Abstract
The nucleus accumbens (NAc) receives converging input from a number of structures proposed to play a role in affective disorders. In particular, the basolateral amygdala (BLA) provides an affective input that overlaps with context-related information derived from the ventral subiculum (vSub) of the hippocampus. We examined how stimulation of the BLA is modulated by, and in turn affects, vSub inputs to this region. In vivo extracellular recordings were performed in the NAc of anesthetized rats. The effect of high frequency (theta-burst) stimulation (HFS) of the BLA on both BLA and vSub-evoked responses was tested. In addition, the involvement of dopamine D2 receptors in BLA-induced plasticity in the NAc was examined by pre-treatment with sulpiride (5 mg.kg, i.v.). Finally, tetrodotoxin (TTX) was used to inactivate the vSub and the effect on BLA-evoked responses was assessed. We found that HFS of the BLA causes hetereogeneous patterns of plasticity, depression and potentiation, respectively, in the rostral and caudal subregions of the NAc that is disrupted following D2 receptor antagonist treatment. In addition, inactivating the vSub with TTX attenuates the ability of the BLA to drive spike firing in the NAc. Thus, the vSub is required for the activation of the NAc by the BLA.
These data support a model whereby the amygdala can coordinate reward-seeking and fear-related behaviors via its differential regulation of NAc output. In addition, the hippocampus inappropriately dominates the information processing within this circuit, potentially contributing to the overwhelming focus on internal emotional states in disorders such as depression.
Keywords: nucleus accumbens, hippocampus, amygdala, dopamine, depression, plasticity
Introduction
The nucleus accumbens (NAc) integrates limbic and cortical inputs arising from monosynaptic glutamatergic projections that originate in the ventral subiculum of the hippocampus (vSub), basolateral amygdala (BLA), and prefrontal cortex (PFC) (French and Totterdell, 2003; Groenewegen et al., 1987; McDonald, 1991; O’Donnell and Grace, 1995b; Petrovich et al., 1996; Shinonaga et al., 1994). Each of these regions is believed to supply a different mode of input to the NAc, with the BLA involved in affective responses (LeDoux, 2000), the vSub in context dependency (Anagnostaras et al., 2001), and the mPFC in behavioral flexibility (Sesack and Grace, 2010). In addition, dopamine supplied by the ventral tegmental area is a potent modulator of NAc responses to both BLA and vSub inputs (Charara and Grace, 2003; Goto and Grace, 2005; Johnson et al., 1994; Totterdell and Smith, 1989). As part of a limbic/motor interface, the gated activity of the NAc regulates behavioral responses to both rewarding and aversive stimuli via its connections with dopaminergic, thalamic, and brainstem regions via the ventral pallidum (Groenewegen et al., 1983; Mogenson and Nielsen, 1983; Mogenson et al., 1983).
Because of its role in affective responses, abnormal amygdalar activation is believed to underlie the behavioral symptoms characteristic of disorders like depression and anxiety (e.g., anhedonia, anxiety, appetite changes, etc). For example, patients with unipolar depression display increased amygdala activation in response to fearful or sad stimuli (Fu et al., 2004; Keedwell et al., 2005; Peluso et al., 2009; Sheline et al., 2001; Surguladze et al., 2005). One manner by which this could occur is if there is a disruption of the normal processing of emotional input conveyed by the BLA to the NAc in depression, which could impact the normal filtering of NAc responses and consequently behavior.
The NAc is known to be functionally heterogeneous with respect to the regulation of positive and negative affect. Thus, selective disruption of glutamate signaling or GABA activation within the rostral and caudal subregions of the NAc elicits appetitive or fear behaviors, respectively (Basso and Kelley, 1999; Reynolds and Berridge, 2001; Reynolds and Berridge, 2002; Reynolds and Berridge, 2008). Moreover, disruption of dopamine activity with combined D1/D2 receptor antagonist treatment blocks the functional heterogeneity of the NAc subregions (Faure et al., 2008). Furthermore, stimulation of BLA fibers to the NAc can cause increases in NAc dopamine release (Floresco et al., 2001a; Floresco et al., 2001b). Consequently, we propose that the BLA could differentially regulate behavior by selective activation of the rostral and caudal NAc subregions, and that the heterogeneous response of NAc to BLA stimulation may involve a dopamine-dependent mechanism.
Whereas it has been shown previously that the hippocampal and PFC inputs have a dominant influence over the excitability of NAc neurons, the role of the BLA in this interaction is less clear. Anatomically, monosynaptic BLA and vSub projections converge on the distal dendrites and spines of NAc neurons (French and Totterdell, 2003). In vivo intracellular recordings showed that stimulation of the fimbria-fornix, but not the BLA, can facilitate the transition of NAc neurons from a less excitable “down” membrane potential to a more excitable “up” membrane potential (O’Donnell and Grace, 1995a). However, increasing hippocampal activity can actually reduce the response of NAc neurons to subsequent BLA stimulation (Floresco et al., 2001b). In contrast, we demonstrate in the present study that loss of hippocampal input also reduces the efficacy of BLA stimulation in both rostral and caudal subregions of the NAc. Indeed, maximal activation of the BLA following inactivation of the vSub fails to sufficiently engage the NAc. This would suggest that the context information supplied by the vSub is crucial for gating the response of NAc neurons to BLA-mediated emotional input. Consequently, the vSub may be required for orchestrating the complex set of behaviors elicited by emotional stimuli.
Materials and Methods
Animals
Experiments were conducted according to the guidelines described in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Adult male Sprague-Dawley rats (Hilltop) weighing 300–400g were used. Animals were pair-housed in a temperature (22ºC) and humidity (47%) controlled environment with a 12-hour light/dark cycle (lights on at 7 a.m.) with ad libitum access to both food and water. All testing was performed at least one week after animals arrived.
Surgery
Animals were anesthetized with an initial dose of chloral hydrate (400 mg.kg, i.p.) and were supplemented periodically (i.v.) to maintain suppression of the hind limb withdrawal reflex. After placing in a stereotaxic frame (Kopf), rats were implanted with a catheter in the lateral tail vein to allow intravenous injections. Body temperature was maintained at 37ºC with a temperature-controlled heating pad (Fintronics).
Drugs and drug administration
Tetrodotoxin (TTX), sulpiride, and Dulbecco’s phosphate-buffered saline (dPBS) were obtained from Sigma-Aldrich. Sulpiride (5mg/kg, with 1 drop HCL) was dissolved in physiological saline and administered intravenously via tail vein catheter. For local infusions into the vSub, TTX (1μM/0.5μl) was dissolved in dPBS and infused with a 33gaugecannula at a rate of 0.5μl over 1 min using a Hamilton syringe. Control infusions consisted of 0.5 μl of dPBS.
