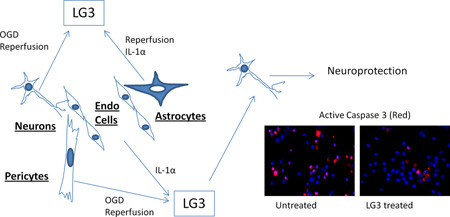
Abstract
Two of the main stresses faced by cells at the NVU as an immediate result of cerebral ischemia are oxygen-glucose deprivation (OGD)/reperfusion and inflammatory stress caused by up regulation of IL-1. As a result of these stresses, perlecan, an important component of the NVU extracellular matrix (ECM), is highly proteolyzed. Here we describe that focal cerebral ischemia in rats results in increased generation of LG3, the c-terminal bioactive fragment of perlecan. Further, in vitro study of the cells of the NVU was performed to locate the source of this increased perlecan-LG3. Neurons, astrocytes, brain endothelial cells and pericytes were exposed to OGD/reperfusion and IL-1α/β. It was observed that neurons and pericytes showed increased levels of LG3 during OGD in their culture media. During in vitro reperfusion, neurons, astrocytes and pericytes showed elevated levels of LG3, but only after exposure to brief durations of OGD. IL-1α and IL-1β treatment tended to have opposite effects on NVU cells. While IL-1α increased or had minimal to no effect on LG3 generation, high concentrations of IL-1β decreased it in most cells studied. Finally, LG3 was determined to be neuroprotective and anti-proliferative in brain endothelial cells, suggesting a possible role for the generation of LG3 in the ischemic brain.
Keywords: Interleukin-1 (IL-1), Oxygen-glucose deprivation (OGD), Perlecan LG3, Cerebral ischemia, Neurovascular unit (NVU), Neurons
INTRODUCTION
Extracellular matrix (ECM) comprises about 20% of the brain (Nicholson & Sykova 1998) and plays a key role in the maintenance of central nervous system (CNS) homeostasis and function. It is composed of proteins including fibronectin, collagen, laminin and heparan sulfate proteoglycans (HSPGs) (Webersinke et al. 1992, Dityatev et al. 2010). One of the most important ECM structures in the brain is the basal lamina-like structure (Dityatev et al. 2010), which forms a thick sheath over pericytes and endothelial cells of the neurovascular unit (Hawkins & Davis 2005) (Farkas & Luiten 2001). An important component of these structures are HSPGs, the most prominent being perlecan, which forms an integral part of the basement membrane of the endothelial cells in mature tissues (Iozzo 1994, Iozzo 1998). Perlecan is also expressed by neurons and astrocytes in addition to endothelial cells (Shee et al. 1998). Thus, perlecan forms an important part of the neurovascular unit (NVU) which consists of neurons, astrocytes, brain endothelial cells, pericytes and ECM (Hawkins & Davis 2005).
Acute brain injury, such as transient cerebral ischemia, damages the NVU by oxygen-glucose deprivation (OGD), and by the reactive oxygen species produced during reperfusion (Chan 2001). This triggers proteolysis of the ECM proteins, especially perlecan, within hours of ischemic insult due to up regulation of various proteases (Fukuda et al. 2004) (Vikman et al. 2007). ECM degradation is accompanied by a local inflammatory response due to up regulation of cytokines (Vikman et al. 2007). One of the main pro-inflammatory cytokines is Interleukin-1 (IL-1) (Allan et al. 2005). Two of the main agonists of the IL-1 family, IL-1α and IL-1β are upregulated within hours of cerebral ischemia (Allan et al. 2005, Hill et al. 1999) and both of them contribute to further ECM degradation, exacerbation of inflammation, and neuronal injury (Thornton et al. 2008).
Perlecan is an extracellular matrix protein which is made up of 5 domains (Bix & Iozzo 2008). Its c-terminal most domain, Domain V (DV), is made up of three laminin globular domains (LG) each separated by two EGF-like repeats (Bix & Iozzo 2008). DV and the fragment resulting from the cleavage of its last laminin globular domain, LG3, have been found to be biologically active (Wright et al. 2010, Bix et al. 2004). Indeed, LG3 has been found to be lowered in the plasma of breast cancer patients, elevated in the urine of patients with end-stage renal failure, and has also been found to be anti-apoptotic for mesenchymal stem cells, thus, suggesting an important physiological role for LG3 (Oda et al. 1996, Chang et al. 2008, Soulez et al. 2010).
Our group recently found that perlecan DV is generated and remains elevated up to 7 days in the brains of mice and rats subjected to transient middle cerebral artery occlusion (MCAo), and that this DV not only has important neuroprotective and proangiogenic effects on the post-stroke brain, but also modulates astrogliosis (Al-Ahmad et al. 2011, Lee et al. 2011). Immunohistochemistry studies using antibodies directed to perlecan Domain V (specifically recognizing the LG3 region) and perlecan Domain IV, which were used to differentiate free Domain V from Domain V attached to full length perlecan, showed that free Domain V is generated mainly at the neurovascular unit (NVU) following cerebral ischemia (Lee et al. 2011). Furthermore, one of the cysteine proteases elevated during cerebral ischemia, cathepsin L, was found to cleave DV and generate LG3 (Cailhier et al. 2008, Fukuda et al. 2004). Thus, we were interested to know if LG3 was also elevated after cerebral ischemia. In the present study, we found that LG3 levels were indeed elevated after transient focal cerebral ischemia in rats. We further investigated the cellular source and mechanisms of LG3 production after cerebral ischemia, and here demonstrate that LG3 production is triggered by OGD/reperfusion insult and IL-1α treatment of the cells of NVU.
MATERIALS AND METHODS
Human LG3 production/purification
Human perlecan LG3 was produced and purified as described previously (Wright et al. 2010). Briefly, LG3 was cloned into the pCEP-pu plasmid which was then transfected into 293-EBNA cells (vector and cells kindly provided by Maurizio Mongiat, Aviano, Italy). Cell-secreted LG3 was purified from the culture supernatant using an added c-terminal 6xHis-tag and Ni-ATA agarose beads (Qiagen). Eluted fractions containing LG3 were combined and dialyzed against Phosphate Buffered Saline (PBS), and the purity of LG3 was confirmed using Sodium Dodecyl Sulphate (SDS) gel electrophoresis (SDS-PAGE) under denatured conditions. LG3 was visualized by staining with Coomassie Brilliant Blue (Sigma Aldrich), silver stain, and western blot with an LG3 antibody (R&D systems, data not shown).
Experimental in vivo stroke model
Middle cerebral artery occlusion (MCAo) was performed on adult male 3-month old Sprague Dawley rats, as approved by Texas A&M College of Medicine IACUC, and as described previously (Lee et al. 2011). Briefly, ischemic injury was performed by tandem common carotid artery and middle cerebral artery occlusion. Animals with diminished perfusion reading (12% to 15% of the initial value), which was confirmed using laser Doppler flow meter (Perimed, Dickinson, TX), were included in further experiments. Occlusions were removed after 60 min and animals were sacrificed 1 day and 3 days post MCAo. Brains were extracted, contralateral and ipsilateral (ischemic) hemispheres were separated, and total homogenates were prepared. Lysates were prepared in RIPA lysis buffer complemented with protease inhibitor cocktail (Calbiochem, EMD Chemicals).
Cell Cultures
All primary cells were extracted from rats or mice as approved by Texas A&M College of Medicine IACUC. All cells were routinely cultured in conditioned incubators (37°C/5% CO2).
Primary fetal cortical neurons were extracted from C57BL6 embryonic day 17–18 mice as previously described (Harris et al. 2007). Briefly, pregnant females were euthanized using cervical dislocation and embryos were dissected in order to obtain cerebral cortices. After mechanical dissociation and treatment with trypsin, neurons were seeded on Poly-D-Lysine (Sigma Aldrich) coated plates in Dulbecco’s modified Eagle Medium (DMEM) high glucose (Invitrogen) containing 2% (v/v) B27 supplement and 1% (v/v) penicillin-streptomycin. Experiments were performed after 3–4 days in vitro (DIV).
Primary astrocytes were prepared from 1 day old Sprague-Dawley rat pups as described previously (Cole & de Vellis 2001, Chow et al. 2001). Briefly, pups were anesthetized using hypothermia and brains were extracted and dissociated by mechanical trituration and trypsin/DNAse I treatment. Cells were seeded on Poly-D-lysine (Sigma Aldrich) coated flasks in DMEM/F12 (Invitrogen) containing 10% (v/v) Fetal Bovine Serum (FBS; Denville Scientific Inc.) and 1% (v/v) penicillin-streptomycin. After 7–9 DIV, the cultures were shaken at 37°C and 180rpm for 24 h to remove contaminating microglia and precursor oligodendrocyte cells. Cultures were then trypsinised and cells were seeded into fish gelatin (Sigma Alderich) coated flasks. Cultures contained 95% astrocytes as assessed by glial fibrillary acid protein (GFAP) immunocytochemistry.
Primary mouse brain endothelial cell (MBEC) cultures were established as described previously (Song & Pachter 2003). Briefly, 4–12 week old C57BL6 mice were euthanized using CO2 inhalation. Brains were extracted and homogenized. Microvessels were separated after density centrifugation in 18% (w/v) Dextran. Microvessels were digested using collagenase/dispase (Roche) solution containing DNAse (Invitrogen) and then suspended in DMEM-F-12 containing 10% (v/v) plasma-derived serum (FirstLink, UK), 10% (v/v) FCS, 100µg/mL endothelial cell growth supplement (BD Biosciences, UK), 100µg/mL heparin, 2mM glutamine, 1% (v/v) penicillin-streptomycin. Cells were then seeded on murine collagen-IV coated (50µg/mL, BD Biosciences) plates, and cultures were used after 14 DIV.
Primary brain pericytes cultures were established as described previously (Dore-Duffy 2003, Al Ahmad et al. 2009). Briefly, adult Sprague-Dawley rats were euthanized using CO2 inhalation. Brains were extracted and homogenized. Microvessels were separated after centrifugation in 20% (w/v) dextran. Microvessels were digested in collagenase/dispase solution (Roche) and seeded on tissue culture plates coated with 250 µg/mL collagen I (BD Biosciences) in DMEM high glucose (Invitrogen) with 20% (v/v) FBS and 1% (v/v) penicillin-streptomycin-amphotericin. After cultures became confluent, cells were trypsinised and seeded on uncoated tissue culture plates, and were cultured in media containing 10% (v/v) FBS and were used at passage 2 (P2). Cultures contained 95% pericytes as assessed by vimentin-positive, Von-Willenbrand factor-negative and GFAP-negative immunostaining as described previously (Kim et al. 2006).
Human brain vascular endothelial cells (hCMEC/D3, kindly provided by Pierre-Olivier Couraud, Institute Cochin Institute, University of Paris, France) were used as a model for human brain endothelial cells (Weksler et al. 2005). hCMEC/D3 were cultured on fish gelatin coated plates in EBM-2 (Lonza) containing 10% (v/v) FBS, 1% (v/v) Penicillin-streptomycin, 1.4 µM hydrocortisone, 5µg/ml ascorbic acid, 1:100 chemically defined lipid concentrate, 1ng/ml basic fibroblast growth factor (bFGF) and 10mM HEPES. They were used from passages 29–38. The rat brain endothelial cell line, RBE4, was also used as a model for rat brain endothelial cells in some experiments (Roux et al. 1994), and were cultured on fish gelatin (Sigma) coated plates from passages 28–35 in Minimum Essential Media alpha (MEM-α) containing nucleosides and ribonucleosides (Invitrogen) and F10 media (Invitrogen) in the ratio of 1:1, and containing 1% (v/v) penicillin-streptomycin.
