Abstract
Parental, particularly maternal, smoking increases the risk of childhood allergic asthma and infection. Similarly, in a murine allergic asthma model, prenatal plus early postnatal exposure to secondhand cigarette smoke (SS) exacerbates airway hyperreactivity and Th2 responses in the lung. However, the mechanism and contribution of prenatal versus early postnatal SS exposure on allergic asthma remains unresolved. To identify the effects of prenatal and/or early postnatal SS on allergic asthma, BALB/c dams and their offspring were exposed gestationally and/or 8–10 weeks post-birth to filtered air or SS. Prenatal, but not postnatal SS strongly increased methacholine and allergen (Aspergillus)-induced airway resistance, Th2-cytokines levels and atopy, and activated the Th2 polarizing pathway GATA3/Lck/ERK1/2/STAT6. Either prenatal and/or early postnatal SS downregulated the Th1-specific transcription factor T-bet and, surprisingly, in spite of high levels of IL-4/IL-13, dramatically blocked the allergen-induced mucous cell metaplasia, airway mucus formation, and the expression of mucus-related genes/proteins: Muc5ac, GABAA-receptors, and SPDEF. Given that SS/nicotine exposure of normal adult mice promotes mucus formation, the results suggest that fetal and neonatal lung are highly sensitive to cigarette smoke. Thus, while the gestational SS promotes Th2 polarization/allergic asthma, it may also impair and/or delay the development of fetal and neonatal lung, affecting mucociliary clearance and Th1 responses. Together, this may explain the increased susceptibility of children from smoking parents to allergic asthma and childhood respiratory infections.
Keywords: Environmental (secondhand) tobacco smoke, airways hyperreactivity, allergic asthma, Th2 polarization, airway mucus
INTRODUCTION
Asthma is a heterogenous disease (1), characterized by airway hyperreactivity (AHR), episodic wheezing, airway inflammation, and mucus secretion (2). Allergic asthma is the most common form of asthma in children. It starts in early life and is associated with AHR, airway inflammation, and atopy (3). Th2 polarization, the hallmark of asthma, is seen in human (3) and animal models of allergic asthma (4, 5).
Deleterious effects of tobacco smoke on human health are well established (6, 7), and increasing epidemiological evidence suggests that environmental factors such as secondhand tobacco smoke and polycyclic aromatic hydrocarbons are important contributors in the development of childhood asthma (8–10). Exposure to cigarette smoke during fetal development and in the early years of a child’s life is a strong risk factor for pulmonary dysfunction, including asthma and COPD (11). Parental smoking, particularly maternal smoking is strongly linked to allergic asthma and infection in children (12–14). Similarly, mice exposed to secondhand cigarette smoke (SS) during early postnatal life develop exacerbated respiratory infections (15). However, results from epidemiological studies are equivocal in identifying the developmental stage at which the fetus and child are vulnerable to the pro-asthmatic effects of maternal smoking. Thus, a link between maternal smoking and childhood asthma has been suggested when both parents were asthmatic (16), smoke exposure occurred during early childhood (17), during both prenatal and early postnatal life (18, 19), during pregnancy only (20–22), or either during prenatal or early postnatal life (23, 24). A possible explanation for these diverse results is that asthma is a heterogenous disease (1) and, in humans, it is difficult to control all the confounders of childhood asthma, such as genetics, birth weight, β2-adrenergic receptor, breastfeeding, and air pollution.
To simulate maternal exposure to cigarette smoke in animal models of allergic asthma, we and others have shown that prenatal plus early postnatal exposure to mainstream or SS exacerbates allergic asthma (5, 25, 26); however, as in humans, individual contributions of prenatal and postnatal smoke exposure on the development of early allergic asthma is not clearly resolved. Moreover, the potential mechanism by which cigarette smoke exposure promotes allergic asthma is not known. In this study we used an established mouse model of allergic asthma to isolate the effects of gestational and early postnatal SS exposures on the development of allergic asthma. We show for the first time that gestational exposure to SS is by far the overwhelming risk factor for exacerbated AHR, Th2 polarization, and atopy, and is associated with activation of GATA3/Lck/ERK1/2/STAT6. Surprisingly, unlike the pro-mucoid effects of cigarette smoke/nicotine in adult humans and animals (27–29), both prenatal and early postnatal exposure to SS suppressed the various parameters of airway mucus response.
MATERIALS AND METHODS
Animals
Pathogen-free BALB/c mice from the Frederick Cancer Research Facility (Frederick, MD) were housed in shoebox-type plastic cages with hardwood chip bedding and conditioned to whole-body exposure in exposure chambers (H1000: Hazleton Systems) for 2 weeks before exposure to SS for breeding (5). The chamber temperature was maintained at 26 ± 2°C, and lights were set to a 12-h on/off cycle. Food and water were provided ad libitum. The Animal Care and Use Committee, IACUC, of Lovelace Respiratory Research Institute approved all animal protocols.
Antibodies and reagents
All reagents, unless stated otherwise, were purchased from Sigma-Aldrich (St. Louis, MO).
Cigarette smoke generation and exposure
Mice were exposed to whole-body secondhand smoke (SS; smoke released from the burning end of a cigarette) or filtered air (FA) for 6 h/day, 7 days/wk as described (5). Briefly, a smoking machine (AMESA Type 1300, AMESA Technologoes, Geneva, Switzerland) generated two 70-cm3 puffs/min from a research cigarette (type 2R1; Tobacco and Health Research Institute, Lexington, KY), and the smoke was captured from the lit end of the cigarettes with a plastic manifold placed above it. This level (total particulate matter: 1.52 ± 0.41 mg/m3) of exposure simulates the conditions at which a pregnant woman would be exposed to environmental tobacco smoke for 3h/day in a smoking bar (5). Adult (3–4 month old) male and female mice were separately acclimatized to SS or FA for 2 wk prior to mating. After ascertaining pregnancy by vaginal smear, pregnant mice were housed singly in plastic cages and continued to receive SS or FA until the pups were born. The mothers and pups continued to be exposed to either FA or SS starting from day 1 of birth until the pups were weaned at 3 wk of age, and then the pups continued to be exposed FA or SS postnatally until sacrifice between 8–10 wk of age. This led to four groups of experimental animals, receiving the following combination of prenatal/postnatal exposures: FA/FA, FA/SS, SS/FA, and SS/SS.
Sensitization with allergen
The allergen used in the study was a lyophilized culture filtrate preparation of Aspergillus fumigatus (Af) the filtrates were stored at −70° C until the use (kindly provided by Dr. John M. Routes, Dept. of Pediatrics, Children’s Hospital, Wisconsin Medical College, Milwaukee). Mice were immunized intratracheally (i.t.) with Af (50 μg/0.1 ml endotoxin-free sterile saline or sterile saline alone) and subsequently challenged i.t. with the Af extracts (100 μg/0.1 ml) three times at 5-day intervals (5).
Total serum IgE
Total serum IgE levels were determined on diluted (1:100) serum using a mouse-specific serum IgE ELISA kit (MD Bioproducts, St. Paul, MN) according to manufacturer’s instruction. The sensitivity of the assay was <2.0 ng/ml; the cross reactivity with IgG was <0.01%.
Airway resistance
Forty-eight hours after the last Af or saline challenge, airway resistance (RL) was measured by the FlexiVent system (SCIREQ, Montreal, Quebec, Canada) as described (5). The peak RL response at the nebulized Af (200 μg/ml), and at each methacholine (MCh) concentration was used for data analysis.
Bronchoalveolar lavage fluid (BALF) collection, cell differentials, and cytokine analysis
Established protocols were followed to obtain BAL from the animals (5). Briefly, mice were anesthetized and killed by exsanguination at 48 h post last Af challenge. Before excision of the lungs, the trachea was surgically exposed, cannulated and, while the left lung lobe was tied off with a silk thread, the right lobe was lavaged twice with 1 ml sterile Ca 2+/Mg2+ free PBS (pH 7.4). Aliquots were pooled from individual animals. Cell differentials and cytokines assays were performed as described (5). Macrophage, neutrophil, lymphocyte, and eosinophil (Eos) numbers were determined microscopically by counting atleast 300 cells/sample. Lavage cytokines were assayed using the Mouse Cytokine MultiPlex ELISA kit (Biosource-Invitrogen, Camarillo, CA) according to the manufacturer’s directions. The sensitivity of the assay was <10 pg/ml.
Immunohistochemistry
For immunohistochemical (IHC) detection of airway mucus, formaldehyde-fixed left lung sections (5 μm) were stained with Alcian Blue-periodic acid Schiff (AB-PAS) as described previously (28). By this procedure, the mucus-producing cells stained distinctive pink and were examined microscopically at 40x magnification.
Analysis of mucosubstances in lung airways
The volume of mucous cells and the density(Vs) of mucosubstances in the airway epithelium was quantitated by a semiautomatic image analysis system (28), using the public domain National Institutes of Health (NIH) Image program (http//rsb.info.nih.gov/nih.gov/nih-image). Morphometry was performed blinded, and the data were expressed as the mean ± SD Vs (nl/mm2 basal lamina).
