Abstract
Our previous studies in bovine eyes demonstrated that the structural correlate to the increase in outflow facility after either Rho-kinase inhibitor Y-27632 (Y27) treatment or washout appeared to be separation between the juxtacanalicular tissue (JCT) and inner wall (IW) of the aqueous plexus, the bovine equivalent of Schlemm's canal (SC). While these findings suggest that Y27 and washout may increase outflow facility through a similar mechanism, the anatomy of bovine outflow pathway differs considerably from both the human and monkey outflow pathway; however, only the human eye does not exhibit washout. In light of this, we compared the effects of Y27 and washout on outflow facility, hydrodynamic patterns of outflow, and the morphology of the IW and JCT in monkey eyes, given that their anatomy is closer to human eyes.
Twelve freshly enucleated monkey eyes were used in this study. Eyes were perfused with Dulbecco's PBS containing 5.5 mM glucose (GPBS) to establish a baseline facility at 15 mmHg. Four eyes were perfused for a short-duration (30 min) as a control, 4 eyes for a long-duration (180 min) to induce washout, and 4 eyes with GPBS+50 μM Y27 for 30 min. All eyes were then perfused with fluorescent microspheres (0.5μm; 0.002%) to label the hydrodynamic patterns of outflow and then perfusion-fixed. Confocal images of frontal sections were taken along the IW of SC. The total length (TL) and the tracer decorated length (FL) of the IW were measured to calculate the average percent effective filtration length (PEFL=FL/TL). Sections with SC were examined by light and electron microscopy. The TL of the IW and the length exhibiting separation (SL) in the JCT were measured to calculate the average percent separation length (PSL= SL/TL).
Outflow facility increased 149.2% (p<0.01) from baseline after washout during long-duration perfusion, and 114.9% (p=0.004) after Y27 treatment, but did not change significantly after short-duration perfusion in control eyes (p=0.46). Distribution of the tracer labeling appeared punctate along the IW of control eyes, while a more uniform pattern was observed after washout and Y27 treatment. PEFL in washout (83.4±2.1%) and Y27 treated eyes (82.5±1.6%) was 3.4-fold larger compared to controls (24.2±4.2%, P<0.001). The JCT appeared distended with loss of connections between JCT cells and between JCT cells and their extracelluar matrix in eyes with washout or after Y-27 treatment. PSL in the JCT was 2.3-fold larger in washout eyes (77.4±3.3%) and 2.2-fold larger in Y27 treated eyes (75.2±5.3%) versus controls (33.5±5.3%, p=0.001). Significant positive correlations were found between outflow facility and PEFL, facility and PSL and between PEFL and PSL.
Our data demonstrated that similar hydrodynamic and morphological changes occurred in the aqueous humor outflow pathway of monkey eyes after induction of washout and Y27 treatment. Both Y27 and washout increase outflow facility by redistributing aqueous outflow through a larger area in the JCT. These hydrodynamic changes are likely driven by morphologic changes associated with a decrease in cell-cell and cell-matrix connections in the JCT.
Keywords: monkey eye, aqueous outflow facility, Y27632, washout, Schlemm's canal, juxtacanalicular tissue, trabecular meshwork, light microscopy, electron microscopy, confocal microscopy
Introduction
The mechanism responsible for the generation of aqueous humor outflow resistance remains unknown in both normal and glaucomatous human eyes. The elevated intraocular pressure (IOP) associated with primary open-angle glaucoma (POAG) occurs due to a malfunction or impairment of aqueous humor filtration within the trabecular meshwork[Epstein 1987; Johnson et al., 2000; Lutjen-Drecoll 1999]. Lack of a thorough understanding of the mechanism involved in regulating trabecular outflow resistance has hindered the development of an effective anti-glaucoma therapy that aims at increasing trabecular outflow. Although the mechanisms involved in governing outflow resistance are unclear, several studies have localized the primary resistive site to a region including the juxtacanalicular tissue (JCT) and inner wall endothelium of Schlemm's Canal (SC)[Grant 1958, 1963; Maepea et al., 1989, 1992].
Drugs that disrupt the cytoskeleton or its contractility have been shown to reduce aqueous humor outflow resistance and IOP. Such drugs include cytochalasin[Johnson 1997; Kaufman et al., 1982; Tian et al., 1999], latrunculin[Ethier et al., 2006; Sabanay et al., 2006], H-7[Bahler et al., 2004; Sabanay et al., 2004], HA 1077[Honjo et al., 2001] and Y27632 [Honjo et al., 2001; Rao et al., 2001; Tian et al., 2005; Waki et al., 2001], and are therefore important candidates for the next generation of glaucoma therapy. Y27632 (Y27) is a protein kinase inhibitor selective for Rho-associated kinase (ROCK) isoforms ROCK-I and ROCK-II[Davies et al., 2000; Ishizaki et al., 2000; Uehata et al., 1997], which regulate the phosphorylation of the regulatory myosin light chain (MLC) to promote actomyosin-driven cell contractility. Inhibiting ROCK with Y27 decreases MLC phosphorylation by promoting MLC-phosphatase activity[Kaibuchi et al., 1999; Rosenthal et al., 2005], leading to cell relaxation and disassembly of actin stress fibers and focal adhesions in many cell types[Rao et al., 2001], including human trabecular meshwork and Schlemm's canal endothelial cells in vitro[Honjo et al., 2001; Rao et al., 2001]. In our previous study in bovine eyes, perfusion with 50 μM Y27 significantly increased outflow facility [Lu et al., 2008]. This result appeared to coincide with separation between the JCT and inner wall, which positively correlated with an increase in effective filtration length along the inner wall of SC.
“Washout” is the progressive increase in aqueous outflow facility observed during prolonged perfusion of non-human eyes[Barany et al., 1954; Barany et al., 1955; Erickson-Lamy et al., 1990; Melton et al., 1960; Van Buskirk et al., 1978; Yan et al., 1991]. Interestingly, the structural changes associated with the increase in outflow facility during washout were found to be similar to those after Y27 treatment (i.e. associated with separation between the JCT and inner wall, which was positively correlated with the increase in effective filtration length[Overby et al., 2002; Scott et al., 2007; Scott et al., 2009]). These findings suggest that inner wall/JCT separation may be a critical morphological mechanism for drug-induced increase in outflow facility. Similarly, Y27 and washout may share a common mechanism in increasing outflow facility.
