Abstract
Regulatory T cells (Treg) play a critical role in the immune system to regulate peripheral tolerance and prevent autoimmunity. However the relative importance of different mechanisms of Treg function remains obscure. Here, we reveal a limited role for programmed cell death pathways in mediating Treg suppression of conventional T cells (Tconv). We show that Tregs are able to suppress the proliferation of Tconv cells that are resistant to apoptosis (Bim−/−, Bim−/−Puma−/−, Bcl2 Tg) or RIPK-dependent necrosis (also referred to as regulated necrosis or necroptosis) (Ripk3−/−) in several in vitro and in vivo assays. These data suggest that programmed cell death pathways, such as apoptosis and RIPK-dependent necrosis, are not required for Treg-mediated suppression.
Keywords: T cells, Suppression, Apoptosis, Necrosis, Necroptosis, Bim, Puma, Bcl-2, Ripk3
Introduction
Regulatory T cells (Treg)3 are potent mediators of immune regulation and play a key role in maintaining peripheral tolerance. A number of Treg populations have been identified, primarily based on their origin of development (thymus versus periphery) and the factors which induce their development (Foxp3, TGFβ, IL-2, retinoic acid, IL-10, IL-35) (1–3). Although a broad array of suppressive mechanisms have been proposed to mediate Treg function, the relative contribution and importance of these mechanisms remains controversial. It has been proposed that Tregs suppress Tconv cells by causing IL-2 deprivation-mediated apoptosis (4). High IL-2 receptor (CD25) expression on Tregs may lead to increased IL-2 ‘consumption’ effectively depleting the local surroundings and thereby starving Tconv cells of this important growth factor that is required for their survival. However, the relative contribution of this mechanism is controversial as more recent studies have shown that IL-2 depletion alone is not required for the suppression of human T cells (5,6). Furthermore, the general contribution of cell death pathways in mediating Treg cell function remains unclear.
Two forms of programmed cell death have been described: apoptosis and RIPK-dependent necrosis. Apoptosis in response to a variety of stimuli is regulated by members of the B cell lymphoma 2 (Bcl-2) family (7). Cells from mice overexpressing Bcl-2, an anti-apoptotic molecule that inhibits the mitochondrial death pathway, are resistant to apoptosis induced by growth factor and cytokine deprivation, radiation exposure, and treatment with glucocorticolds, phorbol esters, ionomycin and sodium azide (8,9). The pro-apoptotic molecule Bim (encoded by the Bcl2l11 gene), in its active state, binds to Bcl-2 in response to stress signals, such as growth factor deprivation, thereby priming the mitochondrial pathway of apoptosis (10). Bim−/− T cells are resistant to apoptosis induced by cytokine or growth factor withdrawal, particularly IL-2 (11). The BH-3 only gene Puma (encoded by the Bbc3 gene) is a transcriptional target of the tumor suppressor p53 (12,13). Lymphocytes from Puma−/− mice are highly resistant to DNA damaging drugs and γ irradiation. These cells also have decreased sensitivity to p53-independent cell death stimuli such as growth factor deprivation and treatment with dexamethasone and phorbol esters (13). Analysis of Bim−/−Puma−/− mice show that these two proteins cooperate in mediating apoptosis of T cells during development, following activation (14,15)and upon cytokine withdrawal (16,17). RIPK-dependent necrosis (also referred to as regulated necrosis or necroptosis) (18), is a recently described novel form of programmed cell death that requires the receptor-interacting serine-threonine kinases Ripk1 and Ripk3 (16,19,20). While the mitochondrial pathway of apoptosis is a major mechanism of mammalian cell death, there are other relevant apoptotic and non-apoptotic cell death pathways. These include the death receptor, inflammasome and caspase-2 pathways of apoptosis, and active necrosis mediated by the mitochondrial permeability transition and by receptor interacting protein kinases (21).
