Abstract
Recent advances in understanding the role of bone in the systemic regulation of energy metabolism indicate that bone marrow cells, adipocytes and osteoblasts, are involved in this process. Marrow adipocytes store significant quantities of fat and produce adipokines, leptin and adiponectin, which are known for their role in the regulation of energy metabolism, whereas osteoblasts produce osteocalcin, a bone-specific hormone that has a potential to regulate insulin production in the pancreas and adiponectin production in fat tissue. Both osteoblasts and marrow adipocytes express insulin receptor and respond to insulin-sensitizing anti-diabetic TZDs in a manner, which tightly links bone with the energy metabolism system. Metabolic profile of marrow fat resembles that of both, white and brown fat, which is reflected by its plasticity in acquiring different functions including maintenance of bone micro-environment. Marrow fat responds to physiologic and pathologic changes in energy metabolism status by changing volume and metabolic activity. This review summarizes available information on the metabolic function of marrow fat and provides hypothesis that this fat depot may acquire multiple roles depending on the local and perhaps systemic demands. These functions may include a role in bone energy maintenance and endocrine activities to serve osteogenesis during bone remodeling and bone healing.
Introduction
Extensive evidence indicates that bone is an integral part of a system which governs energy metabolism. Identification that central regulators of energy metabolism are critical for control of bone mass created groundwork for conceptual change in our understanding of regulation of bone homeostasis. Hypothalamic regulation of energy metabolism by leptin-, NPY-, CART-, and αMSH-dependent signaling appears to be critical for regulation of bone mass (1, 18, 20, 41). In addition, bone is under control of adrenergic signaling, which is activated by sympathetic nervous system (21, 25). Both types of adrenergic receptors, α and β, are expressed in bone where, in a type-specific manner, they regulate bone formation and bone resorption. Thus, α2-AR deficiency results in high bone mass phenotype, increased bone length and decreased expression of osteoclast-specific gene markers suggesting that this type of adrenoceptors negatively regulate bone growth and increase bone resorption by acting directly on osteoclasts (25). On the other hand, β1-AR and β2-AR regulate osteoblasts function, whereas β3-AR is expressed in marrow adipocytes and may control lipolysis and energy production (7, 21, 48, 59). It has been demonstrated that β2-AR positively regulates RANKL production in osteoblasts and osteoblast development by synergizing with BMP-2 signaling, whereas β1-AR regulates osteoblasts proliferation (21, 82). Tissue-specific ablation of these receptors in rodents, or use of beta-blockers in humans, is associated with increased bone mass (9, 88). Fig. 1 summarizes a contribution of factors regulating energy metabolism on bone cell development and bone remodeling.
Figure 1.
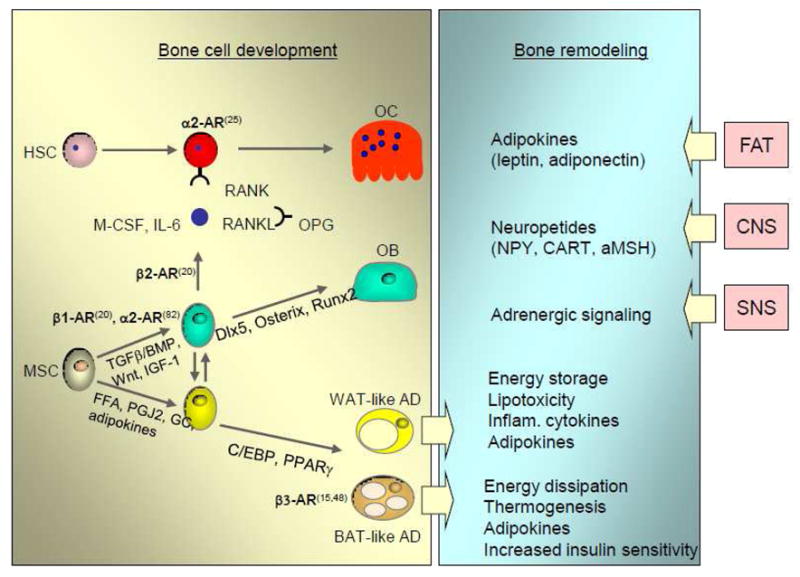
Contribution of factors regulating systemic energy metabolism on bone cell development and bone remodeling. The role of β-adrenergic signaling on osteoblast and osteoclasts development, and metabolic function of adipocytes highlighted by bold letters and referenced. Abbreviations: FFA – free fatty acids, PGJ2 – prostaglandin J2, GC – glucocorticoids.