Single-Unit extracellular recording in nucleus accumbens
The shell subregion of the NAc is involved in coordinating reward-and fear-related behaviors (Basso and Kelley, 1999; Faure et al., 2008; Reynolds and Berridge, 2001). In addition, the NAc shell is involved in learning discriminatory responses to conditioned stimuli (Bradfield and McNally, 2010; Kerfoot et al., 2007). There is substantial evidence for a high degree of convergence of monosynaptic projections from the PFC, BLA, and vSub onto individual medium spiny neurons in the NAc shell (Mulder et al., 1998; O’Donnell and Grace, 1993). It has been shown previously in slice preparations that integration of input from the BLA and vSub is potently modulated by DA (Charara and Grace, 2003). Based on this prior evidence, electrophysiological recordings were restricted to the shell subregion of the NAc. No data was recorded from neurons encountered that were responsive to stimulation of only a single brain region, BLA or vSub. Consequently, an exact proportion of NAc neurons excluded from analysis could not be obtained. However, based on anecdotal observations, it was estimated that <10% of neurons were responsive to stimulation of only a single brain region. Therefore, the data summarized in the manuscript represent the responses of the majority of medium spiny neurons in the NAc.
Single glass microelectrodes (WPI; impedance 7–10MΩ) were filled with 2% Pontamine sky blue dye in 2M NaCl and positioned using a hydraulic microdrive (Narishige) in the shell subregion of the right NAc according to coordinates in a rat brain atlas (Paxinos and Watson, 1996) [anteroposterior (AP) 1.65 or 1.25 mm anterior to bregma; lateral (L), 1.0 mm to the midline; dorsoventral (DV), 5.5–7.5 mm from the top of dura]. Neural activity was amplified and filtered (500–5000Hz, Fintronics) prior to transference to a computer with custom-designed acquisition and analysis software (Neuroscope). Spontaneous activity was monitored on an oscilloscope and single-unit responses were recorded when the signal to noise ratio was at least 3:1.
Stimulation of BLA and vSub
Concentric bipolar electrodes (NEX-100X; Rhodes Medical Instruments) were implanted in the BLA (AP, −3.6 mm; L, 5.0 mm; DV, −7.5 mm) and vSub (AP, −5.5mm; L,4.5mm; DV, 7.5mm). For experiments in which the vSub was inactivated with TTX at the stimulation site, a chemotrode (Plastics One, Inc.) was used. A dual output stimulator (S8800; Grass Technologies) was used to generate single pulses of current (duration, 0.20 msec; intensity 500 μA) in the BLA and vSub (50 msec intervals, every 2 sec). The recording microelectrode was advanced slowly into the NAc until single-unit monosynaptic responses were observed to stimulation of both BLA [latency, 8–16 msec] and vSub [latency, 14–21 msec].
Upon identifying a responsive neuron, the current intensity of BLA and vSub stimulation was adjusted to obtain approximately 50% evoked spike probability (intensity range, 160–900μA). Baseline evoked responses were recorded for 15 min. prior to TTX infusion or theta burst stimulation. TTX was infused into the vSub and BLA-evoked responses were recorded for at least 40 minutes. In a subset of experiments, after the baseline recording period, theta burst stimulation (3 bouts of 10×100 Hz bursts with a 200 ms interburst interval; intensity, 1mA) was applied to the BLA. After theta burst stimulation, the intensity of BLA stimulation was returned to baseline and BLA-and vSub-evoked responses were recorded for 40 min. In another set of experiments, theta burst stimulation was applied to the BLA 20 min after TTX was infused in the vSub and BLA-evoked responses were recorded for an additional 40 min. For experiments in which sulpiride was administered (i.v.), changes in evoked responses were recorded for 5 min prior to theta burst stimulation of the BLA.
Data Analysis
Evoked spike probabilities were calculated by dividing the number of stimuli that evoked an action potential by the total number of stimuli administered. All data were normalized to the average spike probabilities obtained during the baseline period. Subsequently, data are shown and analyzed as the % change in spike probabilities (5 min bins). A Shapiro-Wilk test was used to determine whether the population response of NAc neurons was normally distributed. When responses were separated based on increases or decreases in spike probability, a >15% change in spike probability from baseline had to occur for at least 2 consecutive 5-min bins. A one-way repeated measures ANOVA (within-subject factor, time) followed by the Holm-Sidak post-hoc analysis was used to detect changes in spike probability. Multiple comparisons were analyzed with a two-way ANOVA followed by the Holm-Sidak post-hoc analysis using treatment as the between-subject factor and time as the within-subject factor.
Histology
At the end of each experiment, the location of the recording electrode was marked by ejecting Pontamine sky blue dye from the tip of the electrode using an iontophoresis pump (Kation Scientific; -25μA constant current, 30 min). Small lesions were made at the tip of each stimulation electrode (250μA, 10 sec current pulse) and visualized by adding potassium ferrocyanide during post-fixation. Animals were euthanized with an overdose of chloral hydrate (400 mg.kg, i.v.) and decapitated. The brain was removed and fixed for at least 48 hr. in 8% w/v paraformaldehyde (in PBS) and cryoprotected in 25% w/v sucrose (in PBS). Brains were then sectioned (60 μm coronal sections), placed on gelatin-chromalum-coated slides, and stained with cresyl violet for histochemical verification of the recording/stimulation electrode placements.
Results
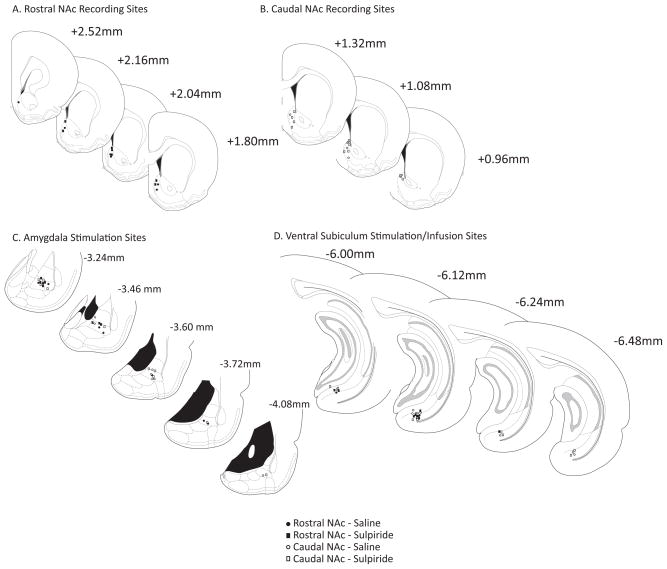
Stimulation electrodes were placed in the lateral and basolateral subregions of the amygdala as well as the ventral subiculum of the hippocampus (Fig. 1c and 1d). Electrophysiological recordings were restricted to the rostral or caudal shell subregion of the NAc (Fig. 1a and 1b). The coordinates used for comparisons of NAc activity along a rostral-caudal gradient were selected based on the reported heterogeneous behavioral effects resulting from local disruption of glutamate or GABA activity in the NAc (Faure et al., 2008; Reynolds and Berridge, 2001; Reynolds and Berridge, 2002); i.e., infusions of a glutamate receptor antagonist, DQNX, or GABA(A) agonist, muscimol, rostrally ( >+2.0 mm relative to bregma) elicited eating behaviors, while more caudal infusions (<+1.4mm relative to bregma) increased fear and defensive behaviors. Histological verification of placement of stimulation electrodes in the BLA and vSub did not support a pattern of preferential activation of rostral NAc neurons by more anterior BLA or vSub stimulation.