In vitro Oxygen Glucose Deprivation (OGD)
Oxygen Glucose Deprivation (OGD) was performed using a previously described setup (Tauskela et al. 2003). Non-neuronal cells were serum-starved overnight by changing culture media with DMEM high glucose (Invitrogen) with 1% (v/v) FBS and 1% (v/v) penicillin-streptomycin. For OGD, both neuronal and non-neuronal cultures were washed twice with OGD media, composed of DMEM with no glucose (Invitrogen) containing 1% (v/v) FBS and 1% (v/v) penicillin-streptomycin, with the addition of B27 for neuronal cultures. Cultures were then incubated with OGD media and transferred into a hypoxic chamber (Billups-Rothenberg) which was flushed with anoxic gas (90% N2, 5% CO2 and 5% H2) for 5 min, according to the manufacturer’s instructions. After OGD, the media was collected for western blot analysis and cultures were incubated with oxygenated DMEM high glucose with 1% (v/v) FBS and 1% (v/v) penicillin-streptomycin (and B27 for neuronal cultures). After 24 h, reperfusion media was collected for western blot analysis.
IL-1α and IL-1β treatment
Stocks of IL-1α and IL-1β (R&D Systems) were diluted in 0.1 % (w/v) BSA / 0.9 % (w/v) NaCl. For non-neuronal cells, 100ng/ml IL-1α and IL-1β were prepared in normal culture media of the cells (described above) and lower treatment concentrations were prepared by serial dilution. The media used for neurons contained 5% (v/v) FBS in addition to their normal culture media. Cells were washed once with their normal culture media before being treated with IL-1α or IL-1β. Treatment was carried out for 24 hours and supernatants were collected for western blot analysis.
Western blot analysis
Western blot analyses were performed on brain lysates, which were prepared in RIPA lysis buffer complemented with protease inhibitor cocktail (Calbiochem, EMD chemicals), or were performed on cell culture supernatants. Culture media was also added to empty, acellular wells and was analyzed by western blot. Cell culture supernatants were concentrated 5–10 fold using vacuum centrifugation (Speedvac, Savant, Thermo Scientific). Bradford assays (Bio-Rad) were used to determine protein concentration, and equal amounts of protein were separated on SDS-PAGE and transferred to nitrocellulose membranes. Non-specific binding sites were blocked in 5% (w/v) non-fat milk in Tris buffer saline containing 1:1000 Tween20 (TBS-T). Membranes were then incubated overnight with anti-endorepellin (1:1000, R&D Systems), hereby referred to as anti-LG3, followed by incubation with horseradish peroxidase-conjugated secondary antibody (1:5000, Genetex) for 2h. Membranes were then developed using ECL kit (Thermo Scientific). Bands were quantified using Image J software (NIH) as described by Luke Miller (http://lukemiller.org/journal/2007/08/quantifying-western-blots-without.html). Ponceau staining (Sigma Aldrich) was carried out using 0.1% (w/v) Ponceau S. solution and was used to normalize the blots obtained from supernatants (Romero-Calvo et al. 2010). GAPDH was used to normalize the blots from brain lysates.
Viability Assay
Fetal cortical neurons were seeded on Poly-D-Lysine (Sigma) coated 96 well plates and used at 3–4 DIV. Cultures were washed twice with DMEM no glucose (Invitrogen) containing 1% (v/v) penicillin-streptomycin (OGD media). They were then incubated with OGD media in a hypoxic chamber for 2h, as described above, to expose them to OGD. Media was then changed to DMEM high glucose (Invitrogen) containing 1% (v/v) L-glutamine and 1% (v/v) penicillin-streptomycin. Cultures were treated with PBS vehicle (control) or with 150 nM LG3, a dose that has previously been demonstrated to be biologically active (Wright, Parham et al, 2010) (Bix, Fu et al, 2004). After 2 days, Alamar blue (AbD Serotec) was added to the wells and fluorescence readings were taken after 24 h with excitation at 560 nm and emission at 590 nm using a florescent plate reader (Victor X3, Perkin Elmer), according to manufacturer’s instructions.
Immunocytochemistry
Fetal cortical neurons were seeded on Poly-D-Lysine coated glass chambers (Lab-Tek, Nunc). At 3–4 DIV, they were exposed to OGD and treated with LG3 or PBS vehicle control, as described above. After 72 h, the cells were fixed in 4% (w/v) paraformaldehyde and permeabilized with 0.1% (v/v) Triton-X 100 solution. Cells were then blocked with 5% (w/v) BSA in PBS at room temperature for 2 h and were subsequently incubated with anti-active caspase 3 antibody (Abcam; 1:125 dilution) overnight at 4°C, followed by washes with 1% (w/v) BSA and incubation with goat anti-rabbit alexa secondary conjugated with Alexa 576 (Invitrogen) for 45 min at room temperature. Cells were mounted in Vectashield with DAPI (Vector Labs). Samples were observed on an Axiovision A1m microscope (Carl Zeiss). Images were captured using a Retiga-SRV camera (Qimaging) and iVision software (BioVision technologies) on an Apple Macintosh computer. Approximately 200 cells (recognized by DAPI nuclear florescence and phase contrast imaging) were counted in randomly selected high power fields, 10 fields per treatment condition, in order quantify the number of the cells showing active caspase-3 staining as a percentage of total cells.
Proliferation Assay
hCMEC/D3 cells were seeded on uncoated wells at a density of 5000 cells/well in 96-well plates in hCMEC/D3 cell media (see above) containing 10% (v/v) FBS. After 24 h, the media was changed to hCMEC/D3 media containing 1% (v/v) FBS with PBS vehicle (control) or 150 nM LG3 (treated). After 48 h, MTS (Cell titer96, Promega) was added to the cultures and the absorbance was read after 4 h at 590 nm using a spectrophotometer (Phoenix sunrise, Tecan).
Statistical Analysis
All experiments were performed at least 3 independent times, each time in duplicate or more unless otherwise stated. The data is presented as mean ± standard error of the mean (SEM). Comparison between groups was performed using two-tailed Student’s t-test, one-way ANOVA with Tukey as a post test, or two-way ANOVA, as appropriate, using GraphPad Prism 4.0. Significance was defined as P<0.05.
RESULTS
Focal ischemia induces the release of LG3
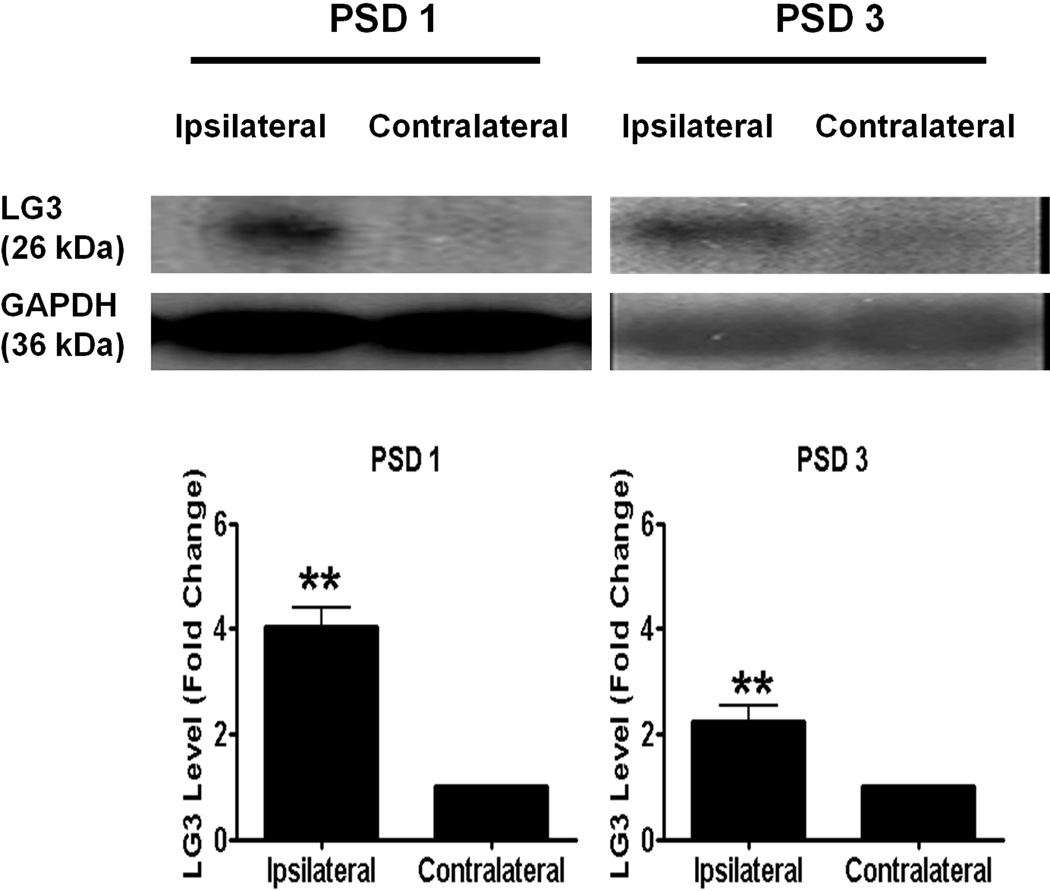
Since our group recently demonstrated the active generation of perlecan DV following stroke injury (Lee et al. 2011), we wanted to investigate if perlecan LG3 is similarly increased after MCAo. As expected, we detected increased LG3 levels in the ipsilateral, ischemic hemisphere as compared to the contralateral hemisphere (of the same animal) at post stroke day (PSD) 1 (Fig. 1a). No LG3 was detected in sham surgical controls (data not shown). This increase was sustained, although slightly diminished at PSD 3.
Figure 1.
Perlecan LG3 levels are elevated after stroke. Representative anti-LG3 western blots were normalized to their respective GAPDH blots and show that LG3 levels are elevated in ipsilateral hemisphere (stroked) when compared to contralateral hemisphere (of the same corresponding animal) at post stroke day (PSD) 1 and PSD 3. Data was analyzed using Student’s t-test and is significant at **p<0.015 as indicated.
