 5 min (left panel) or left unstimulated (right panel). Asterisk in the right panel indicates the position of the immunoprecipitating antibody heavy chain. (d) Normalized Luc activity in MCC-specific T hybridoma cells cotransfected with the same vectors as in (a) together with an RE/AP and a β-Gal reporter plasmids. Cells were stimulated as in Fig. 1c, and Normalized Luc activity was determined in triplicates. ** p < .05. Data are from two experiments.
Figure 4.
Importance of the PxxP motif in the V3 domain of PKCθ for IS localization and CD28 interaction. (a) Quantitative PKC-θ localization analysis using Prkcq−/ − OT-II CD4+ T cells infected with retrovirus expressing GFP-tagged PKCθ, or PKCθ-GFP fusion vectors containing the indicated proline mutations. Representative images are shown in Supplementary Fig. 5. ** p < .05. (b) PKC-θ-CD28 association in PKCθ −/ − CD4+ T cells infected with retrovirus expressing WT or proline-mutated PKC-θ. Cells were harvested and restimulated with CD3+CD28 mAbs for 5 min (c) MCC-specific T hybridoma cells were cotransfected with empty pEF vector or the indicated PKCθ mutants together with RE/APβ-Gal reporter plasmids. Cells were stimulated, and normalized Luc activity was determined as in Fig. 1c. ** p < .05. (d–f) T cell activation in PKC-θ-reconstituted BM chimeras. Prkcq−/ − BM cells transduced with retrovirus expressing empty pMIG vector, or the indicated PKCθ vectors were used to reconstitute Rag−/ − mice. Sorted GFP+ CD4+ T cells were left unstimulated or stimulated overnight with anti-CD3 plus anti-CD28 mAbs to determine the expression of CD69 or CD25 (d); or for 48 h to determine the production of IL-2 (f), and proliferation (g). Data are from two experiments.
Figure 6.
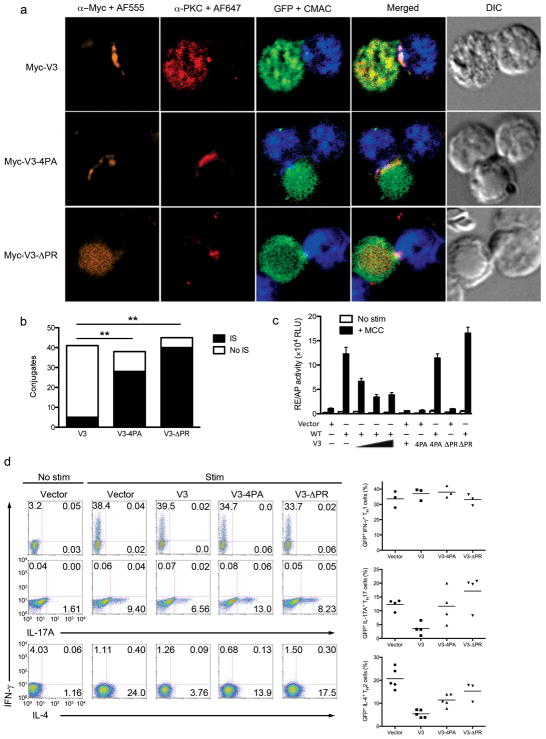
The V3 domain interferes with PKCθ-mediated signaling and T cell differentiation. (a) PKC-θ and V3 localization in OT-II CD4+ T cells infected with retrovirus expressing Myc-tagged non-mutated or proline-mutated V3 vectors. Infected cells (green) were harvested, stimulated, and fixed. Conjugates were stained with anti-Myc plus a secondary Alexa 555-coupled antibody (orange), and anti-PKCθ plus a secondary Alexa 647-coupled antibody (red). Cells were analyzed as in Fig. 1a. (b) Quantitative analysis of the results shown in (a) as in Fig. 1b. ** p < .05. (c) Reporter gene activation in MCC- specific T hybridoma cells cotransfected with indicated PKC-θ and/or V3 vectors together with RE/AP- Luc and β-Gal reporter plasmids. Cells were stimulated and analyzed as in Fig. 1c. (d) TH differentiation in naïve CD4+ T cells from B6 mice stimulated with anti-CD3 plus anti-CD28 mAbs, cultured under TH1-, TH2- or TH17-polarizing conditions, and retrovirally transduced with empty pMIG vector, or with the indicated PKC-θ V3 vectors. Cytokine-producing cells were analyzed by intracellular staining 8 h after restimulation. Right panels represent cumulative data showing percentage of cytokine-producing cells. Data are from six experiments.
We further analyzed the ability of PKC-θ replacement mutant to activate primary T cells by generating bone marrow (BM) chimeras in irradiated Rag1−/ − mice that were reconstituted with Prkcq−/ −BM cells infected in vitro with bicistronic GFP retroviruses expressing wild-type PKC-θ or PKC-θ+δV3. Transduced (GFP+) T cells were analyzed 8 weeks later. Anti-CD3 and -CD28 costimulation of wild-type PKC-θ-reconstituted CD4+ T cells greatly upregulated the expression of both CD69 and CD25, two activation markers regulated by PKC-θ6; however, the ability of PKC-θ+δV3 to induce CD69 or CD25 expression was reduced by ~50% (Fig. 1d,e). Both wild-type PKC-θ and PKC-θ+δV3 were expressed at similar levels in the transduced cells (Fig. 1e, bottom panels). Furthermore, in contrast to wild-type PKC-θ-reconstituted CD4+ T cells, which proliferated and produced interleukin 2 (IL-2) in response to anti-CD3 and -CD28 stimulation in a dose-dependent manner, the PKC-θ+δV3-reconstituted T cells failed to proliferate and produce IL-2 (Fig. 1f, g), similarly to CD4+ T cells from Prkcq−/ − mice or empty vector-reconstituted BM chimera T cells. These data establish that the V3 domain of PKC-θ is critical for the activation of PKC-θ-dependent TCR-CD28 signaling pathways important for T cell activation and, furthermore, that this is a non-redundant function that cannot be replaced by the V3 domain of the closely related PKC-δ.