Figure 1.
Histological verification of electrophysiological recording and stimulation sites (A). Schematic of coronal sections of the rat brain (Paxinos and Watson, 1996) representing recording sites in rostral subregion of the NAc shell (s-NAc), (B). Recording sites in caudal subregion of the s-NAc, (C). Stimulation location in BLA, (D). Stimulation location in vSub. (BLA = basolateral amygdala, vSub = ventral subiculum of the hippocampus, c-NAc = NAc core).
A total of 84neurons were recorded in 84rats. Medium spiny neurons receive convergent monosynaptic input from both the BLA and vSub (French and Totterdell, 2003; O’Donnell and Grace, 1995b). All neurons (N= 84) used in the present study were excited by stimulation of both the BLA and vSub. The average monosynaptic response latencies to BLA and vSub stimulation were 14.5 ± 0.4ms (N = 84) and 16.8 ± 0.5ms(N = 84), respectively, which is consistent with previous reports (Mulder et al., 1998; O’Donnell and Grace, 1995b).
BLA theta burst stimulation induces differential plasticity in rostral and caudal subregions of NAc
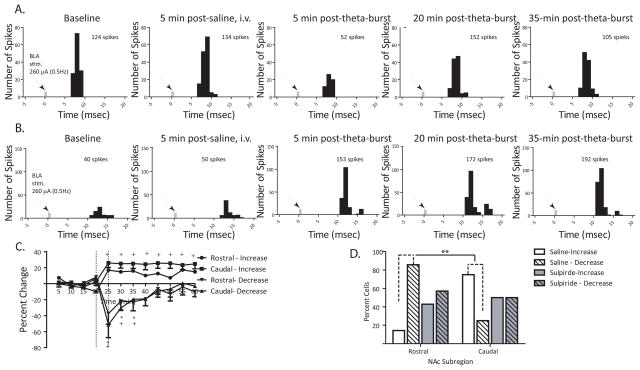
Given the reported heterogeneity of rostral and caudal subregions of the NAc, we tested whether theta burst stimulation of the BLA had disparate effects on plasticity in these subregions. After recording stable baseline activity (≤10% variation)and evoked response probability for 15 min, theta burst stimulation was applied to the BLA. In both the rostral and caudal subregion, the population response to theta burst stimulation was not normally distributed as determined by the Shapiro-Wilk test. As a result, the data was divided based on the predominate response patterns observed. Neurons from the rostral subregion (N=7) of the NAc exhibited either a long-lasting increase orashort-duration decrease in BLA-evoked responses (F = 3.474, p = .001; Fig. 2c), with the majority of neurons (86%) in the rostral NAc exhibiting a short-duration decrease (<20 min) in BLA-evoked responses prior to returning to baseline (Fig. 2d). Similar to what was observed in the rostral subregion, neurons in the caudal subregion (N=8) exhibited either a potentiation or a brief inhibition of BLA-evoked responses following theta burst stimulation (F = 13.681, p =<.001 and F = 7.880, p = .002, respectively; Fig.2c). Unlike the rostral subregion, the majority of neurons in the caudal subregion (75%) exhibited enhanced BLA-evoked activity lasting more than 40 min following theta burst (Fig. 2d). Therefore, the two subregions varied significantly in their predominate response to theta burst stimulation (Fisher exact test, p = 0.04).
Figure 2.
Subregions of the NAc demonstrate differential levels of plasticity in response to BLA theta burst stimulation (A) Representative peristimulus time histogram (5 minsamples, 150 sweeps) illustrating short-duration decrease in BLA-evoked responses in NAc following theta burst stimulation of the BLA, (B) Representative peristimulus time histogram (5 min samples, 150 sweeps) illustrating long lasting potentiation of BLA-evoked responses in NAc following theta burst stimulation of the BLA, (C) Mean percentage change (± SEM) of the BLA-evoked spike probability in the rostral and caudal subregions of the NAc normalized to the baseline spike probability before saline injection and theta burst (time of injection and stimulation represented by vertical lines), (D) Bars represent the number of neurons in rostral and caudal subregions of the NAc exhibiting either an increase or decrease in BLA-evoked responses following saline or sulpiride administration (5 mg/kg, i.v.) and subsequent BLA theta burst. (* denotes p<.05 for rostral neuron responses compared to baseline, + denotes p<.05 for caudal neural responses compared to baseline, repeated measures ANOVA, Holm-Sidak post-hoc; **denotes p<.05 for rostral vs caudal comparisons after saline injection, Fisher exact test)
Previous studies showed that high frequency stimulation of the BLA causes a biphasic increase in dopamine release in the caudal NAc in addition to enhanced BLA-evoked responses (Floresco, Blaha et al. 2001; Floresco, Blaha et al. 2001). After close examination of the recording locations within NAc from these studies, it can be concluded that the potentiation of BLA-evoked responses in the caudal subregion may be dopamine-mediated. It has been demonstrated previously that tetanic stimulation of the fimbria can induce a transient reduction (<2min) in hippocampal-induced activity in the NAc prior to a subsequent long-term potentiation (Belujon and Grace, 2008), and this reduction in activity was reported to be regulated by dopamine D2 receptor activation. In the present study, the effects of D2-receptor antagonist treatment (sulpiride, 5mg/kg, i.v.) administered 5 min prior to BLA theta burst was tested on the heterogeneous responses observed in the rostral and caudal NAc. Sulpiride treatment did not block the occurrence of long-term potentiation or short-term depression of BLA-evoked responses in the rostral and caudal subregions. However, following sulpiride treatment, there was no longer a distinction between rostral and caudal subregions with regard to the predominant response observed, i.e., either excitation or inhibition, to BLA theta burst (Fisher exact test, p >.05; Fig. 2d). Thus, 57% of neurons were briefly inhibited and 43% were excited following BLA theta burst. In the caudal NAc, a similar pattern was observed with 50% of neurons exhibiting an increase in BLA-evoked responses and 50% exhibiting a decrease in evoked responses. Therefore, the response of the rostral and caudal subregions to increased activation of amygdala-dependent affective input is determined partly by dopamine D2 receptor activation.
