Abstract
We have observed that conditioning for hematopoietic transplantation by lethal irradiation induces a proteolytic microenvironment in bone marrow (BM) that activates the complement cascade (CC). As a result, BM is enriched for proteolytic enzymes and the soluble form of the terminal product of CC activation, the membrane attack complex C5b-C9 (MAC). At the same time proteolytic enzymes induced in irradiated BM impair the chemotactic activity of α-chemokine stromal derived factor-1 (SDF-1). Because SDF-1 is considered a crucial BM chemoattractant for transplanted hematopoietic stem/progenitor cells (HSPCs), we sought to determine whether other factors that are resistant to proteolytic enzymes play a role in this process, focusing on proteolysis-resistant bioactive lipids. We found that the concentrations of sphingosine-1-phosphate (S1P) and ceramide-1-phosphate (C1P) increase in BM after conditioning for transplantation and that both S1P and, as we show here for the first time, C1P are potent chemottractants for HSPCs. Next, we observed that C5-deficient mice that do not generate MAC show impaired engraftment of HSPCs. In support of a role for MAC in homing and engraftment, we found that soluble MAC (sMAC) enhances in a CR3 (CD11b/CD18)-dependent manner adhesion of HSPCs to BM stromal cells and increases the secretion of SDF-1 by BM stroma. We conclude that an increase in the BM levels of proteolytic enzyme-resistant S1P and C1P and activation of CC, which leads to the generation of MAC, plays an important and previously underappreciated role in the homing of transplanted HSPCs.
Keywords: MAC, C1P, S1P, SDF-1, CXCR4, stem cell homing
Introduction
Hematopoietic stem progenitor cells (HSPCs) are retained in bone marrow (BM) niches due to the stromal-derived growth factor-1 (SDF-1)–CXCR4 receptor axis and interactions between Very Late Antigen-4 (VLA-4, also known as α4β1 integrin) and its ligand, Vascular Adhesion Molecule-1 (VCAM-1, also known as CD106). While HSPCs express CXCR4 and VLA-4, their corresponding ligands, SDF-1 and VCAM-1, are expressed by cells in the BM microenvironment (e.g., osteoblasts and fibroblasts) 1, 2. We have also reported that activation of the complement cascade (CC) may play an important role in stem cell homing, where iC3b - a solid phase product of CC activation, deposited on BM endothelium and stromal cells tethers CR3 (CD11b/CD18)+ HSPCs to stroma damaged by myeloablative conditioning for transplantation 3.
It is well known that SDF-1, when employed at supra-physiological concentrations, is a potent in vitro chemoattractant for HSPCs. However, because, as we have observed, myeloablative conditioning for hematopoietic transplantation induces a highly proteolytic microenvironment in BM, and SDF-1 is extremely sensitive to degradation by proteolytic enzymes, SDF-1 secreted by stromal cells and osteoblasts is rapidly degraded under these conditions4, 5. Therefore, while a role for the SDF-1–CXCR4 axis in retention of HSPCs in BM under normal conditions is undisputed, its role following myeloablative conditioning is somewhat less certain and some redundant homing mechanisms probably exist. This latter notion is supported by several observations, such as that i) CXCR4−/− fetal liver HSPCs home to BM in an SDF-1-independent manner 5,6, ii) homing of murine HSPCs made refractory to SDF-1 by incubation and co-injection with a CXCR4 receptor antagonist (AMD3100) is normal or only mildly reduced 7, and iii) HSPCs in which CXCR4 has been knocked down by means of an SDF-1 intrakine strategy are able to engraft, even in lethally irradiated recipients 8.
Therefore, we began a search for potential chemoattractants that could direct trafficking of HSPCs, with bioactive lipids as strong candidates, because, lacking peptide bonds, they are resistant to degradation by proteases. We focused in particular on ceramide-1-phosphate (C1P) and sphingosine-1-phosphate (S1P), which are products of membrane lipid metabolism. It is known that S1P is secreted from cells, while C1P is retained intracellularly and is typically released following cell damage 9–13. Our mass spectrometry (MS) analysis revealed that the major isoforms of C1P and S1P were detected at higher concentrations in supernatants harvested from irradiated BM than supernatants from non-irradiated BM, which suggests that these bioactive lipids and chemoattractants are released from “leaky” BM cells damaged by myeloablative irradiation.
Furthermore, we observed that conditioning for hematopoietic transplantation by lethal irradiation activates the complement cascade (CC) in the BM microenvironment, with the accumulation of soluble C5b-C9 membrane attack complex (MAC). The role of CC and soluble MAC (sMAC) in homing was further supported by the fact that C5-deficient mice, which do not generate MAC, exhibit impaired engraftment of HSPCs compared to normal littermates. In support of a role for sMAC in homing and engraftment, we observed (and describe here) that sMAC i) activates signaling in HSPCs, ii) enhances adhesion of HSPCs to BM stromal cells through the HSPC-expressed CR3 (CD11b/CD18) receptor, and iii) increases secretion of SDF-1 from BM stroma.
Based on the foregoing, we propose a novel paradigm whereby an increase in BM of proteolytic enzyme-resistant S1P and C1P and activation of CC with generation of MAC, plays an important and previously underappreciated role in homing of transplanted HSPCs. Thus, modulation of the BM levels of bioactive lipids and CC could become a novel strategy for controlling homing of HSPCs to BM and perhaps other organs as well.
Material and Methods
Animals
Pathogen-free, C57BL/6 mice were purchased from the National Cancer Institute (Frederick, MD) for bone marrow nuclear cell and bone marrow-derived stromal cell isolation. Four to six-week-old C5−/− and C5+/+ (same background as control) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). All mice were allowed to adapt for at least 2 weeks and used for experiments at age 7–9 weeks. Animal studies were approved by the Animal Care and Use Committee of the University of Louisville (Louisville, KY).
Isolation of bone marrow cells and conditioned media
Bone marrow mononuclear cells (BMMNCs) were isolated by flushing the femurs and tibias of pathogen-free C57BL/6 mice. Whole bone marrow cells were separated by Ficoll-Paque gradient, washed, and resuspended in RPMI (Thermo Fisher Scientific, South Logan, Utah, USA) containing 0.5% BSA (Sigma-Aldrich). Conditioned media of lethally irradiated (1000 Gy) mice were prepared as follows. After isolation of whole bone marrow, cells were incubated in RPMI with 0.5% BSA for 1 hr or 6 hrs at 37°C in a 5% CO2 incubator. The supernatant (conditioned medium) was then harvested.
Zymography
To evaluate matrix metalloproteinase 9 (MMP-9) secretion by bone marrow cells, conditioned media harvested from these cells were analyzed by zymography, as described previously 14.
MAC deposition
Immunolocalization of each MAC in the bone marrow of non- and irradiated mice was achieved using the immunoperoxidase detection system 15. Briefly, 4-μm paraffin sections, after deparaffinization and rehydration, were treated with 0.3% hydrogen peroxide in absolute methanol for 30 min to quench endogenous peroxidase activity and incubated in 10% normal goat serum for 1 hr to block non-specific binding. Following overnight incubation with rabbit anti-murine C5b-9 antibody (1:100, Abcam cat# ab55811), sections were exposed to HRP-conjugated goat anti-rabbit IgG antibody (1:1000, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for 1 hr. Immunoreactivity was visualized using the liquid DAB Substrate Chromogen System (Sigma-Aldrich, St Louis MO, USA). Sections were rinsed thoroughly in tap water, lightly counterstained with hematoxylin, dehydrated through a graded ethanol series, cleared in xylene, and mounted using mounting media.
Proteolytic degradation of recombinant SDF-1 (CXCL12)
Recombinant human SDF-1 (rhSDF-1, 100ng) purchased from R&D Systems (Minneapolis, MN, USA) was exposed to each of four kinds of proteases in the absence or presence of specific inhibitors as follows: recombinant human matrix metalloproteinase 2 (MMP-2, 100ng, R&D Systems) and its inhibitors, SB-3CT (100ng, Sigma-Aldrich) or ARP101 (100ng, Sigma-Aldrich); MMP-9 (100ng, R&D Systems) and its inhibitor, SB-3CT; human cathepsin G (100ng, Athens Research & Technology, Athens, GA, USA) and its inhibitor, α1-antichymotrypsin (100ng, Sigma); and human neutrophil elastase (100ng, Calbiochem, Gibbstown, NJ, USA) and its inhibitor, elastase inhibitor II (CMK 50ng, Calbiochem). MMP-2 and MMP-9 were dissolved or diluted with TCNB buffer (50mM Tris, 10mM CaCl2, 150mM NaCl, 0.05% Brij-35, pH 7.5) and activated overnight in the presence of p-aminophenylmercuric acetate (APMA, Sigma-Aldrich) before adding to the reaction mixture. rhSDF-1 (100ng/μl) was pre-incubated with each inhibitor or buffer for 1 hr and subsequently exposed to proteases overnight at 37°C.
