Abstract
Objective
Nascent high-density lipoprotein (HDL) particles form from cellular lipids and extracellular lipid-free apolipoprotein AI (apoAI) in a process mediated by ATP-binding cassette transporter A1 (ABCA1). We have sought out compounds that inhibit nascent HDL biogenesis without affecting ABCA1 activity.
Methods and Results
Reconstituted HDL (rHDL) formation and cellular cholesterol efflux assays were used to show that two compounds that bond via hydrogen with phospholipids inhibit rHDL and nascent HDL production. In rHDL formation assays, the inhibitory effect of compound 1 (methyl 3α-acetoxy-7α,12α-di[(phenylaminocarbonyl)amino]-5β-cholan-24-oate), the more active of the two, depended on its ability to associate with phospholipids. In cell assays, compound 1 suppressed ABCA1-mediated cholesterol efflux to apoAI, the 18A peptide, and taurocholate with high specificity, without affecting ABCA1-independent cellular cholesterol efflux to HDL and endocytosis of acetylated low-density lipoprotein (AcLDL) and transferrin. Furthermore, compound 1 did not affect ABCA1 activity adversely, as ABCA1-mediated shedding of microparticles proceeded unabated and apoAI binding to ABCA1-expressing cells increased in its presence.
Conclusions
The inhibitory effects of compound 1 support a three-step model of nascent HDL biogenesis: plasma membrane remodeling by ABCA1, apoAI binding to ABCA1, and lipoprotein particle assembly. The compound inhibits the final step, causing accumulation of apoAI in ABCA1-expressing cells.
Keywords: nascent HDL, rHDL, ABCA1, apoAI, reverse cholesterol transport
Introduction
Biogenesis of nascent high-density lipoprotein (HDL) particles marks the origins of the critically important antiatherogenic reverse cholesterol transport (RCT) pathway, which removes excessive cholesterol from peripheral tissues, such as macrophages, and carries it to the liver.1 Nascent HDL particles form from extracellular lipid-free apolipoprotein AI (apoAI) and cellular lipids in a process mediated by ATP-binding cassette transporter A1 (ABCA1).2 In vitro studies suggest that the process of nascent HDL biogenesis consists of at least three steps. The first step is apparent when cells express ABCA1, but apoAI is not present in the medium. Even without the apolipoprotein, the transporter creates significant changes in the plasma membrane organization. In particular, it induces redistribution of phosphatidylserine (PS) to the cell surface and drives production of apoAI-free microparticles.3–5 Upon addition of apoAI to ABCA1-expressing cells, the apolipoprotein rapidly binds to the plasma membrane, and newly formed nascent HDL particles appear in the medium at a detectable level in 15 minutes.6 At 21°C, apoAI still binds to ABCA1-expressing cells, but formation of nascent HDL particles completely ceases.6 The difference in sensitivity to temperature suggests that apoAI binding to the ABCA1-remodeled plasma membrane and apoAI and lipid assembly into lipoprotein particles are distinct – second and third, respectively – steps of nascent HDL biogenesis.
In addition to nascent HDL, apoAI can form reconstituted HDL (rHDL) particles in the absence of ABCA1 from liposomes made of synthetic short-chain phospholipids or certain physiologically-relevant lipid mixtures.7,8 rHDL particles are similar in size and shape to nascent HDL. For a large group of apoAI mutants with widely divergent abilities to form rHDL and nascent HDL, the efficiency of rHDL formation positively correlates with the efficiency of nascent HDL biogenesis.8 This suggests that the two processes – i.e., apoAI and synthetic lipid assembly into rHDL and apoAI and cell lipid assembly into nascent HDL (step 3 in nascent HDL biogenesis) – share substantial mechanistic similarities.
In order to unambiguously show the sequential nature of nascent HDL biogenesis, we have sought out chemicals that inhibit rHDL and nascent HDL formation without affecting ABCA1 activity. Our attention was drawn to a group of compounds called synthetic chemical phospholipid translocases/scramblases, which had been designed to form hydrogen bonds with the phosphate residue and carboxyl group of phospholipids.9,10 The original purpose of these chemicals was to facilitate phospholipid trans-bilayer flip-flop by concealing the negative charges inside a hydrophilic pocket.11 As phospholipid translocases, these compounds turned out to be ineffective in nucleated cells.12 Nonetheless, we hypothesized that large hydrogen-bonded translocase-phospholipid complexes could interfere with the apoAI-lipid assembly into lipoprotein particles. Here, we show that a representative member of the translocase group, methyl 3α-acetoxy-7α,12α-di[(phenylaminocarbonyl)amino]-5β-cholan-24-oate (referred to in the following as compound 1), inhibits rHDL and nascent HDL formation, causes accumulation of apoAI in ABCA1-expressing cells and thus resolves the final stages of nascent HDL biogenesis into individual steps in cultured cells under normal physiological conditions.
Methods
Methyl 3α-acetoxy-7α,12α-di[(phenylaminocarbonyl)amino]-5β-cholan-24-oate (compound 1) and N-[2-((4-nitrophenylaminocarbonyl)amino)ethyl)]-N,N-di[2-((4-methylphenylsulfonyl)amino)ethyl]amine (compound 2) were synthesized in-house as previously described.9,10,13 rHDL formation assays were performed by reacting dimyristoylphosphatidylcholine (DMPC) multilamellar vesicles (MLVs) and human apoAI in either Tris-buffered saline EDTA (TBS-ETDA, pH 7.4)7 or glycine-HCl (pH 3.0)14 buffer at ambient instrument temperature (24.3–25.6°C) in the presence of the vehicle (dimethyl sulfoxide, DMSO) or one of the compounds. DMPC MLV solubilization by apoAI was monitored by measuring sample turbidity (absorbance) at 325 nm. For steady-state 1,6-diphenyl-1,3,5-hexatriene (DPH) anisotropy measurements, DMPC MLVs spiked with DPH to 0.2 mole% were extruded 19 times through a polycarbonate membrane with 100 nm pores (Whatman) using a mini-extruder (Avanti Polar Lipids) to derive large unilamellar vesicles (LUVs); the LUVs were incubated with DMSO or one of the compounds for 30 min, followed by measurements of DPH anisotropy at 357 nm excitation and 427 nm emission wavelengths at a range of temperatures in a water jacketed spectrofluorimeter. A previously-described6 four-day protocol was followed to assess effects of the compounds on cellular cholesterol and phospholipid efflux to apoAI from cells either uninduced or induced to express ABCA1. The endocytosis assay was performed as the following: DMSO, compound 1 or dynasore15-pretreated cells were exposed to [3H]cholesterol-labeled acetylated low-density lipoprotein (AcLDL) for 15–20 min at either 37°C or 20°C in the presence of these same compounds, washed with an acidic buffer (0.15 mol/L NaCl, pH 3.0) and extracted with hexane/isopropanol. AcLDL endocytosis was calculated by subtracting the 3H cell associated counts at 20°C from those at 37°C. For the microparticle production assay, cells were labeled with [3H]cholesterol, treated with or without cAMP to induce ABCA1, pretreated with DMSO, compound 1 or wheat germ agglutinin (WGA)/DMSO4 for one hour and further exposed to the same treatment for eight hours. Then, cells and media were collected and analyzed for 3H counts as in the cholesterol efflux assay; and, microparticle production was expressed as the percentage of cellular [3H]cholesterol released to media without any lipid acceptors. Fluorescent microscopy has been previously described.6 Detailed protocols of all methods are included in the Supplemental Material.