SPDEF (SAM pointed domain-containing Ets-like factor) staining
Paraffin embedded lung tissue (5 μm) was deparaffinized and rehydrated by placing slides on a slide warmer at 56° for20 minutes followed by three 10 minute incubations in xylene, a series of alcohol washes, followed by PBS. Antigen retrieval was performed by immersing the slides in 10 mM citrate buffer at 90°C for 15 minutes. Endogenous peroxidase quenching was done by placing the slides in 3% H2O2 in methanol for 15 minutes at room temperature (RT), washed (5x in PBS), and blocked with 10% goat serum for 2 hours at RT. The slides were incubated overnight at 4°C with a guinea pig anti-mouse polyclonal SPDEF antibody (GP954, a generous gift from Dr. Jeffrey Whitsett, Cincinnati Children’s Hospital) at 1:2500 dilution. Slides were washed 5x with buffer and incubated at RT for 30 minutes with a 1:200 dilution of a goat anti-guinea pig biotinylated IgG (cat# BA-1000; Vector Lab, Inc. Burlingame, CA). Slides were washed in PBS and incubated for 30 minutes in the Immunoperoxidase Kit (cat# PK-6100; Vector) as per manufacturer’s instructions. Slides were washed 5x in buffer and incubated for 2 minutes at RT in peroxidase substrate (cat# SK-4100; Vector) without nickel. Slides were then counterstained with hematoxylin and dehydrated. Slides were examined at 40x and SPDEF stains brown.
Quantitative PCR (qPCR)
Total RNA was extracted from the frozen lung samples using TRI reagent (Molecular Research Center, Cincinnati, OH) and quantified as per manufacturer’s instructions. The lung expression of the airway mucin Muc5ac, the Th1 transcription factor T-bet, the Th2 transcription factor GATA3, and the housekeeping gene GAPDH was determined using primer/probe sets (Applied Biosystems, Foster City, CA). The relative expression (test mRNA/GAPDH) was calculated (5).
Western blot analysis
Lung tissues were homogenized in RIPA buffer (20 mM Tris, 150 mM NaCl, 20 mM β-glyceryl-phosphate, 1% Triton-X, 10 mM NaF, 5 mM EDTA, 1 mM Na3VO4,) containing protease inhibitors (1 mM PMSF; 1 μg/ml each of aprotinin, antipain, and leupeptin) at 4°C. Protein content and WB analysis were performed as described (5). NF-κB (p65), GATA3, STAT5, STAT6, and Lck were determined by probing the blots with anti-phospho-NF-κB-p65 (Ser529; rabbit polyclonal; Abcam Inc., San Francisco, CA), anti-phospho-GATA3 (Ser308; rabbit polyclonal; Abcam), anti-phospho-STAT5, (Tyr694/699, mouse monoclonal; Abcam), anti-phospho-STAT6 (Tyr641, rabbit polyclonal; Cell Signaling Tech. Inc., Danvers, MA), and anti-phospho-Lck (Tyr394; rabbit polyclonal; Cell Signaling Tech). GABAAR and T-bet expression was determined by probing the blot with anti-GABAAR (mouse monoclonal, Millipore, Temecula, CA) and anti-T-bet antibody (mouse monoclonal, Abcam). Blots were developed with ECL (Amersham Biosciences, UK) using X-ray photo film. Densitometry: The X-ray films of Western blots were scanned and quantified with GS-800 Calibrated Densitometer using Quantity-One software (Bio-Rad, Hercules, CA).
Data Presentation and Statistical Analysis
All data were analyzed using Graph Pad Prism software 5.03 (Graphpad Software Inc., San Diego, CA). One-way ANOVA was used to compare mean between the groups using Tukey post-hoc test that compares all groups at 95% confidence intervals. Where needed, Bonferroni correction was also used for multiple comparisons. Results are presented as the means ± SD. The differences with p value of ≤0.05 were considered statistically significant.
RESULTS
Prenatal but not postnatal exposure to secondhand smoke increases airway hyperreactivity
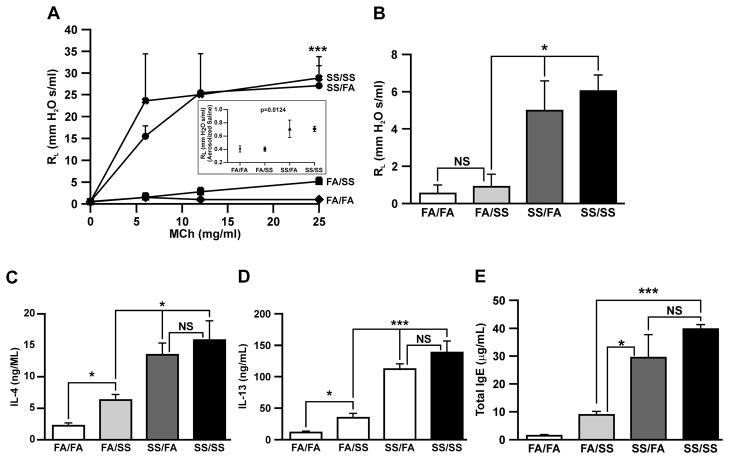
We have previously shown that prenatal plus perinatal exposure to SS increased RL to MCh (5); however, individual contributions of prenatal and postnatal SS exposures on AHR were not addressed. Therefore, dams were exposed to SS or FA as described in Methods and seventeen days prior to sacrifice, four groups of animals (FA/FA, FA/SS, SS/FA, and SS/SS) were sensitized with Af. RL was determined in response to aerosolized MCh (Fig. 1A) or predetermined optimal concentration of aerosolized Af (200 μg/ml) (Fig. 1B). Results demonstrate that, compared to FA/FA, both MCh and Af caused dramatic increases in the RL in prenatally SS-exposed animal groups SS/SS and SS/FA; however, the RL values between FA/FA and FA/SS, and between SS/SS and SS/FA groups were not significantly different, indicating that unlike prenatal, postnatal SS exposures do not affect RL significantly. MCh-induced changes in RL values of mice not sensitized to Af indicated that even in the absence of allergen, prenatally SS-exposed mice (SS/FA and SS/SS) were significantly more sensitive to MCh than FA/FA or FA/SS mice (Supplementary Fig. S1). Even the basal RL of prenatally SS-exposed mice was somewhat higher (statistically significant) than FA/FA or FA/SS mice. These results suggest that while prenatal SS exposure strongly exacerbates MCh- and Af-induced AHR, SS exposure during the early postnatal life had relatively minor effect on this response. Moreover, in this model, changes in AHR reached the level that allowed RL measurements in response to the allergen (Af). RL measurements in non-Af sensitized mice indicated that prenatal exposure to SS exacerbated MCh-induced AHR, however the degree of the response is considerably lower than Af-sensitized mice (Supplementary Fig. S1). Thus, prenatal SS exposure primes the lung for exacerbated AHR response.
FIGURE 1. Prenatal but not postnatal exposure to SS exacerbates airway reactivity, strongly upregulates allergen-induced Th2 cytokine expression, and increases total serum IgE levels.
Lung resistance (RL: cmH2O s/ml) was measured using FlexiVent system in response to: (A), increasing doses of computer-controlled nebulized methacholine (MCh), inset (Fig.1A): shows baseline differences between the groups in response to nebulized saline, and (B), a single nebulized dose (200 μg/ml) of Af. Peak responses at each MCh concentration and response to Af were used for data analysis. (C), IL-4, and (D), IL-13 levels were determined in bronchoalveolar lavage fluid from the Af-sensitized mice by the Mutiplex ELISA kit. (E), Total IgE levels in the serum of Af-sensitized mice were quantitated by using a mouse-specific IgE ELISA kit. All animals were sensitized with Af as described in Methods prior to RL determination. The results are representative of animal responses from two different sets of inhalation exposures. Data are expressed as mean ± SD (n = 5–7/group; * p ≤ 0.05, ** p ≤ 0.01; and *** p ≤ 0.001). FA = filtered air, SS = secondhand cigarette smoke; experimental animal groups of prenatal/postnatal exposure combinations are: FA/FA (pre- FA/postnatal FA); SS/FA (preSS/postnatal FA); FA/SS (pre- FA/postnatal SS); SS/SS (pre- SS/postnatal SS).
Prenatal secondhand smoke exposure induces a strong Th2 cytokine response in the lung
The Th2 cytokines particularly IL-13 and IL-4 play an essential role in allergic asthma and Th2 polarization (30, 31); however, only the AHR and not Th2 responses are ameliorated by the phosphodiesterase4-selective inhibitor rolipram This suggested that AHR and Th2 responses are regulated by different mechanisms (5) and, therefore it was possible that prenatal and early postnatal SS exposures might affect AHR and Th2 polarization differently. To ascertain this possibility, we determined the Af-induced IL-4 and IL-13 production in four groups of mice: FA/FA, SS/FA FA/SS, and SS/SS. Results presented in Fig. 1C & D indicate that compared to control (FA/FA), the early postnatal exposure to smoke (FA/SS) produced a relatively small but significant increase in the lavage levels of the Th2 cytokines IL-4 (Fig. 1C) and IL-13 (Fig. 1D) in response to Af sensitization; however, these levels were much lower than those present in the bronchoalveolar lavage fluids from prenatally SS-exposed animals (SS/FA: 13.48 ± 3.74 ng/ml vs. FA/SS: 6.30 ± 1.92 ng/ml for IL-4, and SS/FA: 111.7 ± 19.82 ng/ml vs. FA/SS: 34.58 ± 15.65 ng/ml for IL-13). Compared to Af-sensitized mice, the levels of IL-4 and IL-13 in non-sensitized mice were very low (Supplementary Fig. S2). These results suggest that prenatal exposure to SS is the predominant risk factor in driving the lung toward Th2 polarization. On the other hand, the levels of IL-4, and IL-13 in mice without Af challenges did not reach statistical significance, indicating that the critical levels of these cytokines are achieved only after allergic sensitization.