In this study we ask if the two forms of programmed cell death, apoptosis and RIPK-dependent necrosis, contribute to the mechanisms used by Treg cells to mediate suppression. This is particularly relevant given previous suggestions that Tregs mediate suppression via cytokine deprivation-mediated apoptosis (specifically IL-2) which is blocked by loss of Bim expression (4). Thus, we asked whether Tregs are capable of suppressing Tconv cells that are resistant to apoptosis (Bim−/−, Bim−/−Puma−/−, Bcl-2 transgenic) and RIPK-dependent necrosis (Ripk3−/−).
Materials and Methods
Mice
C57BL/6 (WT) mice were obtained from The Jackson Laboratory. Bim−/− mice were provided by Andreas Strasser (The Walter and Eliza Hall Institute of Medical Research, Parkville, Australia) (11). Puma−/− mice were provided by Gerard Zambetti (St. Jude Children’s Research Hospital, Memphis, TN) (13). Bcl-2 transgenic mice were provided by John Reed (Salk Institute, La Jolla, CA) (8). Ripk3−/− mice were provided by Vishva Dixit (Genentech Inc., CA) (22). These, as well as Rag1−/−CD3εζ−/− and Rag1−/− mice, were bred and maintained at St. Jude Children’s Research Hospital. All animal experiments were performed in American Association for the Accreditation of Laboratory Animal Care-accredited, specific pathogen-, Helicobacter-, and MNV-free facilities following national, state and institutional guidelines. Animal protocols were approved by the St. Jude Animal Care and Use Committee.
Cell purification and flow cytometry
Spleens and lymph nodes from mice were processed and stained with fluorochrome-conjugated antibodies and purified by FACS (MoFlo; DakoCytomation) using anti-CD4, anti-CD25 and anti-CD45RB (Tconv cells: CD4+CD45RBhiCD25−; Treg cells: CD4+CD45RBloCD25+ in all assays reported). All antibodies used were from BioLegend or eBiosciences.
In vitro suppression assays
The assay was performed as previously described (23). Briefly, Tconv cells (CD4+CD45RBhiCD25−, 2.5 × 104) were cultured with irradiated splenocytes (5 × 104) and anti-CD3 (1 µg/ml) in a 96-well round bottom plate for 68 h with titrating amounts of Tregs (CD4+CD45RBloCD25+). Cells were pulsed with [3H]-thymidine for the last 8 h of culture and proliferation measured. For CFSE dilution, Tconv cells were purified by FACS, washed in serum-free media, resuspended in serum-free media with 0.5 µM CFSE and incubated for 15 min at 37°C. CFSE was quenched with one volume of FBS and cells were washed, resuspended in complete media and incubated with irradiated splenocytes and anti-CD3 with titrating numbers of Tregs for 60 h. Cells were stained with AnnexinV and 7AAD and CFSE dilution was measured by flow cytometry. Percent suppression is calculated as follows: (Tconv c.p.m. – [Tconv + Treg c.p.m.])/Tconv c.p.m. To assay cell contact-independent Treg suppression (24), 1.25 × 104 Tconv cells from WT, Bim−/− and Ripk3−/− mice were cultured with anti-CD3/anti-CD28 coated magnetic beads (Invitrogen Tosyl-activated Microbeads) (4:1 ratio of beads to cells) in the bottom chamber of a Millipore Millicell 96-transwell plate (0.4uM pore size). In the top chamber, 1.25 × 104 Tregs were cocultured with Tconv cells at a 1:4 ratio (Treg:Tconv) with anti-CD3/anti-CD28 coated magnetic beads. Where indicated, some wells were supplemented with 10µg/mL of isotype control, anti-TGFβ (R&D Systems, clone 1D11), anti-IL-10 (BD Pharmingen, #18141D) or anti-IL-35 (anti-Ebi3, clone V1.4F5.25 (25)) antibodies. Transwell plates were incubated for 72 h at 37°C. Cells in the bottom wells were pulsed with [3H]-thymidine for the last 8 h of culture and proliferation measured (25).
