Abstract
Introduction
Signal transducers and activators of transcription (STAT) proteins are transcription factors that when activated, by phosphorylation, regulate gene expression and cellular activity. The aim of this study was to evaluate the local and systemic expression and activation of STAT proteins associated with abdominal aortic aneurysms (AAA).
Methods
Expression and activation of STAT proteins were assessed in aortic wall samples obtained from patients undergoing repair of AAA (N=9) and from non-aneurysmal (NA) donors (N=17). Aortic samples were evaluated for mRNA and protein expression for STAT1, 2, 3, 4, 5a, and 5b using RT-PCR and immunoblot (WB) assays and normalized to β-actin (expressed as arbitrary units). STAT activation was assessed with WB assays using phosphorylated (p)-STAT-specific antibodies. Alterations in STAT activation were calculated by normalizing p-STAT proteins to corresponding total STAT levels. Immunohistochemistry was performed on AAA and NA samples using the total and pSTAT antibodies.Systemic alterations in STAT activation were assessed by evaluating circulating leukocytes for the presence of p-STAT from patients with AAA (AAA, N=8), repaired aneurysm (RA, N=8), or age/gender matched controls with no AAA (CT, N=8). Flow cytometry was performed to assess for circulating levels of STAT1 (pY701), STAT3 (pY705), and STAT5a (pY694) in monocytes, granulocytes, and lymphocytes. Assessments were made at baseline and in response to in vitro stimulation with IFN-gamma (50 ng/mL) or IL-6 (100 ng/mL). Results were analyzed using Student’s T-test and are expressed as mean±SEM.
Results
In AAA tissue compared to NA, STAT-1 (1.08±0.09 v. 0.62±0.07), -2 (0.98±0.07 v. 0.55±0.08), and -4 (0.89±0.12 v. 0.35±0.11) mRNA levels were elevated (P<0.01, all). Corresponding increases in STAT protein were only observed for STAT1 (2.77±0.93 v. 0.93±0.08, P<0.05). Increases in activation were observed in AAA compared to NA in p-STAT2 (0.77±0.1 v. 0.1±0.02, P<0.01), p-STAT3 (1.6±0.3 v. 0.2±0.06, P<0.02) and p-STAT5 (0.57±0.03 v. 0.2±0.03, P<0.05) levels. Phosphorylated STAT1, 2, 3, and 5 were observed in inflammatory cells invading the AAA adventitia. In addition, STAT3 was observed in the media of AAA and NA, but pSTAT3 was only observed in the media of AAA. There were no differences in baseline levels of p-STAT-positive circulating leukocytes. IFN-gamma stimulation decreased STAT-5a (pY694)-positive CT lymphocytes to 40±13% of baseline, but had no effect on AAA or RA lymphocytes (116±35%, 102±19%, respectively; P=0.01). STAT-5a (pY694)-positive CT granulocytes also decreased to 62±18% of baseline compared to AAA or RA granulocytes (122±25%, 126±17%, respectively; P=0.01). Alterations in STAT1 (pY701) and STAT3 (pY705) were not observed in leukocytes following cytokine stimulation.
Conclusions
STAT proteins are important regulators of transcriptional activity and have been linked to cardiovascular disease. The present data suggest that altered levels of phosphorylated STATs are associated with AAA. Understanding their role may provide further insight into the mechanisms of AAA formation and allow for the development of medical treatment options.
Introduction
Abdominal aortic aneurysm (AAA) formation is a multifactorial process that results from the altered homeostasis of the aortic wall matrix protein production and destruction. The AAA wall is characterized by a loss of elastin, increased collagen metabolism, smooth muscle cell apoptosis, and a chronic inflammatory infiltrate. Numerous studies have demonstrated that chronic inflammation plays an important role in AAA formation and progression1–3. The chronic inflammatory nature of the AAA wall provides a cytokine-enriched environment, which have been identified as key signaling mediators of AAA pathogenesis. Specific cytokines that have been postulated to regulate AAA formation include interleukin-1 beta (IL-1β), IL-6, tumor necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-γ)3, 3–10. The underlying molecular mechanisms that regulate this chronic inflammatory process and the subsequent proteolytic process, however, are poorly understood.
Signal transducer and activators of transcription (STAT) proteins are a family of transcription factors that consist of seven members including STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b, and STAT6. These proteins play a dual role in that they both transducer signals through the cytoplasm and function as transcription factors in the nucleus. Cytokine receptors lack enzymatic activity but are associated with tyrosine kinases belonging to the JAK family. Ligand stimulation leads to activation of an associated JAK protein, which leads to recruitment and phosphorylation of STATs. Phosphorylated STAT (pSTAT) proteins, or activated STAT proteins, form homo- or hetero-dimers, and translocate to the nucleus where they regulate gene expression. STATs have been demonstrated to be involved in a variety of processes including immune responses, cell growth and differentiation, cell survival and apoptosis, and oncogenesis; and STAT involvement in these processes is often due to their function in regulating inflammation 11–17.