Enzyme-linked immunosorbent assay (ELISA) for SDF-1
Residual SDF-1 after digestion with proteases was measured by ELISA. Immunoplates (Nunc, Naperville, IL) were coated with mouse anti-human SDF-1 monoclonal antibody (clone 79018, 5μg/ml, R&D systems) and captured overnight at 4°C. After blocking with 1% BSA in PBS for 1 hr at room temperature, SDF-1 mixtures were exposed to proteolytic enzymes +/− inhibitors, diluted (1:100 or 1:500), standards added to duplicate wells, and incubated overnight at 4°C. The wells were washed and incubated with biotinylated mouse anti-human SDF-1 monoclonal antibody (0.2μg/ml, R&D systems) for detection. After incubation for 1 hr at room temperature, plates were washed and incubated with peroxidase-labeled streptavidin (Jackson, Immunoresearch Laboratory) for 30 min at room temperature. Plates were then washed several times and developed with 1.1mM 2,2-azino-bis (3-ethylbenz-thiazoline-6-sulfonic acid) (Sigma-Aldrich) in 0.1M citrate-phosphate buffer (pH 5.0) containing 0.01% H2O2. Standard curves were generated using recombinant mouse or human SDF-1 (R&D Systems).
Prostaglandin E2 (PGE2) EIA
Quantification of PGE2 was analyzed by PGE2 express EIA kit (Cayman Chemical company, Ann Arbor, MI). Mouse stromal cells were isolated from bone marrow and cultured in DMEM with 10% FBS. Stromal cells were starved for 6 hrs before stimulation and stimulated with S1P and C1P for 3hrs. PGE2 concentrations of harvested supernatant were determined according to the manufacturer’s protocol.
Chemotaxis and colony-forming assays
Medium (650μl/well) containing either no chemoattractant, rhSDF-1 (0–500ng/ml), sphingosine-1-phosphate (0–0.5μM, Cayman Chemical, Michigan, USA), or ceramide-1-phosphate (0–100μM, from bovine brain, Sigma-Aldrich) were added to the lower chambers of a Costar Transwell with 5-μm filter, 24-well plate (Costar Corning, Cambridge, MA, USA). Cell suspension (1 × 106 cells/100 μl) was loaded into the upper chambers, and the cultures were incubated (37 °C, 95% humidity, and 5% CO2) for 3 hrs, and subsequently cells in the lower chambers were harvested and counted by FACS. Migrated cells were assayed for the number of colony-forming units of granulocytes/macrophages (CFU-GM). Briefly, cells were resuspended in human methylcellulose base media provided by the manufacturer (R&D Systems), supplemented with granulocyte macrophage colony-stimulating factor (GM-CSF, 25ng/ml) and interleukin-3 (IL-3, 10ng/ml) for CFU-GM, with granulocyte colony stimulating factor (G-CSF, 20ng/ml) for CFU-granulocyte (G), with macrophage colony stimulating factor (M-CSF, 10ng/ml) for CFU macrophage (M), with erythropoietin (EPO, 5 units/ml, Stem cell Tech.) and stem cell factor (SCF, 5ng/ml) for burst-forming units (BFU-E), and with thrombopoietin (TPO, 100ng/ml) for CFU-megakaryocyte (Meg). Cultures were incubated for 7–10 days, at which time they were scored under an inverted microscope for the number of each type of colony. For the colony forming units-spleen (CFU-S) assays, lethally irradiated mice were transplanted with bone marrow cells (1 × 105 cells) pre-treated with soluble MAC (sMAC) for 3 hrs. Twelve days later, spleens were isolated, fixed in Telesyniczky’s solution, and CFU-S colonies were counted on the spleen surface.
FACS analysis
Cell staining was performed in medium containing 2% fetal bovine serum (FBS). All monoclonal antibodies (mAbs) were added at saturating concentrations and the cells were incubated for 30 min on ice, washed twice, resuspended in staining buffer at a concentration of 5 × 106 cells/ml, and analyzed with an LSR II flow cytometer (BD Biosciences, Mountain View, CA). The following monoclonal antibodies were used for detection: phycoerythrin (PE)-conjugated anti-mouse CXCR4 (clone 2B11, BD Pharmingen, San Diego, CA, USA) and PE-conjugated anti-mouse VLA4 (clone 9F10, BD Pharmingen).
Mass spectrometry measurements of C1P and S1P
We have adapted from the work of others and further developed HPLC/ESI/tandem mass spectrometry methods for quantification of S1P and C1P. The method employed uses our API-4000 triple quadrupole mass spectrometer coupled with a Shimadzu Prominence liquid chromatography system. Lipids are separated on a Zorbax Eclipse XDB-C8 column (4.6 × 150mm, 5μm), using methanol/water/HCOOH (75/25/0.5, v/v) with 5mM NH4COOH as solvent A and methanol/water/HCOOH (99/1/0.5, v/v) with 5mM NH4COOH as Solvent B. S1P and C1P were analyzed by HPLC ESI mass spectrometry in positive ionization mode with optimized declustering potentials, collision energies, exit potentials, and monitoring of the following mass/charge (m/z) transitions: C17S1P, 448/388; S1P, 462/402; C12-C1P, 562.436/264.1; and C18-C1P, 644.5/264.4. Calibration of the assay was accomplished using synthetic standards that were independently quantified by phosphorous determination. A known quantity of C17-S1P was added as an internal recovery standard and C12-C1P, C18-C1P, and S1P were quantified by reference to a calibration curve.
RNA quantification for SDF-1
Total RNA was isolated using the RNeasy Mini Kit (Qiagen Inc., Valencia, CA, USA) from whole bone marrow cells of non-irradiated or irradiated mice to evaluate SDF-1 expression level. Messenger RNA was reverse transcribed with 500U of Moloney murine leukemia virus reverse transcriptase primed with oligo-dT. The resulting cDNA fragments were amplified using the SYBR green system (Applied Biosystems, Carlsbad, CA, USA). Primer sequences for β-2 microglobulin (β2 m) were 5′-CAT ACG CCT GCA GAG TTA AGC-3′ (forward) and 5′-GAT CAC ATG TCT CGA TCC CAG TAG-3′ (reverse). For SDF-1, primer sequences were 5′-CGT GAG GCC AGG GAA GAG T-3′ (forward) and 5′-TGA TGA GCA TGG TGG GTT GA-3′ (reverse). For Cox-2, primer sequences were 5′ TGA GCA ACT ATT CCA AAC CAG C (forward), 5′ GCA CGT AGT CTT CGA TCA CTA TC (reverse). For Prostaglandin E2 (PGE2) receptor, EP1, EP2, EP3, and EP4 primer sets were used, as described previously 20. The relative quantity of the target, normalized to an endogenous control gene (β2m) and relative to a calibrator, is expressed as 2−ddCt (fold difference), in which dCt equals the Ct of the target genes minus the Ct of the endogenous control gene, and ddCt equals the dCt of the samples for the target gene minus the dCt of the calibrator for the target gene.
Western blot analysis
BMMN cells were starved overnight in RPMI containing 0.5% BSA at 37°C and stimulated with SDF-1 (0.05 or 0.3μg/ml), S1P (0.02 or 0.1μM), C1P (20 or 100μM), or SC5b-9 (1 or 10μg/ml, Complement Technology, Texas, USA) for 2 or 5 min at 37°C and then lysed for 30 min on ice in M-per lysing buffer (Pierce, Rockford, IL, USA) containing protease and phosphatase inhibitors (Sigma-Aldrich, St Louis, MO, USA). The extracted proteins (30μg) were separated and analyzed for phosphorylation of MAPKp44/42, AKT (Ser473), p38 (Tyr180/Tyr182), Stat-1 (Tyr701), Stat-3 (Tyr705), or Stat-5 (Tyr694) antibodies purchased from Cell Signaling Technology Inc. (Danvers, MA, USA). Equal loading in the lanes was evaluated by stripping the blots and reprobing with the monoclonal or polyclonal antibodies of MAPKp44/42, p38, or Stat-3. The membranes were developed with an enhanced chemiluminescent reagent (Amersham Life Sciences, Arlington Heights, IL, USA) and exposed to film (HyperFilm, Amersham).
Bone marrow transplantation
For transplantation experiments, C5−/− and C5+/+ mice were irradiated with a lethal dose of γ-irradiation (1000 cGy). Twenty-fours hours later, recipient mice were transplanted with 105 wild type BM cells by tail vein injection. Anesthetized transplanted mice were bled at various intervals from the retro-orbital plexus to obtain samples of leukocytes and platelets. To assess the effects MAC in long-term engraftment of hematopoietic stem cells, lethally irradiated mice were transplanted with 105 wild type BMMN cells (sMAC - sC5b-9-primed or unprimed) with 10 mice/group.