Results
Compound 1 and 2 inhibit rHDL formation from DMPC liposomes
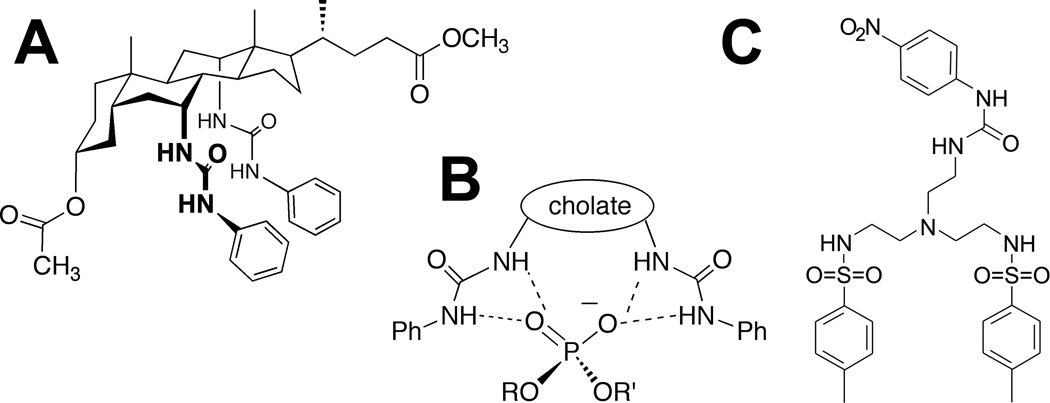
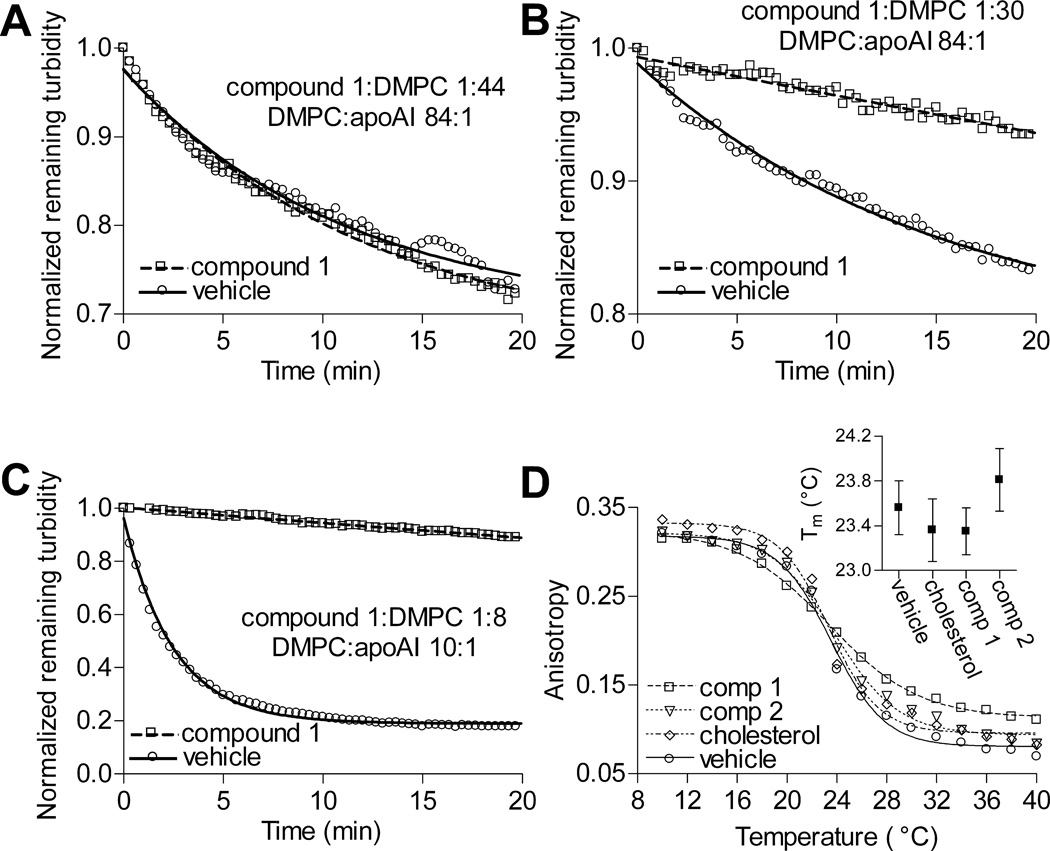
Compound 1 is a derivative of cholate in which the hydroxyl groups at positions C-7 and C-12 are replaced with phenylurea groups for hydrogen bonding with the phosphate residue of phospholipids (Figure 1A and 1B).10 At the DMPC phase transition temperature (Tm), DMPC MLVs and apoAI spontaneously and rapidly form rHDL particles.7 Because MLVs are turbid, while rHDL particles are pellucid, rHDL assembly from MLVs can be monitored by measuring the remaining MLV turbidity.7,16 To determine whether compound 1 affects rHDL formation, DMPC MLVs were incubated with the compound for 30 min to allow it to enter the bilayer, followed by addition of apoAI and immediate commencement of turbidity measurements. The concentration of compound 1 was kept constant at a level (8.3 µmol/L) below its critical micelle concentration to ensure that compound 1 micelles did not contribute to sample turbidity. While the DMPC:apoAI molar ratio was also kept constant at a value (84:1) at which rHDL formation proceeds to completion,16 the absolute concentrations of DMPC and apoAI were varied to achieve different compound 1:DMPC and compound 1:apoAI molar ratios. At compound 1:DMPC molar ratios 1:60 and 1:44 (compound 1:apoAI molar ratios 1:0.71 and 1:0.53, respectively), compound 1 did not affect rHDL formation (Figure 2A and data not shown); however, at compound 1:DMPC molar ratios 1:30, 1:15 and 1:7.4 (compound 1:apoAI molar ratios 1:0.35, 1:0.18 and 1:0.09, respectively), the chemical markedly inhibited the process (Figure 2B and data not shown). Circular dichroism measurements showed that there were only minor differences in the structure of apoAI in the presence of compound 1 at the ineffective compound 1:apoAI molar ratio of 1:44 and the inhibitory compound 1:apoAI molar ratio of 1:30 (apoAI was 50.6 and 53.7 percent helix, respectively), suggesting that the compound 1did not alter apoAI secondary structure. Furthermore, when the compound 1:DMPC molar ratio was 1:8, i.e., within the range in which compound 1 inhibited rHDL formation, while the compound 1:apoAI molar ratio was changed to 1:0.8, i.e., outside of the effective range of the compound, the compound still suppressed rHDL formation (Figure 2C). The latter observation suggests that the inhibitory potency of compound 1 depends on its stoichiometric ratio with DMPC and not with apoAI and thus, that the compound likely impairs the ability of the lipid and not the apolipoprotein to form rHDL.