Our previous studies in bovine eyes suggest a similar mechanism for the increase in outflow facility after washout and Y27 treatment[Lu et al., 2008; Scott et al., 2009]; however, the morphology of the bovine and human outflow pathways is different. The bovine eye has aqueous plexus in place of Schlemm's canal and a reticular meshwork rather than a trabecular meshwork as seen in the human eye[Johnson et al., 1990]. While the monkey eye is morphologically similar to the human eye[Epstein et al., 1991; Hashimoto et al., 1980; Toris et al., 2000], the physiology of the monkey outflow apparatus is comparable to the bovine outflow apparatus because they both exhibit washout[Erickson et al., 1981; Overby et al., 2002; Scott et al., 2007], whereas the human eye does not exhibit washout[Erickson-Lamy et al., 1990; Gong et al., 2009; Scott et al., 2007]. This suggests that the functional anatomy of the human outflow apparatus is physiologically unique when compared with other species that do exhibit a washout effect, including the monkey. The human eye, when compared with bovine and monkey eyes, has a more developed elastic-like cribriform plexus extending from the tendons of the ciliary muscle to the endothelial cells of the inner wall of Schlemm's canal[Gong et al., 1996; Gong et al., 1989; Rohen et al., 1981]. This elastic-like network may provide structural support that inhibits inner wall/JCT distention and contribute to the regulation of steady outflow resistance and may be responsible for the lack of washout in human eyes[Erickson-Lamy et al., 1990]. In light of this, we investigated whether previously observed similarities in bovine eyes treated with Y27 or subjected to washout would differ in the monkey eye, which is significantly closer to the overall anatomy of the human outflow pathway. We hypothesized that Y27 and washout decrease outflow resistance through a similar mechanism by weakening the inner wall/JCT connectivity, inducing the separation between the inner wall and JCT and increasing the available outflow area through the resistive tissue of the JCT and the inner wall. To test our hypothesis, fluorescent microspheres and confocal microscopy were used to visualize the change in the hydrodynamic patterns of outflow after Y27 treatment or induced washout. After confocal microscopy examination, the same tissue was examined using light and electron microscopy to investigate how the facility-increasing effect correlated with changes in the hydrodynamic filtration patterns and morphology within the JCT and inner wall of the monkey eyes.
Materials and Methods
Materials
Twelve enucleated rhesus monkey eyes were obtained from 1) Harvard Medical School New England Regional Primate Research Center, Southborough, MA; 2) University of Wisconsin, Laboratory Animal Center, Madison, WI and delivered on ice within 18 hours after death. Eyes with any discernible damage or accumulated blood in the limbal area or anterior chamber were discarded. Dulbecco's phosphate-buffered saline (Life Technologies, Grand Island, NY) containing 5.5 mM D-glucose (GPBS) was used as mock aqueous humor in all perfusion. Y27632, a selective Rho-Kinase Inhibitor, was obtained from Calbiochem, San Diego, CA (Lot#: B64617). All studies adhered to the ARVO Statement for the Use of Animals and Human Parts in Ophthalmic and Vision Research and were in compliance with Boston University guidelines.
Perfusion Procedure
The mechanical setup of the perfusion system was described previously[Sit et al., 1997; Sit et al., 1997]. Briefly, the perfusion system consists of a perfusion chamber and a collection chamber. The perfusion chamber was linked to a pressure transducer connected electronically by a computer control system (Macintosh G4; Apple Computers, Cupertino, CA). Outflow facility (C=Q/IOP) was measured at 10 Hz, averaged, and electronically recorded every 10 seconds by LabView version 7.0 (National Instrument, USA).
Monkey eyes were cleaned of extraocular tissue and submerged up to the limbus in Dulbecco's phosphate-buffered saline at 34°C. A 23-gauge infusion needle was inserted intracamerally through the peripheral transparent cornea into each eye and connected to the perfusion chamber. Anterior chamber deepening, which can cause an artificial increase in outflow facility, was prevented by inserting the needle tip into the posterior chamber. A second 23-gauge needle was inserted intracamerally into the anterior chamber of each eye and connected to the collection reservoir. During perfusion the collection reservoir tube was clamped. All eyes were perfused at constant pressure (15 mmHg) with GPBS for at least 30 minutes to establish a stable baseline outflow facility. In the Y27 treated group (n=4), the anterior chamber contents were exchanged with 5 ml of GPBS containing 50 μM Y27 and perfused with an additional 0.3 ml of the same solution (which took approximately 25-30 minutes depending on the facility of each eye). In the control group (n=4), the anterior chamber contents were exchanged with 5 ml of GPBS and the eyes were perfused with equal volume of GPBS (0.3 ml) as a control. In washout group (n=4), eyes were continuously perfused with GPBS for 3 hours to induce washout effect. The anterior chamber contents of all eyes were then exchanged with 5 ml of GPBS containing red fluorescent microspheres (0.5μM; 0.002% v/v; Molecular Probes, Ins. Eugene, Oregon) followed by perfusion with an additional 0.3 ml red fluorescent microspheres to label the hydrodynamic outflow patterns. The anterior chamber contents of all eyes were then exchanged with 5 ml of modified Karnovsky's fixative (containing 2.5% glutaraldehyde and 2% paraformaldehyde in phosphate buffer, pH 7.4), and perfusion-fixed with an additional 0.3ml of the fixative. After fixation, an approximate 1 cm cut was made in the equator of each eye to insure fixative into the inside of eyes. Eyes were then immersed in PBS containing 50% Karnovsky's fixative at 4°C to store for further processing.
Confocal Microscopy
All fixed eyes were cut through the equator. The vitreous body and lens of each eye were carefully removed. Anterior segments of the eyes were divided into 4 quadrants (temporal, nasal, superior and inferior). Tissue sections of 1-1.5 mm were cut in two orientations either radial or along a plane tangential to the corneo-scleral limbus and perpendicular to the ocular surface (referred to frontal sections)[Parc et al., 2000]. Sections were then counter-stained in To-pro3 (Molecular Probes, Inc., Eugene, OR, USA.) for 30 minutes to visualize cell nuclei. After 3 successive five-minute washes in PBS, the sections were examined under confocal microscopy (Carl Zeiss 510, Axiovert 100M Laser Scanning Microscope, Heidelberg, Germany). Image scanning, capture and measurement were performed with LSM 510 software version 3.2 PS2 (Carl Zeiss, Heidelberg, Germany). A multi-track channel system was used to visualize the red fluorescent microspheres under 20X magnification. Images were taken along the inner wall of SC with or without accompanying collector channel ostia so that the distribution of the tracer along the inner wall and the trabecular meshwork could be properly analyzed. Since the tracer distribution was dependent on proximity to the collector channel, radial sections that do not contain a collector channel ostium usually have less or no tracer labeling, the total length of the inner wall (TL) and the tracer-decorated length of the inner wall (L) were measured in frontal sections of each eye including all 4 quadrants[Lu et al., 2008; Scott et al., 2009]. A minimum of 16 images per eye were measured and the average percent effective filtration length (PEFL=FL/TL) in each eye was calculated.