In vivo homeostasis assays
The assay was performed as previously described (26). Briefly, Tconv cells (2 × 106) were injected with and without Tregs (0.5 × 106) via the tail vein into Rag1−/−CD3εζ−/− (Bim−/− Tconv recipients) or Rag1−/− mice (Bcl2 transgenic Tconv recipients). Spleens were analyzed 7 days post-transfer.
Inflammatory Bowel Disease
The colitis recovery model of inflammatory bowel disease (IBD) was used as described previously, with modifications (26). Briefly, 0.4 × 106 purified Tconv cells were injected via the tail vein into Rag1−/− mice. Recipients were monitored and upon clinical signs of disease (5–10% body weight loss, approximately 3–4 weeks post-Tconv cell transfer) were given Tregs (0.5 × 106) intraperitonealy. Spleen and lymph node cellularity and colon pathology was analyzed approximately 8 weeks post-Tconv cell transfer.
Results and Discussion
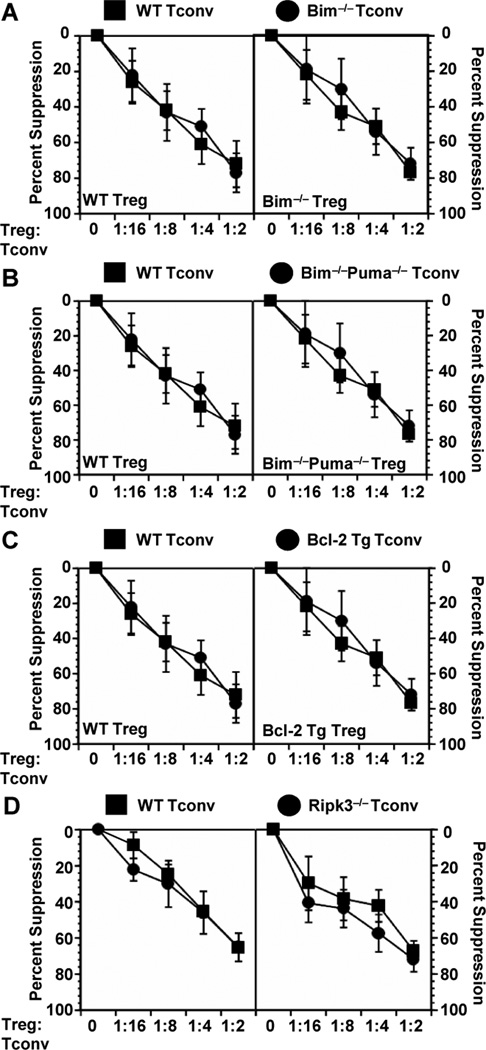
We first assessed the ability of Tregs to suppress Bim−/−, Bim−/−Puma−/−, Bcl-2 transgenic and Ripk3−/− Tconv cells in vitro. Bim−/−Puma−/− mice were included in this analysis as they have been shown to be more resistant to apoptosis induced by cytokine withdrawal than the single knockout mice (17,27). Tconv cells were sorted, incubated with varying concentrations of purified Tregs and proliferation assessed following stimulation by anti-CD3 plus APCs. Tregs from WT mice were able to suppress Tconv cells from Bim−/−, Bim−/−Puma−/−, Bcl-2 transgenic and Ripk3−/− mice in a manner indistinguishable to WT Tconv cells as shown by [3H]-thymidine uptake (Fig. 1). Tregs were also equally capable of suppressing WT, Bim−/− and Ripk3−/− Tconv cells as determined by CFSE dilution (Fig. 2A). Furthermore, no functional defects were observed in the capacity of Tregs from any of the mouse strains used to suppress their corresponding Tconv cell population or WT Tconv cells (Fig. 1). To exclude the possibility that Tconv cell death was occurring in these Treg co-cultures, we stained Tconv cells with AnnexinV and 7AAD. There is no difference in the percentage of live Tconv cells among any of the groups, with an average of just ~3–8% percent of Tconv cells undergoing cell death in the presence of Tregs (Fig. 2B). These results suggest that in vitro Treg-mediated suppression is not mediated via cell death induced by cytokine or growth factor withdrawal as these cells are resistant to death by such mechanisms.