The PKC-θ V3 domain interacts with CD28
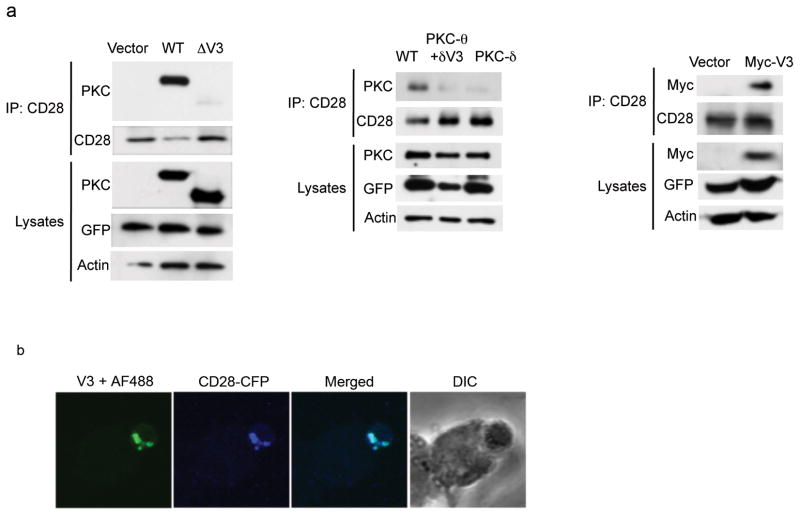
We hypothesized that the importance of the PKC-θ V3 domain for the enzyme’s IS localization and function reflects a critical association of the V3 domain with a ligand that recruits it to the IS. Given the reported colocalization of PKC-θ and CD28 in the IS25,26 and the phorbol ester-induced association between the two26, we examined whether anti-CD3-CD28 costimulation will cause PKC-θ to associate with CD28. Indeed, PKC-θ coimmunoprecipitated with CD28 from anti-CD3-CD28-stimulated T cells (Supplementary Fig. 3a). Maximal association was observed after 5 min of costimulation, and it declined thereafter but was still seen up to 30 min of stimulation. To identify the PKC-θ region required for this interaction, we transfected Jurkat T cells with a series of PKC-θ deletion mutants (Supplementary Fig. 3b). Upon anti-CD3-CD28 costimulation, wild-type PKC-θ as well as a deletion mutant of the N-terminal C2 domain (ΔC2), previously shown to negatively regulate the activation of PKC-θ27, coimmunoprecipitated with CD28 (Supplementary Fig. 3c). However, deletion of the V3 domain abolished this interaction. Deletion of both the C2 and C1a domains of PKC-θ (ΔC2+C1a) reduced, but did not abolish the interaction. We repeated this analysis in primary Prkcq−/ − CD4+ T cells, which were transduced with different PKC-θ- or PKC-δ-expressing retroviruses. The ΔV3 mutant (Fig. 2a, left panel) as well as the PKC-θ+δV3 mutant or wild-type PKC-δ (Fig. 2a, middle panel) did not coimmunoprecipitate with CD28. Thus, the V3 domain of PKC-θ is required for the inducible interaction with CD28. Moreover, the V3 domain was sufficient for this interaction since retrovirus-transduced Myc-tagged V3 coimmunoprecipitated with endogenous CD28 from Prkcq−/ − primary CD4+ T cells (Fig. 2a, right panel). The V3 domain also colocalized with CFP-tagged CD28 in the IS of cotransfected Jurkat T cells (Fig. 2b). Therefore, the PKC-θ V3 domain is necessary and sufficient for the CD3-CD28-induced interaction of PKC-θ with CD28.
Figure 2.
The PKCθ V3 domain is required and sufficient for CD28 interaction. (a) Association of PKC-θ-V3 with CD28 in Prkcq−/ − CD4+ T cells infected with retrovirus expressing empty pMIG vector, or with the indicated PKCθ or PKCδ vectors. Cells were harvested and restimulated with CD3+CD28 mAbs for 5 min. Cell lysates were immunoprecipitated with anti-CD28 mAb, resolved by SDS-PAGE, and immunoblotted with the indicated Abs. (b) Jurkat T cells cotransfected with Myc-tagged PKCθ-V3 plus CFP-tagged CD28 were mixed with SEE-pulsed Raji B cells at a 1:1 ratio. Conjugates were fixed and stained with a rabbit anti-Myc Ab plus a secondary Alexa 488-coupled antibody. Data are from four experiments.
A PR motif in the V3 domain is critical for PKC-θ recruitment
Inspection of the PKC-θ V3 domain revealed a PR motif corresponding to amino acid 328–336 of human PKC-θ, consisting of the sequence Ala-Arg-Pro-Pro-Cys-Leu-Pro-Thr-Pro (ARPPCLPTP). This motif was phylogenetically conserved, especially the two internal Pro residues, in PKC-θ enzymes from multiple species (Supplementary Fig. 4), but absent from the hinge domains of other PKCs. Since PR motifs are known to bind SH3 and WW domains28, we examined whether this motif is important for the localization and function of PKC-θ by inserting it into the V3 domain of PKC-δ (which does not translocate to the IS). Stimulation of Prkcq−/ − OT-II T cells with conjugated Ova-pulsed APCs induced translocation of transduced wild-type PKC-θ and endogenous talin to the cSMAC or the pSMAC, respectively, whereas transduced wild-type PKC-δ did not translocate to the IS (Fig. 3a,b). However, when the PR motif was introduced into PKC-δ (PKC-δ+θPR), it displayed a similar IS localization to wild-type PKC-θ, suggesting that the PR motif in PKC-θ-V3 can mediate IS/cSMAC localization.
Similar to wild-type PKC-θ, but unlike wild-type PKC-δ, PKC-δ+θPR also coimmunopreciptated with CD28 when transduced Prkcq−/ − CD4+ T cells were costimulated with anti-CD3-CD28 antibodies (Fig. 3c, left panel). This association was dependent on T cell stimulation, since it was not observed in similar immunoprecipitates from unstimulated T cells (Fig. 3c, right panel). Similarly, the PKC-δ+θPR mutant was capable of stimulating the activity of the RE/AP (Fig. 3d) or NFAT (Supplementary Fig. 5) reporters in stimulated MCC-T hybridoma cells to a degree approaching (~70–80%) that of wild-type PKC-θ, but significantly higher than that of wild-type PKC-δ. Therefore, the PR motif in PKC-θ-V3 is required for the inducible association with CD28 and the activation of PKC-θ-dependent signaling.