Since the control of energy metabolism directly involves fat tissue metabolism and the fact that fat occupies significant portion of bone marrow cavity, there is a need to review available information on metabolic profile of bone fat and any evidence indicating that this particular fat depot is linked to systemic energy metabolism. The main function of fat tissue is to regulate energy homeostasis through the maintenance of energy storage and its dissipation, and production of circulating hormones which regulate energy metabolism in other organs. In contrast to extramedullar fat depots (e.g. visceral and subcutaneous fat), the function of marrow fat is largely unknown. Historically, it was considered to have supportive role in hematopoiesis by producing the necessary cytokines and energy in the form of heat for hematopoietic cell development (31, 79). Recent evidence indicates that the support for hematopoiesis is provided rather by adipo-osteogenic progenitors, which are essential for the maintenance of hematopoietic niche within the marrow (66). In contrast, the presence of lipid-filled adipocytes in the marrow cavity correlates inversely with hematopoiesis, while their absence, due to targeted suppression of mesenchymal cell differentiation toward adipocytes, correlates with increased hematopoiesis, indicating that mature marrow fat cells are negative regulators of hematopoietic micro-environment (68). However, there is certain paucity in information on metabolic activity of marrow fat. It has been hypothesized that marrow fat participates in lipid metabolism by clearing and storing circulating triglycerides, thereby acting as a localized energy reservoir for emergency situations requiring, for example, de novo osteogenesis (31). Indeed, gene expression profile of marrow adipocytes suggests that they possess activity of energy dissipation as well as endocrine activities, which may modulate local marrow environment supporting bone remodeling (48, 75, 76).
Marrow adipocytes originate in a mesenchymal stem cell (MSC) compartment that also produces osteoblasts. The commitment of MSCs toward either the osteoblast or adipocyte lineage is determined by a combination of extracellular and intrinsic factors that regulate cellular activities of the Wnt, TGFβ/BMP, and IGF-1 signaling pathways and lead to activation of lineage-specific transcriptional regulators including Runx2, Dlx5, and osterix for osteoblast, and PPARγ2 and a family of CAAT enhancer binding proteins for adipocytes (51, 57, 65, 71). Activation of the PPARγ2 isoform with either natural (fatty acids and ecosanoids) or artificial (TZD) ligands directs MSC differentiation toward the adipocyte lineage at the expense of osteoblast formation and results in decreased bone mass (50, 54, 65). Moreover, activation of PPARγ2 in cells of the osteoblast lineage converts them to terminally differentiated adipocytes and irreversibly suppresses their phenotype (52, 53). Marrow adipocytes are under the same transcriptional control and express similar set of genes involved in carbohydrate and lipid metabolism as extramedullar fat cells, indicating that marrow fat response to the environmental and hormonal factors might be similar to other fat depots (75). However, their localization in bone may determine their unique function and unique response to the factors modulating systemic energy metabolism.
Marrow fat metabolic functions
From the energy metabolism perspective there are two types of fat tissue. White fat or WAT, represented by subcutaneous and visceral fat, is characterized by a low number of mitochondria and serves as a primary site for triglyceride/energy storage (81). WAT also functions as endocrine tissue by producing hormones regulating energy balance including leptin and adiponectin. Brown fat or BAT, which appears as discrete tissue located along the neck, and in supraclavical, paravertebral, and perirenal regions, is rich in mitochondria and functions in basal and inducible energy expenditure (29). This is mediated by uncoupling protein 1 (UCP1), which stimulates proton leak from the mitochondrial membrane to uncouple respiration from ATP synthesis to produce heat. BAT thermogenic activity is controlled by the central nervous system via catecholamines and β-adrenergic signaling, and deiodinase 2 (Dio2)-mediated thyroid hormone conversion from thyroxine (T4) to triiodothyronine (T3). Along with its role in adaptive thermogenesis, BAT also has a function in protecting against obesity, insulin resistance and diabetes (13, 22, 44–46).
