Abstract
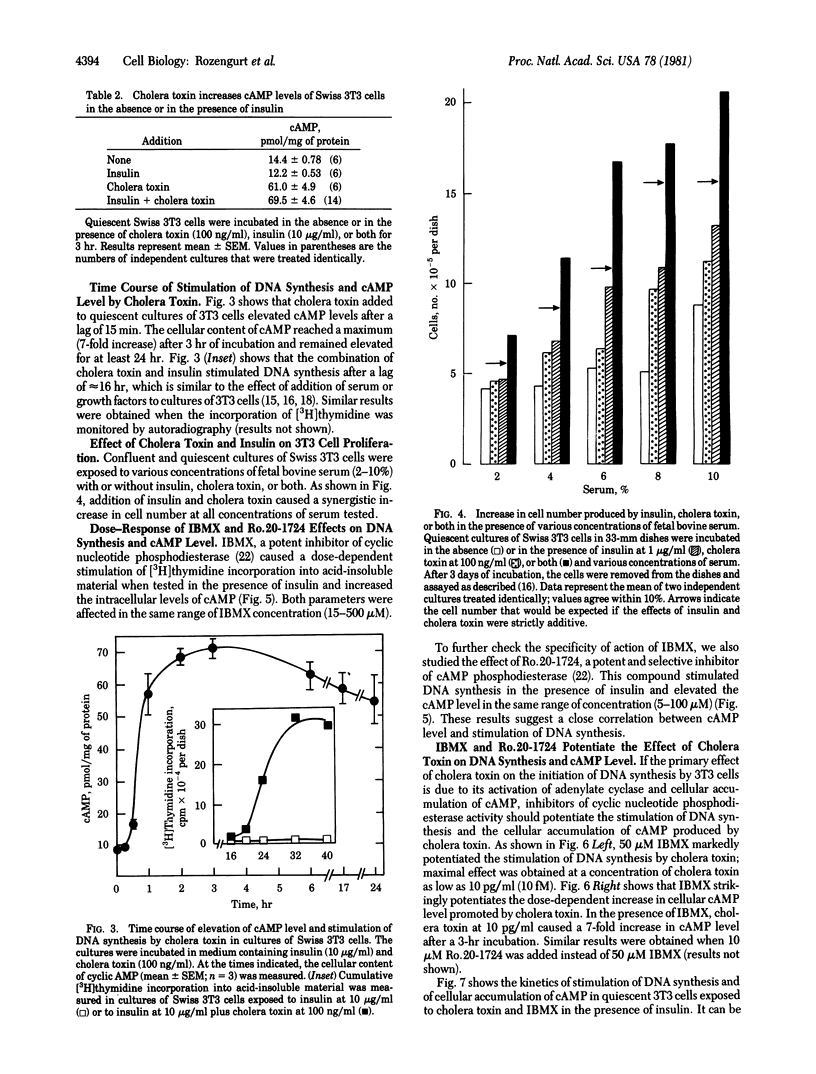
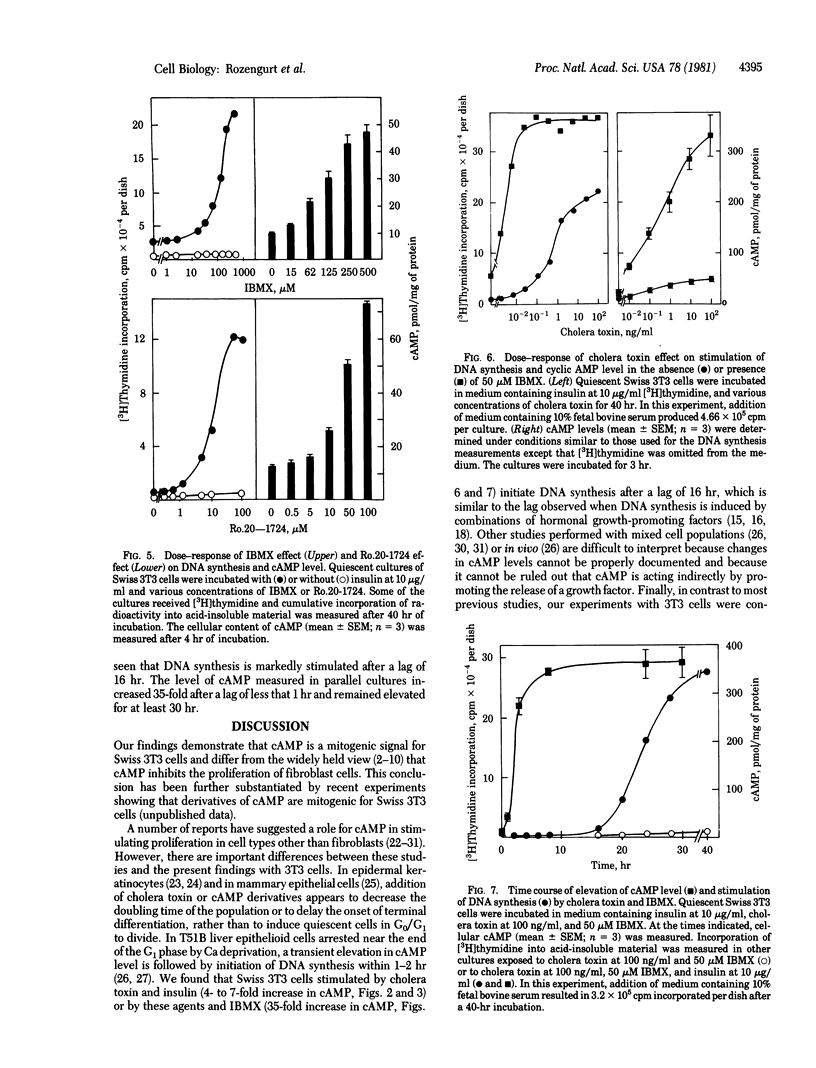
Addition of cholera toxin (100 ng/ml) to quiescent cultures of Swiss 3T3 cells acts synergistically with serum (2-4%), insulin, phorbol esters, epidermal growth factor, and fibroblast-derived growth factor to stimulate DNA synthesis. In the presence of insulin, cholera toxin caused a dose-dependent increase in cumulative [3H]thymidine incorporation into acid-insoluble material and in the intracellular cyclic AMP (cAMP) level. The dose--response curves for the two processes were similar. Furthermore, addition of 1-methyl-3-isobutylxanthine (15--500 microM) or of 4-(3-butoxy-4-methoxybenzyl)-2-imidazolidinone (5--100 microM), both of which are potent inhibitors of cyclic nucleotide phosphodiesterase which are potent inhibitors of cyclic nucleotide phosphodiesterase activity, stimulated DNA synthesis and increased cAMP levels in Swiss 3T3 cells. These compounds strikingly potentiated the effect of cholera toxin on DNA synthesis and on cAMP levels. When quiescent Swiss 3T3 cells were exposed to cholera toxin (100 ng/ml) and insulin at 10 micrograms/ml (4- to 7-fold increase in cAMP level) or to these agents and 1-methyl-3-isobutyl xanthine at 50 microM (35-fold increase in cAMP level), DNA synthesis began after a lag of 16 hr. These results indicate that cAMP acts as a mitogenic signal for Swiss 3T3 cells and differ from the widely held view that cyclic AMP inhibits the proliferation of fibroblast cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergstrand H., Kristoffersson J., Lundquist B., Schurmann A. Effects of antiallergic agents, compound 48/80, and some reference inhibitors on the activity of partially purified human lung tissue adenosine cyclic 3',5'-monophosphate and guanosine cyclic 3',5'-monophosphate phosphodiesterases. Mol Pharmacol. 1977 Jan;13(1):38–43. [PubMed] [Google Scholar]
- Bombik B. M., Burger M. M. c-AMP and the cell cycle: inhibition of growth stimulation. Exp Cell Res. 1973 Jul;80(1):88–94. doi: 10.1016/0014-4827(73)90278-4. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Rozengurt E. An 18,000 molecular weight polypeptide induces early events and stimulates DNA synthesis in cultured cells. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4555–4559. doi: 10.1073/pnas.73.12.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton A. L., Whitfield J. F. The cyclic AMP-dependent initiation of DNA synthesis by T51B rat liver epithelioid cells. J Cell Physiol. 1979 Oct;101(1):139–148. doi: 10.1002/jcp.1041010116. [DOI] [PubMed] [Google Scholar]