Figure 1.
Tregs can suppress Tconv cells with defects in apoptosis and RIPK-dependent necrosis in vitro. Tconv cells were sorted from spleen and lymph nodes of C57BL/6 (WT), Bim−/− (A), Bim−/−Puma−/− (B), Bcl-2 transgenic (C) and Ripk3−/− (D) mice, and cultured with varying concentrations of Treg for 68 h. Cells were pulsed with [3H]-thymidine for the last 8 h of culture and proliferation measured. Average c.p.m. for Tconv cells alone are as follows: WT = 43000, Bim−/− = 28000, Bcl2 Tg = 13000, Bim−/−Puma−/− = 9000, Ripk3−/− = 81000. Data in each panel depict mean percent suppression ± s.e. from 3 independent experiments with each group assayed in triplicate.
Figure 2.
Tregs can suppress Tconv cells with defects in apoptosis and RIPK-dependent necrosis in the absence of cell death via TGFβ and IL-35. (A) Tconv cells sorted from spleen and lymph nodes of C57BL/6 (WT), Bim−/− and Ripk3−/− mice were labeled with CFSE and cultured with WT Tregs at a 1:2 ratio (Tconv:Treg) for 68 h. Analysis of CFSE dilution was measured by flow cytometry. Data are representative of 3 separate experiments. (B) The Tconv cell populations depicted were stained with fluorochrome-conjugated antibodies to CD4 and AnnexinV, and the viability dye 7AAD and the percent cell death determined by flow cytometry. Data are the mean ± s.e. of 3 independent experiments with each group assayed in triplicate. (C) Tconv cells from WT, Bim−/− and Ripk3−/− mice were cultured with anti-CD3/anti-CD28-coated magnetic beads in the bottom chamber of a 96-transwell plate. In the top chamber, 1.25 × 104 WT Tregs and WT Tconv cells were stimulated with anti-CD3/anti-CD28-coated magnetic beads for 72 h [Note that WT Tconv cells are required in the top chamber to potentiate maximal Treg activity (24). Cultures were supplemented with 10µg/mL of either isotype control, anti-TGFβ, anti-IL-10 or anti-IL-35 antibodies as indicated. Tconv cells in the bottom wells were pulsed with [3H]-thymidine for the last 8 h of culture and proliferation measured. Data are the mean ± s.e. of 3 independent experiments with each group assayed in triplicate.
While it is clear that WT and knockout Tconv cells are being suppressed without undergoing cell death, it is possible that different suppressive mechanisms are being used. To probe this issue, we restricted analysis to contact-independent Treg-mediated suppression via inhibitory cytokines by using Transwell plates in which the Tregs and Tconv cell targets are separated by a permeable membrane. We have previously shown that maximal Treg activity in this setting requires contact with Tconv cells (24) which were included in the top chamber with Tregs, while the WT and mutant target Tconv cells are placed in the bottom chamber. The contribution of TGFβ, IL-10 and IL-35 (see refs (25,28) was assessed by the inclusion of neutralizing antibodies. Comparable Treg-mediated suppression of WT, Bim−/− and Ripk3−/− Tconv cells was observed across the permeable Transwell membrane (Fig. 2C). Furthermore, suppression of all three Tconv cell populations was mediated comparably by TGFβ and IL-35, but not by IL-10, suggesting that genetically induced resistance to apoptosis or RIPK-dependent necrosis did not affect the sensitivity of Tconv cells to inhibitory cytokines and thus the mechanisms used by Tregs to mediate suppression.