To determine which Pro residue(s) in the PR motif is important for the IS localization, CD28 association, and reporter activation, we mutated, to alanine, the two external (P330-6A), two internal (P331-4A), or all four (4PA) Pro residues in the PKC-θ PR motif (P330PxxPxP336; numbers refer to amino acid residues of the complete human PKC-θ). The IS/cSMAC localization of the transduced P330-6A mutant in Ova-stimulated Prkcq−/ − OT-II T cells was intact and similar to that of wild-type PKC-θ (Fig. 4a and Supplementary Fig. 6). In contrast, the P331-4A and 4PA mutants failed to localize to the IS and remained largely cytosolic, suggesting that Pro331 and Pro334 are essential for the antigen-induced IS recruitment of PKC-θ.
Similar results were obtained when these PKC-θ mutants were analyzed for their ability to associate with CD28 and to activate reporter genes. Thus, the P331-4A and the 4PA mutants, but not the P330-6A mutant, did not coimmunoprecipitate with CD28 (Fig. 4b) and displayed reduced ability to activate the RE/AP (Fig. 4c) or NFAT (Supplementary Fig. 7) reporters.
We further established the importance of the PxxP motif in CD4+ T cells from BM chimeras on a Prkcq−/ − background reconstituted with the same proline mutants. Unstimulated T cells from PKC-θ-reconstituted mice expressed very low levels of CD69 and CD25 (Fig. 4d). Wild-type PKC-θ- or P330-6A-reconstituted, anti-CD3-CD28-stimulated T cells dramatically upregulated the expression of these activation markers. However, in cells reconstituted with the P331-4A or 4PA mutants, upregulation of CD69 and CD25 was reduced by ~40–50% (Fig. 4d,e). These results were paralleled when proliferation (Fig. 4e) and IL-2 production (Fig. 4f) were measured in the same cells, although CD4+ T cells expressing PKC-θ-P331-4A or -4PA did produce some IL-2 (Fig. 4f). Wild-type or mutated PKC-θ proteins were expressed at similar levels in the transduced T cells (not shown; and see Fig. 1e). Therefore, primary T cell activation also critically depends on the Pro331 and Pro334 residues of PKC-θ.
Lck mediates the PKC-θ-CD28 interaction
The identification of the PxxP motif, a potential SH3-binding motif, in the PKC-θ V3 domain as being essential for CD28 association was intriguing because our mapping analysis of the CD28 cytoplasmic tail revealed that a C-terminal PR motif in murine CD28, i.e. a P206YAP209 motif, was required for the CD28-PKC-θ interaction (data not shown). This is the same CD28 motif required for PKC-θ-CD28 colocalization in the cSMAC, for IL-2 mRNA stabilization, and for lipid raft reorganization26,29–31, as well as for PKC-θ-dependent TH2- and TH17-mediated inflammatory responses13–17,21. Since direct association between the PR motifs in PKC-θ and CD28, respectively, is unlikely, we surmised that this interaction requires an intermediary molecule, with Lck kinase representing a strong candidate. Indeed, we found that the interaction between CD28 and V3 was absent in Lck-deficient Jurkat (JCam1.6) cells and was restored upon transfection with wild-type Lck, which was also included in the V3-CD28 complex (Fig. 5). The V3 domain associated with SH2-inactivated (R154K) Lck, but CD28 was not present in this complex. When JCam1.6 cells were reconstituted with SH3-inactivated (W97A) Lck, PKC-θ-V3 failed to precipitate both CD28 and Lck (Fig. 5). These findings suggest that Lck mediates the interaction between PKC-θ and CD28, with its SH3 domain binding the PR motif in PKC-θ-V3 and its SH2 domain binding phosphorylated Tyr-207 in the CD28 P206YAP209 motif30,32. This mode of a tripartite interaction (Supplementary Fig. 8) is consistent with findings that the SH2 domain of Lck has a much higher affinity than its SH3 domain for the phosphorylated CD28 PYAP motif33 and, conversely, that in stimulated T cells, the Lck SH3 domain is considerably more effective than the SH2 domain in binding PKC-θ34.
Figure 5.
Interaction between CD28 and the V3 of PKCθ is Lck-dependent. PKC-θ-Lck-CD28 association in Lck-deficient (JCam1.6) Jurkat cells cotransfected with Myc-tagged PKCθ-V3 plus WT Lck or its indicated mutants, Transfected cells were stimulated with anti-CD3 and anti-CD28 for 5 min, immunoprecipitated with an anti-Myc Ab, and immunoblotted for Lck and endogenous CD28. Data are from three experiments.
Ectopic V3 domain expression interferes with T cell activation
Given the critical role of the V3 domain in the PKC-θ association with CD28, IS localization and T cell activation, we examined whether ectopic expression of the isolated V3 domain would interfere with the localization and function of PKC-θ by functioning as a CD28-associated “decoy” (Fig. 2a, right panel) and to sequester endogenous PKC-θ from CD28 and the IS. We infected OT-II CD4+ T cells with a bicistronic GFP retrovirus expressing Myc-tagged V3 alone and examined the localization of the transduced V3 protein and endogenous PKC-θ following Ova stimulation. The V3 domain translocated to the IS, but endogenous PKC-θ was sequestered from the IS and found mostly in the cytoplasm (Fig. 6a,b). However, when the proline-mutated V3 domain (4PA) or a V3 domain with a deletion of the whole PR motif (ΔPR) were transduced, they did not localize anymore in the IS and, moreover, they did not interfere with the IS localization of endogenous PKC-θ (Fig. 6a,b).
We next studied the effects of V3 on reporter gene activation. As expected, expression of wild-type PKC-θ alone significantly increased the antigen-induced activity of both RE/AP (Fig. 6c) and NFAT (Supplementary Fig. 9) reporter genes relative to control transfectants. Expression of the V3 alone did not activate these reporters. However, coexpression of the V3 domain together with wild-type PKC-θ resulted in a dose-dependent inhibition of the activity of the PKC-θ-dependent reporter gene. This inhibitory activity was eliminated when the critical PR motif was mutated or deleted (Fig. 6c and Supplementary Fig. 9), resulting in intact PKC-θ-induced reporter activity.