Light Microscopy and Transmission Electron Microscopy
Tissue sections containing SC and/or collector channel ostia, which had been previously examined by confocal microscopy, were then processed for light and transmission electron microscopy. Sections were post-fixed in 2% osmium tetroxide (Electron Microscopy Sciences, Hatfield, PA) in 1.5% potassium ferrocyanide (Fisher Scientific Company, New Jersey) for 2 hours, dehydrated in an ascending series of ethanols, and embedded in Epon-Araldite (Electron Microscopy Sciences, Hatfield, PA). Semi-thin sections (3 μm) were cut and stained with 1% Toluidine Blue (Fisher Scientific Co. USA) to identify the outflow pathway and the inner wall of SC. Light micrographs were taken at an original magnification of 20X to determine whether expansion or separation occurred in the JCT region. The total length of inner wall (TL) and the length exhibiting JCT separation (SL) were measured in frontal sections of each eye including all 4 quadrants[Lu et al., 2008; Scott et al., 2009]. A Minimum of 16 images per eye were measured and the average percentage expansion length (PSL= SL/TL) was calculated in each eye.
Ultrathin sections (90 nm) were cut with an ultramicrotome, counterstained with uranyl acetate (Fisher Scientific Company, New Jersey), and examined using transmission electron microscopy (Model 300; Phillips, Eindhoven, The Netherlands). Micrographs from two to four different quadrants were taken along the inner wall of SC at an original magnification of 3300X.
Statistics
A two-tailed Student's t-test and linear regression analysis were applied (Systat for the Macintosh, ver. 5.2.1; SPSS, Chicago, IL) with a required significance level of p<0.05.
Results
Outflow Facility
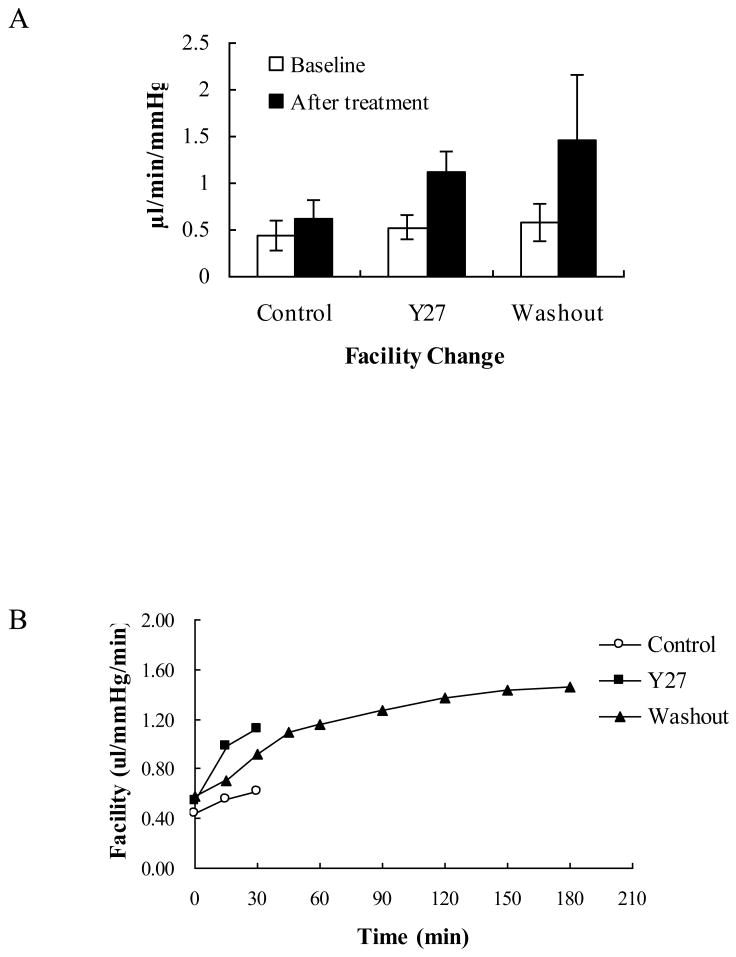
Changes in outflow facility among short-duration, long-duration and Y27 treated groups are shown in Figures 1A and B. In the short-duration (control) group, outflow facility increased 44.3±9.7% (±SEM) from baseline (from 0.44±0.14 to 0.62±0.16 μl/min/mmHg, but did not reach a statistical significance p=0.46). In the long-duration (washout) group, outflow facility increased significantly (149.2±53.2%) from baseline (from 0.58±0.18 to 1.46±0.61 μl/min/mmHg, p<0.01). In the Y27 treated group, the outflow facility displayed a significant increase of 114.9±14.9% from baseline (from 0.54±0.13 to 1.12±0.22 μl/min/mmHg, p=0.004).
Figure 1. Change in Outflow Facility.
A: Outflow facility changed from 0.44±0.16 to 0.62±0.21 (±SEM) μl/min/mmHg (p=0.46) in control eye (with 30 min perfusion), increased from 0.54±0.13 to 1.12±0.22 μl/min/mmHg in the Y27 treated eyes (p=0.004, with 30 min perfusion) and increased from 0.58±0.20 to 1.46±0.70 μl/min/mmHg in washout eyes, (p<0.01, with 3 hours perfusion).
B: This graph shows the changes in outflow facility versus time in control (circles), Y27 treated (black squares) and washout eyes (black triangles).
Confocal Microscopy
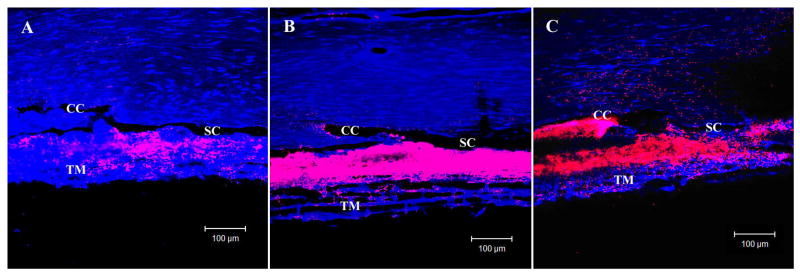
A segmental distribution of tracer in the trabecular meshwork was found by confocal microscopy in control eyes, with punctate labeling along the inner wall that clustered near collector channel ostia (Fig 2A). In contrast, tracer distribution was more uniform throughout the trabecular meshwork in washout and Y27 treated eyes with more extensive labeling along the inner wall of SC (Fig 2B and C).