Prenatal exposure to SS increases leukocytic infiltration in the lung after Af sensitization
Elevated numbers of Eos and neutrophils in the lung are associated with allergic asthma (5). To determine whether pre- and/or postnatal exposure to SS differentially affected the leukocytic infiltration in the lung in response to an allergic challenge, Af-sensitized FA/FA, FA/SS, SS/FA and SS/SS mice were challenged i.t. with Af extracts. The volume of BALF recovered was not significantly different between the groups. BAL cells were collected, cytospinned, and stained to obtain the differential cell count. Prior to Af sensitization, the total number of leukocytes in the BAL in both FA and SS groups was similar (6–8 ± 2.6 × 104); however, after Af challenge (+), the number of cells in FA/FA+ and FA/SS+ rose to 51–54 ± 8.3 × 104. The number increased to 92–98 ± 12.8 × 104 in SS/FA+, and SS/SS+ animals. Thus, Af promoted leukocytic infiltration in the lungs and the infiltration was significantly more pronounced in the animals exposed to SS prenatally; postnatal SS did not make a significant difference in the total number of cells in the BAL (Table I).
Table I.
Leukocytic composition of BAL cells
| Leukocyte subtypes | FA/FA (pre- and postnatal FA) | FA/FA+ (pre- and postnatal FA) + Af | FA/SS+ (postnatal SS) +Af | SS/FA+ (prenatal SS) +Af | SS/SS+(pre-and postnatal SS) +Af |
|---|---|---|---|---|---|
| Total BAL leukocytes | 6.2 ± 2.6×104 | 51.4 ± 8.3×104 | 54.0 ± 6.9×104 | 92.4 ± 11.3×104 | 97.8 ± 12.6×104 |
| Macrophages, % | 90.8 ± 4.41 | 95 ± 1.58 | 92.1 ± 2.65 | *31.6 ± 4.18 | *38.0 ± 5.54 |
| Neutrophils, % | 3.8 ± 2.06 | 4.5 ± 1.48 | 11.5 ± 2.11 | †35.0 ± 4.62 | †38.1 ± 6.46 |
| Lymphocytes, % | 0.7 ± 0.60 | 0.2 ± 0.24 | 2.1 ± 0.53 | ‡1.9 ± 0.33 | ‡2.9 ± 0.37 |
| Eosinophils, % | 0 ± 0.00 | 0.3 ± 0.24 | 1.3 ± 0.34 | §34.3 ± 4.02 | §36.6 ± 5.11 |
FA, filtered air; SS, secondhand cigarret smoke; Af, Aspergillus extract. Data are presented as mean ± SD (n= 5–6). Macrophage
p < 0.05 compared with FA/FA+, and FA/SS+; neutrophils
p < 0.05 compared with FA/FA+, and FA/SS+; for lymphocytes
not significant (ns) compared with FA/FA+, and FA/SS+; and eosinophils
p < 0.05 compared with FA/FA+, and FA/SS+.
The differential BAL cell count (Table I) indicated that macrophages were the predominant cell population in the BAL fluids from FA/FA, FA/FA+, and FA/SS+, comprising 90.8 ± 4.4%, 95 ± 1.6% and 92.1 ± 2.6%, respectively. Thus, while the Af challenge strongly increased the total number of BAL cells, the percentages of macrophages between these groups did not vary significantly; nonetheless, animals exposed postnatally to SS (FA/SS) had small but significantly higher numbers of neutrophils. On the other hand, prenatally SS-exposed groups (SS/FA and SS/SS) showed a dramatic decrease in the proportion of macrophages with concomitant increases in neutrophils and Eos. There results suggest that prenatal exposure strongly primes the lung for neutrophilic and eosinophilic inflammation.
Prenatal secondhand smoke increases total serum IgE levels
Atopy is a strong risk factor for allergic asthma in children (32). To ascertain whether prenatal and/or postnatal SS exposure affects atopy differentially, we measured the serum levels of IgE in the four groups of mice after Af sensitization. Results (Fig. 1E) indicate that, compared to FA/FA, prenatal (SS/FA) and early postnatal (FA/SS) exposures independently elevated total serum IgE levels; however, the IgE level in SS/FA (prenatally SS-exposed animals) of 29.50 ± 14.41 μg/ml was significantly higher than the IgE level of 8.81 ± 2.49 μg/ml in FA/SS (SS exposure only during the early postnatal period). Although, the mean IgE concentration of 39.83 ± 2.75 μg/ml in SS/SS (prenatally and postnatally SS exposure) was higher than prenatally SS-exposed animals, it did not reach statistical significance. These results suggest that while prenatal and early postnatal exposures are independent risk factors for atopy, prenatal exposure is a substantially stronger risk factor.
Prenatal SS activates GATA3, ERK1/2, LCK, AND STAT6
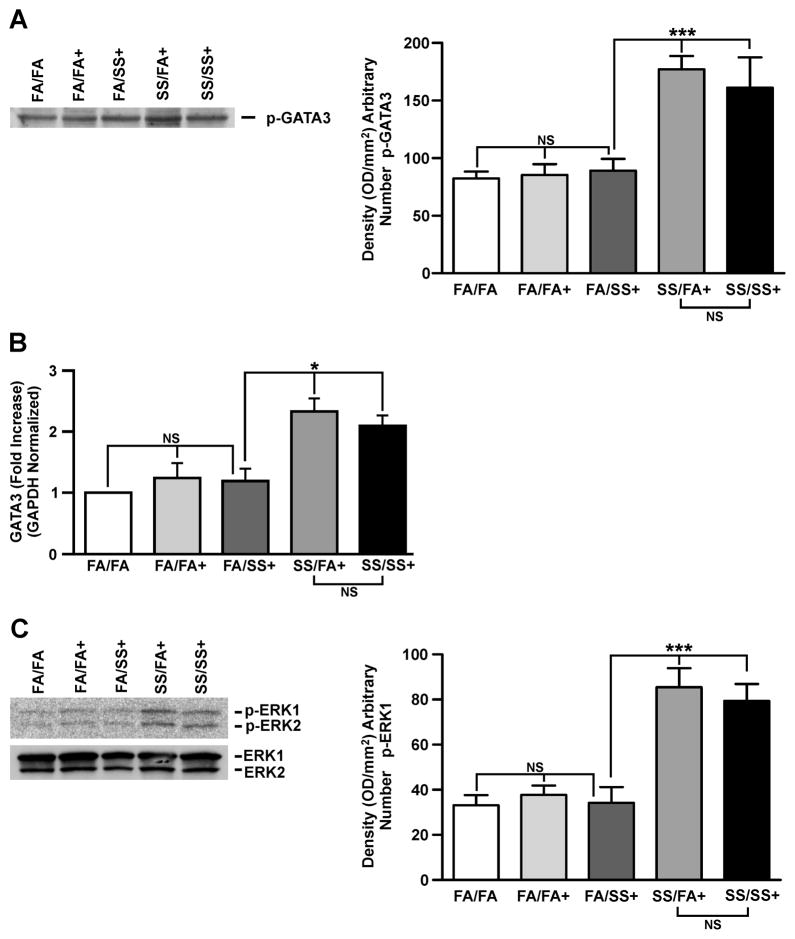
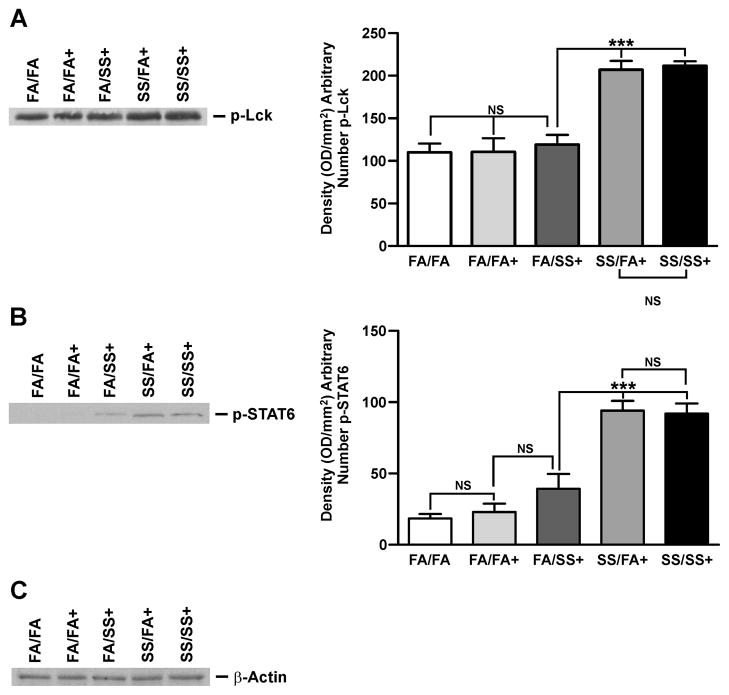
Th2 cytokines are intimately associated with allergic asthma, and coincident with Th2 development is activation of the IL-4/STAT6 pathway that enhances the expression of GATA3 (34). GATA3 is the master transcription factor for Th2 differentiation and, under physiological conditions it is selectively expressed in Th2 but not Th1 cells, and induces Th2 cytokine gene expression (33, 34). Lck, ERK, and STAT6 regulate GATA3 activity (33, 35, 36). Therefore, we examined the lung expression of GATA3, Lck, ERK, and STAT6 by quantitative PCR and/or Western blot analysis. Fig. 2A (left and right panels) shows a representative response of various groups of SS-exposed mice. Mice exposed gestationally to SS (SS/FA and SS/SS) and subsequently exposed to Af exhibited significantly higher levels of activated (phosphorylated) GATA3. Increased GATA3 expression in prenatally SS-exposed animals was also seen by qPCR analysis (Fig. 2B). Moreover, prenatal, but not postnatal exposure to SS activated (phosphorylated) ERK1/2 (p-ERK1/2); total ERK1/2 was not affected by either pre or postnatal SS (Fig. 2C: left and right panels). Similarly, prenatal but not postnatal SS, increased activation (phosphorylation) of Lck (Fig. 3A: left and right panels) and STAT6 (Fig. 3B: left and right panels); neither pre- nor postnatal SS affected the expression of actin (Fig. 3C). These results suggest that prenatal but not postnatal SS activates the Th2 signaling pathway (GATA3/Lck/ERK/STAT6). Af sensitization is critical for the visualization of SS-induced exaggerated Th2 and AHR responses; without Af sensitization, the baseline phosphorylation of GATA3/STAT6 (Supplementary. Fig. S3) and Lck/ERK1/2 (not shown) was comparable between various groups. Thus, activation of GATA3/Lck/ERK/STAT6 is associated with an exacerbated allergen-induced asthmatic phenotype following gestational exposure to SS.