TH2 and TH17 immune responses are compromised in Prkcq−/ − mice, whereas the TH1 response remains relatively intact13–15. Therefore, we determined whether ectopic V3 expression would similarly inhibit TH differentiation. Preactivated B6 CD4+ T cells were infected with retroviruses expressing wild-type or mutated V3 domains, and cultured in vitro under TH1, TH2 or TH17 differentiation conditions. Consistent with previous findings13–15, differentiation into the TH1 lineage was unaffected by any of the ectopically expressed V3 vectors. In contrast, the non-mutated V3 domain inhibited TH17 and TH2 differentiation by ~75%. This inhibition was completely (TH17) or partially (~60–75%; TH2) reversed when the PR motif was mutated or deleted (Fig. 6d). Hence, the V3 domain can act as a decoy to block the localization of endogenous PKC-θ to the IS and thus attenuate its associated signaling and TH differentiation.
PKC-θ V3 inhibits TH2- but not TH1-mediated airway inflammation
We further analyzed the effect of PKC-θ-V3 in vivo in an airway inflammation model using a T cell adoptive transfer system. Mice receiving OT-II TH2 cells transduced with an empty vector developed an inflammatory response by comparison with PBS-challenged control mice, as evidenced by increased number of infiltrating leukocytes in the bronchoalveolar lavages (BAL) fluid (Fig. 7a) and augmented levels of signature TH2 cytokines, IL-4 (Fig. 7b) and IL-5 (Fig. 7c). Introduction of V3 into the transferred TH2 cells ameliorated the disease by decreasing the levels of infiltrating cells and TH2 cytokines to basal levels (Fig. 7a,b,c). However, expression of the PR motif-deleted V3 domain failed to inhibit the inflammatory response. The 4PA mutant partially rescued the inhibition, most likely due to the fact that surrounding amino acid residues in addition to the critical Pro residues also contribute to the regulatory function of the V3 domain.
Figure 7.
V3 inhibits TH2-, but not TH1-mediated, lung inflammation. TH difefrentiation of anti-CD3- plus anti-CD28-stimulated OT-II CD4+ T cells cultured under TH2 (a–c) or TH1 (d, e) -polarizing conditions and retrovitrally transduced with the same PKCθ V3 vectors as in Fig. 6. Sorted GFP+ populations were adoptively transferred into naïve B6 mice, which were challenged with Ova. Total mononuclear cell infiltration in BAL fluid (a, d) and cytokine expression (b, c, e) were analyzed 1 d later. Data are from two experiments.
Adoptive transfer of transduced TH1 effector cells similarly induced lung inflammation manifested by leukocyte infiltration (Fig. 7d) and increased interferon-γ (IFN-γ) levels (Fig. 7e). The native V3 domain (as well as its mutated variants) did not inhibit the inflammatory response (Fig. 7d,e), consistent with the fact that TH1-mediated lung inflammation is relatively PKCθ-independent13–15. Thus, the PKC-θ V3 domain can also function in vivo in a dominant negative manner to block PKC-θ-dependent inflammation.
DISCUSSION
PKC-θ is a critical mediator of TCR-CD28 cosignaling in T cells. Although the catalytic activity of PKC-θ is undoubtedly required for its downstream signaling functions, its IS3 and lipid raft35 localization, which is mediated by its regulatory region, is also critical. However, despite circumstantial evidence4,36, the relationship between the cSMAC localization of PKC-θ and its signaling functions, and whether the former is required for the latter, has not been known, nor have the structural determinants that dictate this unique localization been identified. Here, we showed that the V3 (hinge) domain of PKC-θ and, specifically, a PR motif in V3, is required for this localization via its physical association with CD28 and consequently for PKC-θ-dependent T cell activation and TH2- or TH17-mediated inflammation. This is the first direct evidence that the cSMAC localization of PKC-θ and its ability to activate downstream targets are functionally linked, both residing within a defined structural motif. The signaling events associated with CD28 are not entirely understood, and it remains controversial whether CD28 induces unique signals or simply amplifies TCR signals37,38. Hence, our finding of an inducible association between PKC-θ and CD28 is important since it implies a novel, CD28-specific signaling module that is not shared with TCR signals per se.
Previous studies have circumstantially linked the IS localization and function of PKC-θ to CD28. First, efficient PKC-θ-mediated transcription factor activation depends on CD28 costimulation8,10. Second, CD28 is necessary for the cSMAC localization of PKC-θ36 and this requirement was mapped to a C-terminal P206YAPP motif in CD2839. Third, we and others documented the colocalization of PKC-θ and CD28 in IS-resident microclusters25,26. Furthermore, in the mature IS PKC-θ colocalizes with CD28 in a newly defined peripheral subdomain of the cSMAC in a manner dependent on the same P206YAP motif, and coimmunoprecipitates with CD28 in phorbol ester-stimulated T cells26. Results herein establish that this association is also induced by physiological stimulation with peptide-pulsed APCs, and is dependent on a highly conserved PR motif found in the V3 domain of PKC-θ, but not in other PKCs. This unique motif defines a novel function of the PKC-θ-V3, i.e. costimulation-dependent recruitment of the enzyme to the IS. However, the requirement for the PR motif was not absolute since proline-mutated PKC-θ retained the ability to partially induce CD69 and CD25 upregulation. This likely reflects the fact that some aspects of T cell activation such as Ca2+ signaling12 and CD69 upregulation are only partially dependent on PKC-θ. Indeed, although PKC-θ plays a role in CD69 upregulation40, CD69 expression is also strongly dependent on Ras signaling41.
Since there is no known precedent for a direct interaction between two PR motifs, our findings that PR motifs in both PKC-θ and CD28 are required for their interaction led us to postulate an intermediary protein that mediates this interaction. Previous studies30,32 and results herein support the notion that Lck mediates this interaction through two distinct domains, with its SH2 and SH3 domains binding the CD28 phosphorylated P206Y*AP209 motif or the PKC-θ P331xxP334 motif in V3, respectively. This notion is consistent with the reported differences between the SH2 and SH3 domains of Lck with regard to their binding efficiency to phosphorylated CD28 vs. PKC-θ33,34. However, this model does not exclude the possibility that some other protein(s) acts as a bridge between CD28 and PKC-θ.