Next, we performed in vivo homeostasis assays to determine if Bim-mediated apoptosis, or mechanisms that can be blocked by Bcl-2, play a role in the control of Tconv cell homeostatic expansion. Tconv cells were purified from Bim−/− and Bcl-2 transgenic mice and transferred either alone or with WT Tregs into lymphopenic mice and splenic T cell numbers assessed one week later. Both Bim−/− and Bcl-2 transgenic Tconv cells expanded following transfer (Fig. 3A,B). Importantly, the capacity of Tregs to suppress the homeostatic proliferation of Bim−/− and Bcl-2 transgenic Tconv cells was comparable to WT Tconv cells.
Figure 3.
Tregs can control the homeostatic expansion and colitis caused by Tconv cells with defects in apoptosis. Splenic T cells were sorted from C57BL/6 (WT), Bim−/− (A) and Bcl-2 transgenic (B) mice. Tconv cells were injected into Rag1−/−CD3εζ−/− (A) or Rag1−/− (B) mice, with and without Tregs. Spleens from recipients were analyzed 7 days later. Average percent suppression of Tconv cells is depicted above each group. Each graph is representative of 2 experiments with 4–7 mice per group. *p= <0.5, **p= <0.05, ***p=<0.001. (C-E) Tconv cells were sorted from the spleen and lymph nodes of C57BL/6 (WT) or Bim−/− mice and injected i.v. into the tail vein of Rag1−/− recipients. Loss of body weight was monitored on a weekly and biweekly basis. Approximately 3–4 weeks post-Tconv cell transfer, upon clinical signs of sickness, Tregs were injected i.p. into the Tconv cell recipients. (C) Body weight was monitored an additional 4 weeks. (D−E) Scores and representative histology of the large intestines of the T cell recipients approximately 8 weeks post-Tconv cell transfer and 4 weeks post-Treg transfer are shown. Data are the mean ± s.e of 3 independent experiments with 13–15 (Treg recipients) and 4–6 (controls, no Tregs) mice per group. **p= <0.05.
Lastly, Tregs have been shown to control colitis, a mouse model of IBD, initiated by the transfer of naive Tconv cells into lymphopenic Rag1−/− recipients. We used the recovery method of colitis induction to compare the ability of Tregs to ameliorate disease caused by WT versus Bim−/− Tconv cells (29). This method represents a more physiologic environment in which Tregs would act to exert suppression. WT or Bim−/− Tconv cells were transferred into Rag1−/− recipients and monitored for clinical signs of sickness (weight loss). At approximately 3 to 4 weeks post-Tconv cell transfer, mice began to lose weight and were injected with Tregs and monitored for clinical recovery (weight gain) and histological recovery (using a semi-quantitative grading scheme to score pathology). Control mice that did not receive Tregs continued to lose weight and had a marked increase in large intestine pathology (Fig. 3C,D). However, Bim−/− Tconv cell recipients that received Tregs recovered to the same extent, if not better, than WT Tconv cell recipients receiving Tregs. Bim−/− Tconv cell recipients had restored appetite and resumed weight gain by two weeks post-Treg transfer and had a marked decrease in large intestine pathology as shown by reduction in mean pathology score (Fig. 3D) and reduced inflammation and goblet cell destruction (Fig. 3E). These results demonstrate that Tconv cells that are resistant to apoptosis induced by cytokine and growth factor withdrawal can be suppressed in a disease setting.