Bone marrow fat is often referred as yellow adipose tissue (YAT), because of it’s yellowish appearance due to a moderate number of mitochondria, and it is suspected to have mixed white and brown fat phenotype (30, 48). Historically, marrow fat was merely considered a cellular component of bone that served a passive role by occupying space no longer needed for hematopoiesis. Marrow cavities of newborn mammals contain active hematopoietic tissue, known as “red” marrow. In the first decade of age, “red” marrow undergoes gradual replacement by fatty or “yellow” marrow, which by the second decade of human life fills almost entire cavity of long bone (63). The process begins in the periphery of the skeleton, in the terminal phalanges, it progresses to the distal and proximal long bone, and finally it occurs in the flat bone and vertebral body of the central skeleton (37, 63). In a single bone, conversion of “red’ to “yellow” marrow starts in the diaphyseal region and extends toward proximal and distal metaphysis (37). Fat distribution in human skeleton is site, age, and gender specific. In adults, long bone marrow cavity is entirely filled with fat, while in iliac crest marrow constitutes 40% of fat and 60% of hematopoietic cells (78). Men have more bone marrow fat as compared to age-matched women (49), and over the adult lifespan, men and women can have more than a twofold increase in bone-marrow fat (40, 49, 61, 89). The process of fat accumulation in murine bone differs from that in human bone in respect to age and skeletal location. In C57BL/6 mice, adipocytes start to be visible in tibia and femora after peak bone mass is achieved (4–6 mo of age) (50). Moreover and in contrast to humans, they start to accumulate in proximal and/or distal metaphyseal region, and extend to the diaphyseal part of bone (Fig. 2).
Figure 2.
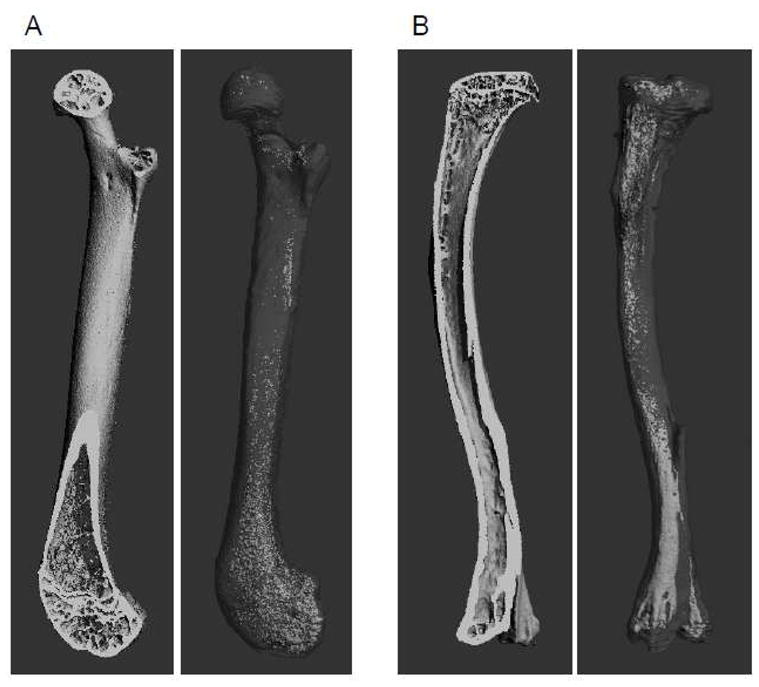
mCT renderings of murine femur (A) and tibia (B) representing longitudinal cross section of mineralized bone tissue (left panel) and fat in the same bone after decalcification and staining with osmium tetroxide (right panel).