Figure 2. Confocal Microscopy and Percent Effective Filtration Length (PEFL).
In control eyes (A) tracer distribution was segmental in the trabecular meshwork (TM) with punctuate labeling along the inner wall (IW) of Schlemm's canal (SC) that clustered near collector channel (CC) ostia. In both Y27 treated eyes (B) and washout eyes (C), tracer distribution was more uniform throughout the TM with extensive labeling along the IW.
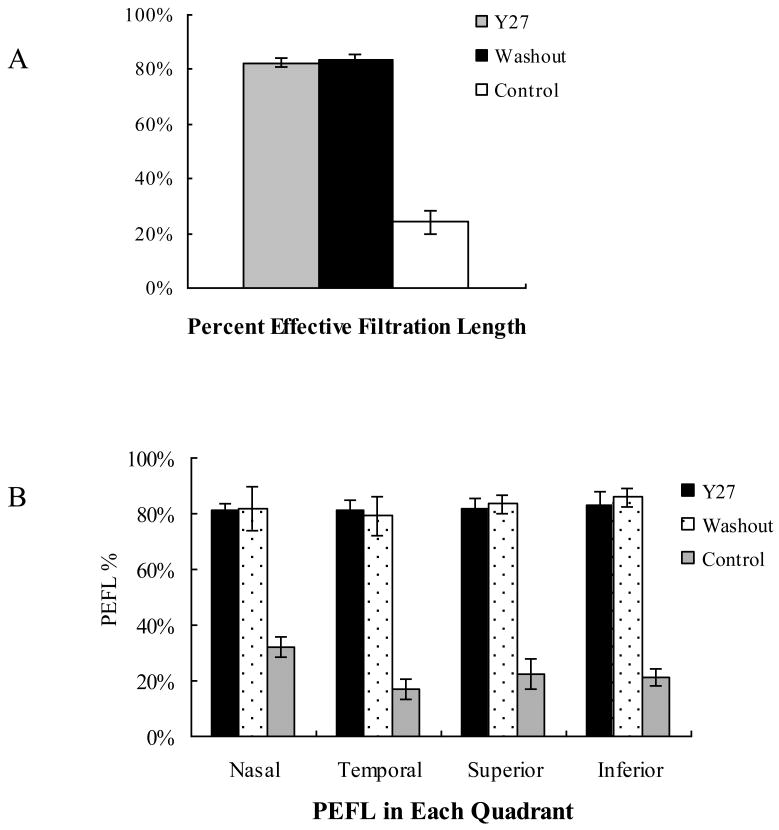
The percent effective filtration length (PEFL) in washout eyes (83.4±2.1%) and in Y27 treated eyes (82.5±1.2%) were both 3.4-fold larger than that of control eyes (24.2±4.2%, p<0.001) (Fig 3A). We further analyzed the PEFL in each quadrant (nasal, temporal, superior and inferior) of control eyes and found that PEFL was 32.2±3.7% in nasal, 16.9±3.8% in temporal, 26.8±5.5% in superior, 21.0±3.1% in inferior. A significant difference was found between the nasal and temporal quadrant (p=0.007) and between the nasal and inferior quadrant (p=0.026); but no significant difference between nasal and superior quadrants (p=0.43). After Y27 treatment, the PEFL increased in all quadrants (nasal: 81.0±2.4%, temporal: 81.1±3.7%, superior: 81.9±3.6%, inferior: 82.8±5.2%) with no significant difference between quadrants. In long-perfused eyes (washout eyes), the PEFL increased in all quadrants (nasal: 81.9±7.9%, temporal: 79.3±7.1%, superior: 83.4±3.5%, inferior: 85.9±3.3%) with no significant difference between quadrants (Figure 3B). This is similar with the Y27 treated eyes.
Figure 3. Measurement of Percent Effective Filtration Length (PEFL).
A: The average PEFL (D) was 3.4-fold larger in both washout eyes (83.4±2.1%) and Y27 treated eyes (82.5±1.6%) compared to control eyes (24.2±4.2%, P<0.001).
B: In control eyes, a significant difference of PEFL was found between the nasal (32.2±3.7%) and temporal quadrants (16.9±3.8% p=0.007) and between the nasal and inferior quadrants (21.0±3.1% p=0.026); but no significant difference between nasal and superior quadrants (26.8±5.5%, p=0.43). After Y27 treatment, the PEFL increased in all quadrants (nasal: 81.0±2.4%, temporal: 81.1±3.7%, superior: 81.9±3.6%, inferior: 82.8±5.2%) with no significant difference between quadrants. In long perfused eyes (washout), the PEFL increased in all quadrants (nasal: 81.9±7.9%, temporal: 79.3±7.1%, superior: 83.4±3.5%, inferior: 85.9±3.3%) with no significant difference between quadrants.
Light Microscopy
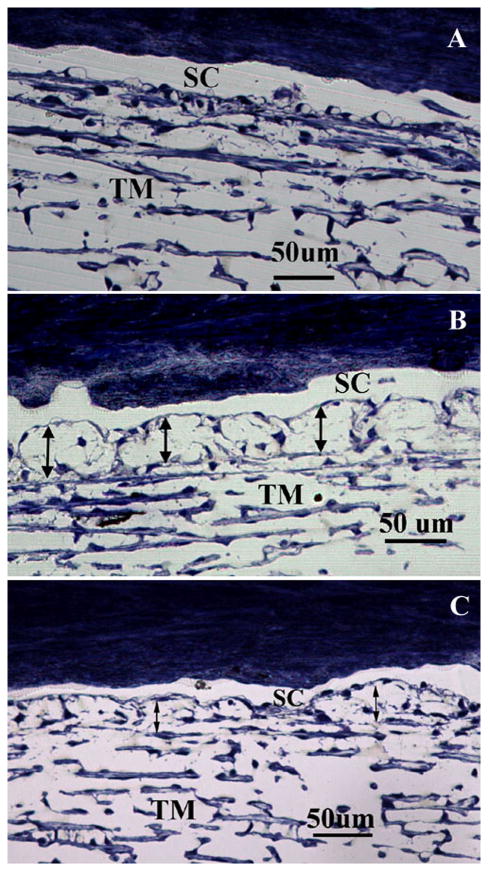
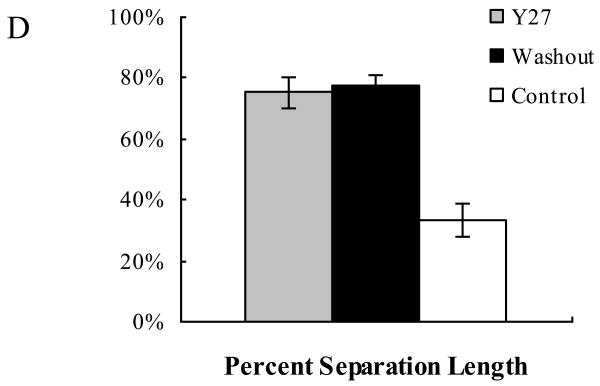
Light microscopy analysis revealed that the inner wall of SC appeared mostly in contact with the underlying JCT matrix in control eyes (Fig 4A). In contrast, a separation between the inner wall and JCT was observed in both washout and Y27 treated eyes (Fig 4B and C). In some areas the inner wall appeared to be ballooned towards the outer wall and sometimes in direct contact with it. The percentage separation length (PSL) was 2.3-fold larger in washout eyes (77.4±3.3%) and 2.2-fold larger in Y27 treated eyes (75.2±4.0%) than in control eyes (33.5±5.3%, p<0.001) (Fig 4D).