FIGURE 2. Prenatal SS exposure induces GATA3 and ERK activation.
Representative result of Western blot (WB) analysis of lung homogenate (70 μg protein) probed with (A), anti-phospho (p)-GATA3 antibody, right panel: densitometry analysis of blots from three separate experiments, (B), lung GATA3 mRNA expression by quantitative PCR as described in Methods (n = 5/group, NS = not significant), and (C), representative of three WB analysis of lung homogenates probed with phosphorylated (p)-ERK1/2 antibody, and total ERK1/2 (lower panel). Right panel: densitometry analysis of blots from three separate experiments (+ indicates Af sensitized; bars represent the mean ± SD; * p ≤ 0.05; *** p ≤ 0.001; NS = not significant).
FIGURE 3. Prenatal SS activates Lck and STAT6.
Lung homogenates (70 μg) were analyzed by Western blot. The blots were probed (A), with anti phospho (p)-Lck antibody, right panel: densitometry analysis of blots from three separate experiments, (B), with anti p-STAT6 antibody, right panel: densitometry analysis of blots from three separate experiments, and (C), with anti-β-actin antibody. The figures are representative of three separate analysis. (+ indicates Af-sensitized, bars represent the mean ± SD; *** p ≤ 0.001, and NS = not significant)
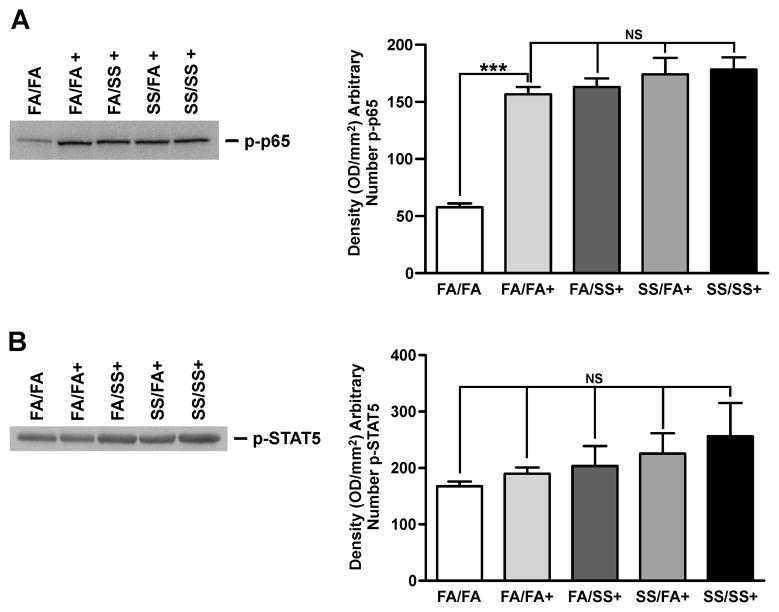
Prenatal SS does not affect allergen-induced NF-κB and STAT5 activation
NF-κB, a ubiquitously expressed transcription factor plays a vital role in inflammatory responses and is activated in some chronic lung diseases such as COPD (37). NF-κB has also been implicated in IL-13-induced lung pathology (38). Serine phosphorylation of NF-κB-p65 subunit has been shown to be important in the function of NF-κB as a transcription factor (39); therefore we determined the level of phosphorylated NF-κB-p65 in the lung extracts as an index of NF-κB activation. As seen in Fig. 4A (left and right panels), although allergic sensitization increased p-NF-κB-p65, neither prenatal nor postnatal SS exposure significantly affected the magnitude of this activation; therefore, it is unlikely that exacerbated allergic asthma associated with prenatal SS exposure is linked to activation of NF-κB. The early production of the Th2 cytokine IL-4 is regulated by phosphorylation of the transcription factor STAT5 (40); however, results presented in Fig. 5B (left and right panels) show that neither pre- nor postnatal SS significantly altered activation (phosphorylation) of STAT5. Together, these results suggest that NF-κB and STAT5 are not strongly associated with the SS-induced exacerbated Th2 responses.
FIGURE 4. SS does not affect NF-κB or STAT5 activation.
Lung homogenates (75 μg protein) were analyzed by Western blot, and the blots were probed with anti-p-p65 antibody, (A), or anti p-STAT5 antibody (B), Data are representative of three separate sets of analyses. Right panel: densitometry analysis of blots from three separate experiments. (+ indicates Af sensitized; bars represent the mean ± SD; *** p ≤ 0.001; NS = not significant)
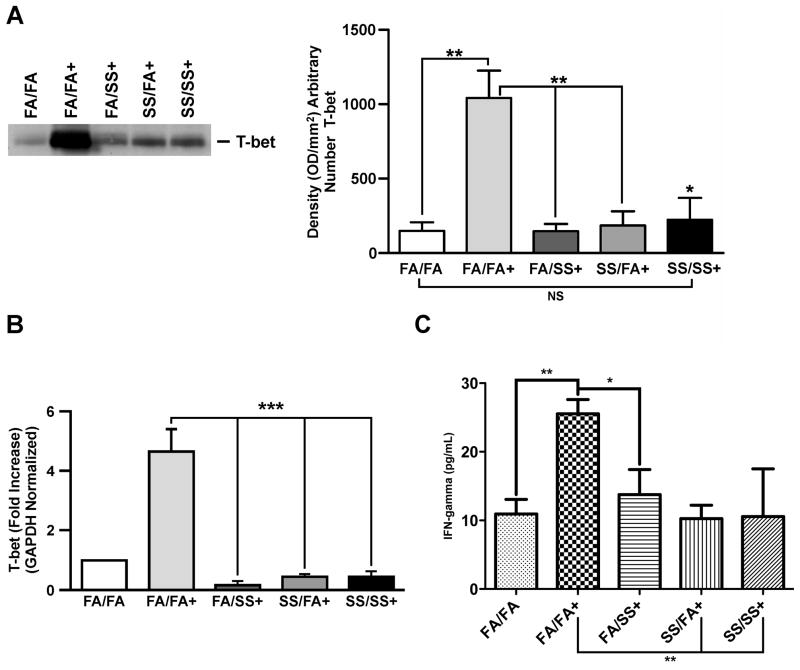
FIGURE 5. Prenatal and/or postnatal SS suppresses T-bet, and IFN-γ.
Representative results of lung homogenates (75 μg protein) analysis by Western blot, and probed with (A), anti-T-bet antibody, Right panel: densitometry analysis of blots from three independent experiments. (B), T-bet mRNA expression in the lung tissue analyzed by quantitative PCR as described in the Methods. (C), IFN-γ level in the BALF as described in the Methods. (n = 5/group; + indicates Af-sensitized mice; bars represent the mean ± SD; * p ≤ 0.05; ** p ≤ 0.01, *** p ≤ 0.001, and NS = not significant).
Prenatal and early postnatal secondhand smoke exposure strongly suppresses the allergen-induced T-bet expression in the lung
GATA3 and T-bet are two opposing transcription factors for Th1 and Th2 development, respectively (40, 41). Results (Fig. 5) clearly show that sensitization of control (FA/FA) animals with Af (FA/FA+) strongly increased the level of T-bet in the lung; however, animals exposed to SS either prenatally (SS/FA) or postnatally (FA/SS) exhibited a dramatic reduction in the Af-induced T-bet protein levels (Fig. 5A: left and right panels). Western blot data presented in the Supplementary Fig. S3 (top panel) shows that exposure to an allergen is essential to upregulate T-bet expression. Quantitative PCR data indicating that prenatal and early postnatal exposure to SS downregulated the expression of T-bet mRNA (Fig. 5B) also support theses results. Thus, prenatal and early postnatal exposure to SS suppresses the allergen-induced T-bet expression, which might impair the development of Th1 responses in the lung. To evaluate the effect of SS-exposure on the prototype Th1 cytokine, IFN-γ levels were assayed in the BAL fluids of the experimental animals. While the various groups did not exhibit significant differences in the basal (unsensitized) levels of IFN-γ (Supplementary. Fig. S2C), FA/FA animals exhibited a significant increase in the BAL levels of IFN-γ after Af sensitization. On the other hand, the IFN-γ levels in animals exposed either prenatally and/or postnatally to SS, failed to upregulate the BAL levels of IFN-γ after the Af challenge (Fig. 5C). Thus, SS exposures affected T-bet and IFN-γ similarly.