Given the chronic inflammatory process that is involved with the development of AAA, it is likely thatSTAT proteins have a potential role in regulating this process. It is not clear from available data what cytokine profile specifically promotes aneurysm formation, but this profile may be better understood by evaluating their downstream effects, such as STAT activation. We hypothesized that activation of STATs, in particular STAT1 and STAT3, promote a pro-inflammatory state that results in matrix degradation. There is a paucity of data, however, evaluating the role of STATs in the process of aneurysmal degeneration. The aim of this current study was to evaluate for alteration in STAT proteins associated with the presence of AAA in humans.
Materials and Methods
Aortic Tissue
Abdominal aortic segments were collected from patients undergoing surgical repair for AAA (N=9), while non-aneurysmal (NA) aortic samples were obtained from organ donors at the time of organ procurement (N=17). Aortic tissues were rinsed with iced phosphate buffered saline (PBS), snap frozen in liquid nitrogen, and stored at −80°C until further analysis. Some samples, or segments of samples, were placed in 10% buffered formalin for 24 hours, followed by reagent alcohol (70% volume/volume) for subsequent permanent section after being embedded in paraffin. Patients undergoing repair of AAA were older (75±3 yrs v. 47±7 yrs, P<0.05) and comprised of more males (100% v. 53%, P<0.05) than the non-aneurysmal controls, but were similar with regard to race (100% v. 82% white). Additional demographic and clinical information are not available. This protocol was approved by the Cleveland Clinic Institutional Review Board (IRB#06-631).
Quantitative real-time reverse transcriptase-polymerase chain reaction
Expression of messenger RNA (mRNA) levels of β-actin (QuantumRNA Beta-actin Internal Standards), human STAT1 (Hs01014001_m1), human STAT2 (Hs00374138_g1), human STAT3 (Hs00376832_m1), human STAT4 (Hs00162389_m1), human STAT5a (Hs00234181_m1), and human STAT5b (Hs00273500_m1) were assayed using quantitative reverse transcriptase polymerase chain reaction (RT-PCR) using Taq-man Gene Expression Assays (Applied Biosystems, Foster City, CA) as previously described18. STAT6 was not evaluated in this study. Briefly, frozen aortic tissue was crushed under liquid nitrogen using a Bio-Pulverizer (Research Products International Corp, Mt. Prospect, IL). Resulting powders were homogenized using TRIzol reagent (Invitrogen, Carlsbad, CA). Messenger RNA was reverse transcribed, and the resultant complementary DNA was amplified by Taq Polymerase (Promega, Madison, WI). Gene-specific assays were performed and all data were normalized and analyzed using Applied Biosystems 7500 operating software. Final data are presented as expression of indicated molecules relative to expression of internal control (β-actin) probe sets as outlined in the system’s instructions for use (Applied Biosystems).
Protein Immunoassay
Tissue samples were prepared according to the methods described above. Frozen aortic tissues were crushed under liquid nitrogen and resulting powders underwent protein extraction using Cell-Lytic M (Sigma, St. Louis, MO) lysis buffer. Total aortic protein was determined with the bicinchoninic acid (BCA) protein assay (Pierce, Rockford, IL). Immunoblotting analysis of aortic protein extracts was performed to investigate STAT protein expression with standard procedures. Briefly, protein extracts (20μg/lane) were subjected to electrophoresis on a 10% Tris-glycine gel (Invitrogen), transferred to a nitrocellulose membrane, and blocked in 5% milk protein. Membranes were then incubated with antibodies for STAT2 (1:2000), STAT4 (1:2000), STAT5(1:2000), and p(Y690)-STAT2 (1:1000), STAT3 (1:2000), p(Y705)-STAT3 (1:2000), p(S727)-STAT3 (1:1250), p(Y694)-STAT5 (1:1000) (Cell Signaling Technology, Beverly, MA), and p(Y693)-STAT4 (1:1000) (Abcam, Cambridge, MA). followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody and detection using ECL Advance™ Westernblotting detection kit (Amersham Bioscience, Piscataway, NJ). Membranes were stripped and reprobed with either an antibody to β-actin or a specific total STAT protein. Protein expression levels in aortic wall samples were normalized by β-actin. Phosphorylated STAT proteins were normalized to either β-actin or to their corresponding total STAT protein. Films were scanned using an Epson Perfection 3200 Scanner (Epson America, Long Beach, CA), and band density was analyzed using Image J software (National Institutes of Health, Bethesda, MD).