- Brooker G., Harper J. F., Terasaki W. L., Moylan R. D. Radioimmunoassay of cyclic AMP and cyclic GMP. Adv Cyclic Nucleotide Res. 1979;10:1–33. [PubMed] [Google Scholar]
- Burger M. M., Bombik B. M., Breckenridge B. M., Sheppard J. R. Growth control and cyclic alterations of cyclic AMP in the cell cycle. Nat New Biol. 1972 Oct 11;239(93):161–163. doi: 10.1038/newbio239161a0. [DOI] [PubMed] [Google Scholar]
- Chlapowski F. J., Kelly L. A., Butcher R. W. Cyclic nucleotides in cultured cells. Adv Cyclic Nucleotide Res. 1975;6:245–338. [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Phorbol esters and vasopressin stimulate DNA synthesis by a common mechanism. Nature. 1980 Oct 16;287(5783):607–612. doi: 10.1038/287607a0. [DOI] [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Stimulation of DNA synthesis by tumour promoter and pure mitogenic factors. Nature. 1978 Dec 14;276(5689):723–726. doi: 10.1038/276723a0. [DOI] [PubMed] [Google Scholar]
- Friedman D. L., Johnson R. A., Zeilig C. E. The role of cyclic nucleotides in the cell cycle. Adv Cyclic Nucleotide Res. 1976;7:69–114. [PubMed] [Google Scholar]
- Gill D. M. Mechanism of action of cholera toxin. Adv Cyclic Nucleotide Res. 1977;8:85–118. [PubMed] [Google Scholar]
- Green H. Cyclic AMP in relation to proliferation of the epidermal cell: a new view. Cell. 1978 Nov;15(3):801–811. doi: 10.1016/0092-8674(78)90265-9. [DOI] [PubMed] [Google Scholar]
- Johnson G. S., Pastan I. Role of 3',5'-adenosine monophosphate in regulation of morphology and growth of transformed and normal fibroblasts. J Natl Cancer Inst. 1972 May;48(5):1377–1387. [PubMed] [Google Scholar]
- Kram R., Mamont P., Tomkins G. M. Pleiotypic control by adenosine 3':5'-cyclic monophosphate: a model for growth control in animal cells. Proc Natl Acad Sci U S A. 1973 May;70(5):1432–1436. doi: 10.1073/pnas.70.5.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelo C. L. Differential effects of cAMP and cGMP on in vitro epidermal cell growth. Exp Cell Res. 1979 Apr;120(1):201–210. doi: 10.1016/0014-4827(79)90550-0. [DOI] [PubMed] [Google Scholar]
- Mierzejewski K., Rozengurt E. Vitamin B12 enhances the stimulation of DNA synthesis by serum in resting cultures of 3T6 cells. Exp Cell Res. 1977 May;106(2):394–397. doi: 10.1016/0014-4827(77)90187-2. [DOI] [PubMed] [Google Scholar]
- Otten J., Johnson G. S., Pastan I. Regulation of cell growth by cyclic adenosine 3',5'-monophosphate. Effect of cell density and agents which alter cell growth on cyclic adenosine 3',5'-monophosphate levels in fibroblasts. J Biol Chem. 1972 Nov 10;247(21):7082–7087. [PubMed] [Google Scholar]