Subregional NAc differences in vSub-evoked responses following BLA theta burst stimulation
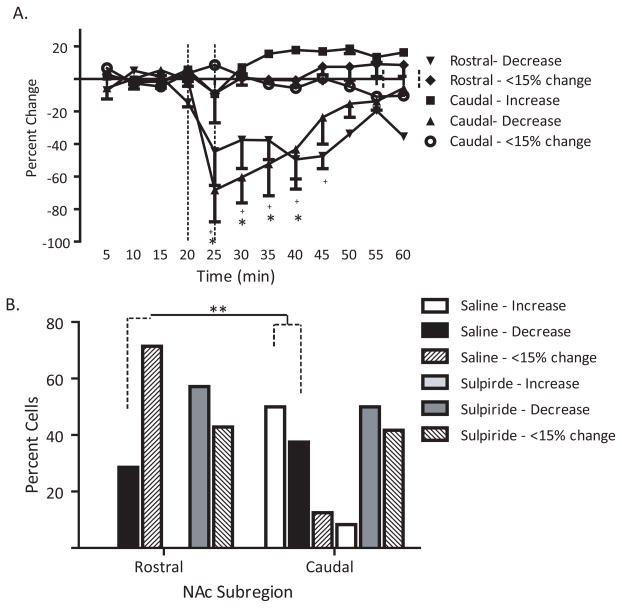
It has been demonstrated previously that high-frequency stimulation of the fimbria can cause a secondary long-term reduction in BLA-evoked responses that is D1receptor -dependent in addition to potentiating hippocampal-evoked responses in the caudal NAc (Floresco et al., 2001b). Less clear is how BLA theta burst stimulation would affect the response of the rostral and caudal subregions of the NAc to the vSub input. The same neurons reported above were also examined for alterations in vSub-evoked activity. Three patterns of vSub-evoked responses (excitation, inhibition, less than15% change in spike probability) were observed following theta burst stimulation of the BLA (Fig. 3a). In the rostral subregion of the NAc, the majority of neurons (71%) exhibited <15% change in vSub-evoked responses (F = 1.19, p > .05), with the remaining 2 neurons demonstrating a decrease in vSub-evoked responses (F = 5.441, p =.005). A different pattern was observed in the caudal subregion where 50% (N=4/8) of neurons displayed an increase in vSub-evoked responses (F = 2.549, p = .019), 38% of neurons had decreases in vSub-evoked responses (F = 5.084, p < .001), and only a single neuron was unaffected by BLA theta burst stimulation. Thus, the pattern of changes in vSub-evoked responses was significantly different between the rostral and caudal subregions (Chi-square = 6.83, p = .03).
Figure 3.
Administration of the D2 antagonist sulpiride eliminates the differential responses between rostral and caudal subregions of the NAc following BLA stimulation. (A) Mean percentage change (±SEM) of the vSub-evoked spike probability in the rostral an caudal subregions of the NAc normalized to the baseline spike probability before saline injection and theta burst of the BLA, (B) Bars represent the number of neurons in rostral and caudal subregions of the NAc exhibiting either an increase, decrease, or <15% change in vSub-evoked responses following saline or sulpiride administration (5 mg/kg, i.v.) and subsequent BLA theta burst. (* denotes p<.05 for rostral neuron responses compared to baseline, + denotes p<.05 for caudal neural responses compared to baseline, repeated measures ANOVA, Holm-Sidak post-hoc; ** denotes p<.05 for rostral vs caudal comparisons after saline injection, Chi-square test)
Blockade ofD2 receptors caused a similar attenuation in the heterogeneous response of rostral and caudal NAc to vSub input following BLA theta burst stimulation. Thus, following sulpiride injection, a greater percentage of neurons in the rostral NAc (57%; N=4/7) displayed a short-lasting decrease (<30 min) in vSub-evoked responses (F = 4.854, p < .001) in addition to those demonstrating less than15% in evoked responses (43%; F = .505, p = .873). In addition, more neurons in the caudal NAc also displayed either a decrease in vSub-evoked responses (50%; F = 3.289, p = .002) or <15 % change in their spike probabilities (45%; F = .969, p =.492) following sulpiride treatment prior to BLA theta burst. Overall, there was a homogeneous response of the rostral and caudal NAc to vSub input following sulpiride treatment and theta burst stimulation of the BLA (Chi-square = .628, p = .731). These data suggest that dopamine is enabling the diverse responses of NAc to both emotional and contextual input via the BLA and the vSub, respectively.
Inactivation of the vSub reduces the efficacy of BLA stimulation in the caudal NAc
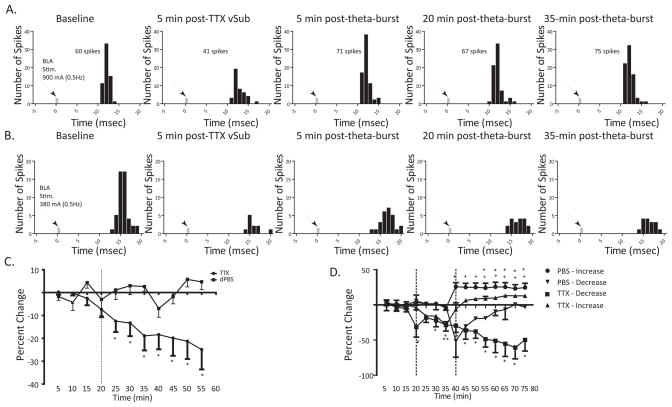
Previous studies demonstrated that increases in hippocampal input to the NAc mediated by the fimbria-fornix can reduce the response of NAc neurons to emotional information conveyed by the BLA (Floresco et al., 2001b). After a stable baseline recording period, TTX (1μM) or dPBS (0.5μl) was infused into the vSub to determine whether a reduction in hippocampal activation would also alter the response of NAc neurons to BLA input. The dominate response to theta burst stimulation of the BLA was a short-term depression of evoked responses in the rostral NAc. Consequently, It was anticipated that a loss of the permissive vSub input (Belujon and Grace, 2008) would cause a reduction of evoked responses that would further obscure the already-present decrease that resulted from theta burst stimulation of the BLA. However, in the caudal subregion, the impact of the loss of vSub input on the potentiated BLA-responses was of greater interest both in terms of measuring the impact, and on its influence on amygdala-related fear responses. For these reasons and to be consistent with previous studies, recordings were restricted to the caudal NAc. Following dPBS infusions (N=7), there was no change in the BLA-evoked spike probabilities (F = .988, p = .466). After infusion of TTX into the vSub (N=25), there was a progressive decrease in BLA-evoked responses that began approximately 10 minutes following termination of the infusion (F = 5.362, p <0.001; Fig.4c).
Figure 4.