The importance of the V3 domain in targeting PKC-θ to CD28 and the IS is not inconsistent with the established importance of the C1 domain in recruiting PKC-θ to the plasma membrane or IS. The PKC-θ C1 domain was reported to localize in the center of the IS42 (although this study did not formally distinguish between the cSMAC and pSMAC), likely reflecting high-level accumulation of diacylglycerol (DAG), the PKC-mobilizing second messenger, at the IS. However, DAG accumulation does not sufficiently explain the unique cSMAC localization of PKC-θ since other PKCs that contain a functional DAG-binding C1 domain do not stably localize at the IS. Hence, there must be some other PKC-θ specific feature that is responsible for its IS/cSMAC localization. We propose that the V3 domain, via its CD28 binding, is responsible for this highly selective, sustained and high stoichiometry3 PKC-θ localization following the initial binding of the C1 domain to membrane DAG, an event that by itself may be highly dynamic and of low stoichiometry. Indeed, PKC-θ-ΔV3, despite containing an intact C1 domain, displayed cytosolic localization in stimulated T cells. This stable, long-term recruitment to the cSMAC allows PKC-θ to mediate its functions. In contrast, other PKCs containing a functional C1 domain, e.g., the PKC-θ-related PKC-δ that has a similar DAG affinity to that of PKC-θ27,43, may also localize at DAG-rich membrane sites but will fail to activate sustained signaling because of their transient low stoichiometry membrane recruitment and/or substrate specificity distinct from PKC-θ. In support of this notion, the PKC-θ C1 domain localized only transiently at the IS, whereas the IS localization of full-length PKC-θ was prolonged and stable44. Similarly, protein kinase D (PKD), which also contains a DAG-binding C1 domain, translocates transiently to the T cell IS42. In addition to the physical PKC-θ-CD28 association reported here, other regulatory events that may contribute to the selective IS/cSMAC localization of PKC-θ and its functions include its tyrosine phosphorylation in the N-terminal C2 domain, which relieves C2-mediated negative regulation10,27,34, autophosphorylation at Thr-219 in the C1 domain45, and/or specific C1 domain-mediated protein-protein interactions46.
Given the requirement of PKC-θ in TH2- and TH17-mediated inflammation and GvH disease, but not in TH1 antiviral or GvL responses, PKC-θ is an attractive drug target for a plethora of diseases. Small molecule selective inhibitors of the catalytic activity of PKC-θ47 have recently been reported. However, the catalytic domains of PKCs are highly conserved and, furthermore, kinase inhibitors generally suffer from lack of sufficient specificity and hence, potential toxic side effects. Our demonstration of a novel approach to attenuate the function of PKC-θ by blocking its obligatory interaction with CD28 suggests that such blockade could serve as a basis for the development of novel therapeutic agents that would selectively suppress undesired T cell-mediated inflammation while, at the same, preserving desired immunity such as antiviral responses.
METHODS
Antibodies (Abs) and Reagents
Monoclonal antibodies (mAbs) specific for mouse CD3 (Clone 145-2C11), CD28 (clone 37.51), IL-4 (clone 11B11), IFN-γ (clone XMG1.2), and anti-IL-12/IL23 p40 were purchased from Biolegend, as were flourophore-conjugated anti-IL-4, anti-IFN-γ, anti-17A, anti-CD69 and anti-CD25. Anti-PKC-θ Abs were obtained from BD Transduction Laboratories and Cell Signaling Technology. A C-terminus-specific anti-PKC-θ Ab cross-reactive with PKC-δ (Clone C-18) and a rabbit polyclonal anti-CD28 Ab were from Santa Cruz Biotechnology. Anti-talin was from Sigma. Cell tracker blue (CMAC), Alexa-647-conjugated anti-mouse Ig and Alexa-555-conjugated anti-rabbit Ig were obtained from Molecular Probes. Recombinant mouse IL-3, IL-4, IL-6, IL-12, stem cell factor (SCF), and TGF̃β were purchased from PeproTech. Digitonin was purchased from EMD Chemicals. Ova (323–339) and MCC (88–103) peptides were from Genescript.
Plasmids
Retroviral plasmids of full-length human PKC-θ and PKC-δ were generated via PCR amplification and subcloned into the pMIG retroviral vector48. PKC-θ-ΔV3 (deletion of aa 282–379), PKC-θ+δV3 (replacement of PKC-θ V3 with aa 282–358 of PKC-δ) and PKC-δ+θPR (insertion of aa 328–336 of human PKC-θ between amino acid I312 and Y313 of PKC-δ) were constructed using overlapping PCR. PKC-θ point mutants were generated using Quikchange II Site-directed Mutagenesis Kit (Stratagene). The PKC-θ V3 expression vector was constructed by in-frame subcloning of amino acid 282–379 of human PKC-θ into a pMIG vector containing an N-terminal Myc tag. V3-4PA and V3-ΔPR were generated via site-directed mutagenesis and overlapping PCR, respectively, of pMIG-V3. Vectors encoding PKC-EGFP fusion proteins were generated by PCR and subcloned into the retroviral vector pMX26. The fluorescent tag was attached to the C-terminus of each PKC via a polyglycine linker (LESGGGGSGGGG).