Adhesion assay
Culture plates (48-well) were seeded with bone marrow-derived stromal cells. Sca-1+ cells were sorted by AutoMACS (Miltenyi Biotec Inc., Auburn, CA) and pre-treated with SDF-1 (30ng/ml), S1P (0.1μM), C1P (100μM) or SC5b-9 (10μg/ml) for 30 min, washed twice, and loaded onto stromal cells. Fifteen minutes later, cell cultures were washed to remove non-adherent cells, and stromal cells with attached CFU-GM were isolated by trypsinization. These cells were subsequently tested in methylcellulose cultures for the presence of CFU-GM clonogenic progenitors, as described above. In blocking studies, cells were coated with recombinant VCAM-1 (R&D systems) overnight at 4°C and then treated with anti-VLA4 Ab (R1–2, BD Pharmingen) for 30 min prior to VCAM-1 adhesion assay. To assess the effects of the PI3K/Akt inhibitor LY294002 (Sigma) or the MAPK inhibitor U0126 (Calbiochem) on cell adhesion, cells were pre-incubated with each of these compounds for 30 minutes before stimulation. In some experiments, BMMNC were obtained from CR3−/− and C3aR−/− mice, and their Sca-1+ cells were isolated. Adherent cells from wt littermates were expanded to grow BM stroma, and adhesion assays were performed as described 3.
Statistical analysis
Arithmetic means and standard deviations were calculated using Instat 1.14 software (Graphpad, San Diego, CA). Statistical significance was defined as P<0.05. Data were analyzed using Student’s t-test for unpaired samples and the t-test for paired samples.
Results
Conditioning for hematopoietic transplantation by lethal irradiation induces a proteolytic microenvironment in BM and activates the complement cascade
It has been reported that mobilization of HSPCs induces a highly proteolytic microenvironment in BM and that several enzymes released during this process impair SDF-1–CXCR4 interactions by degrading SDF-1, which leads to the release of HSPCs into peripheral blood (PB) 16. In addition, as we demonstrated previously, mobilization of HSPCs also activates the complement cascade (CC) in BM and some CC cleavage fragments (e.g., C3a) modulate migration of HSPCs. Based on these findings, we became interested in whether conditioning for transplantation by lethal irradiation also induces a proteolytic microenvironment in BM and activates CC.
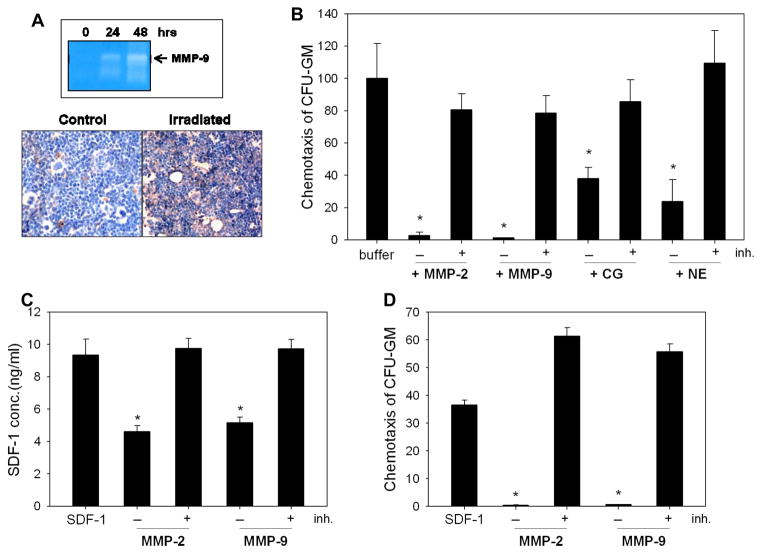
Our zymography data (Figure 1 panel A, top) show that at 24 and 48 hours after irradiation, the concentration of MMP-9 is upregulated in conditioned media harvested from BM cells. This was paralleled by activation of CC and deposition of C5b-C9 (MAC) in the BM microenvironment, as demonstrated by immunohistochemical staining (Figure 1 panel A, bottom).
Figure 1. Myeloablative conditioning for hematopoietic transplantation by lethal irradiation induces a proteolytic microenvironment in murine BM.
Panel A: Zymography revealed that the activity of MMP-9 is increased at 24 and 48 hours in conditioned media harvested from BM cells after lethal (1000 cGy) γ-irradiation (upper panel). The complement cascade becomes activated and MAC is deposited in the BM microenvironment of mice that were conditioned for transplantation by lethal γ-irradiation (lower panel). Because it is a peptide, SDF-1 was disrupted by proteolytic enzymes that are elevated in irradiated BM. Panel B: Chemotactic activity of SDF-1 against clonogenic progenitors decreases after exposure to MMP-2, MMP-9, cathepsin G (CG), or neutrophil elastase (NE). Disruption of SDF-1 was prevented by addition of protease-specific inhibitors. Panel C: SDF-1 was exposed to MMP-2 (100ng) or MMP-9 (100ng) with or without inhibitor (ARP101, 100ng) pre-incubation, and SDF-1 concentration was evaluated by ELISA. Panel D: The SDF-1 samples from Panel C were tested for chemotactic activity against BM-derived CFU-GM. The data shown in panels B–D represent the combined results from three independent experiments carried out in triplicate per group. * p<0.0001.
The induction of a proteolytic microenvironment in BM could degrade the biological activity of SDF-1 4, 5. In fact, Figure 1 panel B shows that several proteolytic enzymes released by BM cells, such as MMP-2, MMP-9, cathepsin G (CG), and neutrophil elastase (NE), neutralize SDF-1 chemotactic activity against HSPCs, and that this is prevented by the presence of enzyme-specific inhibitors. More importantly, Figure 1 panel C demonstrates that even when truncated SDF-1 is still detected by ELISA after exposure to MMP-2 or MMP-9, this chemokine is no longer effective as a chemoattractant in chemotactic assays (Figure 1 panel D).
While the SDF-1 level becomes downregulated in BM after lethal irradiation, the concentrations of S1P and C1P increase
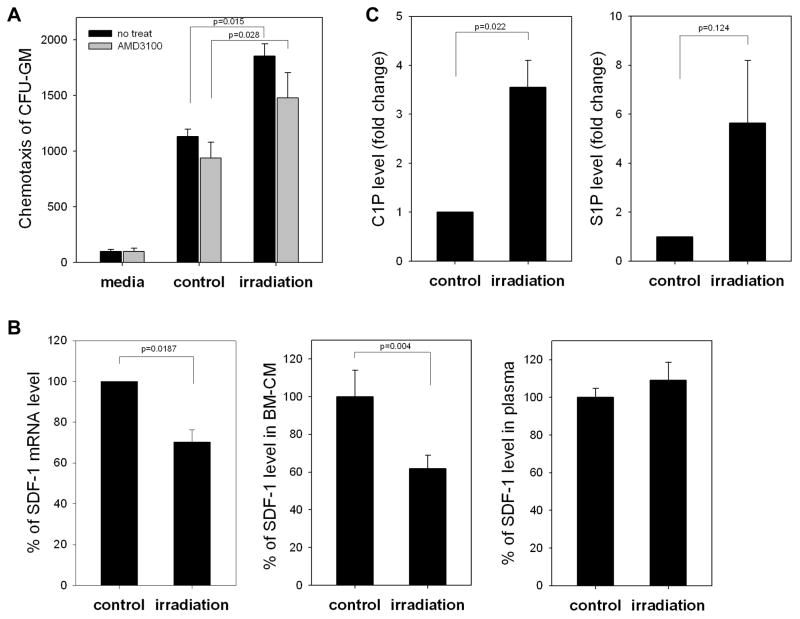
It is widely accepted that irradiation induces expression of SDF-1 in BM 16, 17. Therefore, we became interested in the SDF-1-mediated chemotactic activity of conditioned media (CM) harvested from BM irradiated cells. Figure 2 panel A shows that CM harvested from BM cells 24 hours after lethal irradiation chemoattracts CFU-GM. However, we also observed that exposure of BM cells to the CXCR4 antagonist AMD3100 before chemotaxis did not significantly affect chemotactic responsiveness of tested cells to these CM. This suggests that factors other than SDF-1 are involved in this process. In fact, Figure 2 panel B shows that both SDF-1 mRNA and SDF-1 protein are downregulated in lethally irradiated BM. Of note, the SDF-1 level remained unchanged in PB of irradiated mice, as in non-irradiated control animals.
Figure 2. SDF-1 level decreases in irradiated bone marrow (BM).
Panel A: Conditioned media from irradiated BM cells enhance chemotactic activity of normal clonogeneic progenitors (black bars), but this effect was partially disrupted by blocking CXCR4 with AMD3100 (1μM, gray bars). Panel B: SDF-1 at both the mRNA level (real-time PCR, left panel) and protein level (ELISA, middle panel) decreases after 24 hours in lethally irradiated BM (1000 cGy). At the same time, we did not observe changes in the SDF-1 level in PB of irradiated mice (right panel). Panel C: Mass spectrometry analysis shows that ceramide-1-phosphate (C1P) and sphingosine-1-phosphate (S1P) become upregulated in murine BM after conditioning for hematopoietic transplantation by lethal irradiation. The data shown in panels A–C represent the combined results from three independent experiments carried out in triplicate per group.