The localization of bone fat to the trabecular area, where the active bone remodeling process occurs, suggests that marrow fat may be involved in this process, perhaps by providing energy for hematopoietic and mesenchymal marrow compartment (19). Indeed, an analysis of molecular signature of marrow fat showed that this fat depot expresses gene transcripts characteristic for BAT and involved in energy production (48). Relative expression of thermogenic regulators PGC1α and deiodinase 2 (Dio2) is at the levels characteristic for BAT (48). Bone marrow thermogenic activity and its response to low temperature were recognized long time ago (reviewed in (30)). It has been acknowledged that the distribution of fat in bone correlates with a body temperature gradient and is higher in the appendages, which have lower body temperature, than in the axial skeleton (31). Interestingly, in humans the fat fraction in the heel constitutes nearly 90% even at early age and does not increase with age, whereas, the fat fraction in the spine occupies 30% of marrow cavity at early age and increases to 70% at 60 years of age (58). In mice, we observe dense fat cell concentration in the distal and the proximal parts of murine tibia (Fig. 2B). Although it is a pure speculation at this point, however one can correlate the differences in fat distribution with metabolic activity of bone and body temperature gradient. Hence, the presence of fat in the trabecular region with extensive bone remodeling and its presence in the relatively inactive distal region, which is naturally exposed to the lower temperature than the proximal part, may suggest that these two fat depots may differ metabolically.
Marrow fat may also function in a manner similar to WAT, which includes clearing and storing circulating triglycerides. The analysis of the marrow fat response to antidiabetic TZDs, which improve energy metabolism by increasing cell sensitivity to insulin and increasing lipid storage in adipocytes, suggests that YAT may function as an insulin-sensitive tissue which is involved in fatty acids metabolism (Table 1) (53, 75). Consequently, in marrow adipocytes TZD rosiglitazone upregulates the expression of genes essential for fatty acid metabolism, including fatty acid synthase, fatty acid-binding proteins, hormone-sensitive lipase, and cholesterol transporter CD36. Interestingly, although apparently a large number of genes involved in carbohydrate metabolism is upregulated, there is no change in the expression of any of the important insulin-dependent glucose transporters, including GLUT4 (75). This observation suggests that marrow fat functions in rather lipid than glucose metabolism (75). Most importantly, rosiglitazone induces in marrow adipocytes the expression of genes involved in insulin signaling, among them the insulin receptor, insulin receptor substrate-1 and FoxO1, while suppressing the expression of negative regulators of this signaling network such as Socs3 (Table 1). This profile suggests that marrow fat responds positively to insulin sensitizing conditions. In addition, TZDs upregulate in marrow fat an expression of BAT-specific gene markers (UCP1, PGC1α, Dio2, β3AR, Prdm16 and FoxC2) indicating a potential of marrow fat to provide energy. Similarly, a response of visceral fat to TZDs constitutes an increased expression of BAT-specific gene markers and increased lipid oxidization and energy production (83).
Table 1.