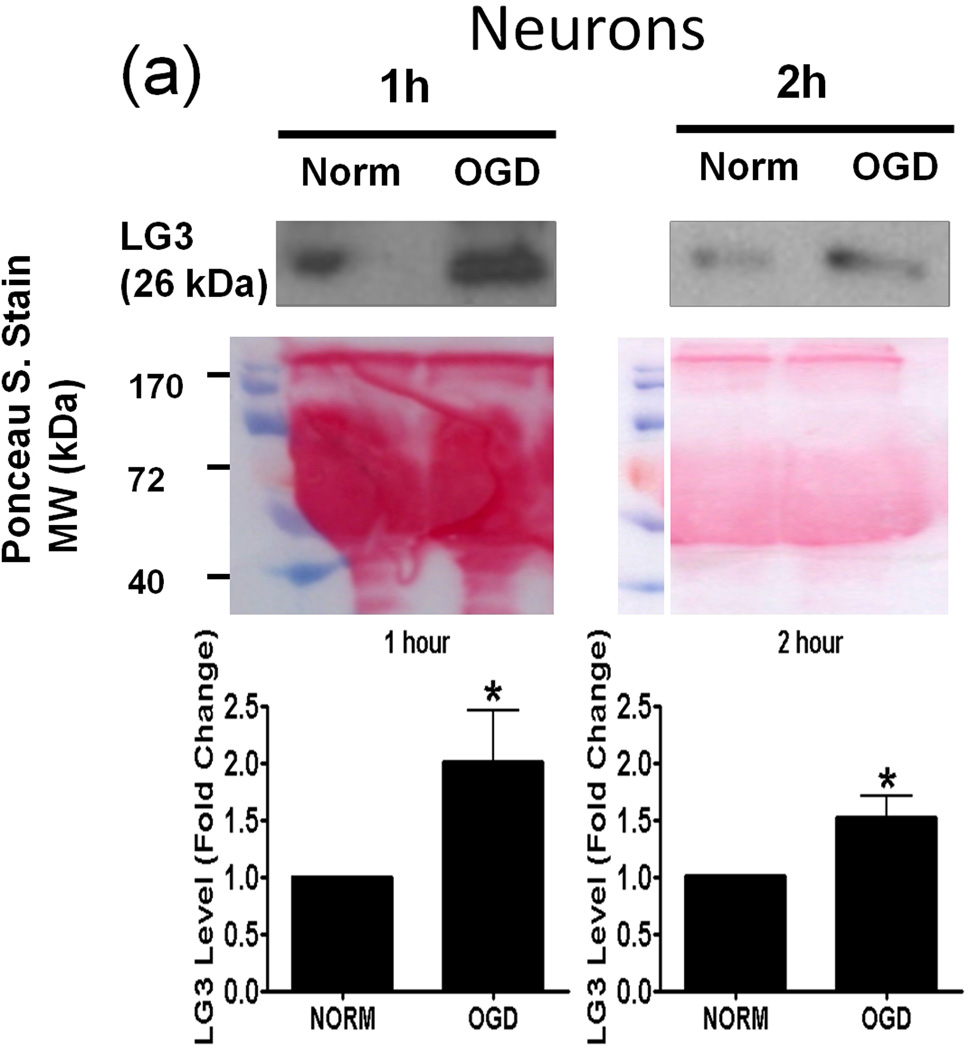
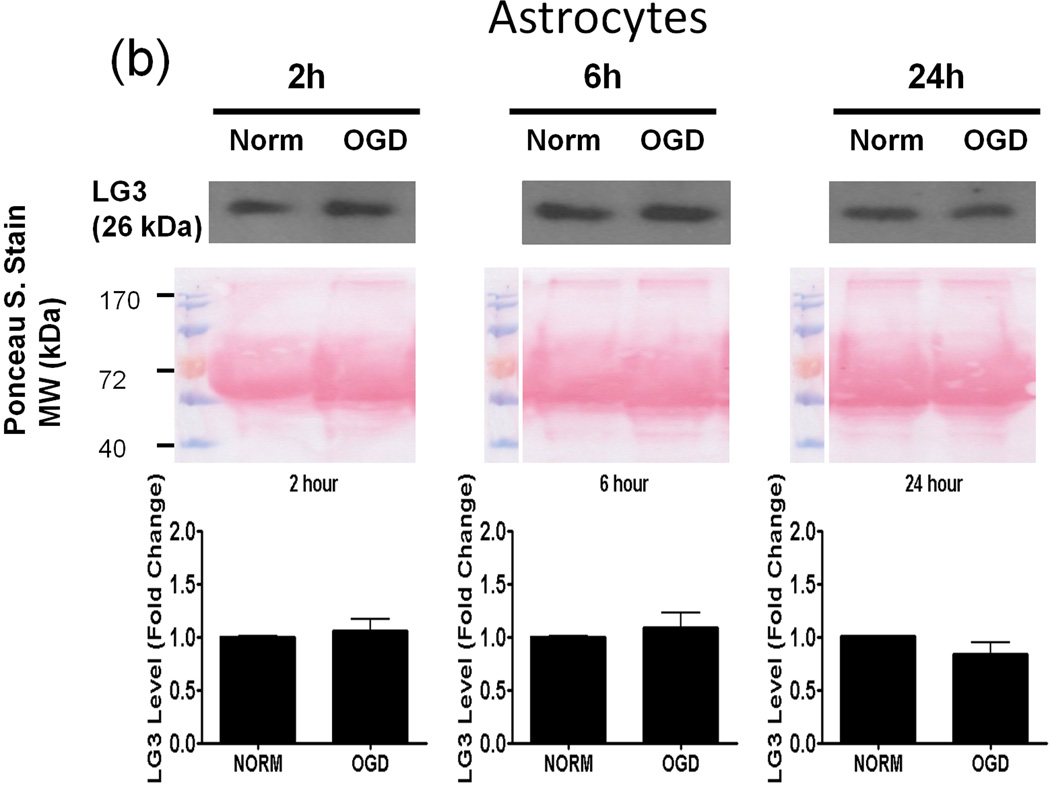
Oxygen-Glucose Deprivation (OGD) causes neurons and pericytes, but not astrocytes, to release more LG3
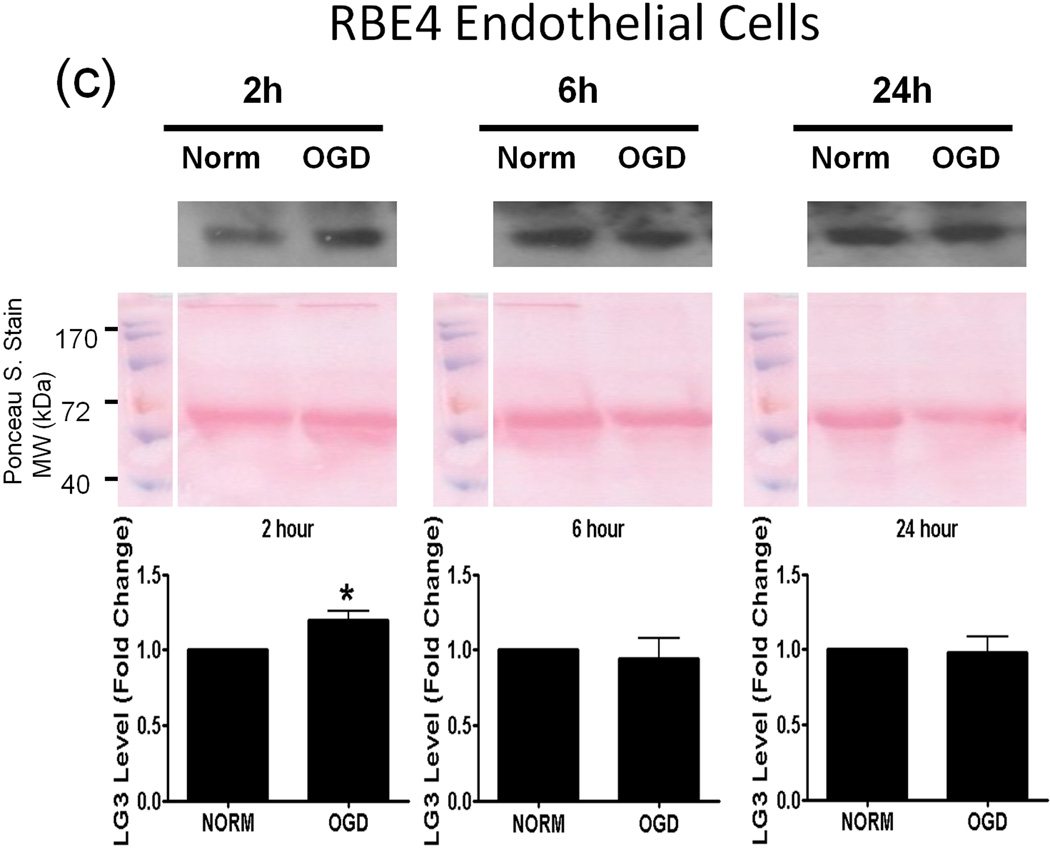
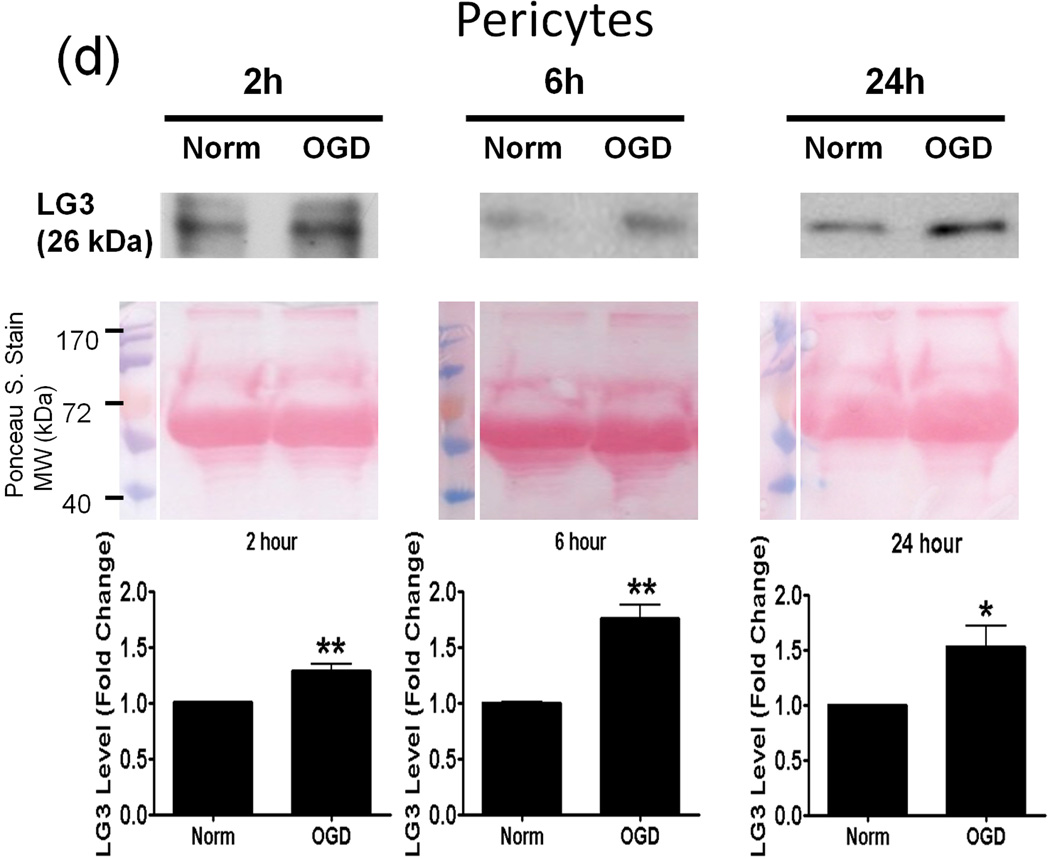
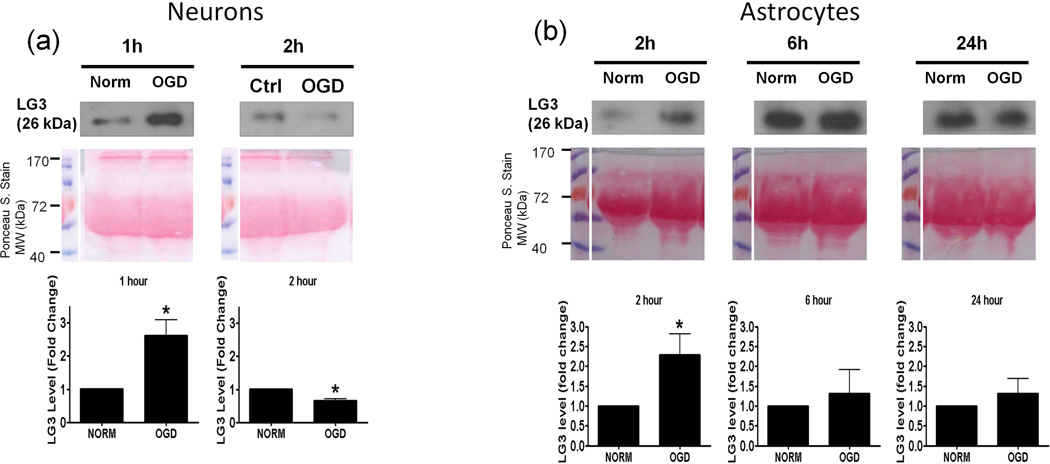
Next, we investigated the possible source(s) of increased LG3 levels seen after MCAo in vivo. To this end, we chose to perform an in vitro analysis, as this approach affords the ability to study cellular components of the NVU individually. Since OGD is the primary stimulus injuring cells during cerebral ischemia, we assessed LG3 levels in the media of neurons, astrocytes, RBE4 brain endothelial cells and pericytes exposed to OGD. The cells were changed to OGD media and were incubated in hypoxic conditions for various durations, after which the OGD media was collected and analyzed for LG3 levels by western blot. These were compared to the LG3 levels present in the media collected from control cultures (normoxic-high glucose) after the same durations. LG3 levels strongly and significantly increased in the media of neurons exposed to 1h and 2h OGD (Fig. 2a). Exposure of primary astrocyte cultures to OGD for 2h, 6h or 24h had no effect on LG3 levels (Fig. 2b), while 2 h OGD, but not 6 or 24 h, induced a slight increase in LG3 levels in the media of brain endothelial cells (Fig. 2c). In contrast, significantly increased levels of LG3 were detected in the media of pericyte cultures exposed to OGD for 2 h, 6 h or 24 h, with maximum release observed during 6h OGD (Fig. 2d). Finally, culture media that had been added to empty, acellular wells did not contain any detectable levels of LG3 (data not shown), demonstrating that the media itself does not appreciably contribute to detectable LG3.