Mice and primary cell cultures
C57BL/6 (B6) mice were housed, maintained under specific pathogen-free conditions, and manipulated according to a protocol approved by the LIAI Animal Care Committee. Prkcq−/ − OT-II TCR-Tg mice were generated by intercrossing OT-II TCR-Tg mice and Prkcq−/ − mice (a gift from D. Littman). CD4+ T cells were isolated by positive selection with anti-CD4 (L3T4) mAb-coated beads (Miltenyi Biotec), and were cultured in RPMI-1640 medium (Mediatech Inc.) supplemented with 10% heat-inactivated fetal bovine serum, 2 mM glutamine, 1 mM sodium pyruvate, 1 mM MEM nonessential amino acids, and 100 U/ml each of penicillin G and streptomycin (Life Technologies). Differentiation into TH1, TH2 or TH17 effector cells was performed as described49,50. Briefly, naïve CD4+ T cells were pre-activated with plate-bound anti-CD3 (5 μg/ml) and soluble anti-CD28 (2.5 μg/ml) mAbs in the presence of polarizing conditions (T H1: 100 U/ml of IL-2, 20 U/ml of IL-12 and 10 μg/ml of anti-IL-4 mAb; TH2: 100 U/ml of IL-2, 200 U/ml of IL-4, 10 μg/ml each of anti-IL-12 and anti-IFNγ mAbs; TH17: 5 ng/ml of TGFβ, 20 ng/ml of IL-6, 10μg/ml each of anti-IL-4 mAb and anti-IFNγ mAbs) for 48 h. Two rounds of retroviral transductions were carried out before resting in the presence of 100 U/ml of IL-2 for 3 days. On d 7, cells were restimulated for 8 h with plate-bound anti-CD3/CD28 mAbs (10 μg/ml each) without additional cytokines, and intracellular cytokine staining was performed in the presence of GolgiStop (BD Biosciences).
Retroviral Transduction
Platinum-E packaging cells were plated in a 6-well plate in 2 ml RPMI plus 10 % FBS. After 24 h, the cells were transfected with retroviral plasmid DNA (5 μg) using TransIT-LT1 transfection reagent (Mirus Bio). After an overnight incubation, the medium was replaced and cultures were maintained for at least another 24 h. The retroviral supernatants were then harvested, filtered, supplemented with 5 μg/ml of polybrene and 200 U/ml of recombinant IL-2, and then used to infect CD4+ T cells that have been preactivated with plate-bound anti-CD3 (5 μg/ml), soluble anti-CD28 (5 μg/ml) mAb and recombinant IL-2 (200 U/ml). Plates were centrifuged for 1 h at 2,000 rpm and incubated for at least 4 h at 32 °C and for overnight at 37 °C, followed by two additional retroviral infections at daily intervals. After the final infection, cells were washed and cultured in RPMI medium containing 10% FBS and recombinant IL-2 (200 U/ml) for another three d before restimulation with anti-CD3 plus anti-CD28 mAbs.
Immunoprecipitation and Western blotting
Retrovirally transduced CD4+ T cells were stimulated with anti-CD3 and anti-CD28 mAbs for 5 min. Cell lysis in 1% digitonin lysis buffer, immunoprecipitation and Western blotting were carried out as described27.
Luciferase (Luc) reporter assay
The CD28 response element (RE/AP)- or NFAT-Luc reporter genes have been described8,40. MCC-specific hybridoma T cells were transfected using Ingenio™ Electroporation Reagent (Mirus Bio). Transfected cells were stimulated for 6 h with APCs consisting of DCEK fibroblasts stably expressing I-Ek and B7-1, which were prepulsed with MCC peptide (10 μg/ml). The cells were the lysed and Luc activity was quantitated and normalized to the activity of a cotransfected β-galactosidase (β-Gal) reporter gene.
Immunofluorescence microscopy
Full-length human PKC-θ and its derivatives were cloned as a GFP fusion protein in the pMX retroviral vector. Retroviral supernatants were used to infect preactivated CD4+ T cells. Transduced cells were rested for an additional three d. On the day of experiment, APC were prepared from WT B6 splenocytes that were depleted of CD4+ T cells using anti-CD4 (L3T4)-coated beads. T-depleted APC were stained with cell tracker blue (CMAC; 20 μM) and then pulsed with 5 μM Ova peptide, respectively, for 30 min at 37 °C. T-APC conjugation was carried out at 37 °C for 20 min before fixation with 4% paraformaldehyde and permeabilization with PBS supplemented with 1% BSA and 0.1% Triton X-100. Immunostaining was carried out with the indicated Abs in PBS supplemented with 1% BSA, 0.3% saponin for 1 h at room temperature. Immunofluorescene images were captured using a Marianas digital fluorescence-microscopy system (Intelligent Imaging Innovations) as described49.
Bone marrow (BM) chimeras
BM chimeras were produced in irradiated Rag1−/ − mice as described48. Briefly, BM cells were flushed from the femurs and tibias of Prkcq−/ − mice. Lin− bone marrow cells were selected through the Lineage Cell Depletion column (Miltenyi Biotec) and cultured for 24 h in DMEM media (Mediatech Inc) containing 10% FBS, 10 ng/ml of IL-3, 20 ng/ml of IL-6 and 50 ng/ml of SCF. Retroviral infections were carried out for 3 consecutive d. GFP+ cells were sorted, and 2 × 105 cells were intravenously injected into irradiated Rag1−/ − mice. Analyses were performed 6–8 weeks post-transfer.
Adoptive transfer and Ova-induced airway inflammation
CD4+ T cells from OT-II B6 mice were cultured with plate-bound anti-CD3 (5 μg/ml), soluble anti-CD28 (2.5 μg/ml) and IL-2 (100 U/ml) under TH1 or TH2 polarizing conditions, as described above. GFP+ cells were sorted, and 1 × 106 cells were injected intravenously into naïve WT B6 mice. One d later, mice received aerosolized Ova (5 mg/ml in 20 ml PBS) for 30 min, once a day for 3 consecutive d, using ultrasonic nebulization. Mice were sacrificed 24 h after the last challenge and assessed for lung inflammation. Collection of BAL fluid and determination of cytokine levels were carried out as described14.
Statistical analysis
Statistical analyses were performed using one-way-ANOVA with post-hoc Bonfferoni’s corrections. Unless otherwise indicated, data represent the mean ± SEM, with p<0.05 considered statistically significant.
Supplementary Material
Acknowledgments
This is manuscript number 1392 from the La Jolla Institute for Allergy and Immunology. The authors are indebted to T. So, W. Duan and M. Croft for the MCC-specific T hybridoma-DCEK fibroblast system and the advice on the allergic airway inflammation model; to S. Becart, C. Fos, and Y. Harada for continuous discussions and suggestions; and to K. Hayashi for some PKC-θ constructs. The work was supported in part by NIH grant CA35299, by a Grant-in-Aid for Scientific Research on Innovative Areas “Fluorescence Live imaging” (No. 23113521) from The Ministry of Education, Culture, Sports, Science, and Technology, Japan, and by the USA-Israel Binational Science Foundation.