Figure 1.
Chemical structures of compound 1 and 2. A, Chemical structure of compound 1. B, Hydrogen bonding between compound 1 and the phosphate residue of the phospholipid. C, Chemical structure of compound 2.
Figure 2.
Inhibition of rHDL formation by compound 1. A, At the compound 1:DMPC molar ratio 1:44, compound 1 did not have an effect on rHDL formation. B, However, at the compound 1:DMPC molar ratio 1:30, the chemical suppressed the reaction. C, Compound 1 also vigorously suppressed rHDL formation when the compound 1:DMPC molar ratio was high, but the compound 1:apoAI ratio was low. D and Insert, Addition of compound 1 or 2 to DMPC did not significantly change the Tm of the phospholipid. DPH anisotropy data points were fitted with sigmoidal curves to derive phase transition temperatures. Error bars represent 95% confidence limits.
Compound 2 (Figure 1C), a derivative of tris(2-aminoethyl)amine, carries two arylsulfonamide groups and one nitrophenylurea group that can bond via hydrogen with the phosphate residue of phospholipids.9 This compound partitions less readily to the lipid bilayer in comparison with compound 1.10 At compound 2:DMPC molar ratio 1:8 (DMPC:apoAI molar ratio 84:1), compound 2 also inhibited rHDL formation, but less effectively than compound 1 (Supplemental Figure I).
The rate of rHDL formation declines precipitously as the reaction temperature deviates above or below the phospholipid Tm.7 Temperature-dependent changes in steady-state anisotropy of DPH were measured to determine whether addition of compound 1 or 2 affects the Tm of DMPC. DMPC LUVs spiked with DPH were incubated with one of the compounds, cholesterol (inactive control) or the vehicle for 30 min at ambient temperature to allow the compounds and cholesterol to enter the bilayer, followed by DPH anisotropy measurements. The compound/cholesterol:DMPC molar ratio was 1:7.4. The calculated Tm for pure DMPC was 23.6°C, a value similar to those previously published.17 Neither compound significantly shifted the Tm of DMPC toward higher or lower temperatures (Figure 2D). The above observations suggest that compound 1 and 2 suppress rHDL formation by acting on the phospholipid without affecting its Tm.
Hydrogen-bonding between compound 1 and DMPC is essential for inhibition of rHDL formation
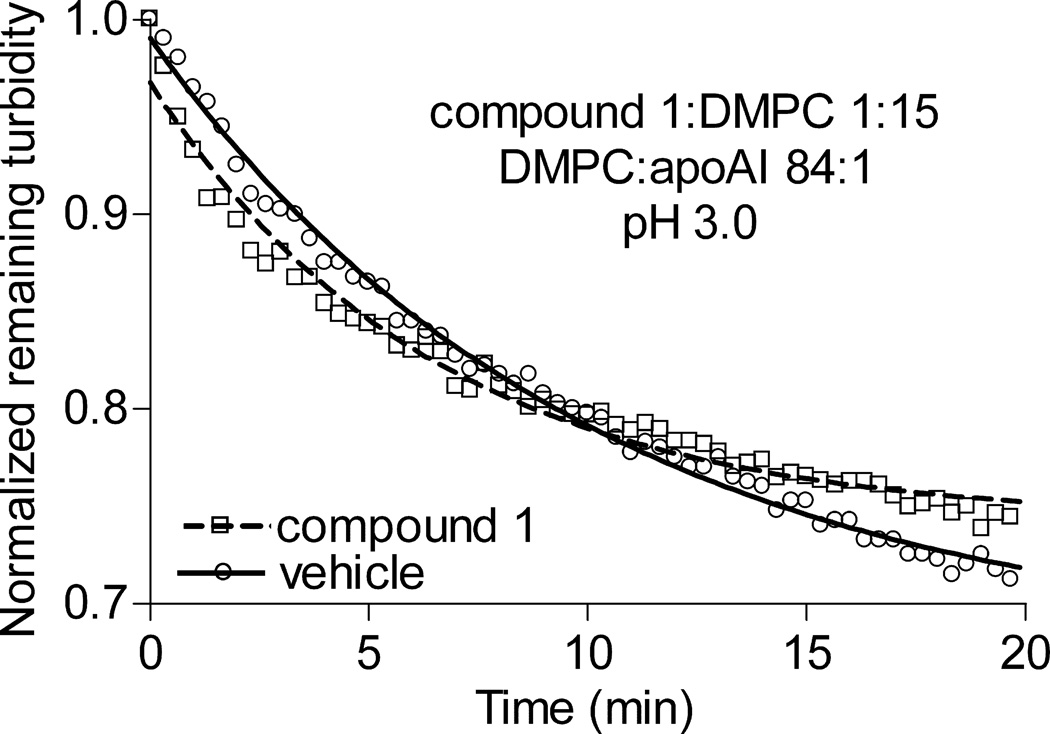
The finding that compounds 1 and 2, which are structurally very divergent, both suppress rHDL formation suggests that hydrogen-bonding with phospholipids – i.e., the intended activity of these chemicals11 – in all probability is responsible for the compounds’ inhibitory action. To further test this conjecture, rHDL formation assays were carried out at pH 3.0, which is below the apparent pKa of 3.5 for the phosphate group of phosphatidylcholine.18 DMPC MVLs and apoAI form rHDL particles just as readily at a low as at a neutral pH.14 However, at pH 3.0 most DMPC molecules would be protonated at the phosphate residue and unable to form hydrogen bonds. Compound 2 is not suitable for this experiment, because it undergoes protonation at the tertiary amine with the pKa of 5.0 and assumes a conformation that binds phospholipids poorly.19 Compound 1 can undergo protonation at the carbonyl oxygen of the urea groups; however, the pKa of protonated urea is close to zero, i.e., much lower than the pH of 3.0.20 Furthermore, it was fortuitously discovered that compound 1 alters the excitation spectrum of DPH, without altering its anisotropic behavior (Supplemental Figure II). DPH is a small cylindrical molecule that resides in the hydrophobic middle of the bilayer.21 In order to interact with DPH, compound 1 must penetrate into the hydrophobic middle as well. At pH 7.4 and 3.0, compound 1 affected DPH excitation spectrum identically, indicating that the low pH did not hinder partitioning of the compound to the lipid bilayer (Supplemental Figure II). At the compound 1:DMPC molar ratio 1:15 (a ratio within the effective range of the compound) and pH 3.0, compound 1 failed to suppress rHDL formation (Figure 3). This suggests that hydrogen bonding between compound 1 and DMPC is essential for suppression of the reaction.