Taken together, using several in vitro and in vivo model systems, we show that Tregs can suppress Bim−/−, Bim−/−Puma−/−, Bcl-2 transgenic and Ripk3−/− Tconv cells in a manner largely indistinguishable from WT Tconv cells. These data show that the absence of Bim/Puma-mediated apoptosis, which underlies cytokine deprivation-induced cell death, or RIPK-dependent necrosis have no effect on the capacity of Tregs to mediated suppression. This was somewhat surprising given a report suggesting that Bim−/− Tconv cells cannot be suppressed, implying that Tregs function in a Bim-dependent manner (4). It is unclear why our data and the published observations differ, but this could be due to differences in the experimental systems used. Differences in the Treg/Tconv cell purification (FACS vs. MACS; cell markers used), media selection, type of stimulation and cell numbers used may have contributed to the discrepancies observed, particularly with the in vitro studies. Nevertheless, it is clear that there is suppression in our study without cell death. A strength of our study is the use of multiple genetic models with deficiencies in cell death pathways which collectively demonstrated in two in vivo systems that Tregs are able to suppress proliferation and pathogenicity in the absence of Tconv cell death. Susceptibility to and recovery from colitis is greatly affected by the recipient’s microbiota, which may also contribute to discrepancies between our observations and those from the Lenardo group (4).
Tregs utilize multiple mechanisms to mediate suppression (1). Thus systems which do not present a challenging environment for Treg function can be of limited value in evaluating the contribution of a specific suppressive mechanism. The colitis recovery model that we use is a more robust method for analyzing Treg suppression during colitis and thus an effective approach for revealing critical mechanisms utilized by Tregs. For instance, while a role for IL-10 and IL-35 in Treg suppression is only marginally revealed by in vitro assays, this recovery model clearly demonstrated a key role for these cytokines in Treg function (1). Thus we would argue that if Bim-mediated apoptosis contributed significantly to Treg function, this would have been revealed in this assay. Nevertheless, we cannot rule out the possibility that this mechanism is used by Tregs but only under unique circumstances or disease scenarios. Furthermore, while Bim and Puma are primary mediators of apoptosis, we cannot rule out the possibility that Tregs use mechanisms of cell death that are Bim-, Puma- and Ripk3-independent and not blocked by Bcl-2. Several studies have shown that Tregs do not simply suppress T cells but also convert them to an induced regulatory population via suppressive cytokines such as TGFb, IL-10 and IL-35 (1). Such mechanisms allow Tregs to expand their regulatory control and potency. In contrast, lytic or apoptotic mechanisms might limit the capacity of Tregs to mediate their suppressive capacity. Further analysis will be required to fully assess the relative contribution of the diverse mechanisms used by Tregs to mediate immune control.
Acknowledgements
We would like to thank Andreas Strasser, Gerard Zambetti and John Reed for knockout mice. We are also very grateful to Creg Workman and Ricardo Weinlich for technical assistance, Richard Cross, Greig Lennon, Stephanie Morgan and Jennifer Rogers for FACS, Karen Forbes, Ashley Castellaw, Amy Krause and Chris Dillon for maintenance, breeding and genotyping of mouse colonies, and the staff of the St. Jude Animal Resource Center for the animal husbandry.
This work was supported by the NIH (AI39480, AI091977), the St Jude Cancer Center Support CORE grant (CA-21765) and the American Lebanese Syrian Associated Charities (ALSAC).