Based on this finding, we began a search for unknown chemoattractants that could be present in CM from irradiated BM cells, with bioactive lipids as strong candidates. This expectation was based on the fact that bioactive lipids, lacking peptide bonds, are resistant to proteolytic degradation. Figure 2 panel C shows our mass spectrometry finding that two bioactive lipids, S1P and C1P, are strongly upregulated in BM after lethal irradiation.
S1P and C1P as novel chemoattractants involved in homing of HSPCs
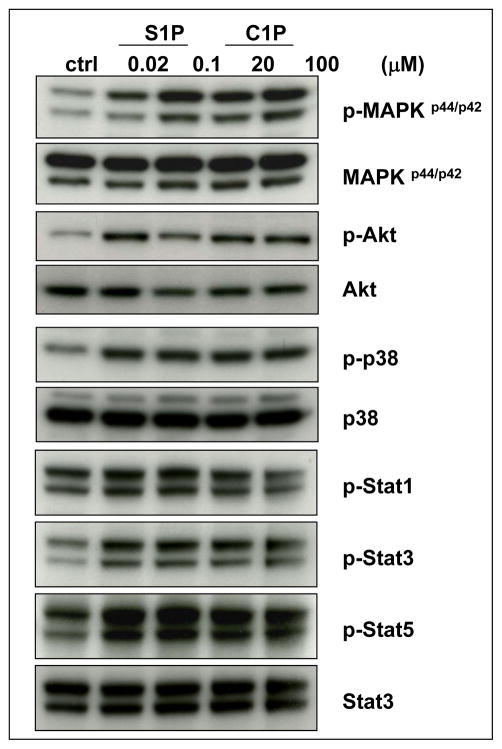
S1P has already been reported to be a strong chemoattractant for HSPCs 15. To our surprise, we found that another bioactive lipid, C1P, in a similar way as S1P, induces several signaling pathways in murine Sca-1+ cells purified by immunomagnetic beads (Figure 3), as well as strongly chemoattacts murine HSPCs (Figure 4 panel A and Supplementary Figure 1). However, both these bioactive lipids and soluble MAC (sMAC), as shown in Supplementary Figure 2 and 3 respectively, do not affect the clonogenicity of murine progenitors for all major hematopoietic lineages. The same result was found for their unphosphorylated forms (data not shown).
Figure 3. Bioactive lipids stimulate signaling pathways in murine Sca-1+ HSPCs.
C1P and S1P activate several signaling pathways in murine BMMNC that are crucial for cell migration and adhesion: MAPKp44/42, Akt, p38, Stat-3, and Stat-5. Before stimulation, cells were starved overnight in RPMI containing 0.5% BSA in an incubator and subsequently stimulated with S1P (0.02μM or 0.1μM) or C1P (20μM or 100μM) for 5 min. Experiments were repeated independently three times with similar results. A representative western blot is shown.
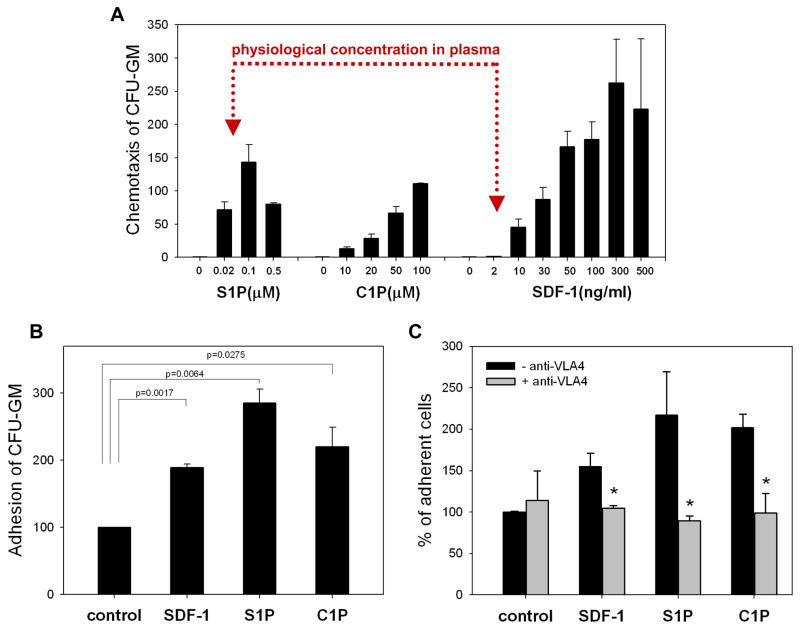
Figure 4. Bioactive lipids show strong chemotactic activity against HSPCs.
Panel A: The chemotatic dose response of S1P, C1P, and SDF-1 against murine clonogenic progenitors is shown. Note that the physiological concentration of SDF-1 in biological fluids (e.g., serum) is approximately 2–5ng/ml, and at this concentration SDF-1 is not effective as a chemoattractant. In contrast, S1P is already a strong chemoattractant for murine BM-derived hematopoietic progenitors at biologically relevant concentrations. Panel B: Both C1P and S1P, like SDF-1, increase adhesion of murine clonogenic progenitors (Sca-1+ cells) to BM-derived stromal cells. The data shown represent the combined results from three independent experiments carried out in triplicate per group. Panel C: In blocking studies, Sca-1+ cells were incubated with 10μg/ml anti-VLA4 integrin mAb for 30 minutes prior to VCAM-1 adhesion assay. The data shown represent the combined results from two independent experiments carried out in quadruplicate per group. * p<0.05.
More importantly, we observed that S1P at physiological serum concentrations is already a much stronger chemoattractant for HSPCs than biologically relevant doses of SDF-1 (Figure 4 panel A). Thus, our data show that while the SDF-1 level decreases in BM due to a highly proteolytic microenvironment, its chemotactic role could be somehow replaced or compensated by an increase in the BM levels of S1P and C1P. Moreover, as shown in Figure 4 panel B, in addition to their strong chemotactic properties, both bioactive lipids, like SDF-1, enhance in a VCAM-1–VLA-4- -dependent manner adhesion of murine CFU-GM to BM stromal cells (Figure 4 panel C). However, at the same time they did not affect expression of CXCR4 and VLA-4 on the surface of HSPCs or secretion of MMP-9 by these cells (data not shown).
Interestingly, we also found that C1P and S1P induce expression of cyclooxygenase-2 (Cox-2) in BM stromal cells, while ELISA data revealed that C1P increases PGE2 secretion by these cells (Supplementary Figure 4 panel A and B). This has important implications because PGE2 has recently been proposed to play an important role in HSPC homing to BM 19,20. Concomitantly, we also observed that C1P upregulates the expression of mRNA for the PGE2 receptor (EP3) in BM-derived Sca-1+Kit+Lin− cells (Supplementary Figure 4 panel C). An increase in PGE2 secretion by irradiated BM stromal cells was further confirmed by ELISA (Supplementary Figure 4 panel D). Based on this, we propose that C1P may additionally increase the PGE2-mediated pro-homing effects 19, 20.
In contrast to S1P, receptor(s) for C1P have not yet been identified, but our preliminary data showing the effect of pertussis toxin suggest that it will also be a Gαi protein-coupled receptor (Supplementary Figure 5).
Transplantation experiments in C5-deficient mice reveal an important role for MAC in engraftment of HSPCs
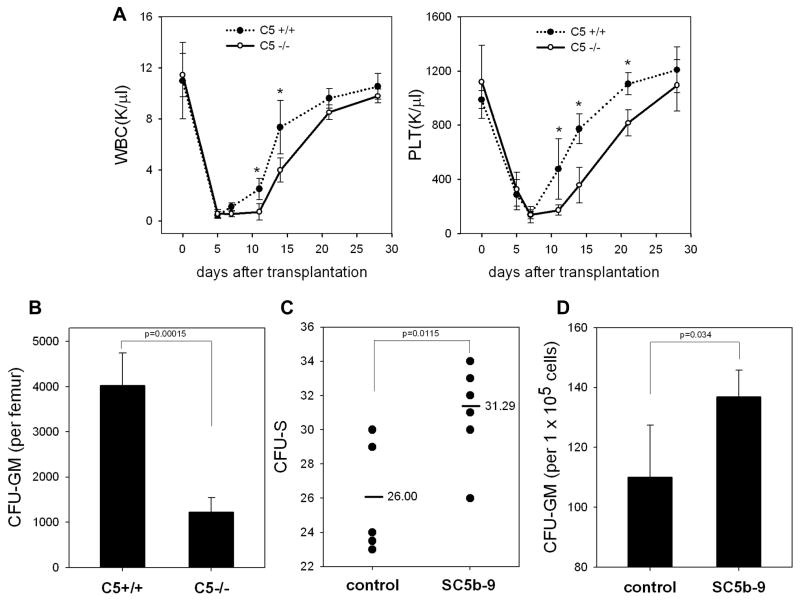
Because our data demonstrated that CC is activated and MAC is deposited in lethally irradiated BM (Figure 1 panel A, right), we asked whether MAC plays a role in homing of HSPCs. To address this question, we transplanted lethally irradiated C5-deficient mice that do not generate MAC, as well as wild type (wt) mice, with wt BM cells. Figure 5 panel A shows that the recovery of leucocytes and platelets was significantly delayed in C5-deficient mice, which do not generate and deposit MAC. In further support of this finding, the bones of mice at day 12 after transplantation exhibited a much lower number of transplant-derived CFU-GM (Figure 5 panel B).