Figure 3.
Compound 1 does not inhibit rHDL formation at pH 3.0. At the compound 1: DMPC molar ratio 1:15 and pH 3.0, compound 1did not have an effect on rHDL formation.
Compound 1 and 2 inhibit specifically nascent HDL formation
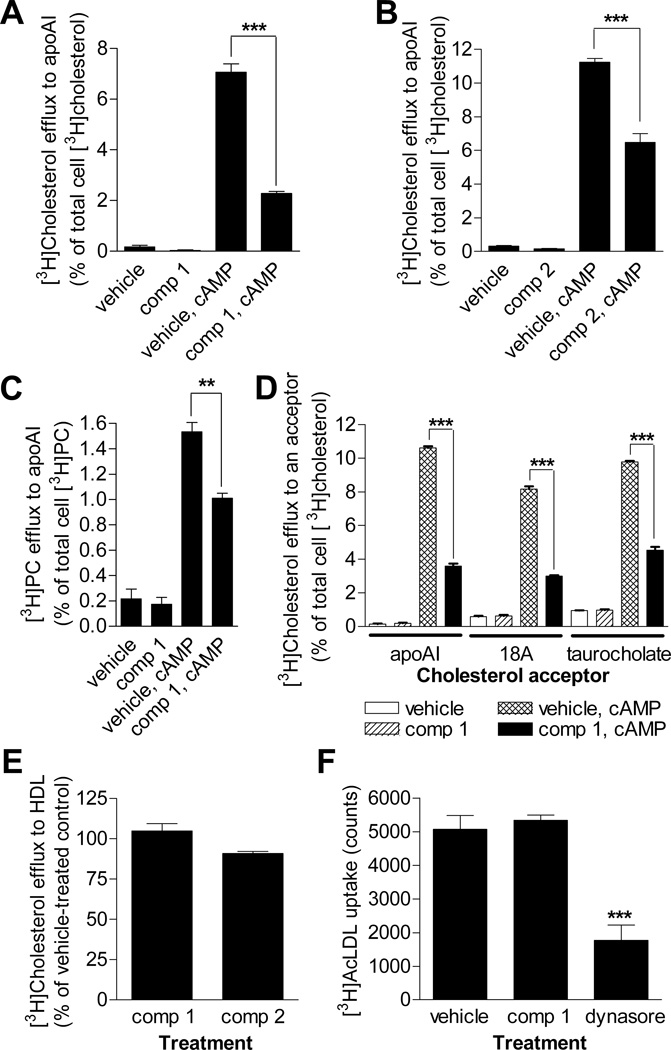
RAW 264.7 murine macrophage cells generate nascent HDL in the presence of exogenous lipid-free apoAI after a treatment with a cAMP analog to induce ABCA1 expression.6 Because cholesterol and phospholipids are incorporated into the lipoprotein concurrently, nascent HDL biogenesis can be readily measured as the percentage of total cell cholesterol or phospholipid that is released to apoAI when ABCA1 is expressed.6 As is evident from the lack of effect on receptor-mediated endocytosis (see below), compound 1 is not toxic to cells at the concentrations used in this study. Compound 2 also does not exhibit cell toxicity at the doses used (LD50 > 100 µmol/L).12 To determine whether compound 1 and 2 inhibit nascent HDL biogenesis, RAW 264.7 cells labeled with [3H]cholesterol and induced to express ABCA1 were pretreated with 10 µmol/L compound 1 for 1 hour or 30 µmol/L compound 2 for 3 hours; thereafter, the pretreatment medium was replaced with fresh medium that contained lipid-free apoAI and the same compound at the same concentration as during the pretreatment, and the cells were allowed to produce nascent HDL for 4 hours. Compound 1 suppressed ABCA1-mediated cholesterol efflux to apoAI by over 70% (Figure 4A). Even through applied at a higher concentration during a longer pretreatment, compound 2 was less effective and suppressed the process by only approximately 50% (Figure 4B). The lower efficacy of compound 2 in this assay is in line with its lower efficacy in the rHDL formation assays (Supplemental Figure I). In addition to RAW 264.7 cells, compound 1 applied at the same concentration with the same treatment scheme significantly inhibited ABCA1-mediated cholesterol efflux to apoAI in BHK-ABCA1 and HEK293-ABCA1 cells (Supplemental Figure III). The 1 hour 10 µmol/L treatment with compound 1 suppressed ABCA1-mediated phospholipid efflux to apoAI in RAW 264.7 cells by approximately 37% (Figure 4C). Nascent HDL particles arise as several species with different lipid composition.22 The greater effect of compound 1 on cholesterol than phospholipid efflux suggests that this inhibitor may restrict production of cholesterol-richer species to a greater degree.
Figure 4.
Compound 1 and 2 suppress nascent HDL formation with high specificity in RAW 264.7 cells. A and B, Inhibition of ABCA1-mediated cholesterol efflux to apoAI by compound 1 (A) and 2 (B). C, Inhibition of ABCA1-mediated phospholipid efflux to apoAI by compound 1. D, Compound 1 inhibits ABCA1-mediated cholesterol efflux irrespective of cholesterol acceptor. Concentrations of apoAI and 18A were 5 µg/mL; sodium taurocholate was applied at 4 mmol/L. Compounds 1 and 2 were applied at 10 and 30 µmol/L, respectively, with a 1 and 3 hour, respectively, pretreatment. 8Br-cAMP (0.3 mmol/L) was used to induce ABCA1 expression. The percentage of [3H]cholesterol efflux to an acceptor was calculated by subtracting the percentage of [3H]cholesterol efflux to medium without this acceptor from the percentage of [3H]cholesterol efflux to medium with the acceptor. Mean ± S.D.; *** - p<0.001 by t-test. E, Compound 1 and 2 applied at 10 and 30 µmol/L, respectively, with a 1 or 3 hour, respectively, pretreatment to RAW 264.7 cells did not have an effect on cellular cholesterol efflux to HDL. The percentage of ABCA1-independent [3H]cholesterol efflux to HDL was calculated by subtracting the percentage of efflux to medium from the percentage of efflux to HDL. The percentage of [3H]cholesterol efflux to HDL under the vehicle treatment was set to 100%. Mean ± S.D.; p>0.5 by t-test. F, Compound 1 at 10 µmol/L with a pretreatment did not alter AcLDL endocytosis in RAW 264.7 cells. Dynasore (160 µmol/L), an inhibitor of dynamin,15 was used as a positive control. Mean ± S.D.; *** - p<0.001 vs. other treatments by ANOVA with Dunnet's multiple comparison posttest.