Immunohistochemical analysis
Tissue sections were deparaffinized and dehydrated through graded xylene and ethanol. For antigen retrieval, slides were incubated in the microwave oven for 20 min in 0.01 mol/L of citrate buffer, pH 6 (Fisher Scientific). To block endogenous peroxidase, the slides were incubated in 3% H2O2 for 10 min at room temperature (RT). Nonspecific binding was blocked by incubation with 5% normal goat serum for 30 min at RT. Incubation with the following primary antibodies occurred for 1 h at RT or overnight at 4°C: STAT1 (1:200) (Santa Cruz Biotechnology); p(Y701)-STAT1 (1:400), STAT2 (1:200), p(Y690)-STAT2 (1:100), STAT3 (1:600), p(Y705)-STAT3 (1:200), STAT4 (1:100), p(Y693)-STAT4 (1:50), STAT5 (1:300), p(Y694)-STAT5 (1:300) (Cell Signaling Technology); and alpha-smooth muscle actin and CD68 (Abcam). After rinsing in PBS-0.1% Tween 20, slides were incubated with an anti-rabbit or anti-mouse biotinylated secondary antibody (1:400 dilution), Vector Laboratories, Burlingame, CA) for 30 min followed by incubation with Vectastain ABC Reagent (Vector Laboratories, Burlingame, CA) for 30 min at RT. Immunoreactive cells were then visualized by addition of diaminobenzidine (DAB, Vector Laboratories, Burlingame, CA) as substrate and counterstained with hematoxylin. Stained slides were observed and analyzed by Olympus BX40 Microscope and Spot2 color digital image analysis software.
Systemic alterations of STAT
To assess for systemic alterations of STAT associated with AAA, blood samples were obtained from patients undergoing evaluation for AAA (AAA, N=8), patients undergoing follow up for a repaired AAA (RA, N=8), and patients undergoing evaluation for carotid artery stenosis (CT, N=8). The patients who had a previous AAA repair were all repaired in an endovascular fashion greater than 24 months prior to the time of enrollment; all had evidence of aneurysm regression, and none had evidence of an endoleak. Control patients undergoing evaluation for carotid artery stenosis all had radiographic demonstration, either by ultrasound or computed tomography, within the preceding 5 years of a normal caliber abdominal aorta. Demographic and clinical data was collected from all patients.
Whole blood was collected from patients, following informed consent, placed in sodium heparin tubes, and placed directly on ice as has previously been described19. Samples were assayed at baseline, or following stimulation with either IL-6 (100 ng/mL) or IFN-γ (50 ng/mL) for 30 minutes. Samples were mixed with 10% formalin and 0.1% Triton X-100 and incubated at room temperature for 30 minutes. Cells were pelleted by centrifugation and resuspended in cold FACS Lyse buffer (Becton Dickinson, Laguna Hills, CA). Cells were pelleted and esuspended in methanol:PBS (1:1 by volume), incubated in 4°C for 10 minutes, and then repelleted and resuspended in cold FACS Lyse buffer. Cells were then incubated at room temperature with antibodies for flow cytometry: CD45 (BD Biosciences, San Jose, CA), Alexa Fluor 488 Mouse Anti-STAT1 (pY701) (BD Bioscienes), Alexa Fluor 647 Mouse Anti-STAT3 (pY705) (BD Biosciences), and Pacific Blue Mouse anti-STAT5 (pY694) (BD Bisciences). Cells were then rewashed and resuspended in FACS Lyse buffer and kept at 4°C until flow cytometry.
Statistical analysis
Statistical analyses were performed using SPSS 15.0 statistics software (SPSS Inc, Chicago, IL). Data were presented either as mean or mean ± standard error of the mean. Continuous and categorical variables were compared by t-test and chi-square/Fisher’s exact test, respectively. A P value of <.05 was considered to be statistically significant.
Results
STAT mRNA was detected in AAA and NA tissues by real time RT-PCR. mRNA expression levels for STAT1, STAT2 and STAT4 were increased 1.76-, 1.79- and 1.53-fold, respectively, in AAA compared with NA (Fig.1, P<0.05). There were no significant changes in mRNA expression of STAT3, STAT5a or STAT5b between the two groups. Only STAT1 had corresponding increases in total protein associated with the increased mRNA (Fig. 2).
Figure 1.
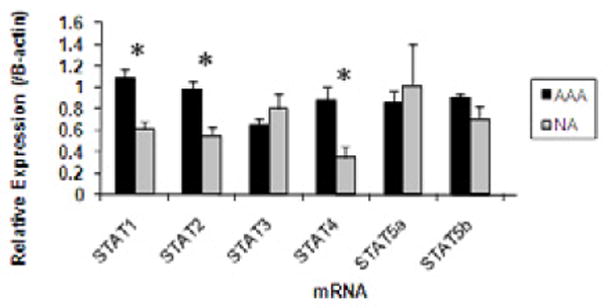
Graphic representation of aortic STAT mRNA obtained from AAA (black bars) or NA (gray bars). STAT mRNA expression was assessed by real time RT-PCR and normalized to β-actin and is expressed in arbitrary units (AU). STAT1, 2, and 4 mRNA were significantly elevated in samples obtained from AAA compared to NA. * P<0.05.