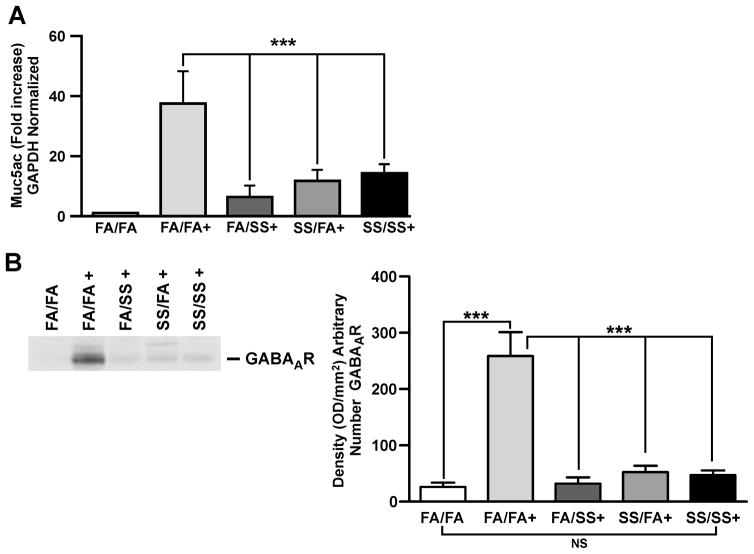
Prenatal and/or early postnatal exposure to secondhand smoke suppresses mucus formation and expression of Muc5ac and GABAARs in the lung
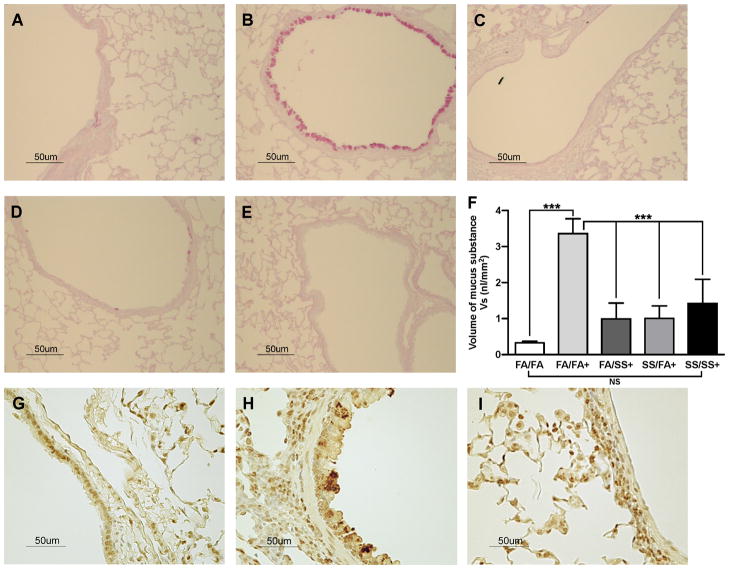
Mucus produced by mucosal epithelial cells in the respiratory tract acts as the first line of defense against inhaled pathogens, which are cleared by the mucociliary apparatus (42, 43). However, dysregulated mucus formation is an important factor in many lung diseases, including asthma. Recent evidence suggests that GABAARs and IL-13 are intimately associated with the airway mucin Muc5ac and mucus formation by bronchial epithelial cells (44, 45). To ascertain the effects of prenatal and postnatal SS exposure on mucus formation, we determined the presence of mucus in the lung (Fig. 7) by immunohistochemistry and the expression of Muc5ac and GABAARs (Fig. 6) by quantitative PCR and Western blot analysis, respectively. As expected, in control (FA/FA) animals, Af sensitization led to significant increases in the airway mucus content (Fig. 7A and B). Surprisingly however, the amount of mucus was dramatically reduced in the airways by SS exposure during early postnatal (Fig. 7D), prenatal (Fig. 7C), and prenatal + postnatal (Fig. 7E) SS exposures. The histopathologic data was validated by determining the volume/density(Vs) of mucosubstances in the mucosal surface epithelium of the lung airways (Fig. 7F). These results were further supported by the observation that prenatal and postnatal SS exposures independently decreased the expression of Muc5ac (Fig. 6A) and GABAARs (Fig. 6B; left and right panels) in the lung. Without allergic (Af) challenge, GABAAR expression is similar in all groups (supplementary Fig. S3 panel 4). Thus, prenatal and/or early postnatal SS exposures suppress mucus production that might adversely impact the mucociliary clearance and defense against inhaled pathogens/noxious particulate agents in these animals.
FIGURE 7. SS exposure suppresses the allergen-induced airway mucus, and SPDEF formation.
Lung sections (5 μm) were stained with AB-PAS for mucus, and SPDEF as described in the Methods. Representative histologic photomicrographs (40x) of the lung sections show the mucus-producing cells in pink, and SPDEF in brown. Mucus staining: (A), FA/FA; (B), FA/FA+; (C), FA/SS+; (D), SS/FA+; (E), SS/SS+; (F), Volume density(Vs) of AB-PAS-stained mucosubstances in the mucosal surfacein the lungepithelium (n = 5/group; bars represent the mean ± SD; *** p ≤ 0.001; and NS = not significant). SPDEF staining: (G), FA/FA; (H), FA/FA+; (I), SS/FA+ (n =3/group); + indicates Af-sensitized.
FIGURE 6. SS exposure suppresses Muc5ac and GABAAR expression.
(A), MUC5AC mRNA expression in the lung tissue analyzed by quantitative PCR (n = 5/group), and (B), Representative of three independent Western blot analysis of lung homogenate (75 μg protein) probed with anti-GABAAR antibody, right panel: densitometry analysis of blots from three separate experiments (+ indicates Af sensitized mice; bars represent the mean ± SD; *** p ≤ 0.001; NS = not significant).
Prenatal exposure to secondhand smoke suppresses SPDEF expression in the lung airways
SPDEF is the transcription factor that plays an important role in the development and differentiation of pulmonary goblet cell and mucus production (46). Because SPDEF is primarily restricted to goblet cells, we ascertained the expression of SPDEF in airway epithelial cells by immunohistochemistry. Results presented in Fig 7. show that the SPDEF is expressed moderately in unsensitized FA/FA airways (Fig. 7G); upon sensitization with Af, FA/FA animals strongly upregulated the expression of SPDEF (Fig. 7H). However, Af sensitization of prenatally SS-exposed animals (SS/FA) failed to increase the expression of SPDEF in the airways; indeed, the expression in SS/FA animals (Fig. 7I) was even lower than the basal levels of SPDEF in control FA/FA animals. Drastic reduction is SPDEF levels was also seen in FA/SS animals (not shown). Thus, the inability of allergens to upregulate the expression of SPDEF in prenatally and/or early postnatally SS-exposed animals may the main cause for impaired differentiation of goblet cells and mucus production in these animals.
DISCUSSION
Th2 bias, atopy, and AHR are the hallmarks of allergic asthma (3–5, 47) and, in animal models, prenatal plus perinatal exposure to mainstream or secondhand cigarette smoke increases AHR and Th2 lung inflammation (25); however, AHR and Th2 polarization do not overlap mechanistically (5). Therefore, it was possible that prenatal and perinatal exposures to cigarette smoke affected AHR and Th2 lung inflammation differentially. Herein, we show that gestational but not early postnatal exposure to SS primes the lung to dramatic increases in allergen-induced airway resistance (RL) as well as Th2 inflammation (increased IL-4 and IL-13 levels) and increased levels of serum IgE. Although the allergen sensitization of gestationally SS-exposed animal caused a significant increase in the serum IgE levels, the IgE may or may not be specific to the allergen. A mild increase in AHR has been reported after early postnatal exposure to SS (48), these studies used Penh values to quantitate AHR. However, the value of Penh in determining lung function and airway resistance has been strongly challenged (49, 50). Therefore, there is no tangible evidence that early postnatal exposure to SS increases airway resistance significantly.
The mechanism by which cigarette smoke exacerbates Th2 responses is largely unknown. Activation of GATA3 is intimately associated with Th2 polarization and suppression of Th1 responses; on the contrary, T-bet promotes Th1 and suppresses Th2 polarization (51–54). We observed that while Af sensitization in normal (FA/FA) animals resulted in an upregulated expression of both GATA3 and T-bet, mice exposed prenatally to SS exhibited a strong upregulated expression of GATA3 and a dramatic suppression of T-bet, indicating while gestational SS promotes Th2 polarization, it simultaneously downregulates Th1 activation. Effects of SS exposure on the Th1 cytokine IFN-γ were similar to those seen with T-bet; thus, exposure to SS causes parallel changes in T-bet and IFN-γ. Although prenatal and/or early postnatal exposure to SS suppressed T-bet, effects of early postnatal SS on GATA3 expression were only weakly higher than control. Thus, while both prenatal and early postnatal SS exposures, downregulate T-bet and IFN-γ, increased GATA3 expression is primarily associated with prenatal SS exposure. GATA3 is known to decrease T-bet expression (54); however, it is not clear whether GATA3 is the only factor that downregulates T-bet in gestationally SS-exposed animals. Nonetheless, given that Th1 responses are important in clearing infections, decreased T-bet could increase the risk for protracted lung infections and, at least in part, explain the increased risk of infections among children exposed to cigarette smoke through parental smoking (55–57).