A dose-response curve for the suppression of ABCA1-mediated cholesterol efflux to apoAI by compound 1 was derived to identify the threshold compound 1:cellular phospholipid molar ratio at which the inhibitor loses its efficacy (Supplemental Figure IV). Based on the previous observations that compound 1 readily enters the cell and freely disperses among the subcellular membranes,12 it was assumed that all cellular phospholipid was available for bonding with the inhibitor. The dosage of compound 1 was expressed as the ratio of moles of this inhibitor added per cell culture well to moles of cell phospholipid in the same well. Compound 1’s efficacy remained essentially unchanged at the compound 1:cellular phospholipid molar ratios from 1:3.4 (10 µM compound 1 in 500 µl of medium per well) to 1:19 (3 µM compound 1 in 300 µl of medium per well), but began declining at the ratios lower than 1:19 (Supplemental Figure IV D). Given the drastic differences between the systems, the threshold compound 1: phospholipid molar ratios at which compound 1 loses its efficacy in DMPC liposomes (1:30) and in cells (1:19) are remarkably similar.
In addition to apoAI, ABCA1 mediates cholesterol efflux to peptide 18A (also called 2F), a small amphipathic peptide,23 and sodium taurocholate.24 At the concentration of 10 µmol/L with a pretreatment, compound 1 suppressed ABCA1-mediated cholesterol efflux to 5 µg/mL 18A, 4 mmol/L taurocholate and 5 µg/mL apoAI to a similar extent (Figure 4D). This shows that the nature of cholesterol acceptor is irrelevant to the inhibitory action of the compound.
RAW 264.7 cells in the absence of ABCA1 induction release cholesterol to HDL.25 The treatment schemes with compound 1 and 2 that dramatically suppressed ABCA1-mediated cholesterol efflux to apoAI did not significantly affect ABCA1-independent efflux of this lipid to HDL (Figure 4E). Effects of compound 1 on receptor-mediated endocytosis of AcLDL and transferrin were also assessed. At the concentration of 10 µmol/L with a pretreatment, compound 1 did not block uptake of either ligand, while the endocytosis inhibitor dynasore15 markedly suppressed AcLDL uptake (Figure 4F and Supplemental Figure V). Finally, compound 1 did not affect ABCA1 expression levels at concentrations sufficient to suppress ABCA1-mediated cholesterol efflux to apoAI (Supplemental Figure VI). Cumulatively, the above observations suggest that compound 1 specifically inhibits nascent HDL biogenesis by acting on cell membranes that are remodeled by ABCA1.
Compound 1 does not affect ABCA1-driven generation of microparticles
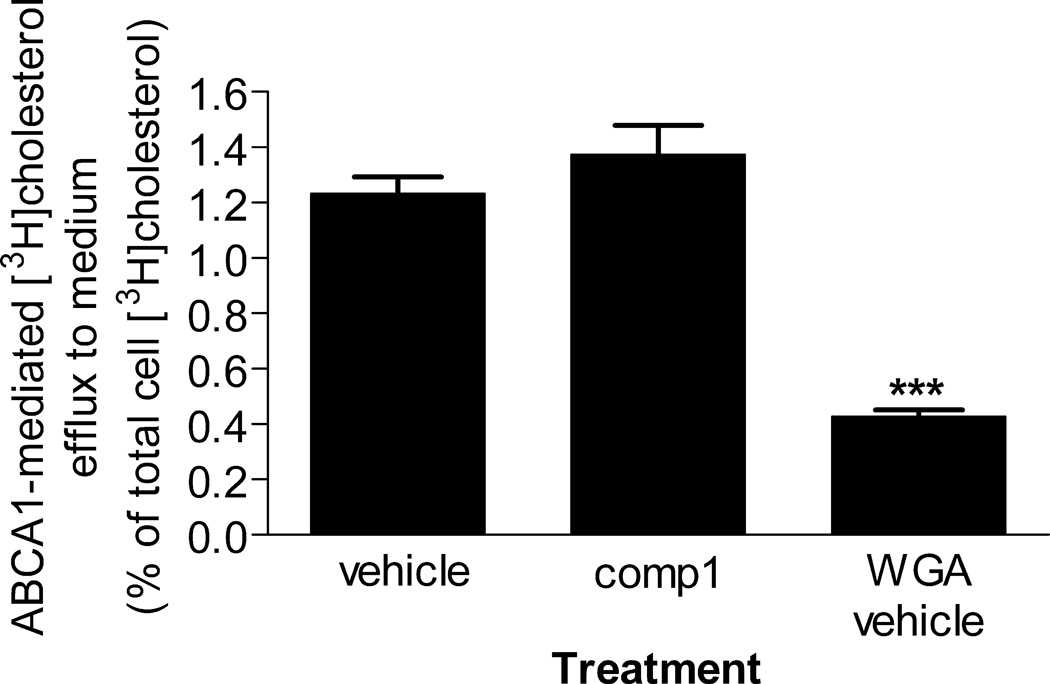
ABCA1 renders the plasma membrane less rigid and induces exposure on the cell surface of plasma membrane PS, which is normally sequestered in the inner leaflet of the organelle.3,4 These ABCA1-driven changes in the plasma membrane organization promote budding of a certain species of microparticles irrespective of presence or absence of apoAI.4 At the concentration of 10 µmol/L with a pretreatment, compound 1 did not affect microparticle formation (Figure 5). In contrast, in line with previous observations,4 WGA significantly inhibited microparticle biogenesis. Using ABCA1-driven production of microparticles as a proxy for ABCA1 activity, this observation suggests that compound 1 does not upset the proper functioning of the transporter.
Figure 5.