Figure 5. C5-deficient mice show an engraftment defect in transplanted HSPCs.
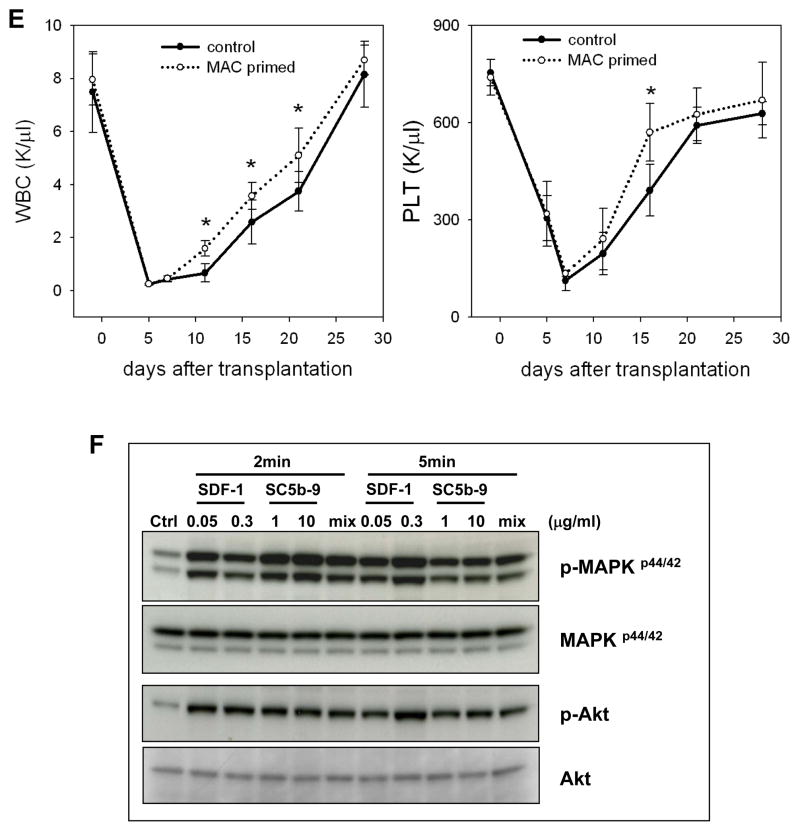
Panel A: Wild type (C5+/+) and C5-deficient (C5−/−) animals, which do not generate MAC, were lethally irradiated and transplanted with wild type bone marrow mononuclear cells. We followed the subsequent recovery of hematopoietic parameters in these animals. C5−/− mice have recovery of peripheral blood leukocytes (upper panel) and platelets (lower panel) delayed by 3–5 days. The data shown represent the combined results from two independent experiments performed with 10 mice/group. * p<0.001. Panel B: By day 12, there is a decrease in the number of CFU-GM in BM of C5−/− mice transplanted with wt BM cells. Panel C: The number of CFU-S colonies formed in the spleens of wt mice were counted after transplantation of wt BMMNC cells, which were pre-stimulated or unstimulated before transplantation by sC5b-9 (10μg/ml), and injected intravenously into lethally irradiated wild type animals. Twelve days later, spleen was isolated and the number of CFU-S evaluated. Panel D: The number of CFU-GM progenitors in BM of wt transplanted mice with SC5b-9-primed or unprimed BM cells. The data shown in panels C and D represent the combined results from three independent experiments carried out in triplicate per group (n=9). Panel E: Wild type mice were lethally irradiated and transplanted with wild type BMMNC after SC5b-9 treatment (primed) or not (unprimed control). We followed the subsequent recovery of hematopoietic parameters in these animals. Mice that were transplanted with SC5b-9-primed BMMNCs have a much faster recovery rate for peripheral blood leukocytes (left panel) and platelets (right panel). * p<0.05. Panel F: Soluble MAC, like SDF-1, activates MAPKp44/42 and Akt in normal murine Sca-1+ cells. “Mix” indicates SDF-1 (0.05μg/ml) plus SC5b-9 (1μg/ml). Experiments were repeated independently three times with similar results. A representative western blot is shown.
To better assess the role of MAC in engraftment of HSPCs, we exposed wt BM cells to sMAC for 30 minutes and subsequently transplanted these sMAC-primed/exposed or unprimed control cells into lethally irradiated wt animals. Figure 5 panel C shows that priming of HSPCs by sMAC significantly enhances engraftment of early progenitor cells, as judged by the formation of spleen colonies (CFU-S). Similarly, priming of BM cells before transplantation by sMAC lead 12 days after transplantation to a presence of a higher number of transplant-derived CFU-GM in long bones (Figure 5 panel D). To address better whether sMAC may affect hematopoietic reconstitution of transplanted mice, normal murine wt BMMNC were exposed ex vivo for 30 min to sMAC and transplanted into lethally irradiated syngeneic recipients (Figure 5 panel E). We observed accelerated recovery of white blood cell- and platelet-counts in animals transplanted with sMAC-exposed (primed) BMMNCs. Finally, Figure 5 panel E shows that sMAC activates signaling (MAPKp44/42 and AKT) in murine Sca-1+ cells purified by immunomagnetic beads, while not affecting clonogenic growth of murine progenitors (Supplementary Figure 3).
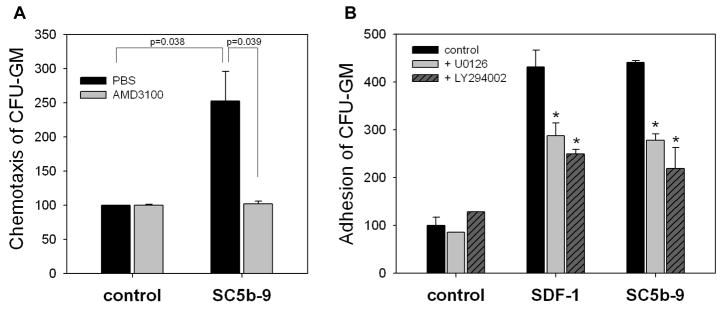
Molecular effects of MAC that direct homing of HSPCs
Finally, we became interested in sMAC-mediated molecular mechanisms that may enhance engraftment of HSPCs. While sMAC did not affect expression of CXCR4 or VLA-4 in murine cells (Supplementary Figure 6), chemotactic responsiveness of BM-derived CFU-GM to supernatants harvested from BM stromal cells (stimulated or unstimulated by sMAC in the presence or absence of AMD3100, Figure 6 panel A), suggest that MAC may enhance SDF-1 secretion by murine BM stroma. Furthermore, sMAC like SDF-1, enhanced adhesion of HSPCs to bone marrow stroma in an AKT- and MAPKp42/44-dependent manner (Figure 6 panel B). However, in contrast to SDF-1, S1P, and C1P (Figure 4 panel C), this pro-adhesive effect of sMAC was not dependent on increasing interactions between VLA-4 and VCAM1 (data not shown).
Figure 6. MAC affects several steps crucial for stem cell homing.
Panel A: Normal murine BM-derived stromal cells were incubated with soluble MAC (sC5b-9, 10μg/ml for 3 hrs) and subsequently conditioned media were harvested from these cells and assayed for their chemotactic activity against normal murine hematopoietic progenitors (black bars). Importantly, this chemotactic effect was totally inhibited after blockage of the CXCR4 receptor by AMD3100 treatment (gray bars). Panel B: Soluble MAC (sC5b-9), like SDF-1, strongly increases adhesiveness of murine hematopoietic progenitor cells (Sca-1+) to murine bone marrow-derived stroma. In inhibition studies, Sca-1+ cells were treated with MAPK inhibitor (U0126, 10μM) or Akt inhibitor (LY294002, 20μM) for 1 h before adhesion assay. The data shown represent the combined results from three independent experiments carried out in triplicate per group.