Figure 4. Light Microscopy and Separation Length.
In control eyes (A) the inner wall (IW) of Schlemm's canal (SC) appeared in contact with the underlying JCT matrix. In Y27 treated eyes (B) and washout eyes (C), the IW and JCT were significantly distended compared to that of control eyes, with discernable separation between the IW and JCT and focal herniations into the lumen of the SC. The PSL (D) in the JCT region was 2.3-fold larger in washout eyes (77.4±3.3%) and 2.2-fold larger in Y27 treated eyes (75.2±5.3%) compared to the control eyes (33.5±5.3%, p=0.001). TM: Trabecular meshwork
Analysis of 64 images in each group showed more giant vacuoles per 100 μm of inner wall in Y27 treated eyes (4.0 ± 0.3) compared to control eyes (2.4 ± 0.3, p = 0.005). But we did not find a significant difference of giant vacuoles between washout eyes (2.4 ± 0.2; N = 71) and control eyes (2.4 ± 0.3; N = 64, p = 0.99).
Transmission Electron Microscopy
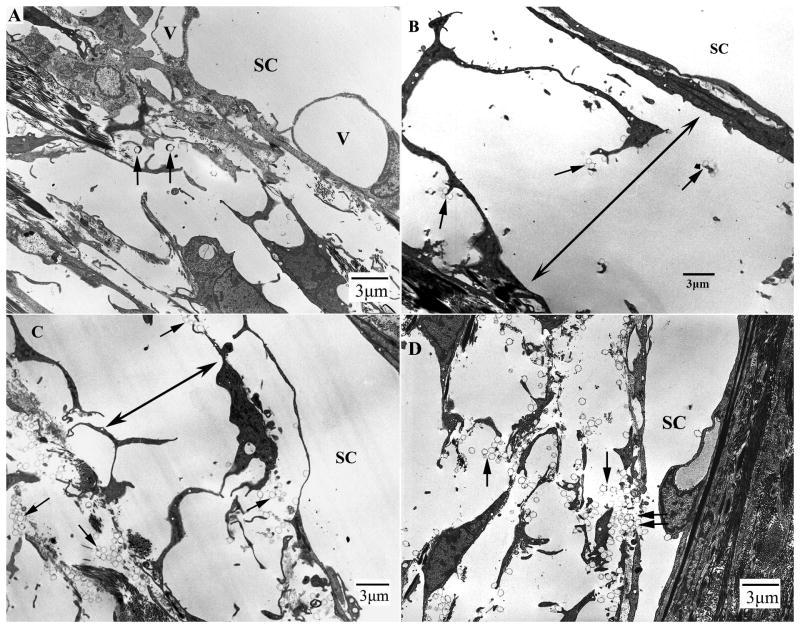
In control eyes, most areas of the inner wall of SC remained in contact with the underlying matrix structures. However, a few areas showed separations between the JCT cells and between the JCT cells and their matrix (not shown). In contrast, significant expansions or separation in the JCT regions were found in both washout and Y27 treated monkey eyes. What appeared to be separations between the inner wall and JCT under light microscopy were actually separations between the JCT cells and between the JCT cells and their matrix in most of areas. In the separated region, the JCT cells appeared stretched with long and slim cell process (Fig 5A, B and C). Microspheres were found in the open spaces of the JCT, and more often found in the JCT underneath the border pores (pores between endothelial cells) or gaps between the inner wall cells. (Fig 5D)
Figure 5. Electron Microscopy.
A: In control eyes, the inner wall (IW) of Schlemm's canal (SC) appeared attached to the underlying JCT structures in most regions. Giant vacuoles (V) were seen along the IW of SC. Microspheres (arrows) were usually seen in the open spaces of the JCT region.
B: In Y27 treated eyes, the IW appeared distended to SC. The JCT appeared loose and disorganized. Significant separations between the IW and JCT were found (double headed arrows). The separation was mainly between JCT cells and the JCT cells and their extracellular matrix. In separated areas, the connections between JCT cells were lost and less extracellular matrix was seen. Microspheres (arrows) were seen in the open spaces of the JCT region.
C: In washout eyes, similar separations between the IW and JCT were found (double headed arrows). The separation was mainly between JCT cells and the JCT cells and their extracellular matrix. In separated areas, the connections between JCT cells were lost. Microspheres (arrows) were seen in the JCT region and under the inner wall.
D: Microspheres (arrows) were found in the open spaces of the JCT, and more often found in the JCT underneath the border pores (pores between endothelial cells or gaps between the inner wall cells (double arrows).