Footnotes
AUTHOR CONTRIBUTIONS
K.-F.K. and A.A. designed the experiments and wrote the manuscript. K.-F.K. generated and analyzed the data. T.S. and T.Y. provided their expertise in cell imaging, T.S. participated in discussion of the data, and T.Y. generated the PKC-GFP and CD28-CFP fusion vectors. N.I. participated actively in discussions leading to this work and in experimental design. A.J.C.-B. performed various experiments and animal work.
COMPETING FINANCIAL INTERESTS
The authors have no competing financial interests.
References
- 1.Dustin ML. The cellular context of T cell signaling. Immunity. 2009;30:482–492. doi: 10.1016/j.immuni.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dustin ML, Chakraborty AK, Shaw AS. Understanding the structure and function of the immunological synapse. Cold Spring Harb Perspect Biol. 2010;2:a002311. doi: 10.1101/cshperspect.a002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monks CR, Kupfer H, Tamir I, Barlow A, Kupfer A. Selective modulation of protein kinase C-θ during T-cell activation. Nature. 1997;385:83–86. doi: 10.1038/385083a0. [DOI] [PubMed] [Google Scholar]
- 4.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 5.Pfeifhofer C, et al. Protein kinase C θ affects Ca2+ mobilization and NFAT cell activation in primary mouse T cells. J Exp Med. 2003;197:1525–1535. doi: 10.1084/jem.20020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun Z, et al. PKC-θ is required for TCR-induced NF-κB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi K, Altman A. Protein kinase C θ (PKCθ): a key player in T cell life and death. Pharmacol Res. 2007;55:537–544. doi: 10.1016/j.phrs.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coudronniere N, Villalba M, Englund N, Altman A. NF-κB activation induced by T cell receptor/CD28 costimulation is mediated by protein kinase C-θ. Proc Natl Acad Sci USA. 2000;97:3394–3399. doi: 10.1073/pnas.060028097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baier-Bitterlich G, et al. Protein kinase C-θ isoenzyme selective stimulation of the transcription factor complex AP-1 in T lymphocytes. Mol Cell Biol. 1996;16:1842–1850. doi: 10.1128/mcb.16.4.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altman A, et al. Positive feedback regulation of PLCγ1/Ca2+ signaling by PKCθ in restimulated T cells via a Tec kinase-dependent pathway. Eur J Immunol. 2004;34:2001–2011. doi: 10.1002/eji.200324625. [DOI] [PubMed] [Google Scholar]
- 11.Lin X, O’Mahony A, Geleziunas R, Greene WC. Protein kinase C θ participates in NF-κB/Rel activation induced by CD3/CD28 costimulation through selective activation of IκB β (IKKβ) Mol Cell Biol. 2000;20:2933–2940. doi: 10.1128/mcb.20.8.2933-2940.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manicassamy S, Sadim M, Ye RD, Sun Z. Differential roles of PKC-θ in the regulation of intracellular calcium concentration in primary T cells. J Mol Biol. 2006;355:347–359. doi: 10.1016/j.jmb.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 13.Marsland BJ, Soos TJ, Spath G, Littman DR, Kopf M. Protein kinase C θ is critical for the development of in vivo T helper Th2 cell but not Th1 cell responses. J Exp Med. 2004;200:181–189. doi: 10.1084/jem.20032229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salek-Ardakani S, So T, Halteman BS, Altman A, Croft M. Differential regulation of Th2 and Th1 lung inflammatory responses by protein kinase C θ. J Immunol. 2004;173:6440–6447. doi: 10.4049/jimmunol.173.10.6440. [DOI] [PubMed] [Google Scholar]
- 15.Salek-Ardakani S, So T, Halteman BS, Altman A, Croft M. Protein kinase Cθ controls Th1 cells in experimental autoimmune encephalomyelitis. J Immunol. 2005;175:7635–7641. doi: 10.4049/jimmunol.175.11.7635. [DOI] [PubMed] [Google Scholar]
- 16.Anderson K, et al. Mice deficient in PKC θ demonstrate impaired in vivo T cell activation and protection from T cell-mediated inflammatory diseases. Autoimmunity. 2006;39:469–478. doi: 10.1080/08916930600907954. [DOI] [PubMed] [Google Scholar]
- 17.Tan SL, et al. Resistance to experimental autoimmune encephalomyelitis and impaired IL-17 production in protein kinase C θ-deficient mice. J Immunol. 2006;176:2872–2879. doi: 10.4049/jimmunol.176.5.2872. [DOI] [PubMed] [Google Scholar]
- 18.Berg-Brown NN, et al. PKCθ signals activation versus tolerance in vivo. J Exp Med. 2004;199:743–752. doi: 10.1084/jem.20031022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giannoni F, Lyon AB, Wareing MD, Dias PB, Sarawar SR. Protein kinase C θ is not essential for T-cell-mediated clearance of murine gammaherpesvirus 68. J Virol. 2005;79:6808–6813. doi: 10.1128/JVI.79.11.6808-6813.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marsland BJ, et al. Innate signals compensate for the absence of PKCθ during in vivo CD8+ T cell effector and memory responses. Proc Natl Acad Sci USA. 2005;102:14374–14379. doi: 10.1073/pnas.0506250102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marsland BJ, et al. TLR ligands act directly upon T cells to restore proliferation in the absence of protein kinase C-θ signaling and promote autoimmune myocarditis. J Immunol. 2007;178:3466–3473. doi: 10.4049/jimmunol.178.6.3466. [DOI] [PubMed] [Google Scholar]
- 22.Valenzuela JO, et al. PKCθ is required for alloreactivity and GVHD but not for immune responses toward leukemia and infection in mice. J Clin Invest. 2009;119:3774–3786. doi: 10.1172/JCI39692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keranen LM, Newton AC. Ca2+ differentially regulates conventional protein kinase Cs’ membrane interaction and activation. J Biol Chem. 1997;272:25959–25967. doi: 10.1074/jbc.272.41.25959. [DOI] [PubMed] [Google Scholar]