Figure 2.
Analysis of perlecan LG3 levels in OGD media of the cells of the NVU. Representative anti-LG3 western blots were normalized to their respective Ponceau S. stains and represent the fold change in LG3 released by a) Neurons b) Astrocytes c) RBE4 brain endothelial cells and d) Brain pericytes during various durations of OGD when compared to LG3 released by control cultures (Norm). Data was analyzed by Student’s t-test and is significant at *p<0.05 and **p<0.01 when compared to respective control culture LG3 levels.
Reperfusion stress causes neurons, astrocytes and pericytes to generate more LG3 only after brief durations of OGD
Following OGD, another stress which the cells of the NVU face is reperfusion, which results in the generation of reactive oxygen species (ROS) (Chan 2001). Therefore, we were interested to see how reperfusion media of cultures treated with OGD would differ in LG3 levels when compared to that of control cultures. Apart from the reoxygenation induced stress, we were also interested in examining the amounts of LG3 released by OGD-damaged cells when they return to normal conditions.
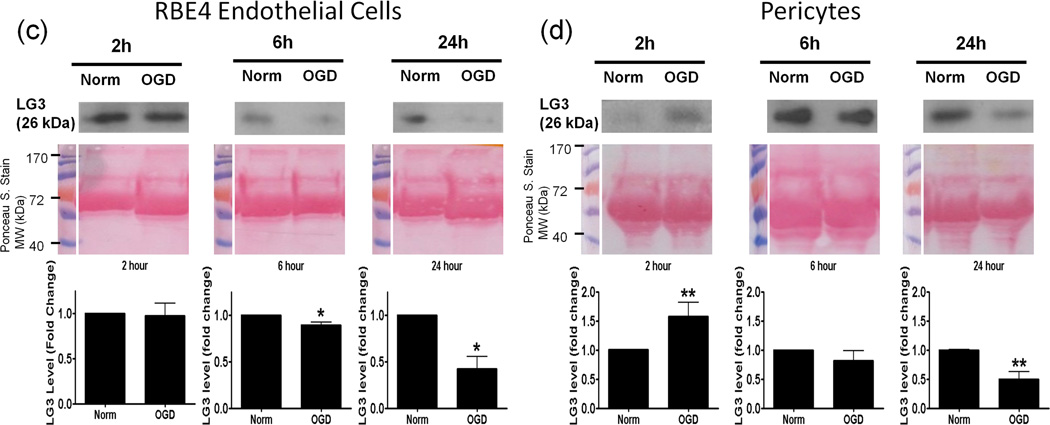
For cultures exposed to OGD, media was changed to normoxic-high glucose media, which was collected after 24 h and termed reperfusion media, as this can be considered to be an in vitro model for in vivo reperfusion. The reperfusion media was analyzed for LG3 levels and compared to LG3 levels in the media from control cultures which were also given a similar change of media. Neurons, astrocytes and pericytes showed an increase in LG3 levels only when OGD was of shorter durations (Fig. 3a, 3b and 3d). A decrease in the release of LG3 was observed after longer durations of OGD exposure to neurons, brain endothelial cells and pericytes (Fig. 3a, 3c and 3d).
Figure 3.
Analysis of perlecan LG3 levels in the reperfusion media of the cells of the NVU. Representative anti-LG3 western blots were normalized to their respective Ponceau S. stains and represent the fold change in LG3 levels by a) Neurons b) Astrocytes c) RBE4 brain endothelial cells and d) Brain pericytes after various durations of OGD (during 24 hours of reperfusion) when compared to LG3 released by control cultures (Norm). Data was analyzed by Student’s t-test and is significant at *p<0.05 and **p<0.01 when compared to respective control culture LG3 levels.
Neurons reperfused after 1 h OGD, showed the strongest significant increase in LG3 levels (Fig. 3a). This increase was lost when neurons were reperfused after a 2 h OGD, with LG3 levels being significantly lower than control values. LG3 levels were significantly increased in reperfusion media of astrocyte cultures exposed to 2h OGD, but this increase was lost in media of astrocytes exposed to 6 or 24 h OGD (Fig. 3b). Although RBE4 endothelial cells showed no change in LG3 levels in their reperfusion media after a 2 h OGD, LG3 levels progressively and significantly decreased after 6 h and 24 h OGD (Fig. 3c). Pericytes, on the other hand, accumulated significantly more LG3 in their reperfusion media after a 2h OGD, when compared to control cultures (Fig. 3d). However, longer durations of OGD induced a progressive decrease in the LG3 levels in the media of pericytes, which became significantly lower after 24 h OGD / reoxygenation.
Interleukin-1α and -1β differentially regulate LG3 generated by neurons, astrocytes, brain endothelial cells and pericytes
The central neuroinflammatory response driven by IL-1 plays a key role in the pathogenesis of cerebral ischemia, and is a key regulator of ECM degradation and remodeling in the brain. As the effect of IL-1 on LG3 production by brain cells is unknown, we investigated whether IL-1α and IL-1β could induce changes in LG3 produced by neuron, astrocyte, brain endothelial cell and pericyte cultures.
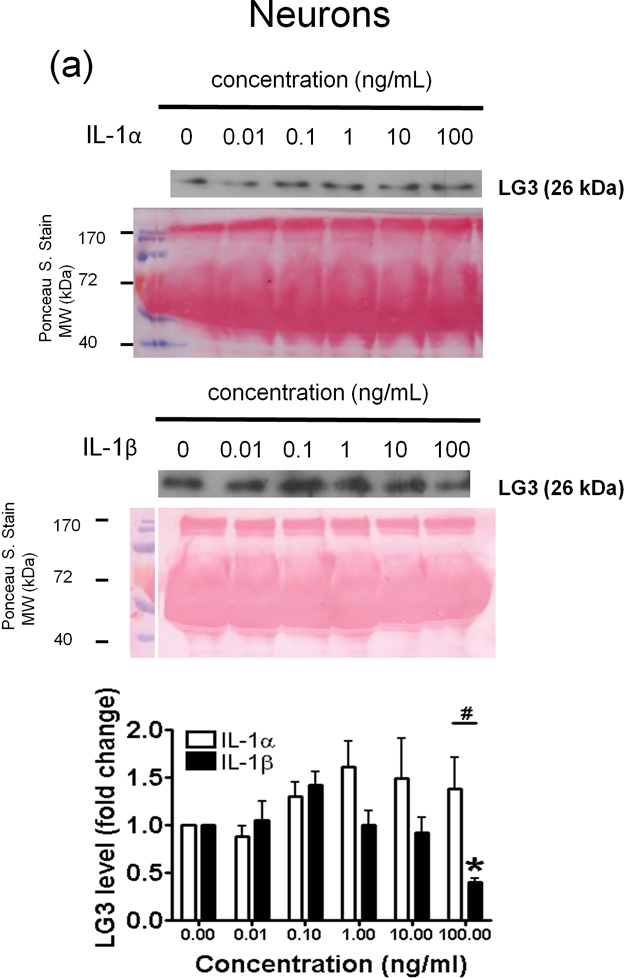
In neurons, IL-1α (0.1 – 100 ng/mL) induced an increase in LG3 levels in the culture media, although this was not found to be statistically significant (Fig. 4a). On the other hand, IL-1β, at 0.1ng/ml, caused an increase in the amount of LG3 released, but, higher concentrations progressively caused a decrease in the amount of LG3 released in culture media, which became significant at 100ng/ml of IL-1β (Fig 4a). As a result, a statistically significant difference was found between the effects of IL-1α and IL-1β at 100ng/mL treatment.