Compound 1 does not affect ABCA1-driven production of microparticles. ABCA1-mediated efflux of cellular [3H]cholesterol to the medium in the absence of apoAI was taken as a measure of microparticle production. To calculate ABCA1-specific efflux, the percentage of [3H]cholesterol efflux from cells not induced to express ABCA1 was subtracted from the percentage of [3H]cholesterol efflux from ABCA1-expressing cells. WGA was used as a positive control known to reduce microparticle production.4 Mean ± S.D.; *** - p<0.001 by t-test.
Compound 1 promotes retention of bound apoAI in ABCA1-expressing cells
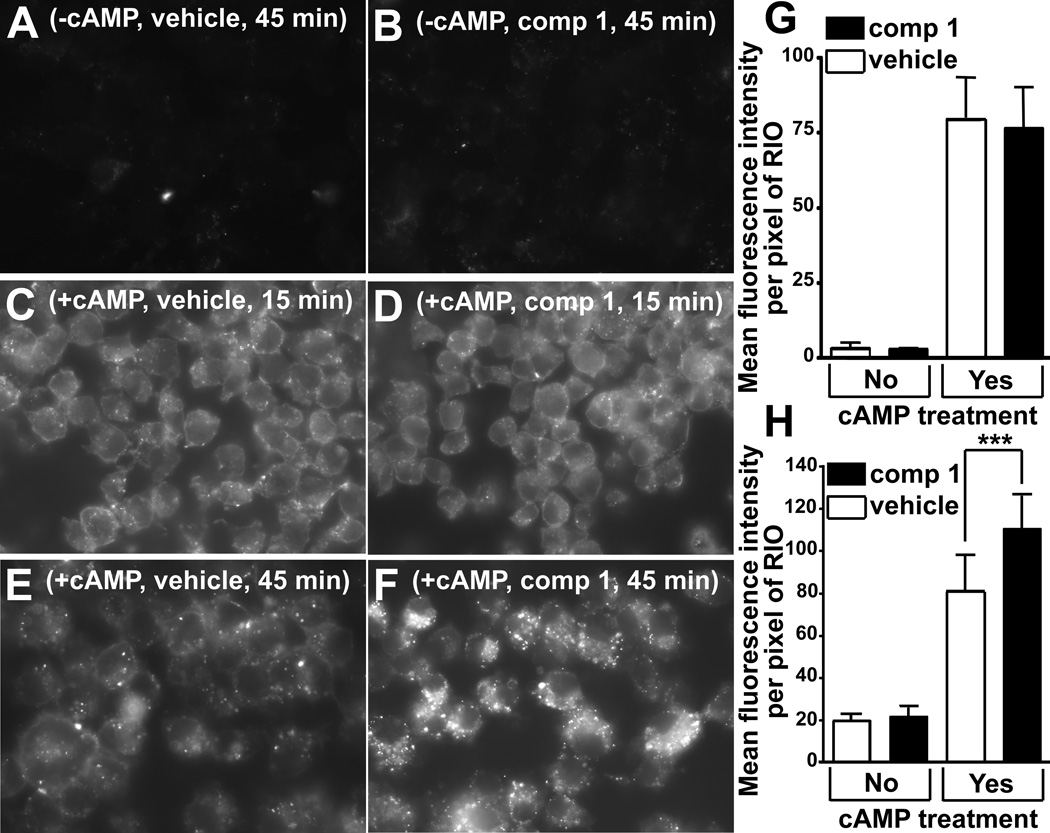
Lipid-free apoAI binds to the plasma membrane of ABCA1-expressing cells prior to the appearance of nascent HDL in the medium.6 A fraction of cell-bound apoAI is further taken up into subcellular compartments.26 To determine whether compound 1 affects the apoAI binding step, RAW 264.7 cells induced to express ABCA1 were pretreated with the compound at 10 µmol/L for 1 hour and thereafter exposed to fluorescently-labeled apoAI and simultaneously treated with compound 1 for 15 or 45 min. A detectable level of nascent HDL first appears in the medium of ABCA1-expressing cells about 15 min after addition of the apolipoprotein.6 Compound 1 and vehicle-treated ABCA1-expressing cells bound and internalized similar amounts of the labeled apoAI after a 15 min incubation period (Figure 6C, 6D, 6G). However, after a 45 min incubation with the labeled apoAI, compound 1-treated cells bound and internalized significantly more of the label than vehicle-treated cells (Figure 6E, 6F and 6H). Furthermore, after the 15 min incubation, the amount of punctate fluorescence in subcellular compartments appeared to be similar in the compound treated and untreated cells (Figure 6C and 6D). But after the 45 min incubation – 30 min after the onset of apoAI release, compound 1-treated cells appeared to contain more and brighter subcellular fluorescent foci (Figure 6E and 6F). Complex formation between ABCA1 and apoAI is required for nascent HDL biogenesis and for partial protection of the transporter from degradation in the intracellular compartment.27,28 At 10 µmol/L with a pretreatment, compound 1 did not suppress cross-linking between ABCA1 and apoAI, suggesting it did not interfere with the complex formation (Supplemental Figure VII). The above observations indicate that compound 1 does not affect apoAI binding to ABCA1-expressing cells or to ABCA1, but, by shutting down formation of nascent HDL and thus halting release of cell-bound apoAI, promotes accumulation of the apolipoprotein in subcellular compartments.
Figure 6.
Compound 1 promotes retention of apoAI bound to ABCA1-expressing cells. A and B, RAW 264.7 cells not induced to express ABCA1 did not bind Alexa Fluor 568 labeled apoAI appreciably. C and D, Cells induced to express ABCA1 and incubated with Alexa Fluor 568 labeled apoAI for 15 min bound and internalized the apolipoprotein robustly regardless of the treatment with compound 1. E and F, Compound 1-treated cells incubated with labeled apoAI for 45 min bound and internalized more of the apolipoprotein than vehicle-treated cells exposed to apoAI for the same duration. G and H, Mean pixel fluorescent intensity within a cell/region of interest calculated from the images represented in A–F (30–40 cells per treatment). G, 15 minute incubation; H, 45 minute incubation; mean ± S.D.; *** - p<0.001 by t-test.
Discussion
The effects of compound 1 on nascent HDL biogenesis are consistent with a three-step model of this process (Supplemental Figure VIII). The first step entails remodeling of the plasma membrane by ABCA1.3 In the second step apoAI specifically binds to ABCA1, as is evident from the apoAI-ABCA1 cross-linking studies,27 and is then brought in contact with the remodeled membrane regions. In vivo these two steps likely occur simultaneously. The third step is the spontaneous assembly of the remodeled-region lipids and apoAI into the nascent HDL lipoprotein particle.