Because we had previously reported that activation of CC in BM leads to deposits of solid-phase iC3b on BM-stromal and endothelial cells and HSPCs are tethered to these deposits in a CR3-dependent manner 3, we isolated Sca-1+ cells from wile type (CR3+) and CR3-deficient mice and evaluated the effect of sMAC on this process (Table 1). Interestingly, we show for the first time that sMAC increases adhesion of clonogeneic progenitors to iC3b deposits in CR3-dependet manner. Finally, however stimulation of murine SKL cells by sMAC induces cells signaling (Figure 5 panel E), this effect seems not to be mediated by a Gαi protein-coupled pertussis toxin-sensitive receptor (Supplementary Figure 5).
Table 1. sMAC enhances iC3b–CR3-dependent adhesion of murine CR3+ hematopoietic progenitor cells to BM stroma.
The number of CFU-GM from Sca-1+ cells from wt and CR3−/− mice that adhered to irradiated wt stroma exposed to wt serum as a source of iC3b deposits was arbitrarily assumed to be 100% in 20-min adhesion assays. Data are pooled from triplicate samples from two independent experiments (n=6).
| Source of Sca-1+ cells | (−) | sMAC added |
|---|---|---|
| WT | 100 +/− 27 | 319+/−49* |
| CR3−/− | 52+/−11 | 54+/−15 |
p<0.0001
Discussion
Hematopoietic stem/progenitor cells (HSPCs) are unrested travelers and circulate in peripheral blood (PB) and lymph during development, moving between major anatomical sites where hematopoiesis is initiated and/or temporarily active (e.g., blood islands in yolk sac, aorta endothelium, and fetal liver) before they reach their final destination in the bone marrow (BM) 21, 22. Later in adult life, a small percentage of HSPCs is continuously released from BM niches into the PB, which may be envisioned as a highway by which HSPCs relocate between distant stem cell niches in order to keep the total pool of BM stem cells in balance 23–25. The number of HSPCs in PB increases also during stress situations (e.g., heart infract and stroke), serious infections, and strenuous exercise 26–29. The release of HSPCs can be enforced by some drugs as seen during pharmacological mobilization, which is employed to harvest HSPCs for transplantation.
It has been demonstrated that HSPCs released into PB during infections may enter tissues and locally give rise to granulocytes and macrophages or even dendritic cells 30. HSPCs that are released from BM may also return to PB and relocate to other niches in BM via the lymphatic system 30. This reverse process, called “homing”, first requires adhesion and immobilization of HSPCs on endothelium and subsequently their active proteolytic enzyme-dependent migration through the blood–bone marrow barrier to the BM microenvironment. A massive migration and homing of HSPCs from PB into BM followed by their engraftment is seen after hematopoietic transplantation. An important step here is conditioning for transplantation by high-dose γ-irradiation or myeloablative chemotherapy, which destroys endogenous hematopoiesis and increases the number of available stem cell niches in BM 16.
The mechanisms that regulate migration of HSPCs out of and back into BM are not well understood. It has been proposed that there is a tug of war over the chemotactic stromal derived factor-1 (SDF-1) gradient between BM and PB that determines whether cells will be released or mobilized from BM into PB or home back from PB to the BM microenvironment. SDF-1, which binds to the Gαi protein-coupled, seven-transmembrane-spanning CXCR4 receptor expressed on HSPCs, plays an unquestioned role in developmental migration of HSPCs during embryogenesis and their subsequent retention in the BM 16, 31. However, observed changes in the SDF-1 gradient between BM and PB do not always support its having a crucial role as a chemoattractant present in PB that directs egress and mobilization of HSPCs or that it is the only chemoattractant for homing of HSPCs back into BM.
For example, as demonstrated by others 8, 32 and us 15, 33, the plasma SDF-1 level does not always correlate with mobilization or egress of HSPCs from BM into PB. Recently, we proposed a novel paradigm where a crucial role in egress of HSPCs into peripheral blood is played by the bioactive lipid sphingosine-1-phosphate, which is released during mobilization by soluble C5b-C9 (sMAC), which is a product of the complement cascade (CC) activation. This mechanism has been recently confirmed by other investigators 34–36.
On the other hand, there is increasing doubt about an exclusive role for SDF-1 in homing of HSPCs into BM. This is based on evidence that i) CXCR4−/− fetal liver HSPCs may home to BM in an SDF-1-independent manner 6, ii) homing of murine HSPCs made refractory to SDF-1 by incubation and co-injection with a CXCR4 receptor antagonist (e.g., the bicyclam AMD3100) is normal or only mildly reduced 7, and finally iii) HSPCs in which CXCR4 has been knocked down by means of an SDF-1 intrakine strategy also engraft in lethally irradiated recipients 8. All this strongly suggests the existence of other factors besides SDF-1 that are involved in the homing of HSPCs. Moreover, SDF-1, which is a well-known and potent chemoattractant for HSPCs is, as a peptide, highly susceptible to degradation by proteases, which are elevated in the BM microenvironment after myeloablative conditioning for transplantation, as we show here for the first time. By employing sensitive ELISA measurements, we found support for this notion with the observation of a decrease in SDF-1 level in murine BM after myeloablative conditioning for transplantation.
Based on these observations, we became interested in other potential factors that, in addition to SDF-1, could chemoattract HSPCs and thus play a role in their homing. Interestingly, we observed that media conditioned by BM cells isolated from lethally irradiated mice show chemotactic activity against HSPCs that is resistant to AMD3100 treatment and heat inactivation, but susceptible to charcoal extraction, which suggests the involvement of bioactive lipids. In support of this notion, it is known that sphingolipids, which are important components of cell membranes, give rise to two bioactive derivatives, sphingosine-1-phosphate (S1P) and ceramide-1-phosphate (C1P), with S1P already identified as a chemoattractant for HSPCs 32 and C1P for monocytes 37, 38. While S1P is released from cells as an important signaling molecule, C1P is an intracellular second messenger and can be released only from “damaged leaky cells” —for example, after myeloablative conditioning of BM for transplantation. The obvious advantage of bioactive lipids as chemoattractants is that, in contrast to SDF-1, they are resistant to proteolytic enzymes.
We report here for the first time that myeloablative conditioning for transplantation not only induces a proteolytic microenvironment in BM but also activates CC, which leads to the deposition not only of iC3b 3 but also to release of sMAC in BM. Furthermore, we also report for the first time that activation of CC correlates with an increase in the BM levels of S1P and C1P. S1P is a well-known chemoattractant for HSPCs 15, 33, but we demonstrated for a frist time that C1P also chemoattracts HSPCs and increases their adhesion to BM-derived fibroblasts. C1P, like S1P, induces activation of MAPKp44/42, p38, Akt, and several Stat proteins. In contrast to S1P, the receptor(s) for C1P is unknown, but because it is sensitive to inhibition by pertussis toxin, as we report here, it is likely to be a Gαi protein-coupled seven transmembrane-span type receptor.
However, despite the fact that the overall SDF-1 level decreases in murine BM after lethal irradiation, the responsiveness of HSPCs to an SDF-1 gradient can be significantly enhanced by some factors such as C3 cleavage fragments 14, prostaglandin E2 (PGE2), 20 or uridine triphosphate (UTP) 44. Since, as already reported irradiated BM is enriched for C3 cleavage fragments 14 and UTP 44, we become interested in effect of irradiation-induced S1P and C1P in BM on the level of PGE2. As we demonstrate here (Supplementary Figure 4), an additional effect of C1P on engraftment of HSPCs may be related to an increase of PGE2 level in BM and PGE2-related pro-homing activities 20,39. In support of this observation, C1P induces activity of cPLA2, which regulates production of arachidonic acid, a substrate for PGE2 synthesis 40. Interestingly, while S1P and C1P are equally effective in inducing Cox-2 mRNA in hematopoietic cells (Supplementary Figure 4 panel A), cells stimulated by C1P produce more PGE2 (Supplementary Figure 4 panel B). This discrepancy requires further study to see, for example, whether S1P increases the half-life of Cox-2.
Moreover, our experiments in C5−/− and normal wt mice have revealed, also for the first time, an important and underappreciated role for CC activation and MAC deposition in the process of BM homing of HSPCs. sMAC has been reported to affect the biology of several cell types 38, 41, but there has, until now, been no report on its effect on HSPCs. The defect in homing and engraftment of HSPCs in the BM of C5-deficient mice described here is supported by the until-now-unrecognized role of sMAC in supporting several steps that facilitate homing of HSPCs. First, sMAC enhances secretion of SDF-1 by BM stromal cells, which probably contributes to ameliorating the drop in BM SDF-1 level in the proteolytic microenvironment induced by conditioning for transplantation. Second, sMAC, as we demonstrate here for the first time, increases the adhesiveness of HSPCs to BM stromal cells in an iC3b–CR3-dependent manner. This is further supported at the molecular level by the ability of sMAC to induce signaling in normal HSPCs. However, if sMAC is employed alone, it does not chemottract HSPCs and, as we have demonstrated here, does not affect expression of CXCR4 and VLA-4 on the surface of HSPCs and does not affect proliferation of clonogenic progenitors. Thus, as we show here for the first time, activation of CC and release of sMAC potentiates the homing properties of bioactive lipids. Of note, while receptors for both C1P and sMAC have not yet been identified, the C1P receptor/s seems to be sensitive to pertussis toxin.