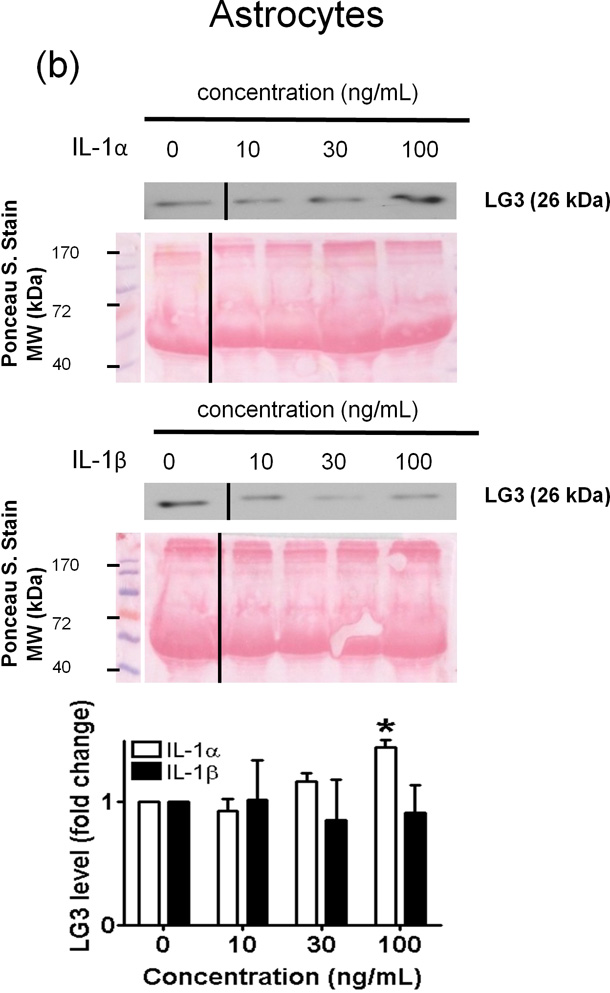
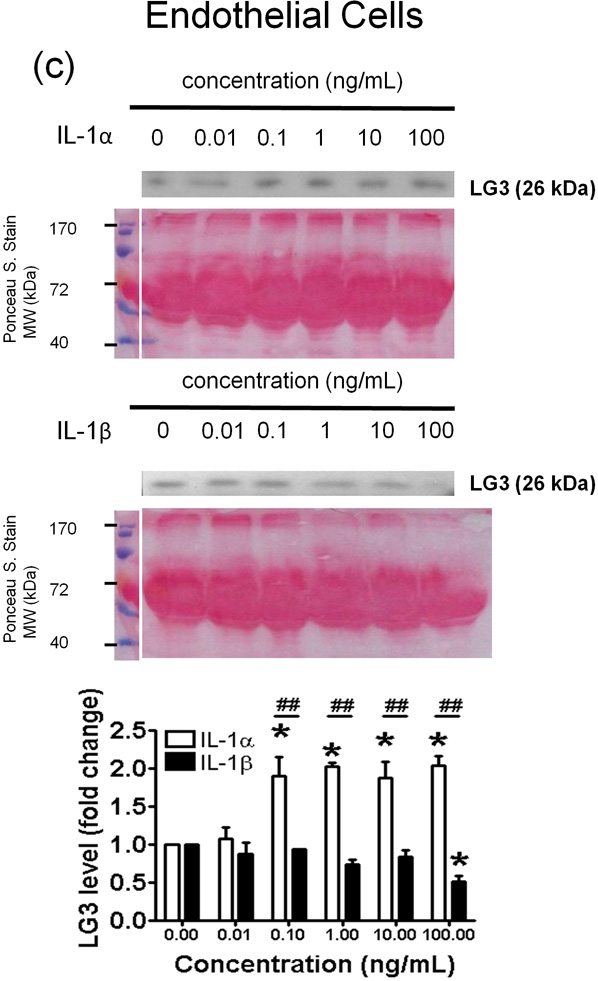
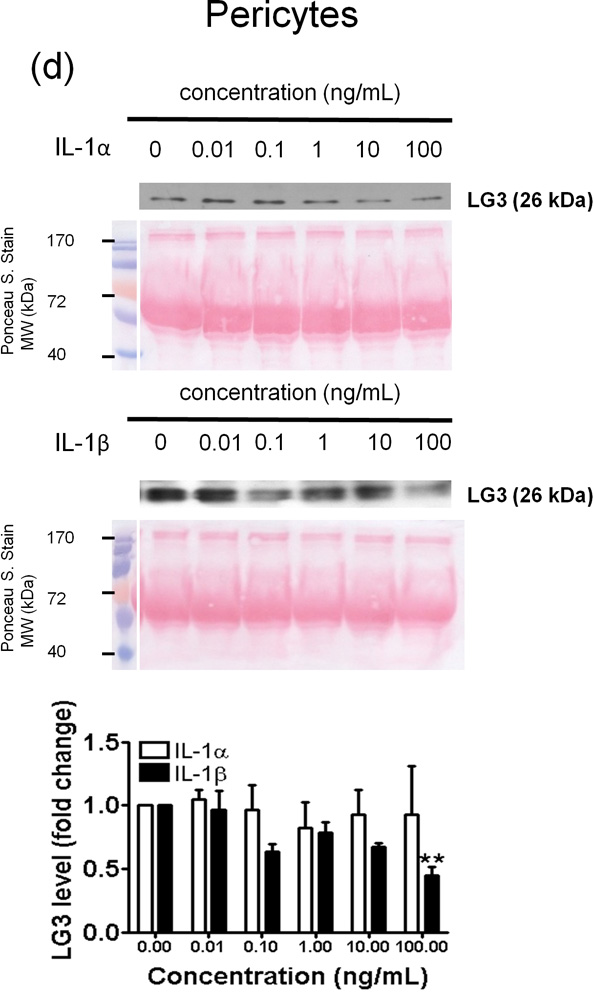
Figure 4.
Effect of IL-1α and IL-1β treatment on the LG3 released by cells of the NVU. Representative anti-LG3 westerns blots were normalized to their respective Ponceau S. Stains and depict LG3 levels in the media of a) Neurons b) Astrocytes c) primary mouse brain endothelial cells and d) brain pericytes after treatment with various concentrations of IL-1α and IL-1β. The data was analyzed by one-way ANOVA and was found to be significant at *p<0.05 and **p<0.01. For each cell, the dose response to IL-1α treatment was compared to the dose response generated to IL-1β treatment by two-way ANOVA and significance was found at #p<0.05 and ##p<0.001.
In astrocytes, IL-1β failed to induce changes in LG3 levels at all of the concentrations tested (Fig. 4b). By contrast, adding increasing concentrations of IL-1α induced a concentration dependent increase in LG3 release, which was significantly higher after a 100 ng/mL treatment.
In mouse brain endothelial cells (MBECs) significant differences were found between the effects of IL-1α and IL-1β at concentrations of 0.1–100ng/ml (Fig. 4c). IL-1α (0.1 – 100 ng/mL) strongly and significantly induced LG3 release from MBECs (Fig. 4c); IL-1β, however, failed to induce changes in LG3 levels at 0.01–10 ng/ml (Fig. 4c), but caused a slight, but significant decrease at high concentration (100 ng/mL). Finally, IL-1α had no effect on LG3 levels released by pericyte cultures, but, IL-1β reduced LG3 release at the highest concentration (100 ng/ml).
LG3 increases neuronal survival after OGD and decreases brain endothelial cell proliferation in vitro
After investigating the cellular source of increased LG3 levels as a result of ischemic / inflammatory stimuli, we investigated whether increased LG3 levels could have biological activity in brain cells after ischemia. Since perlecan domain V, which is elevated after cerebral ischemia, has been found to be neuroprotective and pro-angiogenic (Lee et al. 2011), we tested the hypothesis that LG3 could regulate cell survival and/or proliferation in vitro.
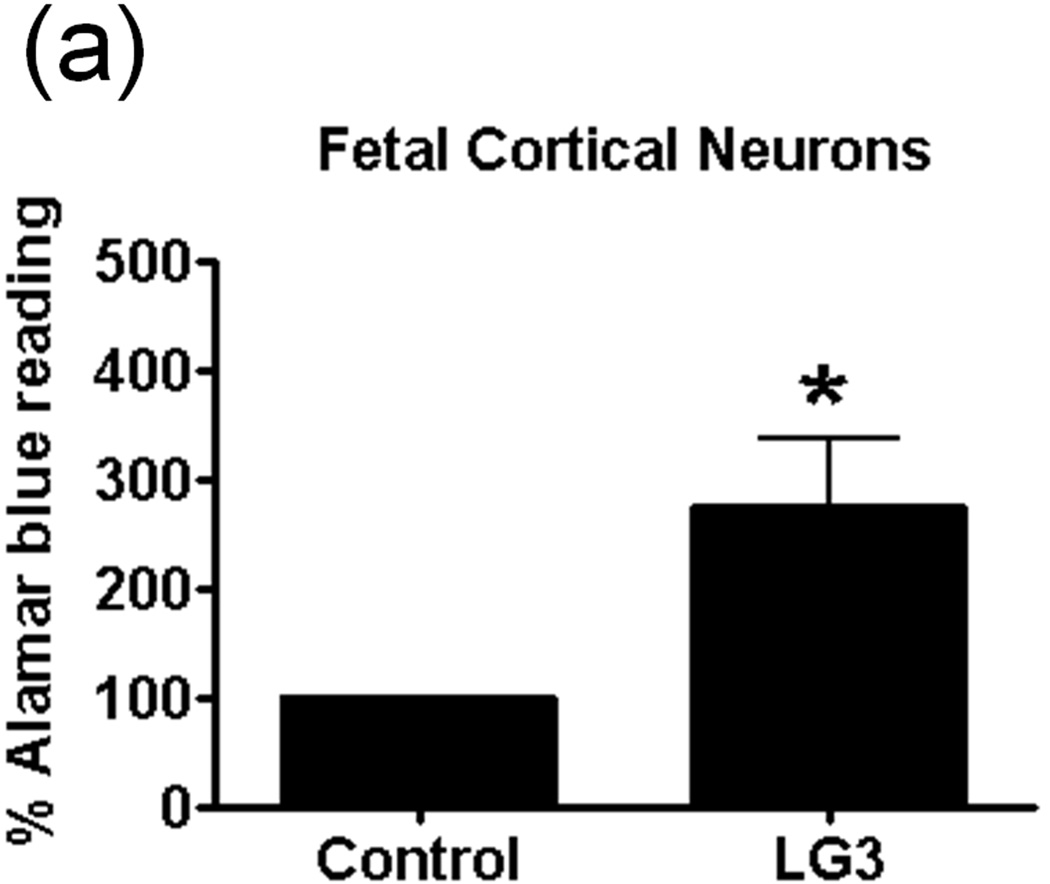
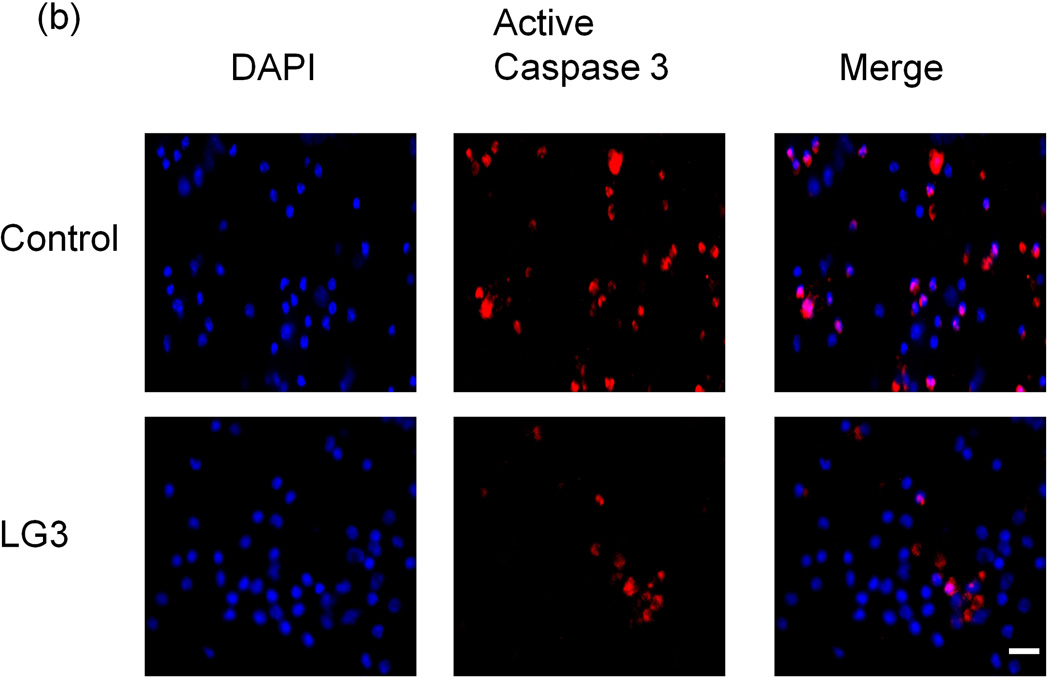
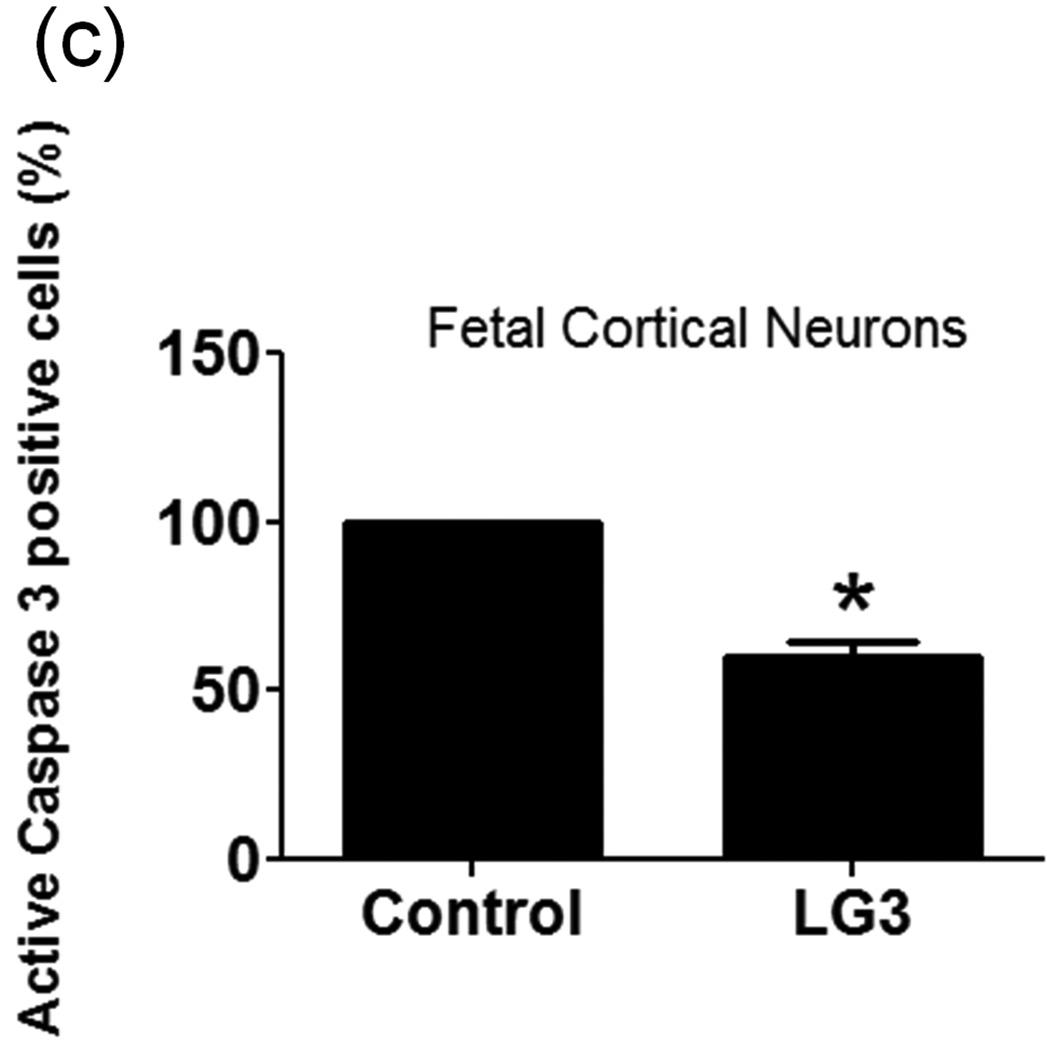