- Pastan I. H., Johnson G. S., Anderson W. B. Role of cyclic nucleotides in growth control. Annu Rev Biochem. 1975;44:491–522. doi: 10.1146/annurev.bi.44.070175.002423. [DOI] [PubMed] [Google Scholar]
- Pawelek J., Halaban R., Christie G. Melanoma cells which require cyclic AMP for growth. Nature. 1975 Dec 11;258(5535):539–540. doi: 10.1038/258539a0. [DOI] [PubMed] [Google Scholar]
- Pruss R. M., Herschman H. B. Cholera toxin stimulates division of 3T3 cells. J Cell Physiol. 1979 Mar;98(3):469–474. doi: 10.1002/jcp.1040980305. [DOI] [PubMed] [Google Scholar]
- Rabinovitch A., Blondel B., Murray T., Mintz D. H. Cyclic adenosine-3',5'-monophosphate stimulates islet B cell replication in neonatal rat pancreatic monolayer cultures. J Clin Invest. 1980 Nov;66(5):1065–1071. doi: 10.1172/JCI109935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Hornby-Smith A., Brockes J. P. Cyclic AMP as a mitogenic signal for cultured rat Schwann cells. Nature. 1978 Jun 22;273(5664):672–673. doi: 10.1038/273672a0. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Legg A., Pettican P. Vasopressin stimulation of mouse 3T3 cell growth. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1284–1287. doi: 10.1073/pnas.76.3.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E. Stimulation of DNA synthesis in quiescent cultured cells: exogenous agents, internal signals, and early events. Curr Top Cell Regul. 1980;17:59–88. doi: 10.1016/b978-0-12-152817-1.50007-9. [DOI] [PubMed] [Google Scholar]
- Schor S., Rozengurt E. Enhancement by purine nucleosides and nucleotides of serum-induced DNA synthesis in quiescent 3T3 cells. J Cell Physiol. 1973 Jun;81(3):339–346. doi: 10.1002/jcp.1040810306. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Purkis P., Fentiman I. S. Cholera toxin and analogues of cyclic AMP stimulate the growth of cultured human mammary epithelial cells. J Cell Physiol. 1980 Mar;102(3):317–321. doi: 10.1002/jcp.1041020306. [DOI] [PubMed] [Google Scholar]
- Wang T., Sheppard J. R., Foker J. E. Rise and fall of cyclic AMP required for onset of lymphocyte DNA synthesis. Science. 1978 Jul 14;201(4351):155–157. doi: 10.1126/science.208147. [DOI] [PubMed] [Google Scholar]
- Whitfield J. F., Boynton A. L., MacManus J. P., Sikorska M., Tsang B. K. The regulation of cell proliferation by calcium and cyclic AMP. Mol Cell Biochem. 1979 Nov 1;27(3):155–179. doi: 10.1007/BF00215364. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Johnson G. S., Pastan I. Control of DNA synthesis and mitosis in 3T3 cells by cyclic AMP. Biochem Biophys Res Commun. 1972 Aug 21;48(4):743–748. doi: 10.1016/0006-291x(72)90669-9. [DOI] [PubMed] [Google Scholar]