Figure 2.

Graphic representation of aortic total STAT protein obtained from AAA (black bars) or NA (gray bars). STAT protein expression was assessed from aortic homogenates using immunoblot assay. Samples were normalized to β-actin and are expressed in arbitrary units. Only STAT1 levels were significantly elevated in AAA samples compared to NA controls (*P<0.01).
STAT proteins become activated once they undergo phosphorylation. Depending on the specific STAT protein, this can occur on a tyrosine or serine residue (or both). To assess for alterations in STAT activation, changes in pSTAT protein levels were also assessed for by immunoblot assay. Changes in total phosphorylated protein was assessed by calculating the ratio of pSTAT to β-actin (pSTAT/β-actin), while alterations in STAT activation were assessed by calculating the ratio of pSTAT to its corresponding total STAT protein level pSTAT/total STAT). Statistical analysis was performed among the groups based on these calculations. The final data, however, is frequently presented as a “fold-change” between the AAA and NA groups, but statistical analysis of this further “normalization” was not performed. We previously demonstrated that STAT1 did not undergo increased rates of phosphorylation at either the serine or tyrosine residue, but there was an increase in total tyrosine phosphorylated STAT1 in direct proportion to the increase in total STAT1 protein18. Alterations in total pSTAT levels (pSTAT/β-actin ) were not observed in any other STAT protein. Increased STAT activation (pSTAT/total STAT) was observed in STAT2, STAT3, and STAT5. Increased activation of STAT2 was observed in AAA compared to NA (P<0.01) demonstrated by a 3.84-fold increase in p(Y690)STAT2 when normalized to total STAT2 (Fig. 3). In addition, p(Y705)-STAT3, when normalized to total STAT3, increased 5.7-fold in AAA compared with NA (P<0.01, Fig. 4). Alterations in p(S727)STAT3 were not observed (1.19-fold AAA/NA, P>0.05). Although there was no significant difference in the protein expression of total STAT5, increased STAT5 activation was observed by a 2.75-fold increase in p(Y694)STAT5 in AAA compared with NA (P<0.01, Fig. 5). There were no significant alterations in STAT 4 phosphorylation.
Figure 3.

(A) Representative immunoblots from aortic homogenates obtained from those without aneurysms (NA) or with aneurysms (AAA). Samples were blotted against p(Y690)STAT-2, total STAT-2 and β-actin. Assessment for STAT2 activation was assessed by analyzing the pSTAT2 versus total STAT2. (B) Graphic representation of STAT2 activation obtained from NA and AAA samples. STAT2 underwent significantly more phosphorylation in samples from AAA compared to those from NA (*P<0.01). There were no significant changes in total STAT2 levels.
Figure 4.
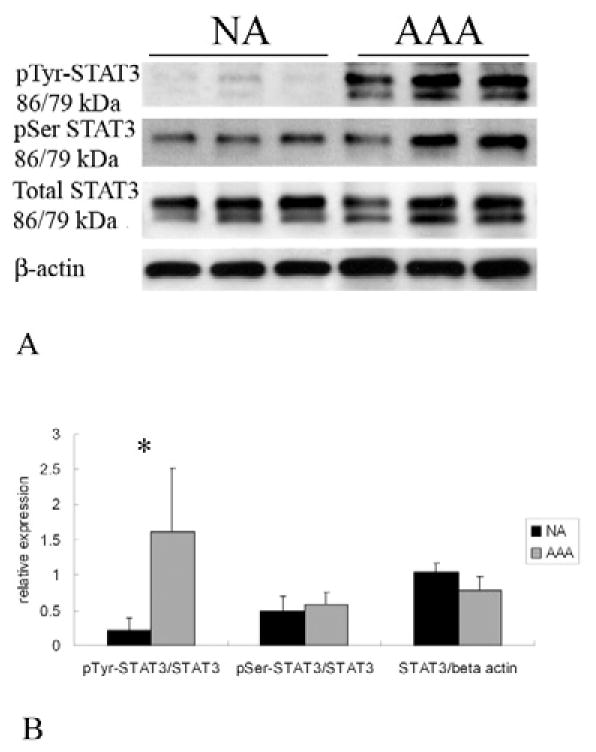
(A) Representative immunoblots from aortic homogenates obtained from those without aneurysms (NA) or with aneurysms (AAA). Samples were blotted with antibodies against p(Y705)STAT-3, p(S727)STAT-3, total STAT3, and β-actin. Assessment for STAT3 activation was assessed by analyzing the different p-STAT-3 versus total STAT-3 (B) Graphic representation of STAT3 activation obtained from NA and AAA samples. STAT3 underwent significantly more activation by tyrosine phosphorylation in AAA compared to NA controls (*P<0.01). There were no significant differences in serine phosphorylation or in total STAT3 levels.
Figure 5.