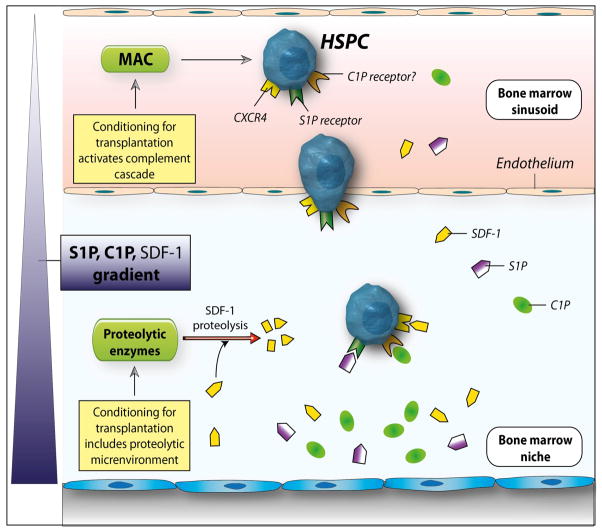
Based on these findings, we propose a new paradigm in which S1P and C1P play an important role in homing of HSPCs. While S1P is a major chemoattractant that directs egress of HSPCs from BM into PB, C1P is released from damaged cells in BM after myeloablative conditioning and, together with SDF-1 and S1P, creates a homing gradient for circulating HSPCs (Figure 7). In addition, the pro-homing effects of both bioactive lipids are positively modulated by the end product of CC activation, which is sMAC. We also propose that S1P, C1P, and sMAC play a more universal role in trafficking of stem cells than previously thought and are involved in regulating the migration of circulating mesenchymal stem cells (MSCs), endothelial progenitor cells (EPCs), and very small embryonic-like (VSEL) stem cells 25, 30, 42, 43. Accordingly, while S1P plays a role in egress of these cells into PB, C1P, together with S1P locally released from damaged cells (e.g., in infarcted myocardium or brain tissue after stroke) and SDF-1 may chemoattract circulating stem cells for organ repair.
Figure 7. The involvement of bioactive lipids in homing and engraftment of HSPCs.
Conditioning for transplantation by radio-chemotherapy induces a proteolytic microenvironment in BM and activates the complement cascade, which leads to generation of soluble MAC. Several proteolytic enzymes are released that decrease the SDF-1 level in BM. At the same time, BM cells damaged by conditioning for transplantation by lethal irradiation release C1P and S1P, which are bioactive lipids resistant to proteolytic enzymes. As potent chemoattractants, both play an important role in homing of stem cells to BM. In addition, MAC generated by CC activation enhances adhesiveness of HSPCs to BM stroma and secretion of SDF-1 by these cells. This increase in SDF-1 secretion may somehow ameliorate the drop in SDF-1 level in such a highly proteolytic microenvironment. What is not shown in this scheme is that CC cleavage fragments, such as C3a and iC3b, also contribute to the homing of HSPCs, as reported by us in the past 3, 14. By increasing the PGE2 level in BM, these CC cleavage fragments level may also modulate the seeding of HSPCs in BM, in addition to the bioactive lipids C1P and S1P.
Supplementary Material
Panel A: The chemotactic activity dose response to S1P and C1P measured by the number of BM-derived CFU-GM is presented. Chemotactic responsiveness to C1P was observed at higher concentrations of this bioactive lipid than for S1P. Panel B: C1P and S1P show some differences in chemoattraction of murine progenitors from different hematopoietic lineages. While SDF-1 at optimal doses chemoattracts all types of progenitors, bioactive lipids show little chemotactic effect against erythroid BFU-E cells. The data shown represent the combined results from three independent experiments carried out in triplicate per group (n = 9).
C1P and S1P do not affect clonogenic growth of murine granulocytic, monocytic, erythrocytic, megakaryocytic, and mixed-lineage progenitor cells. The data shown represent the combined results from three independent experiments carried out in quadruplicate per group (n = 12).
The soluble MAC effect on clonogenic growth of murine granulocytic, monocytic, erythrocytic, megakaryocytic, and mixed-lineage progenitor cells is shown. The data represent the combined results from three independent experiments carried out in quadruplicate per group (n = 12).
Panel A: C1P and S1P increase expression of cyclooxygenase-2 (Cox-2) in BM stromal cells. Panel B: C1P enhances secretion of PGE2 in murine BM stromal cells. Panel C: C1P enhances expression of EP3 mRNA in murine BM-derived SKL cells. Panel D: PGE2 secretion is increased in media harvested from murine BM stromal cells after conditioning for hematopoietic transplantation by lethal irradiation. The data represent the combined results from three (panels A–C) or two independent experiments (panel D).
Panel A: Migration of BMMNCs to C1P and S1P was totally blocked by pre-treatment with pertussis toxin (1μg/ml for 1hr at 37°C). The experiment was repeated three times with similar results. Panel B: Pertussis toxin (PTX) treatment (0.5 μg/ml for 1hr) before stimulation affects phosphorylation of MAPKp44/42, Akt, and Stat-3 by C1P, but not sMAC. One representative study out of two is shown.
Exposure of Sca-1+ cells to MAC does not affect the expression of CXCR4 and VLA-4. One representative study out of three is shown.
Acknowledgments
Supported by NIH grants R01 CA106281 and R01 DK074720, the Stella and Henry Endowment, and European Union structural funds (Innovative Economy Operational Program POIG.01.01.02-00-109/09-00) to MZR.
References
- 1.Levesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001;98:1289–1297. doi: 10.1182/blood.v98.5.1289. [DOI] [PubMed] [Google Scholar]
- 2.Peled A, Grabovsky V, Habler L, Sandbank J, Arenzana-Seisdedos F, Petit I, et al. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34+ cells on vascular endothelium under shear flow. J Clin Invest. 1999;104:1199–1211. doi: 10.1172/JCI7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ratajczak MZ, Reca R, Wysoczynski M, Kucia M, Baran JT, Allendorf DJ, et al. Transplantation studies in C3-deficient animals reveal a novel role of the third complement component (C3) in engraftment of bone marrow cells. Leukemia. 2004;18:1482–1490. doi: 10.1038/sj.leu.2403446. [DOI] [PubMed] [Google Scholar]
- 4.Cho SY, Xu M, Roboz J, Lu M, Mascarenhas J, Hoffman R. The effect of CXCL12 processing on CD34+ cell migration in myeloproliferative neoplasms. Cancer Res. 2010;70:3402–3410. doi: 10.1158/0008-5472.CAN-09-3977. [DOI] [PubMed] [Google Scholar]
- 5.Lévesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111:187–196. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma Q, Jones D, Springer TA. The Chemokine Receptor CXCR4 Is Required for the Retention of B Lineage and Granulocytic Precursors within the Bone Marrow Microenvironment. Immunity. 1999;10:463–471. doi: 10.1016/s1074-7613(00)80046-1. [DOI] [PubMed] [Google Scholar]
- 7.Christopherson KW, 2nd, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of Hematopoietic Stem Cell Homing and Engraftment by CD26. Science. 2004;305:1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 8.Onai N, Zhang YY, Yoneyama H, Kitamura T, Ishikawa S, Matsushima K. Impairment of lymphopoiesis and myelopoiesis in mice reconstituted with bone marrow-hematopoietic progenitor cells expressing SDF-1-intrakine. Blood. 2000;96:2074–2080. [PubMed] [Google Scholar]
- 9.Fyrst H, Saba JD. An update on sphingosine-1-phosphate and other sphingolipid mediators. Nat Chem Biol. 2010;6:489–497. doi: 10.1038/nchembio.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanel P, Andréani P, Graler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 11.Ohkawa R, Nakamura K, Okubo S, Hosogaya S, Ozaki Y, Tozuka M, et al. Plasma sphingosine-1-phosphate measurement in healthy subjects: close correlation with red blood cell parameters. Ann Clin Biochem. 2008;45:356–363. doi: 10.1258/acb.2007.007189. [DOI] [PubMed] [Google Scholar]
- 12.Mitsutake S, Kim TJ, Inagaki Y, Kato M, Yamashita T, Igarashi Y. Ceramide kinase is a mediator of calcium-dependent degranulation in mast cells. J Biol Chem. 2004;279:17570–17577. doi: 10.1074/jbc.M312885200. [DOI] [PubMed] [Google Scholar]
- 13.Lamour NF, Stahelin RV, Wijesinghe DS, Maceyka M, Wang E, Allegood JC, et al. Ceramide kinase uses ceramide provided by ceramide transport protein: localization to organelles of eicosanoid synthesis. J Lipid Res. 2007;48:1293–1304. doi: 10.1194/jlr.M700083-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Reca R, Mastellos D, Majka M, Marquez L, Ratajczak J, Franchini S, et al. Functional receptor for C3a anaphylatoxin is expressed by normal hematopoietic stem/progenitor cells, and C3a enhances their homing-related responses to SDF-1. Blood. 2003;101:3784–793. doi: 10.1182/blood-2002-10-3233. [DOI] [PubMed] [Google Scholar]