Abbreviations used in the paper
- Tconv
conventional T cell
- Treg
regulatory T cell
- WT
wild type
- Tg
transgenic
Reference List
- 1.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev. Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Workman CJ, Szymczak-Workman AL, Collison LW, Pillai MR, Vignali DA. The development and function of regulatory T cells. Cell Mol. Life Sci. 2009;66:2603–2622. doi: 10.1007/s00018-009-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 5.Tran DQ, Glass DD, Uzel G, Darnell DA, Spalding C, Holland SM, Shevach EM. Analysis of adhesion molecules, target cells, and role of IL-2 in human FOXP3+ regulatory T cell suppressor function. J Immunol. 2009;182:2929–2938. doi: 10.4049/jimmunol.0803827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vercoulen Y, Wehrens EJ, van Teijlingen NH, de JW, Beekman JM, Prakken BJ. Human regulatory T cell suppressive function is independent of apoptosis induction in activated effector T cells. PLoS. One. 2009;4:e7183. doi: 10.1371/journal.pone.0007183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marsden VS, Strasser A. Control of apoptosis in the immune system: Bcl-2, BH3-only proteins and more. Annu. Rev. Immunol. 2003;21:71–105. doi: 10.1146/annurev.immunol.21.120601.141029. [DOI] [PubMed] [Google Scholar]
- 8.Strasser A, Harris AW, Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991;67:889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- 9.Ogilvy S, Metcalf D, Print CG, Bath ML, Harris AW, Adams JM. Constitutive Bcl-2 expression throughout the hematopoietic compartment affects multiple lineages and enhances progenitor cell survival. Proc. Natl. Acad. Sci. U. S. A. 1999;96:14943–14948. doi: 10.1073/pnas.96.26.14943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol. Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 12.Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J, MacLean KH, Han J, Chittenden T, Ihle JN, McKinnon PJ, Cleveland JL, Zambetti GP. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4:321–328. doi: 10.1016/s1535-6108(03)00244-7. [DOI] [PubMed] [Google Scholar]
- 13.Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ, Adams JM, Strasser A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 14.Erlacher M, Labi V, Manzl C, Bock G, Tzankov A, Hacker G, Michalak E, Strasser A, Villunger A. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J. Exp. Med. 2006;203:2939–2951. doi: 10.1084/jem.20061552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauer A, Villunger A, Labi V, Fischer SF, Strasser A, Wagner H, Schmid RM, Hacker G. The NF-kappaB regulator Bcl-3 and the BH3-only proteins Bim and Puma control the death of activated T cells. Proc. Natl. Acad. Sci. U. S. A. 2006;103:10979–10984. doi: 10.1073/pnas.0603625103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M, Villunger A, Mak TW. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J. Exp. Med. 2006;203:1657–1663. doi: 10.1084/jem.20060353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, Gottlieb E, Green DR, Hengartner MO, Kepp O, Knight RA, Kumar S, Lipton SA, Lu X, Madeo F, Malorni W, Mehlen P, Nunez G, Peter ME, Piacentini M, Rubinsztein DC, Shi Y, Simon HU, Vandenabeele P, White E, Yuan J, Zhivotovsky B, Melino G, Kroemer G. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death. Differ.Advance online publication. 2011 July 2011;15 doi: 10.1038/cdd.2011.96. (doi:10.1038/cdd.2011.96) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 21.Green DR. A matter of life and death. In: Sever R, Steur R, Schaefer R, editors. Means to an end: apoptosis and other cell death mechanisms. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2010. p. 5. [Google Scholar]
- 22.Newton K, Sun X, Dixit VM. Kinase RIP3 Is Dispensable for Normal NF-{kappa}Bs, Signaling by the B-Cell and T-Cell Receptors, Tumor Necrosis Factor Receptor 1, and Toll-Like Receptors 2 and 4. Mol. Cell. Biol. 2004;24:1464–1469. doi: 10.1128/MCB.24.4.1464-1469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collison LW, Vignali DA. In vitro treg suppression assays. Methods Mol. Biol. 2011;707:21–37. doi: 10.1007/978-1-61737-979-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collison LW, Pillai MR, Chaturvedi V, Vignali DA. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner. J. Immunol. 2009;182:6121–6128. doi: 10.4049/jimmunol.0803646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ, Brown SA, Rehg JE, Jones ML, Ni HT, Artis D, Turk MJ, Vignali DA. IL-35-mediated induction of a potent regulatory T cell population. Nat. Immunol. 2010;11:1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Workman CJ, Collison LW, Bettini M, Pillai MR, Rehg JE, Vignali DA. In vivo treg suppression assays. Methods Mol. Biol. 2011;707:119–156. doi: 10.1007/978-1-61737-979-6_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren D, Tu HC, Kim H, Wang GX, Bean GR, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science. 2010;330:1390–1393. doi: 10.1126/science.1190217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 29.Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol. Rev. 2006;212:256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]