Among the factors that promote Th2 development is the IL-4-STAT6 pathway that enhances the expression of GATA3 and Th2 polarization (33). Although STAT5 has also been implicated in Th2 responses, this transcription factor primarily affects the early IL-4 production (40), and the Af-induced activation STAT5 was not significantly affected by either prenatal or postnatal SS exposures. On the other hand, while prenatal exposure strongly activated STAT6, activation of STAT6 by postnatal SS was much weaker. Thus, activation of STAT6 may play a significant role in activation of GATA3 by prenatal SS-exposure. In addition to STAT6, a number of other factors promote GATA3 activation during Th2 polarization. Lck controls GATA3 and IL-4 production, and Lck−/− Th2-cells have lower levels of IL-4 and GATA3 and a higher expression of T-bet and IFN-γ (36). Moreover, activated ERK inhibits ubiquitination and degradation of GATA3 in Th2 cells (35), thereby increasing the level of activated GATA3. On the other hand, activated Lck and ERK negatively regulate T-bet expression (35, 36). Our results show that Lck and ERK1/2 are activated in the lungs of prenatally SS-exposed animals, and these factors are likely to contribute to the increased GATA3 and decreased T-bet expression. The observation that early postnatal SS only weakly activated Lck/ERK1/2/GATA3 and Th2 polarization also supports a potential correlation between stronger upregulated activation of Lck/ERK1/2/GATA3 and stronger Th2 polarization in gestationally SS-exposed animals. However, while it is likely that STAT6 and Lck/ERK strongly trigger Th2 polarization in prenatally SS-exposed animals, other potential mechanisms of GATA3 activation contributing to Th2 polarization in these animals cannot be ruled out. For example, cigarette smoke has been shown to elevate lung inflammation through epigenetic changes involving chromatin modifications (58), and proinflammatory cytokines, such as IL-6 (59, 60) also activate GATA3.
NF-κB is the transcription factor that regulates proinflammatory cytokine production, and it may also play an important role in IL-13-induced lung pathogenesis (38). Activated NF-κB contains NF-κB-p50 and phosphorylated NF-κB-p65 subunits (61), and our results indicate that although Af sensitization causes a sharp increase in p65, neither prenatal nor early postnatal SS significantly altered the magnitude of this response. Thus, while NF-κB might be important in allergic sensitization, it is unlikely to play a critical role in the SS-induced exacerbated allergic asthma.
Direct methods such as MCh or histamine are commonly used to access AHR; however, this approach has several drawbacks (62). It has been observed that increased AHR detected by direct stimuli such as histamine or MCh is not specific to allergic asthma and may be associated with a number of other lung diseases, such as COPD, sarcoidosis, and bronchiectasis. Moreover, a significant number of normal subjects may also show increased AHR to these agents (reviewed in 63). On the other hand, indirect stimuli (e.g., allergens) act on cell types such as inflammatory and neuronal cells to transmit the signal to effector cells, and are considered a better indicator of asthma and inflammation (63). However, in general allergen-specific AHR changes are much weaker than MCh-induced AHR changes and, therefore, difficult to quantify. To our knowledge, allergen-induced AHR has not been reported in animal models, although the effects of allergens on isolated lungs and tracheal rings have been described (64, 65). Given the magnitude of MCh-induced RL in prenatally SS-exposed animals, we determined whether the prenatal SS-exposure was a strong enough trigger for a significant increase in allergen-induced RL. Indeed, prenatal, but not postnatal, SS exposure strongly increased the Af-induced RL, indicating that prenatal SS exposure is a strong stimulus for allergic asthma and an excellent animal model to test the efficacy of interventions for allergic asthma-associated AHR.
Mucus production is a cardinal feature of bronchial asthma and associated with goblet cell metaplasia (66). IL-13 and IL-4 play a critical role in mucus formation (45, 67); however, in spite of large increases in Th2 cytokines, including IL-13/IL-4 in gestationally SS-exposed animals, prenatal and early postnatal SS exposures dramatically reduced airway mucus formation. While IL-13 may induce lung inflammation and Muc5ac through the ERK1/2/MAPK pathway independent of STAT6 (68; 69), STAT6 also controls Th2 cytokines and goblet cell metaplasia (70). Thus, in spite of the presence of IL-13 and activated STAT6 and ERK1/2, prenatal and early postnatal SS exposures suppress goblet cell formation and mucus production. This observation was counterintuitive, as cigarette smoke is a strong pro-mucus stimulus in humans and adult mice (71). Muc5ac is the major inducible mucin in the airways and its expression is controlled by GABAA receptors in airway epithelial cells (72), and the expression of both Muc5ac and GABAA receptors was strongly down-regulated by pre- and/or early postnatal exposure to SS. Mammalian lung development starts in the fetus and continues for a significant period after the birth (i.e., several weeks to several years in mice and humans respectively; 73). It is possible that SS exposure during this critical period blocks or delays development of type-II airway epithelial cells. The transcription factor SPDEF plays an important role in the growth and differentiation of goblet cells, and our results suggest that the lung expression of this critical transcription factor is downregulated by pre- and/or early postnatal SS exposures. Together these results suggest that SS affects the differentiation of airway epithelial cells into goblet cells. Recently, Fu and Spindel (72) reported an increased number of GABAAR-expressing cells in the pulmonary neuroepithelial bodies from monkeys exposed gestationally to nicotine, suggesting that an increased potential for mucus formation. A likely explanation for this is that cigarette smoke is a very complex mixture of thousands of chemicals and some of these chemicals might affect early lung development/maturation. Prenatal and early postnatal exposure to SS might at least temporarily impair the developmental process and make cells either hypo- or hyperresponsive to various growth and differentiation factors. Indeed, prenatal nicotine was reported to adversely affect cellular communication and normal lung development (74), and early postnatal SS exposure impaired Clara cell secretory protein levels (15). We have some preliminary evidence that gestational exposure to SS affects the lung development and development of type II cells (Singh et al., unpublished observation). Thus, prenatal and/or early postnatal SS exposure may impair/delay the development/differentiation of airway goblet cells and reduce mucus production even in the presence of high levels of IL-13/IL-4 and activated STAT6 and ERK1/2.
The mucociliary apparatus is important in the clearance of pathogens from the respiratory tract, and mucosal epithelial cells act as the first line of defense against respiratory pathogens (42). While excessive mucus production may contribute to the morbidity of some respiratory diseases, diminished mucus formation is likely to encourage respiratory infections. Together with suboptimal Th1 development through decreased T-bet, loss of mucus formation may increase the susceptibility and length of respiratory infections, and explain the increased risk of respiratory infections among children from mothers who smoke. cigarettes. Overall, these studies strongly suggest that a fetus is exceptionally sensitive to cigarette smoke, which may promote the development of childhood allergic asthma and respiratory infections, and every effort should be made to dissuade women from being exposed to cigarette smoke, including environmental tobacco smoke during pregnancy.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. John Routes (Medical College of Wisconsin, Milwaukee) for generous supply of Aspergillus fumigates extracts. We also thank Mr. Steve Randock and Ms. Wendy Piper for graphics, and Ms. Paula Bradley for editorial help.
Resources: Supported in part by grants from NIH (RO1 DA017003, R01 DA04208-17), Flight Attendant Medical Research Institute (FAMRI) grants to SPS and NCM, and funds from Lovelace Respiratory Research Institute.
Footnotes
Disclosures
The authors have no financial conflict of interest in the subject of this manuscript.