- 24.Huang J, Sugie K, La Face DM, Altman A, Grey HM. TCR antagonist peptides induce formation of APC-T cell conjugates and activate a Rac signaling pathway. Eur J Immunol. 2000;30:50–58. doi: 10.1002/1521-4141(200001)30:1<50::AID-IMMU50>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 25.Tseng SY, Waite JC, Liu M, Vardhana S, Dustin ML. T cell-dendritic cell immunological synapses contain TCR-dependent CD28-CD80 clusters that recruit protein kinase C θ. J Immunol. 2008;181:4852–4863. doi: 10.4049/jimmunol.181.7.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yokosuka T, et al. Spatiotemporal regulation of T cell costimulation by TCR-CD28 microclusters and protein kinase C θ translocation. Immunity. 2008;29:589–601. doi: 10.1016/j.immuni.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melowic HR, et al. Mechanism of diacylglycerol-induced membrane targeting and activation of protein kinase Cθ. J Biol Chem. 2007;282:21467–21476. doi: 10.1074/jbc.M700119200. [DOI] [PubMed] [Google Scholar]
- 28.Kay BK, Williamson MP, Sudol M. The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 2000;14:231–241. [PubMed] [Google Scholar]
- 29.Dodson LF, et al. Targeted knock-in mice expressing mutations of CD28 reveal an essential pathway for costimulation. Mol Cell Biol. 2009;29:3710–3721. doi: 10.1128/MCB.01869-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller J, et al. Two pathways of costimulation through CD28. Immunol Res. 2009;45:159–172. doi: 10.1007/s12026-009-8097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez-Lockhart M, et al. Cutting edge: CD28-mediated transcriptional and posttranscriptional regulation of IL-2 expression are controlled through different signaling pathways. J Immunol. 2004;173:7120–7124. doi: 10.4049/jimmunol.173.12.7120. [DOI] [PubMed] [Google Scholar]
- 32.Sadra A, et al. Identification of tyrosine phosphorylation sites in the CD28 cytoplasmic domain and their role in the costimulation of Jurkat T cells. J Immunol. 1999;162:1966–1973. [PubMed] [Google Scholar]
- 33.Hofinger E, Sticht H. Multiple modes of interaction between Lck and CD28. J Immunol. 2005;174:3839–3840. doi: 10.4049/jimmunol.174.7.3839-a. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, et al. Regulation of protein kinase Cθ function during T cell activation by Lck-mediated tyrosine phosphorylation. J Biol Chem. 2000;275:3603–3609. doi: 10.1074/jbc.275.5.3603. [DOI] [PubMed] [Google Scholar]
- 35.Bi K, et al. Antigen-induced translocation of PKC-θ to membrane rafts is required for T cell activation. Nat Immunol. 2001;2:556–563. doi: 10.1038/88765. [DOI] [PubMed] [Google Scholar]
- 36.Huang J, et al. CD28 plays a critical role in the segregation of PKC θ within the immunologic synapse. Proc Natl Acad Sci USA. 2002;99:9369–9373. doi: 10.1073/pnas.142298399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat Rev Immunol. 2003;3:939–951. doi: 10.1038/nri1248. [DOI] [PubMed] [Google Scholar]
- 38.Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. 2009;229:12–26. doi: 10.1111/j.1600-065X.2009.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanchez-Lockhart M, Graf B, Miller J. Signals and sequences that control CD28 localization to the central region of the immunological synapse. J Immunol. 2008;181:7639–7648. doi: 10.4049/jimmunol.181.11.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villalba M, et al. A novel functional interaction between Vav and PKCθ is required for TCR-induced T cell activation. Immunity. 2000;12:151–160. doi: 10.1016/s1074-7613(00)80168-5. [DOI] [PubMed] [Google Scholar]
- 41.D’Ambrosio D, cantrell DA, Frati L, Santoni A, Testi R. Involvement of p21ras in T cell CD69 expression. Eur J Immunol. 1994;24:616–620. doi: 10.1002/eji.1830240319. [DOI] [PubMed] [Google Scholar]
- 42.Spitaler M, Emslie E, Wood CD, Cantrell D. Diacylglycerol and protein kinase D localization during T lymphocyte activation. Immunity. 2006;24:535–546. doi: 10.1016/j.immuni.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 43.Stahelin RV, et al. Mechanism of diacylglycerol-induced membrane targeting and activation of protein kinase Cδ. J Biol Chem. 2004;279:29501–29512. doi: 10.1074/jbc.M403191200. [DOI] [PubMed] [Google Scholar]
- 44.Carrasco S, Merida I. Diacylglycerol-dependent binding recruits PKCθ and RasGRP1 C1 domains to specific subcellular localizations in living T lymphocytes. Mol Biol Cell. 2004;15:2932–2942. doi: 10.1091/mbc.E03-11-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thuille N, et al. Critical role of novel Thr-219 autophosphorylation for the cellular function of PKCθ in T lymphocytes. EMBO J. 2005;24:3869–3880. doi: 10.1038/sj.emboj.7600856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colon-Gonzalez F, Kazanietz MG. C1 domains exposed: from diacylglycerol binding to protein-protein interactions. Biochim Biophys Acta. 2006;1761:827–837. doi: 10.1016/j.bbalip.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Boschelli DH. Small molecule inhibitors of PKCθ as potential antiinflammatory therapeutics. Curr Top Med Chem. 2009;9:640–654. doi: 10.2174/156802609789007372. [DOI] [PubMed] [Google Scholar]
- 48.Holst J, et al. Generation of T-cell receptor retrogenic mice. Nat Protoc. 2006;1:406–417. doi: 10.1038/nprot.2006.61. [DOI] [PubMed] [Google Scholar]
- 49.Becart S, et al. Tyrosine-phosphorylation-dependent translocation of the SLAT protein to the immunological synapse is required for NFAT transcription factor activation. Immunity. 2008;29:704–719. doi: 10.1016/j.immuni.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Canonigo-Balancio AJ, Fos C, Prod’homme T, Becart S, Altman A. SLAT/Def6 plays a critical role in the development of Th17 cell-mediated experimental autoimmune encephalomyelitis. J Immunol. 2009;183:7259–7267. doi: 10.4049/jimmunol.0902573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.