The effect of LG3 on neuronal viability was studied because of particular neuronal susceptibility to damage due to ischemia/reperfusion (I/R). Fetal cortical neurons were exposed to OGD and then were treated with LG3 or PBS vehicle control. Their viability was measured 72 h later using Alamar blue assays. We found that LG3 treatment resulted in about a 300% increase in neuronal viability compared to PBS treated cells (Fig. 5a). To further validate and elucidate the neuroprotective effect of LG3, we also performed active caspase 3 immunocytochemistry of similarly treated fetal cortical neurons (Fig. 5b). We observed that LG3 treatment caused a significant decrease in the percentage of cells staining positive for active caspase-3 (Fig. 5c).
Figure 5.
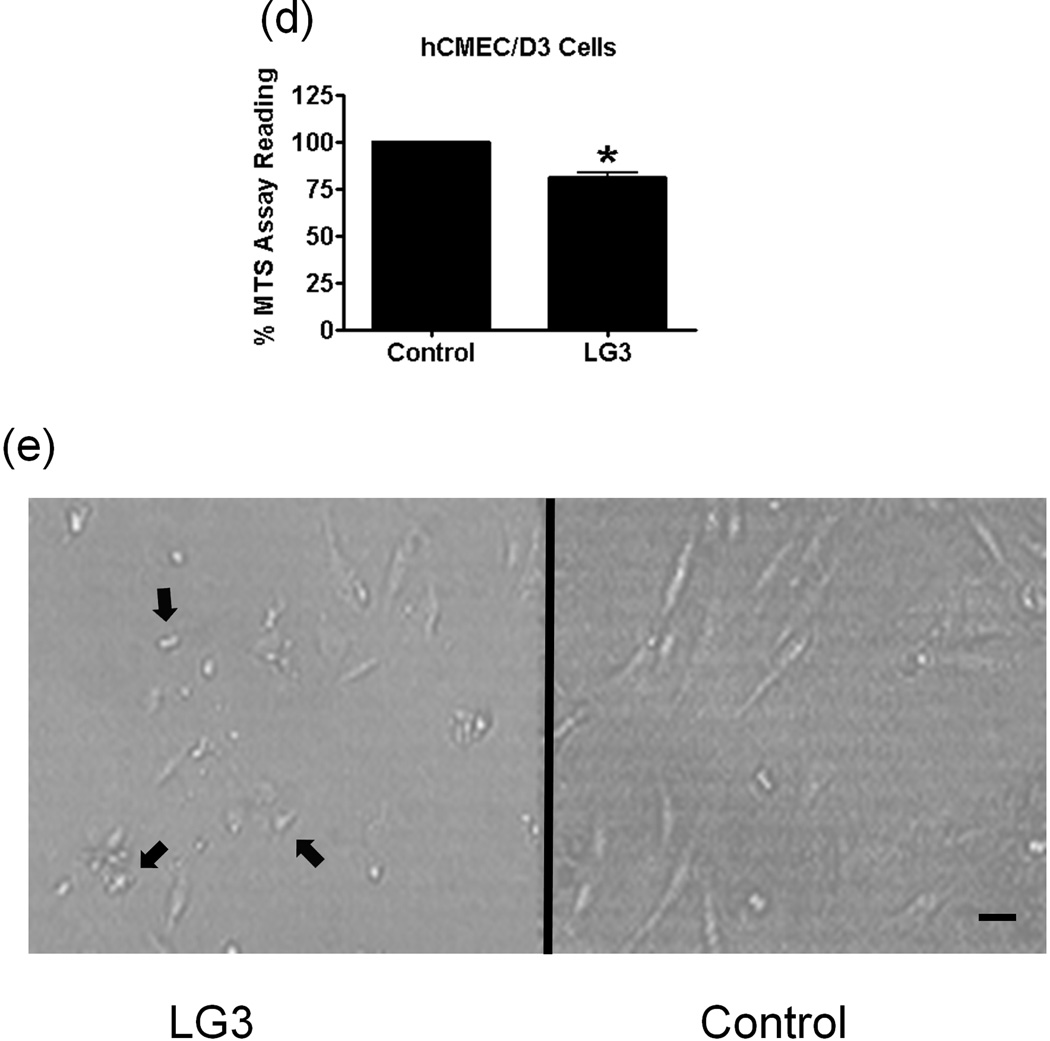
LG3 is neuroprotective and anti-proliferative for brain endothelial cells. a) Fetal cortical neurons were treated with 2 h of OGD and then treated with 150nM LG3 or PBS (control). Alamar blue readings were taken 72 h later and the elevation in Alamar blue readings obtained after LG3 treatment is shown as a percentage of the Alamar blue readings obtained for control cultures. Data was analyzed by Student’s t-test and significance was found at *p<0.05 when compared to control. b) Active Caspase-3 immunocytochemistry on OGD treated neurons. Fetal cortical neurons were treated with 2 h of OGD and then treated with 150nM LG3 or PBS (control). They were fixed and stained with active caspase-3 antibody (red) and DAPI (blue) 72 h later. Scale bar = 20 µm. c) Representative quantification of active Caspase-3 staining on fetal cortical neurons (FCN) showing the active caspase-3 positive cells as a percentage of total cells. The data was analyzed by Student’s t-test and is significant at *p<0.05 d) hCMEC/D3 brain endothelial cells were treated with 150 nM LG3 and their proliferation was analyzed 48 h later using MTS assay. The mean MTS reading of LG3 treated cells is shown as a percentage of mean MTS reading of untreated cells. Data was analyzed by Student’s t-test and is significant at *p<0.05. e) LG3 causes hCMEC/D3 cells to express a ‘shriveled’ morphology (arrows). Cells were treated with LG3 and images were taken after 48 h of LG3 treatment using a light microscope. Scale bar = 10µm.
We also studied the effect of LG3 on the proliferation of the hCMEC/D3 brain endothelial cell line. hCMEC/D3 cells treated with LG3 proliferated significantly less than untreated cells (Fig. 5d). In addition to a decrease in proliferation, the brain endothelial cells also showed a "shriveled" morphology in response to LG3 treatment (Fig. 5e).