References
- 1.Humbert M, Menz G, Ying S, Corrigan CJ, Robinson DS, Durham SR, Kay AB. The immunopathology of extrinsic (atopic) and intrinsic (non-atopic) asthma: more similarities than differences. Immunol Today. 1999;20:528–533. doi: 10.1016/s0167-5699(99)01535-2. [DOI] [PubMed] [Google Scholar]
- 2.Lemanske RF, Jr, Busse WW. 6. Asthma. Review. J Allergy Clin Immunol. 2003;111:S502–S519. doi: 10.1067/mai.2003.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holt PG, Rowe J, Kusel M, Parsons F, Hollams EM, Bosco A, McKenna K, Subrata L, de Klerk N, Serralha M, Holt BJ, Zhang G, Loh R, Ahlstedt S, Sly PD. Toward improved prediction of risk for atopy and asthma among preschoolers: a prospective cohort study. J Allergy Clin Immunol. 2010;125:653–659. doi: 10.1016/j.jaci.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 4.Cates EC, Fattouh R, Johnson JR, Llop-Guevara A, Jordana M. Modeling responses to respiratory house dust mite exposure. Contrib Microbiol. 2007;14:42–67. doi: 10.1159/000107054. [DOI] [PubMed] [Google Scholar]
- 5.Singh SP, Mishra NC, Rir-Sima-Ah J, Campen M, Kurup V, Razani-Boroujerdi S, Sopori ML. Maternal exposure to secondhand cigarette smoke primes the lung for induction of phosphodiesterase-4D5 isozyme and exacerbated Th2 responses: rolipram attenuates the airway hyperreactivity and muscarinic receptor expression but not lung inflammation and atopy. J Immunol. 2009;183:2115–2121. doi: 10.4049/jimmunol.0900826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002;2:372–377. doi: 10.1038/nri803. [DOI] [PubMed] [Google Scholar]
- 7.Henderson AJ. The effects of tobacco smoke exposure on respiratory health in school-aged children. Paediatr Respir Rev. 2008;9(2):1–27. doi: 10.1016/j.prrv.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Pinkerton KE, Joad JP. The Mammalian respiratory system and citical windows of exposure for children’s health. Environ Health Perspect Suppl. 2000;108(3):457–463. doi: 10.1289/ehp.00108s3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar R. Prenatal factors and the development of asthma. Curr Opin Pediatr. 2008;20:682–687. doi: 10.1097/MOP.0b013e3283154f26. [DOI] [PubMed] [Google Scholar]
- 10.Perera FW, Tang Y, Herbstman J, Tang D, Levin L, Miller R, Ho SM. Relation of DNA methylation of 5′-CpG island of ACSL3 to transplacental exposure to airborne polycyclic aromatic hydrocarbons and childhood asthma. PLoS One. 2009;4:e4488–e4502. doi: 10.1371/journal.pone.0004488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children’s health. Pediatrics. 2004;113:1007–1015. [PubMed] [Google Scholar]
- 12.Cook DG, Strachan DP. Health effects of passive smoking – 10: summary of effects of parental smoking on the respiratory health of children and implications for research. Thorax. 1999;54:357–366. doi: 10.1136/thx.54.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheraghi M, Salvi S. Environmental tobacco smoke (ETS) and respiratory health in children. Eur J Pediatr. 2009;168:897–905. doi: 10.1007/s00431-009-0967-3. [DOI] [PubMed] [Google Scholar]
- 14.Dharmage SC, Erbas B, Jarvis D, Wjst M, Raherison C, Norbäck D, Heinrich J, Sunyer J, Svanes C. Do childhood respiratory infections continue to influence adult respiratory morbidity? Eur Respir J. 2009;33:237–244. doi: 10.1183/09031936.00062907. [DOI] [PubMed] [Google Scholar]
- 15.Phaybouth V, Wang SZ, Hutt JA, McDonald JD, Harrod KS, Barrett EG. Cigarette smoke suppresses Th1 cytokine production and increases RSV expression in a neonatal model. Am J Physiol Lung Cell Mol Physiol. 2006;290:L222–L231. doi: 10.1152/ajplung.00148.2005. [DOI] [PubMed] [Google Scholar]
- 16.Keil T, Lau S, Roll S, Grüber C, Nickel R, Niggemann B, Wahn U, Willich SN, Kulig M. Maternal smoking increases risk of allergic sensitization and wheezing only in children with allergic predisposition: longitudinal analysis from birth to 10 years. Allergy. 2009;64:445–451. doi: 10.1111/j.1398-9995.2008.01867.x. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka K, Miyake Y, Sasaki S, Ohya Y, Hirota Y. Maternal smoking and environmental tobacco smoke exposure and the risk of allergic diseases in Japanese infants: the Osaka Maternal and Child Health Study. J Asthma. 2008;45:833–838. doi: 10.1080/02770900802339742. [DOI] [PubMed] [Google Scholar]
- 18.Gergen PJ, Fowler JA, Maurer KR, Davis WW, Overpeck MD. The burden of environmental tobacco smoke exposure on the respiratory health of children 2 months through 5 years of age in the United States: Third National Health and Nutrition Examination Survey, 1988 to 1994. Pediatrics. 1998;101:E8. doi: 10.1542/peds.101.2.e8. [DOI] [PubMed] [Google Scholar]
- 19.Wang C, Salam MT, Islam T, Wenten M, Gauderman WJ, Gilliland FD. Effects of in utero and childhood tobacco smoke exposure and beta2-adrenergic receptor genotype on childhood asthma and wheezing. Pediatrics. 2008;122:e107–e114. doi: 10.1542/peds.2007-3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu FB, Persky V, Flay BR, Zelli A, Cooksey J, Richardson J. Prevalence of asthma and wheezing in public schoolchildren: association with maternal smoking during pregnancy. Ann Allergy Asthma Immunol. 1997;79:80–84. doi: 10.1016/S1081-1206(10)63090-6. [DOI] [PubMed] [Google Scholar]
- 21.Jedrychowski W, Flak E, Mróz E. Cigarette smoking by mothers during pregnancy and pulmonary function of their school age children. Pneumonol Alergol Pol. 1997;65:605–610. [PubMed] [Google Scholar]
- 22.Xepapadaki P, Manios Y, Liarigkovinos T, Grammatikaki E, Douladiris N, Kortsalioudaki C, Papadopoulos NG. Association of passive exposure of pregnant women to environmental tobacco smoke with asthma symptoms in children. Pediatr Allergy Immunol. 2009;20:423–429. doi: 10.1111/j.1399-3038.2008.00820.x. [DOI] [PubMed] [Google Scholar]
- 23.Kulig M, Luck W, Lau S, Niggemann B, Bergmann R, Klettke U, Guggenmoos-Holzmann I, Wahn U. Effect of pre- and postnatal tobacco smoke exposure on specific sensitization to food and inhalant allergens during the first 3 years of life. Multicenter Allergy Study Group, Germany. Allergy. 1999;54:220–228. doi: 10.1034/j.1398-9995.1999.00753.x. [DOI] [PubMed] [Google Scholar]
- 24.Pattenden S, Antova T, Neuberger M, Nikiforov B, De Sario M, Grize L, Heinrich J, Hruba F, Janssen N, Luttmann-Gibson H, Privalova L, Rudnai P, Splichalova A, Zlotkowska R, Fletcher T. Parental smoking and children’s respiratory health: independent effects of prenatal and postnatal exposure. Tob Control. 2006;15:294–301. doi: 10.1136/tc.2005.015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh SP, Barrett EG, Kalra R, Razani-Boroujerdi S, Langley RJ, Kurup V, Tesfaigzi Y, Sopori ML. Prenatal cigarette smoke decreases lung cAMP and increases airway hyperresponsiveness. Am J Respir Crit Care Med. 2003;168:342–47. doi: 10.1164/rccm.200211-1262OC. [DOI] [PubMed] [Google Scholar]
- 26.Joad JP, Munch PA, Bric JM, Evans SJ, Pinkerton KE, Chen CY, Bonham AC. Passive smoke effects on cough and airways in young guinea pigs. Am J Respir Crit Care Med. 2004;169:499–504. doi: 10.1164/rccm.200308-1139OC. [DOI] [PubMed] [Google Scholar]
- 27.Shao MX, Nakanaga T, Nadel JA. Cigarette smoke induces MUC5AC mucin overproduction via tumor necrosis factor-alpha-converting enzyme in human airway epithelial (NCI-H292) cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L420–L427. doi: 10.1152/ajplung.00019.2004. [DOI] [PubMed] [Google Scholar]
- 28.Mishra NC, Rir-Sima-ah J, Langley RJ, Singh SP, Peña-Philippides JC, Koga T, Razani-Boroujerdi S, Hutt J, Campen M, Kim KC, Tesfaigzi Y, Sopori ML. Nicotine primarily suppresses lung Th2 but not goblet cell and muscle cell responses to allergens. J Immunol. 2008;180:7655–7663. doi: 10.4049/jimmunol.180.11.7655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Churg A, Cosio M, Wright JL. Mechanisms of cigarette smoke-induced COPD: insights from animal models. Am J Physiol Lung Cell Mol Physiol. 2008;294:L612–L631. doi: 10.1152/ajplung.00390.2007. [DOI] [PubMed] [Google Scholar]
- 30.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 31.Barnes PJ. Role of GATA-3 in allergic diseases. Curr Mol Med. 2008;8:330–334. doi: 10.2174/156652408785160952. [DOI] [PubMed] [Google Scholar]
- 32.Gelfand EW. Pediatric asthma: a different disease. Proc Am Thorac Soc. 2009;6:278–282. doi: 10.1513/pats.200808-090RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu J, Min B, Hu-Li J, Watson CJ, Grinberg A, Wang Q, Killeen N, Urban JF, Jr, Guo L, Paul WE. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat Immunol. 2004;5:1157–1165. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
- 34.Zhang DH, Cohn L, Ray P, Bottomly K, Ray A. Transcription factor GATA-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gne. J Biol Chem. 1997;272:21597–21603. doi: 10.1074/jbc.272.34.21597. [DOI] [PubMed] [Google Scholar]
- 35.Yamashita M, Shinnakasu R, Asou H, Kimura M, Hasegawa A, Hashimoto K, Hatano N, Ogata M, Nakayama T. Ras-ERK MAPK cascade regulates GATA3 stability and Th2 differentiation through ubiquitin-proteasome pathway. J Biol Chem. 2005;280(2):9409–29419. doi: 10.1074/jbc.M502333200. [DOI] [PubMed] [Google Scholar]