Correlation Analysis
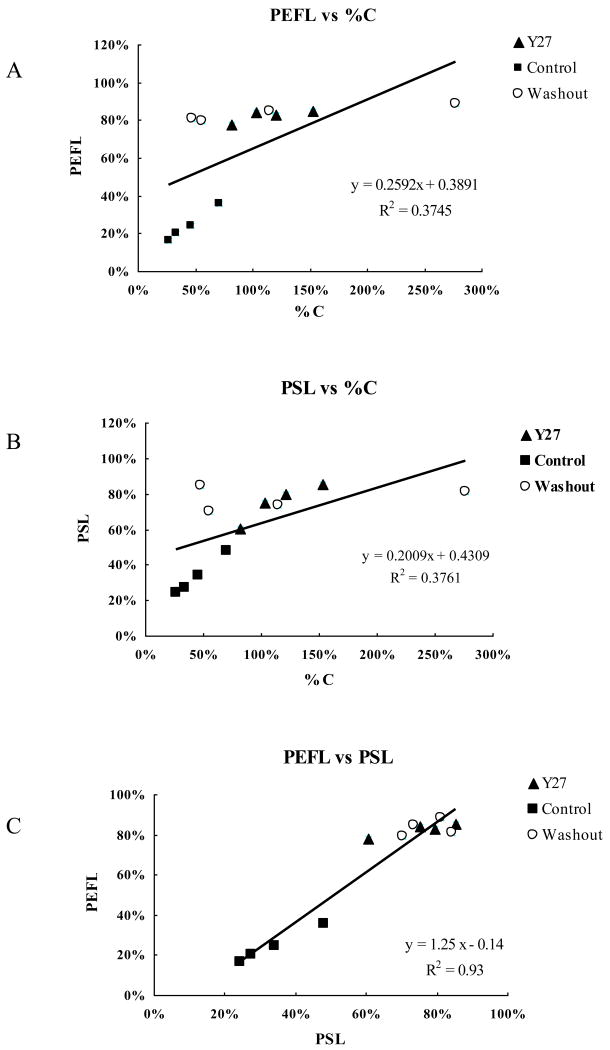
All three groups of eyes were put together in the correlation analysis, including the Y27 treated eyes, control eyes and washout eyes. Significant positive correlations were found between the percent effective filtration length (PEFL) and the percent facility change (%C) (p=0.03, Fig 6A), the percent separation length (PSL) and the percent facility change (%C) (p=0.03, Fig 6B) and between percent effective filtration length (PEFL) and percent separation length (PSL) (p<0.001, Fig 6C).
Figure 6. Correlation Analysis.
There are significant positive correlations between the percent effective filtration length (PEFL) and percent change of outflow facility (%C, p=0.03) (A), percent separation length (PSL) in the JCT and percent change of outflow facility (%C, p=0.03 (B), and between PEFL and PSL (P<0.001) (C).
Discussion
In this study, we investigated the effects of Rho-kinase inhibitor Y-27 and washout on outflow facility, hydrodynamic patterns of outflow, and morphology of the JCT and inner wall of SC to determine whether the monkey eye would respond in the same way as the bovine eye with which it shares little similarity regarding the anatomy of its outflow system[Lu et al., 2008; Scott et al., 2009]. Despite the fact that the outflow pathway of monkeys is far more similar to that of the human eye than to the bovine eye, previous physiologicall studies of outflow have indicated that the monkey eye behaves more like bovine than human eyes[Gong et al., 2009]. In studies of aqueous outflow dynamics both bovine and monkey eyes exhibit a washout effect[Erickson et al., 1981; Kaufman et al., 1988; Overby et al., 2002; Scott et al., 2007], a phenomenon that does not occur in flow studies using human eyes[Erickson-Lamy et al., 1990; Scott et al., 2007]. When the effects of Rho-kinase inhibitor Y-27 and washout were compared in the monkey eye, both led to: (i) a significant increase in outflow facility; (ii) a more uniform tracer distribution along the inner wall of SC and a significant increase in the PEFL; and (iii) a significant increase in the PSL that was characterized by separation between JCT cells and between the JCT cells and their matrix; (iv) significant positive correlations between outflow facility and PEFL, between outflow facility and PSL and between PEFL and PSL. These changes are similar to previous findings in bovine eyes[Lu et al., 2008; Scott et al., 2009]. Although outflow facility increased after both washout induction and Y27 treatment, a greater increase in outflow facility was found after washout compared to Y-27 treatment in both bovine[Lu et al., 2008; Scott et al., 2009] and monkey eyes. This may be due to the shorter time and smaller volume perfused in the Y27 treated group (30 minutes) than washout group (3 hours).
Similar to our previous bovine studies[Lu et al., 2008; Overby et al., 2002; Scott et al., 2007], a positive correlation was found between the outflow facility and PSL in monkey eyes after either Y-27 treatment or induction of washout. However, separation in bovine eyes was found between the basal lamina of the inner wall endothelial cells and the extracellular matrix of the JCT (matrix-matrix separation). In contrast, separation in monkey eyes was found between the JCT cells (cell-cell separation) or between JCT cells and their matrix (cell-matrix separation), a similar morphological change was previously reported after latrunculin-B treatment[Sabanay et al., 2006]. These results suggest that monkey eyes may have a stronger connectivity between the basal lamina of the inner wall cells and the JCT than bovine eyes. Interestingly, both of these morphological changes resulted in a similar increase in PEFL, which in turn positively correlated with an increase in outflow facility. Our data suggest that loss of adhesion or separation between the inner wall and JCT (bovine) or the JCT region (monkey) may be responsible for the hydrodynamic redistribution of outflow patterns and increased facility. Y-27 and washout may act through a similar mechanism by directing the aqueous outflow through a larger and looser area of the JCT. Our previous and current data also suggests that PEFL may be the best anatomical parameter yet found when seeking reliable correlates for physiological parameters related to the outflow system[Battista et al., 2008; Lu et al., 2008; Scott et al., 2009].
In this study, a significant increase in the number of giant vacuoles was found per 100 μm of inner wall in Y-27 treated eyes compared to control eyes (p = 0.005). This finding is consistent with previous observations in porcine [Rao et al., 2001] and bovine eyes[Lu et al., 2008]. As a Rho kinase inhibitor, Y-27 can relax the cytoskeleton and weaken the connection between JCT cells and between JCT cells and their matrix. This has been supported by the light and the transmission electron microscopy in this study. The JCT cells and tissue became looser and expanded after Y-27 treatment possibly making it easier to form giant vacuoles. Similar increases in the number of giant vacuoles was not found in washout eyes although the majority of morphological changes found after washout were similar to those after Y-27 treatment in both bovine and monkey eyes. This may imply that the mechanism to increase outflow facility by Y-27 and washout is different to some extent. Further study is warranted to determine whether Y-27 and washout act through similar or different pathways.
In summary, this study demonstrated that similar hydrodynamic and morphological changes occurred in the aqueous humor outflow pathway of monkey eyes after induction of washout and Y-27632 treatment. Both Y-27 and washout increase outflow facility by redistributing aqueous outflow through a larger area in the JCT. These hydrodynamic changes are likely driven by morphologic changes associated with a decrease in cell-cell and cell-matrix connections in the JCT. A significant positive correlation between the percent effective filtration length (PEFL) and percent facility change (%C) (p=0.03, Fig 6A), percent separation length (PSL) of the inner wall and JCT and percent facility change (%C) (p=0.03, Fig 6B) and between percent effective filtration length (PEFL) and percent separation length (PSL) (p<0.001, Fig 6C) suggests that the outflow facility, outflow hydrodynamics and morphology are likely coupled. It is our belief that these 3 inter-dependent phenomena together provide a mechanism explaining how outflow facility could be influenced in a similar way under different experimental conditions in both bovine[Lu et al., 2008; Scott et al., 2009] and monkey eyes. Since the human eye does not exhibit washout, it would be interesting to study the effects of Y-27 on human eyes in the future, using PSL and PEFL as the prime correlates for outflow kinetics. If Y-27 is able to induce an effect equivalent to washout in the human eye, a new approach to enhancing outflow to reduce intraocular pressure in glaucoma could be an end result.