Discussion
In this study, we observed a significant increase in the generation of the perlecan c-terminal fragment, LG3, in the brain after transient MCAo. The elevation of LG3 levels in the ipsilateral hemisphere occurred within 24 h after ischemia suggesting that events taking place during or immediately after cerebral ischemia cause increased perlecan synthesis/proteolysis. We therefore investigated the effects of ischemia/reperfusion stress and the effect of elevation of cytokines, which takes place within hours of cerebral ischemia (Hill et al. 1999), on LG3 generation from neurons, astrocytes, brain endothelial cells and pericytes in vitro, in an attempt to identify the potential cellular source(s) of the post-stroke generated LG3. Even though we demonstrated increased levels of LG3 both in vivo and in vitro post-ischemic conditions, the exact molecular source(s) of the increased LG3 remains to be identified. The increase in LG3 may result from an increase in cellular perlecan synthesis following ischemic conditions, increased cellular release of proteases cleaving previously secreted perlecan in the ECM, or both. However, the relative persistence of LG3 at 3 days post-stroke could suggest that its source at that time is at least partially from de novo brain cell-generated perlecan. It also remains to be investigated whether matrix can be degraded intracellularly in the brain, a possibility which has been established in HUVECs (Premzl et al. 2006).
Even though we show a significant increase in LG3 levels in rats following MCAo, a similar increase was also observed in stroked mice and non-human primates (data not shown), demonstrating the multi-species similarity in terms of perlecan synthesis/proteolysis. Furthermore, human perlecan domain V (DV) and LG3 also show a high degree (nearly 100%) of amino acid sequence similarity to their rat and mouse counterparts, and have thus been used successfully with mouse, rat, and human cells previously (Mongiat et al. 2003, Bix et al. 2004, Bix et al. 2007, Lee et al. 2011). Thus, these evidences indicate a conserved nature of perlecan and its DV and LG3 fragments, in terms of sequence, metabolism, and function.
In our study, brain lysates were generated from ipsilateral stroked brain hemispheres and contralateral, non-stroked brain hemispheres. Unfortunately, this method does not afford sufficient spatial resolution to determine exactly where LG3 has been generated, i.e. at the ischemic core versus the ischemic border area, or how much has been generated in each area. Therefore, assuming that LG3 is produced at both the ischemic core and the ischemic border area, it is conceivable that LG3 generated in the core would also be neuroprotective. This core-generated LG3 might then contribute to increased heterogeneity/viability within the core such that it could potentially reduce the evolution of "mini-cores" (associated with multiple, more viable "mini-penumbras") within the core proper into a more homogenous core over time (Del Zoppo et al. 2011).
OGD may stimulate the release of cysteine proteases and degradation of perlecan causing release of LG3
A key factor in cerebral ischemia-induced brain injury and inflammation is reduction in oxygen and glucose supply. Hence, we hypothesized that OGD could be a direct inducer of perlecan proteolysis. Neurons and pericytes were highly responsive during OGD in terms of LG3 levels. Brain endothelial cells showed a small increase only during 2 h OGD while astrocytes showed no changes. The increase seen during lower durations of OGD might be due to the release of lysosomal proteases and breakdown of already established matrix instead of de novo protein synthesis of perlecan. These results are also in agreement with a previous in vivo study which showed a significant decrease in perlecan positive microvessels 2 h after MCAo, which was caused by an increase in the levels of cysteine proteases and hence, proteolysis of perlecan (Fukuda et al. 2004). Our study shows that neurons (primarily), pericytes and brain endothelial cells may be responsible for such an increase in proteolysis, and that merely OGD may initiate the release of proteases.
Cells release decreased levels of LG3 after longer exposures to OGD
We also assessed the effects of reperfusion/reoxygenation induced oxidative stress on the generation of LG3. Based on the LG3 levels observed after reperfusion, we could also assess the damage caused to the cells by OGD. Reperfusion of neuron and astrocyte cultures after 1 h and 2 h of OGD, respectively, caused a substantial increase in LG3 levels. This increase in LG3 levels during reperfusion after short durations of OGD, by cells present in large numbers in the brain, may partially account for the increased LG3 levels detected in vivo, 1 day after MCAo. Brain pericytes also showed a significant increase in LG3 levels during reperfusion after 2 h of OGD.
Interestingly, it appears that longer durations of OGD induce a decrease in LG3 levels, as detected in neurons (after 2 h OGD), brain endothelial cells (after 6 and 24 h OGD) and pericytes (after 24 h OGD). Astrocytes, on the other hand, show no such decrease even after exposure to 24 h of OGD. This data for RBE4 endothelial cells and astrocytes is corroborated by the changes in viability seen in these cell types after exposure to various durations of OGD, where astrocytes show no significant decrease in viability even after 24 h of hypoxic OGD (Lee et al. 2009, Schmid-Brunclik et al. 2008). For RBE4 endothelial cells, in our OGD model, the decrease in viability after longer durations of OGD/reperfusion was confirmed by lower metabolism of cell medium, as confirmed by lack of change in color of the phenol red ph indicator in the cell medium (data not shown). For neurons, a decrease in viability 24 h after 1 h of OGD has previously been reported (Jones et al. 2004). On the other hand, we did not see any significant decrease in neuronal viability 24h after a 1 h OGD (data not shown). Given the very short duration of OGD involved, this may represent a difference in experimental setup. Much of cell death caused by OGD is triggered and occurs after the cells are returned to normal conditions (Nakajima et al. 2006). Since we show that dying cells generate lesser amounts of LG3 than healthy cells, our results would seemingly be in contrast with a previous study which showed that apoptotic human umbilical vein endothelial cells (HUVECs) generate greater amounts of LG3 (Laplante et al. 2006). This discrepancy may be due to the difference in the mechanism of OGD/reperfusion mediated cell death. Thus, we can collectively conclude that shorter durations of OGD that stress, but do not kill cells of the NVU, lead to an increase in LG3 levels, while longer periods of OGD that result in cell death lead to decreased LG3 levels.
An increase in LG3 levels may partially be maintained by IL-1α even 3 days after stroke
Given that IL-1 is established as a key mediator of inflammation and neuronal injury, we next investigated whether IL-1 could regulate LG3 production by cells associated with the NVU. Both IL-1α and IL-1β induce similar biological actions by binding to the same signaling receptors (Allan et al. 2005), and therefore, have redundant functions (Boutin et al. 2001). However, IL-1α and IL-1β have also been found to have clear differential effects (Trebec-Reynolds et al. 2010). In agreement with this, we found here that IL-1α and IL-1β have generally opposite effects in neurons and endothelial cells, in that, IL-1α induced increased levels of LG3 while IL-1β induced a decrease in LG3 levels. Our data strongly correlates with a previous study which reported that IL-1α increases global perlecan mRNA in the hippocampus and in astrocytes (Garcia de Yebenes et al. 1999), and we can speculate that IL-1α may cause an increase in LG3 levels via an increase in perlecan synthesis.
Both IL-1α and IL-1β are elevated within hours of cerebral ischemia and IL-1α may stay elevated up to 4 days after stroke (Hill et al. 1999). But, unlike IL-1β, IL-1α is constitutively expressed in the brain (Boutin et al. 2001). Furthermore, IL-1α (but not IL-1β) has been recently shown to be released by blood platelets and induce brain endothelium activation in the ischemic core within 24 h after stroke (Thornton et al. 2010). Since IL-1α has also previously been shown to be transported across blood-brain barrier (Banks et al. 1994), IL-1α is more likely to be present at a higher concentration than IL-1β in the brain. Thus, even though IL-1β is elevated after cerebral ischemia (Hill et al. 1999), high enough concentrations may not be reached for it to produce an effect since it only decreases LG3 levels at high concentrations. IL-1α may, on the other hand, contribute towards maintaining the levels of LG3 generated by brain cells, given the sensitivity of cells, such as brain endothelial cells, to low concentrations of IL-1α, and the possibility of its higher concentrations in the brain, even 3 days after stroke. Additionally, we conclude that brain pericytes express an IL-1 receptor since they respond to IL-1β.
Increased LG3 levels have functional significance
We observed a beneficial effect of LG3 on neuronal survival. Our group previously showed that LG3 blocks neuronal toxicity induced by amyloid beta through α2β1 integrin binding (Wright et al. 2010). Here we demonstrate that LG3 may act as an agonist to promote neuronal survival after OGD induced damage. We further showed that LG3 caused a decrease in active caspase 3 immunostaining following OGD-reperfusion, which suggests that LG3 has anti-apoptotic effects on neurons, which have previously been observed in other cell types (Laplante et al. 2006, Soulez et al. 2010).
LG3 also causes a small decrease in the proliferation of hCMEC/D3 brain endothelial cells. This is in direct contrast to perlecan DV's enhancement of brain endothelial cell proliferation (Lee et al. 2011) and may reflect relative differences in their affinities for the perlecan DV pro-angiogenic receptor α5β1 integrin (Clarke et al. 2011). Furthermore, it is possible that the post-stroke pro-angiogenic activity of DV is regulated by its further proteolytic cleavage yielding the anti-angiogenic LG3.
In summary, we have demonstrated that perlecan LG3 is persistently generated after focal cortical ischemia, provided evidence that specific cell types of the NVU are responsible for the generation of LG3 after OGD or reperfusion, respectively, and demonstrated that IL-1α and IL-1β differentially affect LG3 production. Finally, we provide evidence that this post ischemia generated LG3 is neuroprotective, suggesting its potential relevance to stroke pathophysiology, and hinting at its therapeutic potential.
Acknowledgements
The authors would like to thank Dr. Abraham Al Ahmad (Texas A&M College of Medicine) for his technical assistance and guidance on astrocyte and pericyte cultures. We also thank Sophie Leow-Dyke and Charlotte Allen (Faculty of Life Sciences, University of Manchester) for providing IL-1α/β treated brain endothelial cell samples. We also thank Christi Parham and Courtney Shaw (Texas A&M College of Medicine) for their guidance on neuron cultures, and Maurizio Mongiat (Aviano cancer research center) for his providing of key reagents to clone LG3. The study was funded by the Ted Nash Long Life Foundation, Texas A&M funding, and NIH grant R01NS065842-01A01 (to G. Bix).