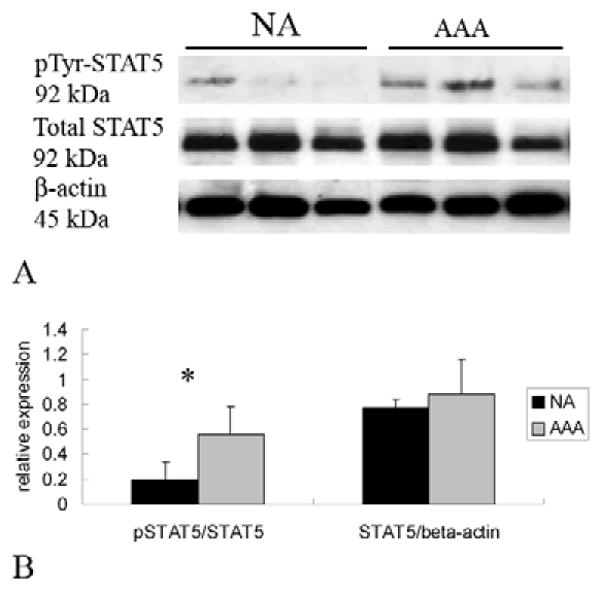
(A) Representative immunoblots from aortic homogenates obtained from those without aneurysms (NA) or with aneurysms (AAA). Samples were blotted with antibodies against p(Y694)STAT5, total STAT5, and β-actin. (B) Assessment of STAT5 activation was determined by assessing the ratio of p-STAT5 to total STAT5. While there was no difference in total STAT5 protein from AAA (gray bar) compared to NA (black bar), there was a significant increase in STAT5 activation demonstrated by the significant increase in the ratio of p-STAT5/STAT5 (*P<0.01).
Immunohistochemical analysis demonstrated staining of total- and phosphorylated- STAT1 (Fig. 6), -STAT2 (Fig. 7), and -STAT5 (Fig. 7) within the adventitial layers of the aorta in samples obtained from AAA but not from NA. Corresponding cells also stained positive for CD68 (Fig. 6) suggesting they are of the monocyte/macrophage lineage. Staining for STAT3 was identified in smooth muscle cells (SMC) from both the non-aneurysmal and aneurysmal tissue (Fig. 8), but staining for p(Y705)STAT3 was only visualized in aneurysmal tissue. Positive staining for both STAT3 and p(Y705)STAT3 was seen in the inflammatory cells of the adventitia of the aneurysm, but again not noted in the non-aneurysmal tissue.
Figure 6.

Representative samples from aortic tissue obtained from non-aneurysmal aorta (A and C) and aneurysmal aorta (B and D). Immunohistochemical analysis failed to show any staining for STAT1 in non-aneurysmal tissue (A (40x) and C (100x)), but was present (arrow) in the adventitial layer in samples from aortic aneurysms (B, 40x). This appeared to localize to inflammatory cells (arrows) within the adventitial layer (D, 100x). Corresponding cells in this layer also stained positive for CD68 (E, 100x) suggesting they are of a monocyte/macrophage lineage.
Figure 7.

Representative sample from aortic tissue from a patient with (B (40x), C (100X) and D (100x)) and without (A (40X)) an AAA. Immunohistochemical analysis with antibodies directed against STAT5 failed to demonstrate any positive staining in the normal aorta (A), but there was positive staining for both STAT5 (B) and pSTAT5 (C) in inflammatory cells infiltrating the adventitia of the aneurysm. Similarly, staining with antibodies to pSTAT2 showed positive cells in the adventitial inflammatory cells (D).
Figure 8.

Representative samples from aortic tissue obtained from non-aneurysmal aorta (A and B (100x)) and aneurysmal tissue (C, D, E (100x)). Immunohistochemical analysis demonstrates that STAT3 staining (brown cells) occurs in both non-aneurysmal (A) and aneurysmal (C) tissue in the medial layer in smooth muscle cells. pSTAT3 staining, however, only occurred in aneurysmal tissue (arrow) (D) and not non-aneurysmal aorta (B). STAT3 (not shown) and pSTAT3 also stained positive in the inflammatory cells within the adventitia of aneurysms (E).
The demographics for patients that underwent analysis for systemic alterations in STAT proteins were similar (Table I) except that the non-aneurysmal controls all had cerebrovascular disease and no radiologic evidence of AAA. There were no alterations in baseline levels of p-STAT-positive circulating leukocytes. IFN-γ stimulation decreased p(Y694)STAT-5-positive lymphocytes to 0.4±0.13-fold of baseline, which was significantly altered compared to samples from AAA (1.16±0.35, P-0.002) or RA (1.02±0.19, P=0.01). p(Y694)STAT-5-positive granulocytes also decreased to 0.618±0.18 of baseline compared to AAA (1.22±0.25, P=0.01) or RA (1.26±0.17, P=0.01). Alterations in p(Y701)STAT1 and p(Y705)STAT3 were not observed in any leukocyte at baseline or following cytokine stimulation.