Research Highlights.
Facility and morphology of outflow were studied after Y27632 treatment and washout.
Both Y27632 treatment and washout increase outflow facility in monkey eyes.
This was positively correlated with an increase of effective filtration area.
This was positively correlated with an increase of separation in the JCT.
This may be caused by a decrease in cell-cell and cell-matrix connections in the JCT.
Acknowledgments
We thank Kristine Erickson for the generous use of her ocular perfusion system, Rozanne Richman, MS for technical assistance and Dr. Patrick Scott for valuable scientific discussion
Grant Support: National Glaucoma Research, a program of the American Health Assistant Foundation, Wing Tat Lee Fund from Boston University School of Medicine, NIH -EY018712; The Massachusetts Lions Eye Research Fund to Boston University and Shanghai Science and Technology Commission, Grant 08JC 140330
Footnotes
Commercial Relationships: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bahler CK, Hann CR, Fautsch MP, Johnson DH. Pharmacologic disruption of Schlemm's canal cells and outflow facility in anterior segments of human eyes. Invest Ophthalmol Vis Sci. 2004;45:2246–54. doi: 10.1167/iovs.03-0746. [DOI] [PubMed] [Google Scholar]
- Barany EH, Scotchbrook S. Influence of testicular hyaluronidase on the resistance to flow through the angle of the anterior chamber. Acta Physiol Scand. 1954;30:240–8. doi: 10.1111/j.1748-1716.1954.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Barany EH, Woodin AM. Hyaluronic acid and hyaluronidase in the aqueous humour and the angle of the anterior chamber. Acta Physiol Scand. 1955;33:257–90. doi: 10.1111/j.1748-1716.1955.tb01208.x. [DOI] [PubMed] [Google Scholar]
- Battista SA, Lu Z, Hofmann S, Freddo T, Overby DR, Gong H. Reduction of the available area for aqueous humor outflow and increase in meshwork herniations into collector channels following acute IOP elevation in bovine eyes. Invest Ophthalmol Vis Sci. 2008;49:5346–52. doi: 10.1167/iovs.08-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DL. Open angle glaucoma. Why not a cure? Arch Ophthalmol. 1987;105:1187–8. doi: 10.1001/archopht.1987.01060090045023. [DOI] [PubMed] [Google Scholar]
- Epstein DL, Rohen JW. Morphology of the trabecular meshwork and inner-wall endothelium after cationized ferritin perfusion in the monkey eye. Invest Ophthalmol Vis Sci. 1991;32:160–71. [PubMed] [Google Scholar]
- Erickson-Lamy K, Rohen JW, Grant WM. Outflow facility studies in the perfused bovine aqueous outflow pathways. Curr Eye Res. 1988;7:799–807. doi: 10.3109/02713688809033211. [DOI] [PubMed] [Google Scholar]
- Erickson-Lamy K, Schroeder AM, Bassett-Chu S, Epstein DL. Absence of time-dependent facility increase (“washout”) in the perfused enucleated human eye. Invest Ophthalmol Vis Sci. 1990;31:2384–8. [PubMed] [Google Scholar]
- Erickson KA, Kaufman PL. Comparative effects of three ocular perfusates on outflow facility in the cynomolgus monkey. Curr Eye Res. 1981;1:211–6. doi: 10.3109/02713688109001851. [DOI] [PubMed] [Google Scholar]
- Ethier CR, Read AT, Chan DW. Effects of latrunculin-B on outflow facility and trabecular meshwork structure in human eyes. Invest Ophthalmol Vis Sci. 2006;47:1991–8. doi: 10.1167/iovs.05-0327. [DOI] [PubMed] [Google Scholar]
- Gong H, Tripathi RC, Tripathi BJ. Morphology of the aqueous outflow pathway. Microsc Res Tech. 1996;33:336–67. doi: 10.1002/(SICI)1097-0029(19960301)33:4<336::AID-JEMT4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Gong H, Freddo TF. The washout phenomenon in aqueous outflow--why does it matter? Exp Eye Res. 2009;88:729–37. doi: 10.1016/j.exer.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong HY, Trinkaus-Randall V, Freddo TF. Ultrastructural immunocytochemical localization of elastin in normal human trabecular meshwork. Curr Eye Res. 1989;8:1071–82. doi: 10.3109/02713688908997400. [DOI] [PubMed] [Google Scholar]
- Grant WM. Further studies on facility of flow through the trabecular meshwork. AMA Arch Ophthalmol. 1958;60:523–33. doi: 10.1001/archopht.1958.00940080541001. [DOI] [PubMed] [Google Scholar]
- Grant WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol. 1963;69:783–801. doi: 10.1001/archopht.1963.00960040789022. [DOI] [PubMed] [Google Scholar]
- Hashimoto JM, Epstein DL. Influence of intraocular pressure on aqueous outflow facility in enucleated eyes of different mammals. Invest Ophthalmol Vis Sci. 1980;19:1483–9. [PubMed] [Google Scholar]
- Honjo M, Inatani M, Kido N, Sawamura T, Yue BY, Honda Y, Tanihara H. Effects of protein kinase inhibitor, HA1077, on intraocular pressure and outflow facility in rabbit eyes. Arch Ophthalmol. 2001;119:1171–8. doi: 10.1001/archopht.119.8.1171. [DOI] [PubMed] [Google Scholar]
- Honjo M, Tanihara H, Inatani M, Kido N, Sawamura T, Yue BY, Narumiya S, Honda Y. Effects of rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Invest Ophthalmol Vis Sci. 2001;42:137–44. [PubMed] [Google Scholar]
- Ishizaki T, Uehata M, Tamechika I, Keel J, Nonomura K, Maekawa M, Narumiya S. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol Pharmacol. 2000;57:976–83. [PubMed] [Google Scholar]
- Johnson DH. The effect of cytochalasin D on outflow facility and the trabecular meshwork of the human eye in perfusion organ culture. Invest Ophthalmol Vis Sci. 1997;38:2790–9. [PubMed] [Google Scholar]
- Johnson M, Johnson DH, Kamm RD, DeKater AW, Epstein DL. The filtration characteristics of the aqueous outflow system. Exp Eye Res. 1990;50:407–18. doi: 10.1016/0014-4835(90)90142-h. [DOI] [PubMed] [Google Scholar]