Inactivation of the vSub potently attenuates BLA drive of the NAc. (A) Representative peristimulus time histogram illustrating reduction in BLA-evoked responses in the NAc following infusion of TTX into the vSub and a subsequent return to baseline following theta burst stimulation of the BLA, (B) Representative peristimulus time histograms illustrating a long-lasting reduction in BLA-evoked responses following inactivation of the vSub and subsequent theta burst stimulation of the BLA, (C) Mean percentage change (± SEM) of the BLA-evoked spike probability in the NAc normalized to the baseline spike probability before TTX or dPBS infusion (represented by vertical line) into the vSub, (D) Mean percentage change (± SEM) of the BLA-evoked spike probability in the NAc normalized to the baseline spike probability before TTX or dPBS infusion into the vSub and subsequent theta burst stimulation of the BLA (time of injection and stimulation represented by vertical lines). (*and **denote p<.05 for responses following TTX infusion in comparison to baseline, + denotes p<.05 following PBS infusion in comparison to baseline, repeated measures ANOVA, Holm-Sidak post-hoc )
As reported above, theta burst stimulation applied to the BLA 20 minutes after infusion of dPBS into the vSub (N=10) caused a long-term potentiation of BLA-evoked responses in a majority (90%) of neurons, with only one neuron exhibiting a short-duration decrease in BLA-evoked responses (F = 21.392, p <.001; Fig 4d). Following inactivation of the vSub (N=10), significantly fewer neurons (60%) showed an increase inBLA-evoked responses following theta burst stimulation of the BLA (F = 2.467, p = .007). In addition, the onset of potentiated BLA-evoked responses occurred approximately 15 min later than what was observed in control neurons. Thus, 40% of neurons exhibited a long-lasting decrease in BLA-evoked responses following inactivation of the vSub and subsequent theta burst stimulation of the BLA (F = 1.921, p = .038). These data suggest that loss of input from the vSub greatly reduces, but does not eliminate, the efficacy of BLA stimulation.
Discussion
Activation of individual neurons in the NAc is subject to the competing drive of converging inputs from prefrontal cortex (PFC), vSub, and BLA (Belujon and Grace, 2008; Goto and Grace, 2005; Mulder et al., 1998; O’Donnell and Grace, 1994; O’Donnell and Grace, 1995b). It has been shown previously that following tetanic stimulation of the fimbria, the hippocampus can dominate over BLA input on NAc neural responses (Floresco et al., 2001b). In contrast, the present study uses inactivation of the vSub with TTX to demonstrate a permissive role of the hippocampus in gating the response of NAc to BLA input. In addition, we show that hyperactivation of the BLA with intact processing from the vSub enables a disparate pattern of plasticity in the rostral and caudal subregions of the NAc that likely forms the basis of the unique behavioral responses attributed to these subregions. Moreover, the dopamine system supports this heterogeneous response of the NAc to both BLA and vSub input.
Differential gating of vSub and BLA input underlies heterogeneous NAc activation
The nucleus accumbens is integral to the neural circuitry mediating associative reward processes as well as conditioned fear responses (Ambroggi et al., 2008; Bradfield and McNally, 2010; Klucken et al., 2009b; Reynolds and Berridge, 2001; Young, 2004). The NAc core and shell subregions exhibit differential activation to both fear and reward associated stimuli (Ambroggi et al., 2008; Ghitza et al., 2003; Nicola et al., 2004). Through its integration of information from the BLA and vSub, the NAc is able to coordinate competing behavioral drives.
Firing patterns of both amygdala and hippocampal neurons correlate with behaviorally salient features of external discrete and contextual stimuli (Green and Arenos, 2007). Pavlovian conditioning procedures that involve the repeated pairing of a neutral and aversive stimuli leads to an increase in amygdala activation in response to the previously neutral stimulus (Maren and Quirk, 2004; Rosenkranz and Grace, 2002; Rosenkranz and Grace, 2003; Rosenkranz et al., 2003). Neurons in the hippocampus display similar increases in activation in response to both discrete conditioned stimuli as well as the conditioning context (Munera et al., 2001). In addition, BLA neurons exhibit differential firing patterns discriminating the value of reward-predictive cues in a discriminative stimulus task (Ambroggi et al., 2008). Inactivation of the BLA can interfere with both the discriminative firing properties of NAc core neurons as well as performance during the discriminative stimulus task. Consequently, selective activation of the BLA and vSub could coordinate approach and avoidance behaviors via connections with the NAc.
In addition to promoting the differential activity of NAc neurons to external stimuli, the BLA and vSub can alter the response of NAc to alternate afferent input. Single-pulse stimulation of the BLA can enhance the response of NAc neurons to PFC input, while train stimulation results in a depression of PFC-evoked responses (McGinty and Grace, 2009a; McGinty and Grace, 2009b). In the current study, we show that high frequency stimulation of the BLA can suppress vSub-evoked responses in both the rostral and caudal subregions of the NAc (Fig.5B). This would suggest that abnormally high amygdala activation could interfere with goal-directed behavior in favor of emotionally-motivated behavior. The enhancing effect of activation of the vSub on gating of the responses of NAc neurons to PFC input has been described previously (O’Donnell and Grace, 1995b). Here we show that inactivation of the vSub not only causes a progressive decrease in the responses of the caudal NAc to single-pulse stimulation of the BLA but also alters the expression of BLA-induced plasticity in the NAc, indicative of a reduction in the efficacy of BLA input (Fig.5C). Indeed, the results of the present study suggest that loss of the permissive input from the vSub reduces heterogeneous activity of NAc neurons. Overall, it appears that putative affective information supplied by the BLA is most effective in influencing NAc responses when corresponding contextual information from the vSub is also available. Indeed, studies show that conditioned responses are typically context-dependent (Kim et al., 1993), which is supported by this BLA-NAc interaction. This interdependency could act to prevent emotion-triggered behavioral responses outside of the relevant context.
Figure 5.
Schematic illustrating the interactions between BLA and vSub in the NAc. (A) Therostral and caudal subregions of the NAc receive convergent monosynaptic, glutamatergic input from vSub and BLA. Each NAc subregion regulates different motivational drives, e.g. appetitive and fear behaviors, respectively (B) Following theta burst stimulation of the BLA, there is a shift in rostral and caudal NAc activation. Both vSub and BLA input to the caudal NAc is potentiated while there is a suppression of evoked responses in the rostral NAc. Consequently, fear generated responses would likely predominate over appetitive behaviors, (C) Following inactivation of the vSub with TTX, the permissive drive onto the NAc is lost and the efficacy of BLA stimulation is greatly reduced. This could reflect the loss of hippocampal-facilitated context-dependency of the fear response. (c-NAc = caudal nucleus accumbens, r-NAc = rostral nucleus accumbens, vSub = ventral subiculum of the hippocampus, BLA= basolateral amygdala)
Modulatory influence of BLA on NAc plasticity is dopamine-mediated
It is already known that dopamine has a profound influence on the gating of NAc responses to its various limbic afferents (Belujon and Grace, 2008; Floresco et al., 2001b; O’Donnell and Grace, 1996; Yang and Mogenson, 1984; Yun et al., 2004). Stimulation of the BLA at frequencies similar to what has been observed in freely moving animals elicits an increase in dopamine release as well as increased BLA-evoked spike probabilities in the region of the NAc corresponding to the caudal NAc in the present study (Floresco et al., 2001a; Pratt and Mizumori, 1998). The effects of D1 and D2 antagonist treatment on BLA-induced changes in neural responses in the NAc are mixed. One study reports a blockade of LTP of BLA-evoked responses in the caudal NAc by the D1 receptor antagonist SCH23390, but not by the D2 receptor antagonist, sulpiride (Floresco et al., 2001a). However, other work suggests that stimulation of the BLA can induce a suppression of PFC-evoked responses in the NAc that is not mediated by either D1 or D2 receptors (McGinty and Grace, 2008). In the present study, D2 antagonist treatment did not completely block plasticity in the NAc. Instead it altered the pattern of plasticity in the rostral and caudal subregions of the NAc, such that a more homogeneous response to BLA stimulation was observed. This pattern of results is consistent with reports that the majority of neurons in the NAc express little overlap between D1-and D2-receptor expressing neurons, suggesting two different populations with presumably disparate responses to DA input (Day et al., 2008; Gerfen et al., 1990; Surmeier et al., 1992).