References
- Al-Ahmad AJ, Lee B, Saini M, Bix GJ. Perlecan domain V modulates astrogliosis In vitro and after focal cerebral ischemia through multiple receptors and increased nerve growth factor release. Glia. 2011 doi: 10.1002/glia.21227. [DOI] [PubMed] [Google Scholar]
- Al Ahmad A, Gassmann M, Ogunshola OO. Maintaining blood-brain barrier integrity: pericytes perform better than astrocytes during prolonged oxygen deprivation. J Cell Physiol. 2009;218:612–622. doi: 10.1002/jcp.21638. [DOI] [PubMed] [Google Scholar]
- Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Ehrensing CA. Blood-borne interleukin-1 alpha is transported across the endothelial blood-spinal cord barrier of mice. J Physiol. 1994;479(Pt 2):257–264. doi: 10.1113/jphysiol.1994.sp020293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bix G, Fu J, Gonzalez EM, et al. Endorepellin causes endothelial cell disassembly of actin cytoskeleton and focal adhesions through alpha2beta1 integrin. J Cell Biol. 2004;166:97–109. doi: 10.1083/jcb.200401150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bix G, Iozzo RA, Woodall B, Burrows M, McQuillan A, Campbell S, Fields GB, Iozzo RV. Endorepellin, the C-terminal angiostatic module of perlecan, enhances collagen-platelet responses via the alpha2beta1-integrin receptor. Blood. 2007;109:3745–3748. doi: 10.1182/blood-2006-08-039925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bix G, Iozzo RV. Novel interactions of perlecan: unraveling perlecan's role in angiogenesis. Microsc Res Tech. 2008;71:339–348. doi: 10.1002/jemt.20562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin H, LeFeuvre RA, Horai R, Asano M, Iwakura Y, Rothwell NJ. Role of IL-1alpha and IL-1beta in ischemic brain damage. J Neurosci. 2001;21:5528–5534. doi: 10.1523/JNEUROSCI.21-15-05528.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cailhier JF, Sirois I, Laplante P, et al. Caspase-3 activation triggers extracellular cathepsin L release and endorepellin proteolysis. J Biol Chem. 2008;283:27220–27229. doi: 10.1074/jbc.M801164200. [DOI] [PubMed] [Google Scholar]
- Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Chang JW, Kang UB, Kim DH, Yi JK, Lee JW, Noh DY, Lee C, Yu MH. Identification of circulating endorepellin LG3 fragment: Potential use as a serological biomarker for breast cancer. Proteomics Clin Appl. 2008;2:23–32. doi: 10.1002/prca.200780049. [DOI] [PubMed] [Google Scholar]
- Chow J, Ogunshola O, Fan SY, Li Y, Ment LR, Madri JA. Astrocyte-derived VEGF mediates survival and tube stabilization of hypoxic brain microvascular endothelial cells in vitro. Brain Res Dev Brain Res. 2001;130:123–132. doi: 10.1016/s0165-3806(01)00220-6. [DOI] [PubMed] [Google Scholar]
- Clarke D, Lee B, Al Ahmad A, Parham C, Auckland L, Fertala A, Kahle M, Bix G. Perlecan Domain V Induces Vascular Endothelial Growth Factor Secretion in Brain Endothelial Cells is Driven Through Integrin α5β1 and ERK-Dependent Signaling Pathways. Blood. 2011 doi: 10.1371/journal.pone.0045257. In Revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R, de Vellis J. Preparation of astroycte, oligodendrocyte, and microglia cultures from primary rat cerebral cultures. In: Fedoroff S, Richardson A, editors. Protocols for neural cell culture. Totowa, N.J.: Humana Press; 2001. pp. 117–127. [Google Scholar]
- Del Zoppo GJ, Sharp FR, Heiss WD, Albers GW. Heterogeneity in the penumbra. J Cereb Blood Flow Metab. 2011 doi: 10.1038/jcbfm.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dityatev A, Seidenbecher CI, Schachner M. Compartmentalization from the outside: the extracellular matrix and functional microdomains in the brain. Trends Neurosci. 2010;33:503–512. doi: 10.1016/j.tins.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Dore-Duffy P. Isolation and characterization of cerebral microvascular pericytes. Methods Mol Med. 2003;89:375–382. doi: 10.1385/1-59259-419-0:375. [DOI] [PubMed] [Google Scholar]
- Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer's disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Fini CA, Mabuchi T, Koziol JA, Eggleston LL, Jr, del Zoppo GJ. Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke. 2004;35:998–1004. doi: 10.1161/01.STR.0000119383.76447.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia de Yebenes E, Ho A, Damani T, Fillit H, Blum M. Regulation of the heparan sulfate proteoglycan, perlecan, by injury and interleukin-1alpha. J Neurochem. 1999;73:812–820. doi: 10.1046/j.1471-4159.1999.0730812.x. [DOI] [PubMed] [Google Scholar]
- Harris J, Lee H, Tu CT, Cribbs D, Cotman C, Jeon NL. Preparing e18 cortical rat neurons for compartmentalization in a microfluidic device. J Vis Exp. 2007:305. doi: 10.3791/305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Hill JK, Gunion-Rinker L, Kulhanek D, et al. Temporal modulation of cytokine expression following focal cerebral ischemia in mice. Brain Res. 1999;820:45–54. doi: 10.1016/s0006-8993(98)01140-8. [DOI] [PubMed] [Google Scholar]
- Iozzo RV. Perlecan: a gem of a proteoglycan. Matrix Biol. 1994;14:203–208. doi: 10.1016/0945-053x(94)90183-x. [DOI] [PubMed] [Google Scholar]
- Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- Jones PA, May GR, McLuckie JA, Iwashita A, Sharkey J. Apoptosis is not an invariable component of in vitro models of cortical cerebral ischaemia. Cell Res. 2004;14:241–250. doi: 10.1038/sj.cr.7290225. [DOI] [PubMed] [Google Scholar]
- Kim JA, Tran ND, Li Z, Yang F, Zhou W, Fisher MJ. Brain endothelial hemostasis regulation by pericytes. J Cereb Blood Flow Metab. 2006;26:209–217. doi: 10.1038/sj.jcbfm.9600181. [DOI] [PubMed] [Google Scholar]
- Laplante P, Raymond MA, Labelle A, Abe J, Iozzo RV, Hebert MJ. Perlecan proteolysis induces an alpha2beta1 integrin- and Src family kinase-dependent anti-apoptotic pathway in fibroblasts in the absence of focal adhesion kinase activation. J Biol Chem. 2006;281:30383–30392. doi: 10.1074/jbc.M606412200. [DOI] [PubMed] [Google Scholar]
- Lee B, Clarke D, Al Ahmad A, et al. Perlecan domain V is neuroprotective and proangiogenic following ischemic stroke in rodents. J Clin Invest. 2011;121:3005–3023. doi: 10.1172/JCI46358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HT, Chang YC, Tu YF, Huang CC. VEGF-A/VEGFR-2 signaling leading to cAMP response element-binding protein phosphorylation is a shared pathway underlying the protective effect of preconditioning on neurons and endothelial cells. J Neurosci. 2009;29:4356–4368. doi: 10.1523/JNEUROSCI.5497-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongiat M, Sweeney SM, San Antonio JD, Fu J, Iozzo RV. Endorepellin, a novel inhibitor of angiogenesis derived from the C terminus of perlecan. J Biol Chem. 2003;278:4238–4249. doi: 10.1074/jbc.M210445200. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Wakasa T, Okuma Y, Inanami O, Nomura Y, Kuwabara M, Kawahara K. Dual inhibition of protein phosphatase-1/2A and calpain rescues nerve growth factor-differentiated PC12 cells from oxygen-glucose deprivation-induced cell death. J Neurosci Res. 2006;83:459–468. doi: 10.1002/jnr.20740. [DOI] [PubMed] [Google Scholar]
- Nicholson C, Sykova E. Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 1998;21:207–215. doi: 10.1016/s0166-2236(98)01261-2. [DOI] [PubMed] [Google Scholar]
- Oda O, Shinzato T, Ohbayashi K, Takai I, Kunimatsu M, Maeda K, Yamanaka N. Purification and characterization of perlecan fragment in urine of end-stage renal failure patients. Clin Chim Acta. 1996;255:119–132. doi: 10.1016/0009-8981(96)06395-4. [DOI] [PubMed] [Google Scholar]
- Premzl A, Turk V, Kos J. Intracellular proteolytic activity of cathepsin B is associated with capillary-like tube formation by endothelial cells in vitro. J Cell Biochem. 2006;97:1230–1240. doi: 10.1002/jcb.20720. [DOI] [PubMed] [Google Scholar]
- Romero-Calvo I, Ocon B, Martinez-Moya P, Suarez MD, Zarzuelo A, Martinez-Augustin O, de Medina FS. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal Biochem. 2010;401:318–320. doi: 10.1016/j.ab.2010.02.036. [DOI] [PubMed] [Google Scholar]
- Roux F, Durieu-Trautmann O, Chaverot N, Claire M, Mailly P, Bourre JM, Strosberg AD, Couraud PO. Regulation of gamma-glutamyl transpeptidase and alkaline phosphatase activities in immortalized rat brain microvessel endothelial cells. J Cell Physiol. 1994;159:101–113. doi: 10.1002/jcp.1041590114. [DOI] [PubMed] [Google Scholar]
- Schmid-Brunclik N, Burgi-Taboada C, Antoniou X, Gassmann M, Ogunshola OO. Astrocyte responses to injury: VEGF simultaneously modulates cell death and proliferation. Am J Physiol Regul Integr Comp Physiol. 2008;295:R864–R873. doi: 10.1152/ajpregu.00536.2007. [DOI] [PubMed] [Google Scholar]
- Shee WL, Ong WY, Lim TM. Distribution of perlecan in mouse hippocampus following intracerebroventricular kainate injections. Brain Res. 1998;799:292–300. doi: 10.1016/s0006-8993(98)00490-9. [DOI] [PubMed] [Google Scholar]
- Song L, Pachter JS. Culture of murine brain microvascular endothelial cells that maintain expression and cytoskeletal association of tight junction-associated proteins. In Vitro Cell Dev Biol Anim. 2003;39:313–320. doi: 10.1290/1543-706X(2003)039<0313:COMBME>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Soulez M, Sirois I, Brassard N, Raymond MA, Nicodeme F, Noiseux N, Durocher Y, Pshezhetsky AV, Hebert MJ. Epidermal growth factor and perlecan fragments produced by apoptotic endothelial cells co-ordinately activate ERK1/2-dependent antiapoptotic pathways in mesenchymal stem cells. Stem Cells. 2010;28:810–820. doi: 10.1002/stem.403. [DOI] [PubMed] [Google Scholar]
- Tauskela JS, Mealing G, Comas T, Brunette E, Monette R, Small DL, Morley P. Protection of cortical neurons against oxygen-glucose deprivation and N-methyl-D-aspartate by DIDS and SITS. Eur J Pharmacol. 2003;464:17–25. doi: 10.1016/s0014-2999(03)01371-2. [DOI] [PubMed] [Google Scholar]
- Thornton P, McColl BW, Greenhalgh A, Denes A, Allan SM, Rothwell NJ. Platelet interleukin-1alpha drives cerebrovascular inflammation. Blood. 2010;115:3632–3639. doi: 10.1182/blood-2009-11-252643. [DOI] [PubMed] [Google Scholar]
- Thornton P, Pinteaux E, Allan SM, Rothwell NJ. Matrix metalloproteinase-9 and urokinase plasminogen activator mediate interleukin-1-induced neurotoxicity. Mol Cell Neurosci. 2008;37:135–142. doi: 10.1016/j.mcn.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Trebec-Reynolds DP, Voronov I, Heersche JN, Manolson MF. IL-1alpha and IL-1beta have different effects on formation and activity of large osteoclasts. J Cell Biochem. 2010;109:975–982. doi: 10.1002/jcb.22476. [DOI] [PubMed] [Google Scholar]
- Vikman P, Ansar S, Henriksson M, Stenman E, Edvinsson L. Cerebral ischemia induces transcription of inflammatory and extracellular-matrix-related genes in rat cerebral arteries. Exp Brain Res. 2007;183:499–510. doi: 10.1007/s00221-007-1062-5. [DOI] [PubMed] [Google Scholar]
- Webersinke G, Bauer H, Amberger A, Zach O, Bauer HC. Comparison of gene expression of extracellular matrix molecules in brain microvascular endothelial cells and astrocytes. Biochem Biophys Res Commun. 1992;189:877–884. doi: 10.1016/0006-291x(92)92285-6. [DOI] [PubMed] [Google Scholar]
- Weksler BB, Subileau EA, Perriere N, et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19:1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- Wright S, Parham C, Lee B, et al. Perlecan domain V inhibits alpha2 integrin-mediated amyloid-beta neurotoxicity. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.10.018. [DOI] [PubMed] [Google Scholar]