ABCA1-mediated membrane remodeling
One feature of ABCA1 remodeling of the plasma membrane is increased cell surface PS exposure, although cell surface PS by itself does not support lipid efflux to apoAI.5 A consequence of ABCA1 remodeling of the plasma membrane is the apolipoprotein-independent release of microparticles.4 Our data show that compound 1 does not impair this function and thus, does not alter the first step of our three-step model. Some particulars of ABCA1-mediated cholesterol efflux to taurocholate suggest that only certain membrane regions – likely those in the vicinity of the transporter – are remodeled. The ability of taurocholate to solubilize bilayers depends on its concentration and on bilayer stability.29 4 mmol/L taurocholate can induce cholesterol efflux (a proxy for membrane solubilization) only when ABCA1 is expressed (Figure 4D); however, 10 mmol/L taurocholate promotes robust ABCA1-independent cholesterol efflux (data not shown). This suggests that ABCA1 renders some regions of the plasma membrane less stable and more susceptible to the bile acid, relative the bulk of the organelle. Compound 1 may accumulate in these remodeled regions and protect the bilayer from low but not high concentrations of taurocholate.
ApoAI binding to ABCA1-expressing cells
ApoAI is readily cross-linked to ABCA1.2 Furthermore, those apoAI mutants that exhibit high affinity for the transporter in the cross-linking assay are also very efficient at formation of nascent HDL.27 A recent study reported that a large portion of cell-bound apoAI cannot be cross-linked to the transporter.30 This observation is consistent with the mechanism in which apoAI first binds to ABCA1 and thereafter associates with ABCA1-modified lipid sites, where it briefly resides before forming nascent HDL. Unimpaired binding of apoAI to ABCA1-expressing cells and to ABCA1 in the presence of compound 1 suggests that the compound does not interfere with this step of nascent HDL biogenesis.
Assembly of apoAI and cellular membrane lipids into nascent HDL
The final step in HDL biogenesis is the assembly of cell-bound apoAI and membrane lipids into nascent HDL particles and particle release from the cell. Several lines of evidence suggest that compound 1 inhibits this final step. First, as outlined above, the first two steps are not impaired by the compound. Second, the bilayer stabilizing activity of compound 1 that protects membranes against solubilization by taurocholate would also likely protect the same membranes against solubilization by apoAI. Third, accumulation of cell-bound apoAI in the presence of the compound indicates that the apolipoprotein fails to form nascent HDL. Fourth, compound 1 inhibits rHDL formation, demonstrating that it can interfere with apoAI and lipid assembly into lipoprotein particles. Solubilization of phosphatidylcholine liposomes by taurocholate begins with adsorption of the bile acid to the bilayer surface via interaction with the charged lipid headgroups.31 Compound 1 likely blocks taurocholate solubilizing activity at this first step by forming hydrogen bonds with the phosphate residue of glycerophospholipids and preventing the bile acid from electrostatically interacting with the lipid charges. Charge interactions are also critical for adsorption to the bilayer surface of cationic amphipathic peptides, such as 18A, and cationic amphipatic helix-containing proteins, such as apoAI.32,33 It is likely that hydrogen bonding of compound 1 to glycerophospholipids forestalls electrostatic interactions between ABCA1-recruited apoAI and ABCA1-remodeled plasma membrane regions.
To our best knowledge, compound 1 is the first known chemical that inhibits both rHDL formation and the lipoprotein assembly step of nascent HDL biogenesis. The finding that the two are susceptible to the same compound (as well as to compound 2) strongly suggests they are mechanistically similar. This implies that after ABCA1 prepares a region of the plasma membrane and brings apoAI in close apposition to it, the apolipoprotein associates with the bilayer without further assistance from the transporter. Thus, rHDL formation is a faithful model of the assembly step of nascent HDL biogenesis. Compound 1 is a very useful tool for investigations of rHDL and nascent HDL assembly. Future research with this compound may aid in further elucidation of the molecular mechanism of apoAI-driven lipoprotein production.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by the NIH grants HL66082 and HL098055 to JDS and the American Heart Association postdoctoral fellowship 10POST3630047 to NNL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Cuchel M, Rader DJ. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation. 2006;113:2548–2555. doi: 10.1161/CIRCULATIONAHA.104.475715. [DOI] [PubMed] [Google Scholar]
- 2.Yvan-Charvet L, Wang N, Tall AR. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol. 2010;30:139–143. doi: 10.1161/ATVBAHA.108.179283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamon Y, Broccardo C, Chambenoit O, Luciani MF, Toti F, Chaslin S, Freyssinet JM, Devaux PF, McNeish J, Marguet D, Chimini G. ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nat Cell Biol. 2000;2:399–406. doi: 10.1038/35017029. [DOI] [PubMed] [Google Scholar]
- 4.Nandi S, Ma L, Denis M, Karwatsky J, Li Z, Jiang XC, Zha X. ABCA1-mediated cholesterol efflux generates microparticles in addition to HDL through processes governed by membrane rigidity. J Lipid Res. 2009;50:456–466. doi: 10.1194/jlr.M800345-JLR200. [DOI] [PubMed] [Google Scholar]
- 5.Smith JD, Waelde C, Horwitz A, Zheng P. Evaluation of the role of phosphatidylserine translocase activity in ABCA1-mediated lipid efflux. J Biol Chem. 2002;277:17797–17803. doi: 10.1074/jbc.M201594200. [DOI] [PubMed] [Google Scholar]
- 6.Smith JD, Le Goff W, Settle M, Brubaker G, Waelde C, Horwitz A, Oda MN. ABCA1 mediates concurrent cholesterol and phospholipid efflux to apolipoprotein A-I. J Lipid Res. 2004;45:635–644. doi: 10.1194/jlr.M300336-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Jonas A. Reconstitution of high-density lipoproteins. Methods Enzymol. 1986;128:553–582. doi: 10.1016/0076-6879(86)28092-1. [DOI] [PubMed] [Google Scholar]