- Johnson M, Erickson K. Mechanisms and routes of aqueous humor drainage. WB Sanders Co.; Philadelphia, USA: 2000. [Google Scholar]
- Kaibuchi K, Kuroda S, Amano M. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu Rev Biochem. 1999;68:459–86. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- Kaufman PL, Erickson KA. Cytochalasin B and D dose-outflow facility response relationships in the cynomolgus monkey. Invest Ophthalmol Vis Sci. 1982;23:646–50. [PubMed] [Google Scholar]
- Kaufman PL, True-Gabelt B, Erickson-Lamy KA. Time-dependence of perfusion outflow facility in the cynomolgus monkey. Curr Eye Res. 1988;7:721–6. doi: 10.3109/02713688809033201. [DOI] [PubMed] [Google Scholar]
- Lu Z, Overby DR, Scott PA, Freddo TF, Gong H. The mechanism of increasing outflow facility by rho-kinase inhibition with Y-27632 in bovine eyes. Exp Eye Res. 2008;86:271–81. doi: 10.1016/j.exer.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutjen-Drecoll E. Functional morphology of the trabecular meshwork in primate eyes. Prog Retin Eye Res. 1999;18:91–119. doi: 10.1016/s1350-9462(98)00011-1. [DOI] [PubMed] [Google Scholar]
- Maepea O, Bill A. The pressures in the episcleral veins, Schlemm's canal and the trabecular meshwork in monkeys: effects of changes in intraocular pressure. Exp Eye Res. 1989;49:645–63. doi: 10.1016/s0014-4835(89)80060-0. [DOI] [PubMed] [Google Scholar]
- Maepea O, Bill A. Pressures in the juxtacanalicular tissue and Schlemm's canal in monkeys. Exp Eye Res. 1992;54:879–83. doi: 10.1016/0014-4835(92)90151-h. [DOI] [PubMed] [Google Scholar]
- Melton CE, DeVille WB. Perfusion studies on eyes of four species. Am J Ophthalmol. 1960;50:302–8. [Google Scholar]
- Overby D, Gong H, Qiu G, Freddo TF, Johnson M. The mechanism of increasing outflow facility during washout in the bovine eye. Invest Ophthalmol Vis Sci. 2002;43:3455–64. [PubMed] [Google Scholar]
- Parc CE, Johnson DH, Brilakis HS. Giant vacuoles are found preferentially near collector channels. Invest Ophthalmol Vis Sci. 2000;41:2984–90. [PubMed] [Google Scholar]
- Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci. 2001;42:1029–37. [PubMed] [Google Scholar]
- Rohen JW, Futa R, Lutjen-Drecoll E. The fine structure of the cribriform meshwork in normal and glaucomatous eyes as seen in tangential sections. Invest Ophthalmol Vis Sci. 1981;21:574–85. [PubMed] [Google Scholar]
- Rosenthal R, Choritz L, Schlott S, Bechrakis NE, Jaroszewski J, Wiederholt M, Thieme H. Effects of ML-7 and Y-27632 on carbachol- and endothelin-1-induced contraction of bovine trabecular meshwork. Exp Eye Res. 2005;80:837–45. doi: 10.1016/j.exer.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Sabanay I, Tian B, Gabelt BT, Geiger B, Kaufman PL. Functional and structural reversibility of H-7 effects on the conventional aqueous outflow pathway in monkeys. Exp Eye Res. 2004;78:137–50. doi: 10.1016/j.exer.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Sabanay I, Tian B, Gabelt BT, Geiger B, Kaufman PL. Latrunculin B effects on trabecular meshwork and corneal endothelial morphology in monkeys. Exp Eye Res. 2006;82:236–46. doi: 10.1016/j.exer.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Scott PA, Overby DR, Freddo TF, Gong H. Comparative studies between species that do and do not exhibit the washout effect. Exp Eye Res. 2007;84:435–43. doi: 10.1016/j.exer.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott PA, Lu Z, Liu Y, Gong H. Relationships between increased aqueous outflow facility during washout with the changes in hydrodynamic pattern and morphology in bovine aqueous outflow pathways. Exp Eye Res. 2009;89:942–9. doi: 10.1016/j.exer.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Sit AJ, Coloma FM, Ethier CR, Johnson M. Factors affecting the pores of the inner wall endothelium of Schlemm's canal. Invest Ophthalmol Vis Sci. 1997;38:1517–25. [PubMed] [Google Scholar]
- Sit AJ, Gong H, Ritter N, Freddo TF, Kamm R, Johnson M. The role of soluble proteins in generating aqueous outflow resistance in the bovine and human eye. Exp Eye Res. 1997;64:813–21. doi: 10.1006/exer.1997.0276. [DOI] [PubMed] [Google Scholar]
- Tian B, Gabelt BT, Geiger B, Kaufman PL. Combined effects of H-7 and cytochalasin B on outflow facility in monkeys. Exp Eye Res. 1999;68:649–55. doi: 10.1006/exer.1998.0647. [DOI] [PubMed] [Google Scholar]
- Tian B, Kaufman PL. Effects of the Rho kinase inhibitor Y-27632 and the phosphatase inhibitor calyculin A on outflow facility in monkeys. Exp Eye Res. 2005;80:215–25. doi: 10.1016/j.exer.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Toris CB, Zhan GL, Wang YL, Zhao J, McLaughlin MA, Camras CB, Yablonski ME. Aqueous humor dynamics in monkeys with laser-induced glaucoma. J Ocul Pharmacol Ther. 2000;16:19–27. doi: 10.1089/jop.2000.16.19. [DOI] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–4. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Van Buskirk EM, Brett J. The canine eye: in vitro dissolution of the barriers to aqueous outflow. Invest Ophthalmol Vis Sci. 1978;17:258–71. [PubMed] [Google Scholar]
- Waki M, Yoshida Y, Oka T, Azuma M. Reduction of intraocular pressure by topical administration of an inhibitor of the Rho-associated protein kinase. Curr Eye Res. 2001;22:470–4. doi: 10.1076/ceyr.22.6.470.5489. [DOI] [PubMed] [Google Scholar]
- Yan DB, Trope GE, Ethier CR, Menon IA, Wakeham A. Effects of hydrogen peroxide-induced oxidative damage on outflow facility and washout in pig eyes. Invest Ophthalmol Vis Sci. 1991;32:2515–20. [PubMed] [Google Scholar]