- 15.Ratajczak MZ, Lee H, Wysoczynski M, Wan W, Marlicz W, Laughlin MJ, et al. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010;24:976–985. doi: 10.1038/leu.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 17.Ponomaryov T, Peled A, Petit I, Taichman RS, Habler L, Sandbank J, et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106:13331–1339. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bastianutto C, Mian A, Symes J, Mocanu J, Alajez N, Sleep G, et al. Local radiotherapy induces homing of hematopoietic stem cells to the irradiated bone marrow. Cancer Res. 2007;67:10112–10116. doi: 10.1158/0008-5472.CAN-07-2192. [DOI] [PubMed] [Google Scholar]
- 19.Hoggatt J, Pelus LM. Eicosanoid regulation of hematopoiesis and hematopoietic stem and progenitor trafficking. Leukemia. 2010;24:1993–2002. doi: 10.1038/leu.2010.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoggatt J, Singh P, Sampath J, Pelus LM. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113:5444–5455. doi: 10.1182/blood-2009-01-201335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baron MH. Embryonic origins of mammalian hematopoiesis. Exp Hematol. 2003;31:1160–1169. doi: 10.1016/j.exphem.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Weissman I, Papaioannou V, Gardner R. Fetal hematopoietic origins of the adult hemolymphoid system. In: Clarkson B, Mark P, Till J, editors. Differentiation of Normal and Neoplastic Cells. New York: Cold Spring Harbor Lab. Press; 1978. pp. 33–47. [Google Scholar]
- 23.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: Present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 24.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 25.Kucia MJ, Wysoczynski M, Wu W, Zuba-Surma EK, Ratajczak J, Ratajczak MZ. Evidence that very small embryonic-like stem cells are mobilized into peripheral blood. Stem Cells. 2008;26:2083–2092. doi: 10.1634/stemcells.2007-0922. [DOI] [PubMed] [Google Scholar]
- 26.Greenbaum AM, Link DC. Mechanisms of G-CSF-mediated hematopoietic stem and progenitor mobilization. Leukemia. 2011;25:211–217. doi: 10.1038/leu.2010.248. [DOI] [PubMed] [Google Scholar]
- 27.Lévesque JP, Helwani FM, Winkler IG. The endosteal ‘osteoblastic’ niche and its role in hematopoietic stem cell homing and mobilization. Leukemia. 2010;24:1979–1992. doi: 10.1038/leu.2010.214. [DOI] [PubMed] [Google Scholar]
- 28.Ratajczak MZ, Kim CH, Wojakowski W, Janowska-Wieczorek A, Kucia M, Ratajczak J. Innate immunity as orchestrator of stem cell mobilization. Leukemia. 2010;24:1667–1675. doi: 10.1038/leu.2010.162. [DOI] [PubMed] [Google Scholar]
- 29.Ratajczak MZ. Spotlight series on stem cell mobilization: many hands on the ball, but who is the quarterback? Leukemia. 2010;24:1665–1666. doi: 10.1038/leu.2010.181. [DOI] [PubMed] [Google Scholar]
- 30.Massberg S, Schaerli P, Knezevic-Maramica I, Köllnberger M, Tubo N, Moseman EA, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dar A, Kollet O, Lapidot T. Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice. Exp Hematol. 2006;34:967–975. doi: 10.1016/j.exphem.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Seitz G, Boehmler AM, Kanz L, Möhle R. The role of sphingosine 1-phosphate receptors in the trafficking of hematopoietic progenitor cells. Ann N Y Acad Sci. 2005;1044:84–89. doi: 10.1196/annals.1349.011. [DOI] [PubMed] [Google Scholar]
- 33.Marquez-Curtis LA, Turner AR, Sridharan S, Ratajczak MZ, Janowska-Wieczorek A. The Ins and Outs of Hematopoietic stem cells: Studies to Improve Transplantation Outcomes. Stem Cell Rev. 2010 doi: 10.1007/s12015-010-9212-8. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allende ML, Tuymetova G, Lee BG, Bonifacino E, Wu YP, Proia RL. S1P1 receptor directs the release of immature B cells from bone marrow into blood. J Exp Med. 2010;207:1113–1124. doi: 10.1084/jem.20092210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pereira JP, Xu Y, Cyster JG. A role for S1P and S1P1 in immature-B cell egress from mouse bone marrow. PLoS One. 2010;5:e9277. doi: 10.1371/journal.pone.0009277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harun Nadia, Thien Marilyn, Juarez Julius G, Bradstock Kenneth Francis, Bendall Linda J. S1P1 Agonists for Use as Adjunct Mobilizing Agents. ASH annual meeting; 2010. Abstract # 826. [Google Scholar]
- 37.Granado MH, Gangoiti P, Ouro A, Arana L, González M, Trueba M, et al. Ceramide 1-phosphate (C1P) promotes cell migration involvement of a specific C1P receptor. Cell Signal. 2009;21:405–412. doi: 10.1016/j.cellsig.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Chen Y, Yang C, Jin N, Xie Z, Tang Y, Fei L, et al. Terminal complement complex C5b-9-treated human monocyte-derived dendritic cells undergo maturation and induce Th1 polarization. Eur J Immunol. 2007;37:167–176. doi: 10.1002/eji.200636285. [DOI] [PubMed] [Google Scholar]
- 39.Pelus LM, Hoggatt J, Singh P. Pulse exposure of hematopoietic grafts to prostaglandin E2 in vitro facilitates engraftment and recovery. Cell Prolif. 2011;44:22–29. doi: 10.1111/j.1365-2184.2010.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pettus BJ, Kitatani K, Chalfant CE, Taha TA, Kawamori T, Bielawski J, et al. The coordination of prostaglandin E2 production by sphingosine-1-phosphate and ceramide-1-phosphate. Mol Pharmacol. 2005;68:330–335. doi: 10.1124/mol.104.008722. [DOI] [PubMed] [Google Scholar]
- 41.Corallini F, Bossi F, Gonelli A, Tripodo C, Castellino G, Mollnes TE, et al. The soluble terminal complement complex (SC5b-9) up-regulates osteoprotegerin expression and release by endothelial cells: implications in rheumatoid arthritis. Rheumatology (Oxford) 2009;48:293–298. doi: 10.1093/rheumatology/ken495. [DOI] [PubMed] [Google Scholar]
- 42.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: Present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 43.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 44.Rossi L, Manfredini R, Bertolini F, Ferrari D, Fogli M, Zini R, et al. The extracellular nucleotide UTP is a potent inducer of hematopoietic stem cell migration. Blood. 2007;109:533–542. doi: 10.1182/blood-2006-01-035634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Panel A: The chemotactic activity dose response to S1P and C1P measured by the number of BM-derived CFU-GM is presented. Chemotactic responsiveness to C1P was observed at higher concentrations of this bioactive lipid than for S1P. Panel B: C1P and S1P show some differences in chemoattraction of murine progenitors from different hematopoietic lineages. While SDF-1 at optimal doses chemoattracts all types of progenitors, bioactive lipids show little chemotactic effect against erythroid BFU-E cells. The data shown represent the combined results from three independent experiments carried out in triplicate per group (n = 9).
C1P and S1P do not affect clonogenic growth of murine granulocytic, monocytic, erythrocytic, megakaryocytic, and mixed-lineage progenitor cells. The data shown represent the combined results from three independent experiments carried out in quadruplicate per group (n = 12).
The soluble MAC effect on clonogenic growth of murine granulocytic, monocytic, erythrocytic, megakaryocytic, and mixed-lineage progenitor cells is shown. The data represent the combined results from three independent experiments carried out in quadruplicate per group (n = 12).
Panel A: C1P and S1P increase expression of cyclooxygenase-2 (Cox-2) in BM stromal cells. Panel B: C1P enhances secretion of PGE2 in murine BM stromal cells. Panel C: C1P enhances expression of EP3 mRNA in murine BM-derived SKL cells. Panel D: PGE2 secretion is increased in media harvested from murine BM stromal cells after conditioning for hematopoietic transplantation by lethal irradiation. The data represent the combined results from three (panels A–C) or two independent experiments (panel D).
Panel A: Migration of BMMNCs to C1P and S1P was totally blocked by pre-treatment with pertussis toxin (1μg/ml for 1hr at 37°C). The experiment was repeated three times with similar results. Panel B: Pertussis toxin (PTX) treatment (0.5 μg/ml for 1hr) before stimulation affects phosphorylation of MAPKp44/42, Akt, and Stat-3 by C1P, but not sMAC. One representative study out of two is shown.
Exposure of Sca-1+ cells to MAC does not affect the expression of CXCR4 and VLA-4. One representative study out of three is shown.