- 8.Vedhachalam C, Duong PT, Nickel M, Nguyen D, Dhanasekaran P, Saito H, Rothblat GH, Lund-Katz S, Phillips MC. Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-I and formation of high density lipoprotein particles. J Biol Chem. 2007;282:25123–25130. doi: 10.1074/jbc.M704590200. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki Y, Shukla R, Smith BD. Facilitated phosphatidylserine flip-flop across vesicle and cell membranes using urea-derived synthetic translocases. Org Biomol Chem. 2004;2:214–219. doi: 10.1039/b314006g. [DOI] [PubMed] [Google Scholar]
- 10.Lambert TN, Boon JM, Smith BD, Pérez-Payán MN, Davis AP. Facilitated phospholipid flip-flop using synthetic steroid-derived translocases. J Am Chem Soc. 2002;124:5276–5277. doi: 10.1021/ja026022y. [DOI] [PubMed] [Google Scholar]
- 11.Boon JM, Smith BD. Synthetic membrane transporters. Curr Opin Chem Biol. 2002;6:749–756. doi: 10.1016/s1367-5931(02)00399-x. [DOI] [PubMed] [Google Scholar]
- 12.DiVittorio KM, Hofmann FT, Johnson JR, Abu-Esba L, Smith BD. Facilitated phospholipid translocation in vesicles and nucleated cells using synthetic small molecule scramblases. Bioorg Med Chem. 2009;17:141–148. doi: 10.1016/j.bmc.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boon JM, Lambert TN, Sisson AL, Davis AP, Smith BD. Facilitated phosphatidylserine (PS) flip-flop and thrombin activation using a synthetic PS scramblase. J Am Chem Soc. 2003;125:8195–8201. doi: 10.1021/ja029670q. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda M, Nakano M, Miyazaki M, Tanaka M, Saito H, Kobayashi S, Ueno M, Handa T. Conformational change of apolipoprotein A-I and HDL formation from model membranes under intracellular acidic conditions. J Lipid Res. 2008;49:2419–2426. doi: 10.1194/jlr.M800287-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Massey JB, Pownall HJ. Cholesterol is a determinant of the structures of discoidal high density lipoproteins formed by the solubilization of phospholipid membranes by apolipoprotein A-I. Biochim Biophys Acta. 2008;1781:245–253. doi: 10.1016/j.bbalip.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonas A, Mason WR. Interactions of dipalmitoyl- and dimyristoylphosphatidylcholines and their mixtures with apolipoprotein A-I. Biochemistry. 1981;20:3801–3805. doi: 10.1021/bi00516a020. [DOI] [PubMed] [Google Scholar]
- 18.Tatulian SA. Ionization and ion binding. In: Cevc G, editor. Phospholipids handbook. New York, New York: Marcel Dekker; 1993. pp. 511–552. [Google Scholar]
- 19.Boon JM, Lambert TN, Smith BD, Beatty AM, Ugrinova V, Brown SN. Structure/activity study of tris(2-aminoethyl)amine-derived translocases for phosphatidylcholine. J Org Chem. 2002;67:2168–2174. doi: 10.1021/jo016416s. [DOI] [PubMed] [Google Scholar]
- 20.Wen N, Brooker MH. Urea protonation: Raman and theoretical study. J Phys Chem. 1993;97:8608–8616. [Google Scholar]
- 21.Lentz BR. Use of fluorescent probes to monitor molecular order and motions within liposome bilayers. Chem Phys Lipids. 1993;64:99–116. doi: 10.1016/0009-3084(93)90060-g. [DOI] [PubMed] [Google Scholar]
- 22.Duong PT, Collins HL, Nickel M, Lund-Katz S, Rothblat GH, Phillips MC. Characterization of nascent HDL particles and microparticles formed by ABCA1-mediated efflux of cellular lipids to apoA-I. J Lipid Res. 2006;47:832–843. doi: 10.1194/jlr.M500531-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Tang C, Vaughan AM, Anantharamaiah GM, Oram JF. Janus kinase 2 modulates the lipid-removing but not protein-stabilizing interactions of amphipathic helices with ABCA1. J Lipid Res. 2006;47:107–114. doi: 10.1194/jlr.M500240-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Nagao K, Zhao Y, Takahashi K, Kimura Y, Ueda K. Sodium taurocholate-dependent lipid efflux by ABCA1: effects of W590S mutation on lipid translocation and apolipoprotein A-I dissociation. J Lipid Res. 2009;50:1165–1172. doi: 10.1194/jlr.M800597-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oram JF, Lawn RM, Garvin MR, Wade DP. ABCA1 is the cAMP-inducible apolipoprotein receptor that mediates cholesterol secretion from macrophages. J Biol Chem. 2000;275:34508–34511. doi: 10.1074/jbc.M006738200. [DOI] [PubMed] [Google Scholar]
- 26.Faulkner LE, Panagotopulos SE, Johnson JD, Woollett LA, Hui DY, Witting SR, Maiorano JN, Davidson WS. An analysis of the role of a retroendocytosis pathway in ABCA1-mediated cholesterol efflux from macrophages. J Lipid Res. 2008;49:1322–1332. doi: 10.1194/jlr.M800048-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chroni A, Liu T, Fitzgerald ML, Freeman MW, Zannis VI. Cross-linking and lipid efflux properties of apoA-I mutants suggest direct association between apoA-I helices and ABCA1. Biochemistry. 2004;43:2126–2139. doi: 10.1021/bi035813p. [DOI] [PubMed] [Google Scholar]
- 28.Lu R, Arakawa R, Ito-Osumi C, Iwamoto N, Yokoyama S. ApoA-I facilitates ABCA1 recycle/accumulation to cell surface by inhibiting its intracellular degradation and increases HDL generation. Arterioscler Thromb Vasc Biol. 2008;28:1820–1824. doi: 10.1161/ATVBAHA.108.169482. [DOI] [PubMed] [Google Scholar]
- 29.Schubert R, Schmidt KH. Structural changes in vesicle membranes and mixed micelles of various lipid compositions after binding of different bile salts. Biochemistry. 1988;27:8787–8794. doi: 10.1021/bi00424a015. [DOI] [PubMed] [Google Scholar]
- 30.Vedhachalam C, Ghering AB, Davidson WS, Lund-Katz S, Rothblat GH, Phillips MC. ABCA1-induced cell surface binding sites for ApoA-I. Arterioscler Thromb Vasc Biol. 2007;27:1603–1609. doi: 10.1161/ATVBAHA.107.145789. [DOI] [PubMed] [Google Scholar]
- 31.Andrieux K, Forte L, Lesieur S, Paternostre M, Ollivon M, Grabielle-Madelmont C. Solubilisation of dipalmitoylphosphatidylcholine bilayers by sodium taurocholate: a model to study the stability of liposomes in the gastrointestinal tract and their mechanism of interaction with a model bile salt. Eur J Pharm Biopharm. 2009;71:346–355. doi: 10.1016/j.ejpb.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Dathe M, Schümann M, Wieprecht T, Winkler A, Beyermann M, Krause E, Matsuzaki K, Murase O, Bienert M. Peptide helicity and membrane surface charge modulate the balance of electrostatic and hydrophobic interactions with lipid bilayers and biological membranes. Biochemistry. 1996;35:12612–12622. doi: 10.1021/bi960835f. [DOI] [PubMed] [Google Scholar]
- 33.Drin G, Antonny B. Amphipathic helices and membrane curvature. FEBS Lett. 2010;584:1840–1847. doi: 10.1016/j.febslet.2009.10.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.