The homogeneous response of rostral and caudal NAc to BLA theta burst stimulation following sulpiride treatment parallels the loss of behavioral heterogeneity of the rostral and caudal subregions following combined D1 and D2 antagonist treatment (Faure et al., 2008). This would suggest that DA, via D1 and D2 receptors, acts locally within the NAc subregions to coordinate motivational behaviors. An in situ hybridization study of the pattern of D1 and D2 mRNA in the rostral and caudal subregions of the NAc shell and core showed that similar proportions of neurons retrogradely labeled from the ventral pallidum express either D1 or D2 mRNA (Lu et al., 1998). In constrast, neurons retrogradely labeled from the VTA in the rostral and caudal NAc shell mainly express D1 mRNA. It is important to note that there was no difference in D2 mRNA expression between the rostral and caudal subregions of the NAc. Consequently, D2 and D1 receptor expressing neurons in the NAc may represent two different populations based on their projection targets, VP or VTA.
Regional NAc differences in processing BLA input
Previous electrophysiological recordings in halothane-anesthetized animals reported a majority of amygdala-evoked responses in the anterior or rostral NAc, suggesting a topographical arrangement of these inputs (Callaway et al., 1991). However, most of the work done on the gating of activity by vSub, BLA, and PFC of individual neurons in the NAc has not differentiated between the rostral and caudal subregions (Belujon and Grace, 2008; Floresco et al., 2001a; Floresco et al., 2001b; French and Totterdell, 2003). This is the first time that electrophysiological responses from both the rostral and caudal subregions of the NAc are reported for comparison. In the present study, a short-duration decrease in BLA-evoked responses in the rostral NAc along with potentiated BLA-evoked responses in the caudal NAc were the predominate responses to theta burst stimulation of the BLA. Regulating appetitive behaviors has been attributed to the anterior or rostral NAc (Basso and Kelley, 1999; Reynolds and Berridge, 2001; Reynolds and Berridge, 2002; Reynolds and Berridge, 2008). In contrast, the posterior or caudal NAc is important for mediating behavioral responses to fearful or aversive stimuli (Kerfoot et al., 2007). It has already been shown that exposure to stressful environments, presumably inducing strong BLA activation, can cause an expansion of the caudal fear-generating zone of the NAc (Reynolds and Berridge, 2008). Although speculative, one interpretation of our data is that the BLA-induced shift in activation between the two NAc subregions may be important for adapting behavioral responses to changes in contextual or emotional stimuli. DA may allow for the shifting of the relative activation between the rostral and caudal subregions of the NAc in coordination with input from the BLA (Faure et al., 2008). As a consequence, the dominant behavioral response may likewise be shifted towards reward-seeking behaviors and away from fear responses. In the present study, alterations in D2 receptor activation following treatment with sulpiride strongly attenuated the dopamine-mediated facilitation of BLA-and vSub-evoked responses in the caudal NAc following BLA theta burst stimulation. This reduction in the response of NAc neurons could interfere with the development of discriminatory firing patterns based on contextual or reinforcement information.
Functional Implications
These data have important implications for both normal learning and pathological conditions. Importantly, the NAc is involved in fear learning as well as goal-directed behaviors (Klucken et al., 2009a; Pezze and Feldon, 2004). We have shown here that both BLA and vSub are potent modulators of NAc activity. Consequently, integration of contextual information from the vSub and affective information from the BLA in the NAc is likely involved in the generation of appropriate behavioral responses to environmental contingencies.
In both major depressive disorder and anxiety disorders there is increased amygdala activation during the evaluation of emotional stimuli (Beesdo et al., 2009; Bremner et al., 2008; Fu et al., 2004; Peluso et al., 2009; Thomas et al., 2001). Indeed, the perceived level of fear is associated with the degree of amygdala activation in response to fearful stimuli in anxious and depressed patients. In addition, contingency awareness in normal individuals, e.g. the association between neutral and aversive stimuli, is related to increased activation of both the amygdala and nucleus accumbens (Klucken et al., 2009a; Klucken et al., 2009b). High frequency stimulation of the BLA can increase DA release in the NAc (Floresco et al., 2001a). Similar increases in DA release in NAc have been reported following stress (Amato et al., 2007; Doherty and Gratton, 2007). It has also been reported that exposure to stress can cross-sensitize with psychostimulants (Antelman et al., 1980; Kalivas and Duffy, 1989; Sorg and Kalivas, 1991). Combined with evidence that increases in amygdala activation occur in response to drug-associated stimuli in cocaine users (Childress et al., 1999), it is possible that aberrant activation of the amygdala could promote stress-induced relapse in addicted individuals via a pathological increase in DA activity within the NAc. There is some indication that localDA in the rostral and caudal shell is important for mediating both positive incentive salience and negative fearful salience, respectively (Faure et al., 2008). Alterations in the normal BLA activation of these subregions in the NAc might account for increases in drug seeking behaviors in addicted individuals.
We demonstrated that theta burst stimulation of the BLA potentiates responses in the NAc subregion implicated in processing fear, thereby facilitating fearful responses when the organism is in a context of repetitive fear-associated stimuli. Consequently, inappropriate BLA hyperactivation likely disrupts the normal processing within the NAc and increases the likelihood of fear responses to neutral stimuli. Antidepressant treatment is effective in normalizing hyperactivation of the BLA, as well as ventral striatum, (Fu et al., 2004; Sheline et al., 2001) and likely accounts for part of the symptomatic improvement.
Acknowledgments
This work was supported by United States Public Health Service Grants DA15408 and MH57440 (A.A.G.) and T32-MH18273-23(K.M.G).We thank Niki MacMurdo for her technical assistance; Brian Lowry for the production, development, and support with the custom-designed electrophysiology software (Neuroscope); and Pauline Belujon for critical reading and helpful discussions.