Table I.
Demographic data for patients undergoing analysis for alterations in STAT proteins in circulation leukocytes.
| AAA (N=8) | RA (N=8) | CT (N=8) | P-Value | |
|---|---|---|---|---|
| Age | 75±3 yrs | 74±2 yrs | 70±2 yrs | NS |
| Aortic Diameter | 54±0.6 mm | 32±2.7 mm | - | P<0.001 |
| CAD | 50% | 25% | 25% | NS |
| DM | 0% | 0% | 0% | NS |
| Current Tobacco | 0% | 12.5% | 25% | NS |
| HTN | 87.5% | 87.5% | 87.5% | NS |
| Renal Disease | 0% | 0% | 0% | NS |
| Pulmonary Disease | 12.5% | 12.5% | 25% | NS |
| Hyperlipidemia | 100% | 75% | 100% | NS |
| CVD | 12.5% | 25% | 100% | P<0.01 |
| ACEI/ARB Use | 62.5% | 25% | 25% | NS |
| Statin Use | 100% | 75% | 75% | NS |
CAD – coronary artery disease, DM – diabetes mellitus, HTN – hypertension, CVD – cerebrovascular disease, ACE – angiotensin converting enzyme inhibitor, ARB – angiotensin receptor blocker
Discussion
Abdominal aortic aneurysm formation is a multifactorial process that results in the altered homeostasis of the aortic wall. Multiple mechanisms are involved in this process, and a better understanding of these pathways could potentially allow the development of medical therapies aimed at reducing or preventing the risk of aneurysm expansion and rupture. A growing body of evidence suggests that transcription factors, which regulate gene expression, have an important role in regulating aneurysm formation, and that manipulation of these factors can attenuate experimental models of aneurysm formation. For example, inhibition of c-Jun-N-terminal kinase, a mitogen activated protein kinase, not only prevents experimental AAA formation but also causes regression of the aneurysms20. In addition, inhibition of transcription factor nuclear factor-κB and ets ameliorate AAA formation in experimental AAA formation21.
STAT proteins are a family of transcription factors that, among other functions, regulate the downstream effects of cytokine signaling. While multiple cytokines have been implicated in the pathogenesis of AAA, little data exist defining the role of STAT proteins in this disease. Our laboratory has recently demonstrated that STAT1 protein is increased in human AAA, and that there are increased levels of tyrosine phosphorylated STAT1 corresponding to the increase in total STAT1 protein levels18. Furthermore, mice deficient in STAT1 develop larger aortic aneurysms that rupture more readily in an experimental model of AAA formation, as compared to STAT1 competent mice 18. Further information about the role of other STAT proteins in the pathogenesis of AAA has not been reported.
Data from the current evaluation demonstrate that STAT expression, as measured by mRNA and protein production, is not significantly upregulated in human AAA tissue. STAT proteins, however, exert their downstream effect once activated through amino acid phosphorylation, and the current data demonstrate alteration in STAT activation in AAA. In addition to STAT1, STAT2, STAT3, and STAT5 have increased rates of phosphorylation in AAA tissue compared to non-aneurysmal controls, which suggests increased activity of these transcription factors. It is not surprising, given the role of STATs in regulating cytokine signaling, that the majority of these phosphorylated proteins were identified in the inflammatory cells infiltrating the adventitia of the AAA. It is intriguing to note, however, that STAT3, and in particular phosphorylated STAT3, also localizes to smooth muscle cells within the media. This is not the first evaluation demonstrating STAT alterations in vascular smooth muscle cells. Potula and colleagues demonstrated that STAT3 activation is important in vascular smooth muscle cell migration 22, while Kakisis et al. demonstrated that STAT3 regulated vascular smooth muscle response to cyclic strain23. The significance of STAT3 activation in both inflammatory cells and resident smooth muscle cells in AAA tissue is not apparent from the data provided, but merits further evaluation.
The results from AAA tissue, however, need to be evaluated with caution. The patient population from which aortic tissue was procured was younger and had a higher percentage of women compared with those with aneurysmal disease. In addition, the state of brain death can incite a systemic inflammatory state that may affect STAT phosphorylation, although controlling for this may have further accentuated our results. Furthermore, additional clinical data from the non-aneurysmal donors was not available; as such, other confounding variables could not be assessed. It is likely, however, given that the majority of changes identified in STAT activation were localized to inflammatory cells within the aortic aneurysm adventitia, that the differences were secondary to the presence of an aneurysm and not some other clinical variable. Additionally, what cannot be discerned from the data is whether the alterations in STAT phosphorylation contribute to aneurysm formation or are the result of the inflammatory response generated by the presence of an AAA. Clearly, based on our previous analysis of STAT118, the upregulation of these STATs may not be causative but may occur in a protective fashion. Much more targeted investigations are currently underway to try to determine the significance of the STAT activation and to understand the mechanisms they may be regulating in AAA pathogenesis.