Expression of transcripts for insulin signaling and fatty acids metabolism in response to treatment of U-33/γ2 cells with insulin sensitizing drug rosiglitazone (75)
| Functional category | Gene name | Gene symbol | Fold expression vs. U-33/c cells |
|---|---|---|---|
| Insulin signaling | Insulin receptor | IR | 3.4 |
| Insulin receptor substrate 1 | IRS1 | 1.9 | |
| Forkhead box O1 | FoxO1 | 1.8 | |
| Suppressor of cytokine signaling 3 | Socs3 | −6.1 | |
|
| |||
| Fatty acids metabolism | Fatty acid synthase | FAS | 5.5 |
| Fatty acid binding protein 4 | FABP4 | 69.6 | |
| Fatty acid binding protein 5 | FABP5 | 82.9 | |
| Lipoprotein lipase | LPL | 3.4 | |
| Hormone-sensitive lipase | Lipe | 74.4 | |
| CD36 antigen, cholesterol transport | CD36 | 351.9 | |
| Cell death-inducing effector c/Fat specific protein 27 | Cidec/FSP27 | 457.2 | |
Marrow fat endocrine/paracrine function
An endocrine activity of fat cells includes production of adipokines, among them leptin and adiponectin, which regulate caloric intake and insulin sensitivity in peripheral tissues, respectively. Both adipokines are also produced in bone, however in smaller quantities as compared to WAT, and their receptors are expressed in bone cells, linking bone and fat metabolism (48). Although strong evidence points to CNS as a mediator of leptin effect on bone, the presence of leptin receptor in osteoblasts and osteoclasts, as well as leptin production by marrow adipocytes suggests receptor-mediated direct effect on bone. Indeed, animal studies demonstrated that leptin increases bone mineral density, bone mineral content, and bone formation rate, while it decreases the number and the size of bone marrow adipocytes when acting on bone peripherally (33, 34). In addition, db/db mice with leptin receptor-deficiency have reduced bone mass and bone strength, indicating that receptor mediated leptin activity can be anabolic for bone, in contrast to its activity through CNS (20, 21, 41, 85). However, human epidemiological studies correlating levels of circulating leptin with bone mass and fracture risk are not conclusive and indicate that this adipokine is rather a poor predictor of skeletal status (5, 39, 56).
In contrast, recent clinical evidence suggests that adiponectin may play an important role in the regulation of skeletal homeostasis. It has been shown that high levels of circulating adiponectin correlate with lower BMD in older men and women (4), and increased fracture risk only in older men, but not in older women (5). Interestingly, increased incidence of fractures in men occurred despite higher hip BMD and lower levels of circulating adiponectin, as compared to women, indicating that association between adiponectin and bone may be influenced by sex hormones.
In contrast to human studies, mice deficient in adiponectin show either transient increase or no effect on bone mass (69, 74, 86). In vitro however, adiponectin inhibits adipocyte formation, stimulates osteoblast phenotype and cell proliferation, and inhibits osteoclastogenesis (6, 86, 90). Moreover, adiponectin increases BMP-2 expression in osteoblastic cells via AdipoR1 receptor signaling pathway which includes activation of AMPK, p38 and NF-kappaB signaling (35). The direct role of adiponectin in regulation of new bone formation was demonstrated recently in the model of distraction osteogenesis (38). Intermittent administration of adiponectin to the mandibular osteodistraction site resulted in significant increase in intramembranous bone formation and an increase in overall rate of bone regeneration (38). Taken together, these results suggest that locally produced adiponectin may have a positive effect on bone, perhaps in emergency situations which require new bone formation.
Marrow fat response to physiological and pathological changes in energy metabolism
The connection between marrow fat and the systemic energy metabolism is reflected in its response to changes in energy balance. Alterations in the efficiency of energy metabolism system during aging, and in overnutrition, malnutrition, and diabetes correlate with changes in the fat volume in bone and changes in its activity. Aging process is associated with decreased efficiency of energy utilization partially due to functional impairment of fat tissue (12). A decline in peripheral fat depots size and function correlate with increased fat accumulation in bone. It is hypothesized that aging causes redistribution of lipids to cells of other organs, like bone marrow, muscle and liver, which may acquire fat-like phenotype without functioning as bona fide adipocytes and can lead to lipotoxicity in these organs (43). Nuclear magnetic resonance analysis of the qualitative changes in fat of lumbar vertebra showed that with aging the level of unsaturated fatty acids decreases (89). Similar changes in fat quality are observed in visceral fat with aging and metabolic diseases, which lead to accumulation of monocytes, production of inflammatory cytokines and development of insulin resistance in fat tissue (81). Despite an increase in bone fat with aging, the phenotype of marrow adipocytes changes toward lower efficiency in energy production as reflected by lower expression of brown fat gene markers: UCP1, Dio2, PGC1α and β3 adrenergic receptor (48). These data suggest that marrow fat metabolism may be subjected and is responding to the same factors which modulate extramedullar fat metabolism during aging (48).