- 36.Kemp KL, Levin SD, Bryce PJ, Stein PL. Lck mediates Th2 differentiation through effects on T-bet and GATA-3. J Immunol. 2010;184:4178–4184. doi: 10.4049/jimmunol.0901282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murugan V, Peck MJ. Signal transduction pathways linking the activation of alveolar macrophages with the recruitment of neutrophils to lungs in chronic obstructive pulmonary disease. Exp Lung Res. 2009;35:439–485. doi: 10.1080/01902140902759290. [DOI] [PubMed] [Google Scholar]
- 38.Chapoval SP, Al-Garawi A, Lora JM, Strickland I, Ma B, Lee PJ, Homer RJ, Ghosh S, Coyle AJ, Elias JA. Inhibition of NF-kappaB activation reduces the tissue effects of transgenic IL-13. J Immunol. 2007;179:7030–7041. doi: 10.4049/jimmunol.179.10.7030. [DOI] [PubMed] [Google Scholar]
- 39.Sasaki CY, Barberi TJ, Ghosh P, Longo DL. Phosphorylation of RelA/p65 on serine 536 defines an I{kappa}B{alpha}-independent NF-{kappa}B pathway. J Biol Chem. 2005;280(3):4538–34547. doi: 10.1074/jbc.M504943200. [DOI] [PubMed] [Google Scholar]
- 40.Christodoulopoulos P, Cameron L, Nakamura Y, Lemière C, Muro S, Dugas M, Boulet LP, Laviolette M, Olivenstein R, Hamid Q. TH2 cytokine-associated transcription factors in atopic and nonatopic asthma: evidence for differential signal transducer and activator of transcription 6 expression. J Allergy Clin Immunol. 2001;107:586–5891. doi: 10.1067/mai.2001.114883. [DOI] [PubMed] [Google Scholar]
- 41.Jenner RG, Townsend MJ, Jackson I, Sun K, Bouwman RD, Young RA, Glimcher LH, Lord GM. The transcription factors T-bet and GATA-3 control alternative pathways of T-cell differentiation through a shared set of target genes. Proc Natl Acad Sci U S A. 2009;106:17876–17881. doi: 10.1073/pnas.0909357106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006;86:245–278. doi: 10.1152/physrev.00010.2005. [DOI] [PubMed] [Google Scholar]
- 43.Evans CM, Koo JS. Airway mucus: the good, the bad, the sticky. Pharmacol Ther. 2009;121:332–348. doi: 10.1016/j.pharmthera.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiang YY, Wang S, Liu M, Hirota JA, Li J, Ju W, Fan Y, Kelly MM, Ye B, Orser B, O’Byrne PM, Inman MD, Yang X, Lu WY. A GABAergic system in airway epithelium is essential for mucus overproduction in asthma. Nat Med. 2007;13:862–867. doi: 10.1038/nm1604. [DOI] [PubMed] [Google Scholar]
- 45.Kuperman DA, Schleimer RP. Interleukin-4, interleukin-13, signal transducer and activator of transcription factor 6, and allergic asthma. Curr Mol Med. 2008;8:384–392. doi: 10.2174/156652408785161032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen G, Korfhagen TR, Xu Y, Kitzmiller J, Wert SE, Maeda Y, Gregorieff A, Clevers H, Whitsett JA. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest. 2009;119:2914–2924. doi: 10.1172/JCI39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Schoor J, Joos GF, Pauwels RA. Indirect bronchial hyperresponsiveness in asthma: mechanisms, pharmacology and implications for clinical research. Eur Respir J. 2000;16:514–533. doi: 10.1034/j.1399-3003.2000.016003514.x. [DOI] [PubMed] [Google Scholar]
- 48.Barrett EG, Wilder JA, March TH, Espindola T, Bice DE. Cigarette smoke-induced airway hyperresponsiveness is not dependent on elevated immunoglobulin and eosinophilic inflammation in a mouse model of allergic airway disease. Am J Respir Crit Care Med. 2002;165:1410–1418. doi: 10.1164/rccm.2106029. [DOI] [PubMed] [Google Scholar]
- 49.Sly PD, Turner DJ, Collins RA, Hantos Z. Penh is not a validated technique for measuring airway function in mice. Am J Respir Crit Care Med. 2005;172:256. doi: 10.1164/ajrccm.172.2.954. [DOI] [PubMed] [Google Scholar]
- 50.Lundblad LK, Irvin CG, Hantos Z, Sly P, Mitzner W, Bates JH. Penh is not a measure of airway resistance. Eur Respir J. 2007;30:805. doi: 10.1183/09031936.00091307. [DOI] [PubMed] [Google Scholar]
- 51.Zhou M, Ouyang W. The function role of GATA-3 in Th1 and Th2 differentiation. Immunol Res. 2003;28:25–37. doi: 10.1385/IR:28:1:25. [DOI] [PubMed] [Google Scholar]
- 52.Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science. 2005;307:430–433. doi: 10.1126/science.1103336. [DOI] [PubMed] [Google Scholar]
- 53.Usui T, Preiss JC, Kanno Y, Yao ZJ, Bream JH, O’Shea JJ, Strober W. T-bet regulates Th1 responses through essential effects on GATA-3 function rather than on IFNG gene acetylation and transcription. J Exp Med. 2006;203:755–766. doi: 10.1084/jem.20052165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu J, Yamane H, Cote-Sierra J, Guo L, Paul WE. GATA-3 promotes Th2 responses through three different mechanisms: induction of Th2 cytokine production, selective growth of Th2 cells and inhibition of Th1 cell-specific factors. Cell Res. 2006;16:3–10. doi: 10.1038/sj.cr.7310002. [DOI] [PubMed] [Google Scholar]
- 55.Dybing E, Sanner T. Passive smoking, sudden infant death syndrome (SIDS) and childhood infections. Hum Exp Toxicol. 1999;18:202–205. doi: 10.1191/096032799678839914. [DOI] [PubMed] [Google Scholar]
- 56.Bradley JP, Bacharier LB, Bonfiglio J, Schechtman KB, Strunk R, Storch G, Castro M. Severity of respiratory syncytial virus bronchiolitis is affected by cigarette smoke exposure and atopy. Pediatrics. 2005;115:e7–e14. doi: 10.1542/peds.2004-0059. [DOI] [PubMed] [Google Scholar]
- 57.Marseglia GL, Avanzini MA, Caimmi S, Caimmi D, Marseglia A, Valsecchi C, Poddighe D, Ciprandi G, Pagella F, Klersy C, Castellazzi AM. Passive exposure to smoke results in defective interferon-gamma production by adenoids in children with recurrent respiratory infections. J Interferon Cytokine Res. 2009;29:427–432. doi: 10.1089/jir.2008.0108. [DOI] [PubMed] [Google Scholar]
- 58.Rajendrasozhan S, Yao H, Rahman I. Current perspectives on role of chromatin modifications and deacetylases in lung inflammation in COPD. COPD. 2009;6:291–297. doi: 10.1080/15412550903049132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 60.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 61.Ahn KS, Aggarwal BB. Transcription factor NF-kappaB: a sensor for smoke and stress signals. Ann N Y Acad Sci. 2005;1056:218–2133. doi: 10.1196/annals.1352.026. [DOI] [PubMed] [Google Scholar]
- 62.Joos GF. Bronchial hyperresponsiveness: too complex to be useful? Curr Opin Pharmacol. 2003;3:233–238. doi: 10.1016/s1471-4892(03)00046-8. [DOI] [PubMed] [Google Scholar]
- 63.Rogers DF, O’Connor BJ. Airway hyperresponsiveness: relation to asthma and inflammation? Thorax. 1993;48:1095–1096. doi: 10.1136/thx.48.11.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Larsen GL, Renz H, Loader JE, Bradley KL, Gelfand EW. Airway response to electrical field stimulation in sensitized inbred mice. Passive transfer of increased responsiveness with peribronchial lymph nodes. J Clin Invest. 1992;89:747–752. doi: 10.1172/JCI115651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Witzenrath M, Ahrens B, Kube SM, Braun A, Hoymann HG, Hocke AC, Rosseau S, Suttorp N, Hamelmann E, Schütte H. Detection of allergen-induced airway hyperresponsiveness in isolated mouse lungs. Am J Physiol Lung Cell Mol Physiol. 2006;291:L466–L472. doi: 10.1152/ajplung.00011.2005. [DOI] [PubMed] [Google Scholar]
- 66.Izuhara K, Ohta S, Shiraishi H, Suzuki S, Taniguchi K, Toda S, Tanabe T, Yasuo M, Kubo K, Hoshino T, Aizawa H. The mechanism of mucus production in bronchial asthma. Curr Med Chem. 2009;16:2867–2875. doi: 10.2174/092986709788803196. [DOI] [PubMed] [Google Scholar]
- 67.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee PJ, Zhang X, Shan P, Ma B, Lee CG, Homer RJ, Zhu Z, Rincon M, Mossman BT, Elias JA. ERK1/2 mitogen-activated protein kinase selectively mediates IL-13-induced lung inflammation and remodeling in vivo. J Clin Invest. 2006;116(1):63–173. doi: 10.1172/JCI25711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kono Y, Nishiuma T, Okada T, Kobayashi K, Funada Y, Kotani Y, Jahangeer S, Nakamura S, Nishimura Y. Sphingosine kinase 1 regulates mucin production via ERK phosphorylation. Pulm Pharmacol Ther. 2010;23(3):6–42. doi: 10.1016/j.pupt.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 70.Fritz DK, Kerr C, Fattouh R, Llop-Guevara A, Khan WI, Jordana M, Richard CD. A mouse model of airway disease: oncostatin M-induced pulmonary eosinophilia, goblet cell hyperplasia, and airway hyperresponsiveness are STAT6 dependent, and interstitial pulmonary fibrosis is STAT6 independent. J Immunol. 2011;186:1107–1118. doi: 10.4049/jimmunol.0903476. [DOI] [PubMed] [Google Scholar]
- 71.Yu H, Li Q, Kolosov VP, Perelman JM, Zhou X. Regulation of Cigarette Smoke-Induced Mucin Expression by Neuregulin1 β/ErbB3 Signalling in Human Airway Epithelial Cells. Basic Clin Pharmacol Toxicol. 2011 doi: 10.1111/j.1742–7843.2011.00686.x. [DOI] [PubMed] [Google Scholar]
- 72.Fu XW, Wood K, Spindel ER. Prenatal nicotine exposure increases GABA signaling and mucin expression in airway epithelium. Am J Respir Cell Mol Biol. 2010 doi: 10.1165/rcmb.2010–010990C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shi W, Bellusci S, Warburton D. Lung development and adult lung diseases. Chest. 2007;132:651–656. doi: 10.1378/chest.06-2663. [DOI] [PubMed] [Google Scholar]
- 74.Rehan VK, Asotra K, Torday JS. The effects of smoking on the developing lung: insights from a biologic model for lung development, homeostasis, and repair. Lung. 2009;187:281–289. doi: 10.1007/s00408-009-9158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.