Footnotes
Statement of Interest: None
References
- Amato JL, Bankson MG, Yamamoto BK. Prior exposure to chronic stress and MDMA potentiates mesoaccumbens dopamine release mediated by the 5-HT(1B) receptor. Neuropsychopharmacology. 2007;32(4):946–954. doi: 10.1038/sj.npp.1301174. [DOI] [PubMed] [Google Scholar]
- Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59(4):648–661. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11(1):8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Antelman SM, Eichler AJ, Black CA, Kocan D. Interchangeability of stress and amphetamine in sensitization. Science. 1980;207(4428):329–331. doi: 10.1126/science.7188649. [DOI] [PubMed] [Google Scholar]
- Basso AM, Kelley AE. Feeding induced by GABA(A) receptor stimulation within the nucleus accumbens shell: regional mapping and characterization of macronutrient and taste preference. Behavioral Neuroscience. 1999;113(2):324–336. doi: 10.1037//0735-7044.113.2.324. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Lau JY, Guyer AE, McClure-Tone EB, et al. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Archives of General Psychiatry. 2009;66(3):275–285. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P, Grace AA. Critical role of the prefrontal cortex in the regulation of hippocampus-accumbens information flow. Journal of Neuroscience. 2008;28(39):9797–9805. doi: 10.1523/JNEUROSCI.2200-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradfield LA, McNally GP. The role of nucleus accumbens shell in learning about neutral versus excitatory stimuli during Pavlovian fear conditioning. Learning and Memory. 2010;17(7):337–343. doi: 10.1101/lm.1798810. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Elzinga B, Schmahl C, Vermetten E. Structural and functional plasticity of the human brain in posttraumatic stress disorder. Progress in Brain Research. 2008;167:171–186. doi: 10.1016/S0079-6123(07)67012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway CW, Hakan RL, Henriksen SJ. Distribution of amygdala input to the nucleus accumbens septi: an electrophysiological investigation. Journal of Neural Transmission General Section. 1991;83(3):215–225. doi: 10.1007/BF01253391. [DOI] [PubMed] [Google Scholar]
- Charara A, Grace AA. Dopamine receptor subtypes selectively modulate excitatory afferents from the hippocampus and amygdala to rat nucleus accumbens neurons. Neuropsychopharmacology. 2003;28(8):1412–1421. doi: 10.1038/sj.npp.1300220. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, et al. Limbic activation during cue-induced cocaine craving. American Journal of Psychiatry. 1999;156(1):11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M, Wokosin D, Plotkin JL, Tian X, et al. Differential excitability and modulation of striatal medium spiny neuron dendrites. Journal of Neuroscience. 2008;28(45):11603–11614. doi: 10.1523/JNEUROSCI.1840-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty M, Gratton A. Differential involvement of ventral tegmental GABA(A) and GABA(B) receptors in the regulation of the nucleus accumbens dopamine response to stress. Brain Research. 2007;1150:62–68. doi: 10.1016/j.brainres.2007.02.081. [DOI] [PubMed] [Google Scholar]
- Faure A, Reynolds SM, Richard JM, Berridge KC. Mesolimbic dopamine in desire and dread: enabling motivation to be generated by localized glutamate disruptions in nucleus accumbens. Journal of Neuroscience. 2008;28(28):7184–7192. doi: 10.1523/JNEUROSCI.4961-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG. Dopamine D1 and NMDA receptors mediate potentiation of basolateral amygdala-evoked firing of nucleus accumbens neurons. Journal of Neuroscience. 2001a;21(16):6370–6376. doi: 10.1523/JNEUROSCI.21-16-06370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG. Modulation of hippocampal and amygdalar-evoked activity of nucleus accumbens neurons by dopamine: cellular mechanisms of input selection. Journal of Neuroscience. 2001b;21(8):2851–2860. doi: 10.1523/JNEUROSCI.21-08-02851.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SJ, Totterdell S. Individual nucleus accumbens-projection neurons receive both basolateral amygdala and ventral subicular afferents in rats. Neuroscience. 2003;119(1):19–31. doi: 10.1016/s0306-4522(03)00150-7. [DOI] [PubMed] [Google Scholar]
- Fu CH, Williams SC, Cleare AJ, Brammer MJ, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Archives of General Psychiatry. 2004;61(9):877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250(4986):1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Fabbricatore AT, Prokopenko V, Pawlak AP, et al. Persistent cue-evoked activity of accumbens neurons after prolonged abstinence from self-administered cocaine. Journal of Neuroscience. 2003;23(19):7239–7245. doi: 10.1523/JNEUROSCI.23-19-07239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopamine-dependent interactions between limbic and prefrontal cortical plasticity in the nucleus accumbens: disruption by cocaine sensitization. Neuron. 2005;47(2):255–266. doi: 10.1016/j.neuron.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Green JT, Arenos JD. Hippocampal and cerebellar single-unit activity during delay and trace eyeblink conditioning in the rat. Neurobiology of Learning and Memory. 2007;87(2):269–284. doi: 10.1016/j.nlm.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen G, Buurman WA, van der Linden CJ, Jeunhomme GM, et al. Cellular cytotoxicity against canine endothelial cells. Analysis of determinants recognized by CTL. Tissue Antigens. 1983;21(2):114–128. [PubMed] [Google Scholar]
- Groenewegen HJ, Vermeulen-Van der Zee E, te Kortschot A, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23(1):103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- Johnson LR, Aylward RL, Hussain Z, Totterdell S. Input from the amygdala to the rat nucleus accumbens: its relationship with tyrosine hydroxylase immunoreactivity and identified neurons. Neuroscience. 1994;61(4):851–865. doi: 10.1016/0306-4522(94)90408-1. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Similar effects of daily cocaine and stress on mesocorticolimbic dopamine neurotransmission in the rat. Biological Psychiatry. 1989;25(7):913–928. doi: 10.1016/0006-3223(89)90271-0. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, et al. The neural correlates of anhedonia in major depressive disorder. Biological Psychiatry. 2005;58(11):843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Kerfoot EC, Agarwal I, Lee HJ, Holland PC. Control of appetitive and aversive taste-reactivity responses by an auditory conditioned stimulus in a devaluation task: a FOS and behavioral analysis. Learning and Memory. 2007;14(9):581–589. doi: 10.1101/lm.627007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short-and long-term contextual fear. Behavioral Neuroscience. 1993;107(6):1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- Klucken T, Kagerer S, Schweckendiek J, Tabbert K, et al. Neural, electrodermal and behavioral response patterns in contingency aware and unaware subjects during a picture-picture conditioning paradigm. Neuroscience. 2009a;158(2):721–731. doi: 10.1016/j.neuroscience.2008.09.049. [DOI] [PubMed] [Google Scholar]
- Klucken T, Tabbert K, Schweckendiek J, Merz CJ, et al. Contingency learning in human fear conditioning involves the ventral striatum. Human Brain Mapping. 2009b;30(11):3636–3644. doi: 10.1002/hbm.20791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lu XY, Ghasemzadeh MB, Kalivas PW. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience. 1998;82(3):767–780. doi: 10.1016/s0306-4522(97)00327-8. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5(11):844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. Neuroscience. 1991;44(1):15–33. doi: 10.1016/0306-4522(91)90248-m. [DOI] [PubMed] [Google Scholar]