In addition to evaluating for alterations in STAT expression in aortic tissue, alterations in circulating phosphorylated STAT levels were assessed for STAT1, 3 and 5. These were performed at baseline and in response to the stimuli IFN-γ and IL-6. Both of these are known to classically upregulate STAT1 and 3, respectively, and have been shown to be important in AAA pathogenesis. STAT5 is classically linked to the GH receptor, and we opted not to assess the response of circulating leukocyte to this hormone as it has not been demonstrated to be significant in human AAA formation. STAT5, however, has been shown to be activated (or inactivated) by IFN-γ and/or IL-6 in a cell-specific fashion. At baseline, no alterations in STAT phosphorylation were identified in circulating leukocytes. These findings suggest that AAA formation is not due to a systemic alteration in STAT activation. It is surprising to observe, however, that none of these groups responded with a significant activation of STAT1 or STAT3 in response to IFN-γ or IL-6 stimulation, respectively. While not previously reported, reactivity of leukocytes to cytokine stimulation may be diminished in the aging population and thus not observed in these patients. Additional testing is necessary to compare the STAT responsiveness in circulating leukocytes from both a young and aged population. Also interesting is the observation that there was reduction in cytokine-induced STAT5 activation in patients with cerebrovascular disease but no aneurysmal disease. This may suggest that one mechanism regulating the differentiation of occlusive disease from aneurysmal disease is the response of the inflammatory cells to cytokines. Furthermore, we observed that in AAA tissue phosphorylated STAT5 localized to inflammatory cells that appear to be of the monocyte/macrophage lineage. Systemic alterations in phosphorylated STAT5, however, were not observed in circulating monocytes. This may be secondary to changes in cell reactivity that occur as they infiltrate the aorta changing from a monocyte to macrophage phenotype.
STAT proteins are key regulators of inflammation and cytokine signaling. These processes are important in the mechanisms involved in aneurysm formation. These current observations provide some insight as to which STAT proteins may be involved in the process of AAA pathogenesis. These observations, however, provide only a preliminary step in evaluating the mechanisms that may be involved in these processes. Certainly more questions were raised than answered. Additional evaluations are necessary to understand the relative importance of these alterations and their downstream effects. This will help to discern whether manipulation of cytokine signaling mechanisms, at the level of STAT proteins, may be beneficial in the treatment of AAA.
Acknowledgments
This research is supported by the Foundation for Accelerated Vascular Research, the American Vascular Association, and the National Institutes of Health (5KO8HL87798).
Footnotes
Presented in part before the Academic Surgical Congress, Huntington Beach, CA, Feburary 1, 2011
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saratzis A, Kitas GD, Saratzis N, Melas N. Can statins suppress the development of abdominal aortic aneurysms? A review of the current evidence. Angiology. 2010;61:137–44. doi: 10.1177/0003319709335514. [DOI] [PubMed] [Google Scholar]
- 2.Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2006;26(5):987–94. doi: 10.1161/01.ATV.0000214999.12921.4f. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu K, Shichiri M, Libby P, Lee R, Mitchell R. Th2-predominant inflammation and blockade of IFN gamma signaling induce aneurysm in allografted aortas. J Clin Invest. 2004;114:168–71. doi: 10.1172/JCI19855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King V, Yin A, Kristo F, Anderson T, Ahluwalia N, Hardy G, et al. Interferon-gamma and the interferon-inducible chemokine CXCL10 protect against aneurysm formation and rupture. Circulation. 2009;119:426–35. doi: 10.1161/CIRCULATIONAHA.108.785949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ocana E, Perez-Requena J, Bohorquez J, Brieva J, Rodriguez C. Chemokine receptor expression on infiltrating lymphocytes from abdominal aortic aneurysms: role of CXCR4-CXCL12 in lymphoid recruitment. Atherosclerosis. 2008;200:264–70. doi: 10.1016/j.atherosclerosis.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 6.Xiong W, Zhao Y, Prall A, Greiner T, Baxter B. Key roles of CD4+ T cells and interferon gamma in the development of abdominal aortic aneurysms in a murine model. J Immunol. 2009;2004:2607–12. doi: 10.4049/jimmunol.172.4.2607. [DOI] [PubMed] [Google Scholar]