- McGinty VB, Grace AA. Selective activation of medial prefrontal-to-accumbens projection neurons by amygdala stimulation and Pavlovian conditioned stimuli. Cerebral Cortex. 2008;18(8):1961–1972. doi: 10.1093/cercor/bhm223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty VB, Grace AA. Activity-dependent depression of medial prefrontal cortex inputs to accumbens neurons by the basolateral amygdala. Neuroscience. 2009a;162(4):1429–1436. doi: 10.1016/j.neuroscience.2009.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty VB, Grace AA. Timing-dependent regulation of evoked spiking in nucleus accumbens neurons by integration of limbic and prefrontal cortical inputs. Journal of Neurophysiology. 2009b;101(4):1823–1835. doi: 10.1152/jn.91162.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, Nielsen MA. Evidence that an accumbens to subpallidal GABAergic projection contributes to locomotor activity. Brain Research Bulletin. 1983;11(3):309–314. doi: 10.1016/0361-9230(83)90166-1. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Swanson LW, Wu M. Neural projections from nucleus accumbens to globus pallidus, substantia innominata, and lateral preoptic-lateral hypothalamic area: an anatomical and electrophysiological investigation in the rat. Journal of Neuroscience. 1983;3(1):189–202. doi: 10.1523/JNEUROSCI.03-01-00189.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder AB, Hodenpijl MG, Lopes da Silva FH. Electrophysiology of the hippocampal and amygdaloid projections to the nucleus accumbens of the rat: convergence, segregation, and interaction of inputs. Journal of Neuroscience. 1998;18(13):5095–5102. doi: 10.1523/JNEUROSCI.18-13-05095.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munera A, Gruart A, Munoz MD, Fernandez-Mas R, et al. Hippocampal pyramidal cell activity encodes conditioned stimulus predictive value during classical conditioning in alert cats. Journal of Neurophysiology. 2001;86(5):2571–2582. doi: 10.1152/jn.2001.86.5.2571. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Yun IA, Wakabayashi KT, Fields HL. Firing of nucleus accumbens neurons during the consummatory phase of a discriminative stimulus task depends on previous reward predictive cues. Journal of Neurophysiology. 2004;91(4):1866–1882. doi: 10.1152/jn.00658.2003. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Physiological and morphological properties of accumbens core and shell neurons recorded in vitro. Synapse. 1993;13(2):135–160. doi: 10.1002/syn.890130206. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Tonic D2-mediated attenuation of cortical excitation in nucleus accumbens neurons recorded in vitro. Brain Research. 1994;634(1):105–112. doi: 10.1016/0006-8993(94)90263-1. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Different effects of subchronic clozapine and haloperidol on dye-coupling between neurons in the rat striatal complex. Neuroscience. 1995a;66(4):763–767. doi: 10.1016/0306-4522(95)00091-v. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. Journal of Neuroscience. 1995b;15(5 Pt 1):3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Dopaminergic reduction of excitability in nucleus accumbens neurons recorded in vitro. Neuropsychopharmacology. 1996;15(1):87–97. doi: 10.1016/0893-133X(95)00177-F. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, editors. The rat brain in stereotaxic coordinates. San Diego: Academic; 1996. [DOI] [PubMed] [Google Scholar]
- Peluso MA, Glahn DC, Matsuo K, Monkul ES, et al. Amygdala hyperactivation in untreated depressed individuals. Psychiatry Research. 2009;173(2):158–161. doi: 10.1016/j.pscychresns.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Risold PY, Swanson LW. Organization of projections from the basomedial nucleus of the amygdala: a PHAL study in the rat. Journal of Comparative Neurology. 1996;374(3):387–420. doi: 10.1002/(SICI)1096-9861(19961021)374:3<387::AID-CNE6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Progress in Neurobiology. 2004;74(5):301–320. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Pratt WE, Mizumori SJ. Characteristics of basolateral amygdala neuronal firing on a spatial memory task involving differential reward. Behavioral Neuroscience. 1998;112(3):554–570. doi: 10.1037//0735-7044.112.3.554. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. Journal of Neuroscience. 2001;21(9):3261–3270. doi: 10.1523/JNEUROSCI.21-09-03261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/”disliking” reactions, place preference/avoidance, and fear. Journal of Neuroscience. 2002;22(16):7308–7320. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nature Neuroscience. 2008;11(4):423–425. doi: 10.1038/nn2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Dopamine-mediated modulation of odour-evoked amygdala potentials during pavlovian conditioning. Nature. 2002;417(6886):282–287. doi: 10.1038/417282a. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Affective conditioning in the basolateral amygdala of anesthetized rats is modulated by dopamine and prefrontal cortical inputs. Annals of the New York Academy of Sciences. 2003;985:488–491. doi: 10.1111/j.1749-6632.2003.tb07107.x. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. Journal of Neuroscience. 2003;23(35):11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35(1):27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, et al. Increased amygdala esponse to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biological Psychiatry. 2001;50(9):651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Shinonaga Y, Takada M, Mizuno N. Topographic organization of collateral projections from the basolateral amygdaloid nucleus to both the prefrontal cortex and nucleus accumbens in the rat. Neuroscience. 1994;58(2):389–397. doi: 10.1016/0306-4522(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Sorg BA, Kalivas PW. Effects of cocaine and footshock stress on extracellular dopamine levels in the ventral striatum. Brain Research. 1991;559(1):29–36. doi: 10.1016/0006-8993(91)90283-2. [DOI] [PubMed] [Google Scholar]
- Surguladze S, Brammer MJ, Keedwell P, Giampietro V, et al. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biological Psychiatry. 2005;57(3):201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Eberwine J, Wilson CJ, Cao Y, et al. Dopamine receptor subtypes colocalize in rat striatonigral neurons. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(21):10178–10182. doi: 10.1073/pnas.89.21.10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, et al. Amygdala response to fearful faces in anxious and depressed children. Archives of General Psychiatry. 2001;58(11):1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Totterdell S, Smith AD. Convergence of hippocampal and dopaminergic input onto identified neurons in the nucleus accumbens of the rat. Journal of Chemical Neuroanatomy. 1989;2(5):285–298. [PubMed] [Google Scholar]
- Yang CR, Mogenson GJ. Electrophysiological responses of neurones in the nucleus accumbens to hippocampal stimulation and the attenuation of the excitatory responses by the mesolimbic dopaminergic system. Brain Research. 1984;324(1):69–84. doi: 10.1016/0006-8993(84)90623-1. [DOI] [PubMed] [Google Scholar]
- Young AMJ. Increased extracellular dopamine in nucleus accumbens in response to unconditioned and conditioned aversive stimuli: Studies using 1 min microdialysis in rats. Journal of Neuroscience Methods. 2004;138(1–2):57–63. doi: 10.1016/j.jneumeth.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Yun IA, Wakabayashi KT, Fields HL, Nicola SM. The ventral tegmental area is required for the behavioral and nucleus accumbens neuronal firing responses to incentive cues. Journal of Neuroscience. 2004;24(12):2923–2933. doi: 10.1523/JNEUROSCI.5282-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]