- 7.Juvonen J, Surcel H-M, Satta J, Teppo A-M, Bloigu A, Syrjala H, et al. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 1997;17:2843–7. doi: 10.1161/01.atv.17.11.2843. [DOI] [PubMed] [Google Scholar]
- 8.Davis V, Persidskaia R, Baca-Regen L, Fiotti N, Halloran B, Baxter B. Cytokine pattern in aneurysmal and occlusive disease of the aorta. J Surg Res. 2001;101:152–6. doi: 10.1006/jsre.2001.6281. [DOI] [PubMed] [Google Scholar]
- 9.Lindeman JH, bdul-Hussien H, Schaapherder AF, van Bockel JH, Von der Thusen JH, Roelen DL, et al. Enhanced expression and activation of pro-inflammatory transcription factors distinguish aneurysmal from atherosclerotic aorta: IL-6- and IL-8-dominated inflammatory responses prevail in the human aneurysm. Clin Sci (Lond) 2008;114:687–97. doi: 10.1042/CS20070352. [DOI] [PubMed] [Google Scholar]
- 10.Flondell-Site D, Lindblad B, Kolbel T, Gottsater A. Cytokines and systemic biomarkers are related to the size of abdominal aortic aneurysms. Cytokine. 2009;46:211–5. doi: 10.1016/j.cyto.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Stritesky GL, Muthukrishnan R, Sehra S, Goswami R, Pham D, Travers J, et al. The Transcription Factor STAT3 Is Required for T Helper 2 Cell Development. Immunity. 2011;28(34):39–49. doi: 10.1016/j.immuni.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh CK, Geba GP, Molfino N. Investigational therapeutics targeting the IL-4/IL-13/STAT-6 pathway for the treatment of asthma. Eur Respir Rev. 2010;19:46–54. doi: 10.1183/09059180.00007609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scharl M, Hruz P, McCole DF. Protein tyrosine phosphatase non-receptor Type 2 regulates IFN-gamma-induced cytokine signaling in THP-1 monocytes. Inflamm Bowel Dis. 2010;16:2055–64. doi: 10.1002/ibd.21325. [DOI] [PubMed] [Google Scholar]
- 14.Wei L, Vahedi G, Sun HW, Watford WT, Takatori H, Ramos HL, et al. Discrete roles of STAT4 and STAT6 transcription factors in tuning epigenetic modifications and transcription during T helper cell differentiation. Immunity. 2010;32:840–51. doi: 10.1016/j.immuni.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei L, Laurence A, O’Shea JJ. New insights into the roles of Stat5a/b and Stat3 in T cell development and differentiation. Semin Cell Dev Biol. 2008;19:394–400. doi: 10.1016/j.semcdb.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gamero AM, Young MR, Mentor-Marcel R, Bobe G, Scarzello AJ, Wise J, et al. STAT2 contributes to promotion of colorectal and skin carcinogenesis. Cancer Prev Res (Phila) 2010;3:495–504. doi: 10.1158/1940-6207.CAPR-09-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luhrmann A, Tschernig T, von der LH, Hecker M, Pabst R, Wagner AH. Decoy oligodeoxynucleotide against STAT transcription factors decreases allergic inflammation in a rat asthma model. Exp Lung Res. 2010;36:85–93. doi: 10.3109/01902140903144138. [DOI] [PubMed] [Google Scholar]
- 18.Eagleton M, Xu J, LLiao M, Parine B, Chisolm G, Graham L. Loss of STAT1 is associated with increased aortic rupture in an experimental model of aortic dissection and aneurysm formation. J Vasc Surg. 2010;51:951–61. doi: 10.1016/j.jvs.2009.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carey R, Jurickova I, Ballard E, Bonkowski E, Han X, Xu H, et al. Activation of an IL-6:STAT3-dependent transcriptome in pediatric-onset inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:446–57. doi: 10.1002/ibd.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyake T, Aoki M, Masaki H, Kawasaki T, Oishi M, Kataoka K, et al. Regression of abdominal aortic aneurysm by simultaneous inhibition of nuclear factor kB and ets in a rabbit model. Circ Res. 2007;101:1175–84. doi: 10.1161/CIRCRESAHA.107.148668. [DOI] [PubMed] [Google Scholar]
- 21.Nakashima H, Aoki M, Miyake T, Kawasaki T, Iwai M, Jo N, et al. Inhibition of experimental abdominal aortic aneurysm in the rat by use of decoy oligonucleotides suppressing activity of nuclear factor kBand ets transcription factors. Circulation. 2004;109:132–8. doi: 10.1161/01.CIR.0000105725.61763.A2. [DOI] [PubMed] [Google Scholar]
- 22.Potula H, Wang D, Van Quyen D, Singh N, Kunumani-Sridharan V, Karpurapu M, et al. Src-dependent STAT-3-mediated expression of monocyte chemoattractant protein-1 is required for 15(S)-hydroxyeicosatetraenoic acid-induced vascular smooth muscle cell migration. J Biol Chem. 2009;284:31142–55. doi: 10.1074/jbc.M109.012526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kakisis J, Prahan S, Cordova A, Liapis C, Sumpio B. The role of STAT-3 in the mediation of smooth muscle cell response to cyclic strain. Int J Biochem Cell Biol. 2005;37:1396–406. doi: 10.1016/j.biocel.2005.01.009. [DOI] [PubMed] [Google Scholar]