Number of studies indicate an inverse relationship between bone mass and fat mass in bone. An increased fat content in bone correlates negatively with bone mass during aging and with decreased bone acquisition during growth (17, 32, 73, 87). The underlying mechanism includes changes in the signaling and transcriptional control of MSCs differentiation leading to their preferential differentiation toward adipocytes and at the expense of osteoblast formation (62, 70). However, it is also possible that changes in bone fat metabolism lead to lipotoxicity, which may contribute to the bone loss with aging. Indeed, increase in oxidative stress and accumulation of oxidized lipids in bone is associated with increased local inflammatory responses, resistance to anabolic effects of PTH and Wnt signaling, and osteoporotic bone loss (2, 28, 42, 72).
Overnutrition and malnutrition are two additional examples of systemic changes in energy metabolism, which affect both, bone fat volume and bone mass. In obese premenopausal women, visceral fat positively correlates with the fat content in vertebra and independently of BMD indicating that obesity and bone marrow fat accumulation are linked through the same mechanism, which is responsible for handling increased calorie intake and accumulation of energy in the form of fat (11). The same studies showed that increased fat in vertebra correlates with decreased BMD, independently of obesity, supporting inverse relationship between adipocyte and osteoblast differentiation (11). Surprisingly, in conditions of decreased calorie intake and decrease in the amount of peripheral fat, the content of fat in bone is also increased (11, 16). Patients with anorexia nervosa have elevated marrow fat mass in vertebra and femur, which is associated with low mineral density and elevated levels of circulating Pref-1, a negative regulator of osteoblast and adipocyte differentiation (10). Similarly, caloric restriction increases fat content in murine bone (16). The 30% reduction in daily caloric intake has a deleterious effect on growing murine bone reflected by decrease in cortical and trabecular bone mass. Changes in bone mass are accompanied by increased fat accumulation in the marrow despite a decrease in peripheral fat mass. Leptin and IGF-1 levels are also decreased suggesting a possible role of these signaling in lipids accumulation in bone (16).
Diabetes is a disease of impaired glucose and fatty acids metabolism due to deficiency in insulin signaling in fat, liver and muscle. Both types of diabetes, insulin-dependent Type 1 and insulin-independent Type 2, are associated with increase of fat volume in bone. In Type 1, characterized by pancreatic β-cell failure to produce insulin, increased quantities of fat in bone correlate with low bone mass and low levels of circulating IGF-1 and deficiency in vitamin D (8, 64, 80). In contrast, in Type 2, which is associated with high levels of serum insulin but inability of peripheral tissue to respond to it, fat mass in bone is increased but this increase is not associated with lower bone mass. In a murine model of hyperinsulinemia due to insulin clearance impairment in the liver, high bone mass coincides with increased fat content (36). An analysis of metabolic profile of marrow fat of diabetic yellow agouti mice showed decreases similar to aging in the expression of brown fat specific genes involved in energy production (48). This suggests that diabetic disease not only affects peripheral fat but also bone fat and changes its function.
Interestingly, in a model of increased energy production due to deficiency in early B-cell factor 1 (Ebf1), increased bone formation and high bone mass correlates with high quantity of fat in bone (26) suggesting a positive, or at least lack of a negative, effect of fat on osteogenesis. Moreover, heterotropic bone formation is associated with accumulation of brown fat cells expressing UCP1, suggesting the role of bone fat in providing energy and supporting hematopoiesis (67). Thus, fat in bone may acquire different metabolic status dictated by systemic energy metabolism status and certain local demands of physiological importance. The above examples suggest that metabolic activity of fat is as important for bone homeostasis as its presence in bone.
Integration of bone metabolism with energy metabolism has been presented recently as a model which links anabolic effect of insulin signaling in osteoblasts with bone turnover and regulation of insulin sensitivity in peripheral organs (24, 27). Thus, in osteoblasts insulin signaling regulates an expression of Runx2 and osteocalcin production. In addition, insulin increases support for osteoclastogenesis by decreasing an expression of OPG, a decoy receptor for RANKL. As a result, insulin increases bone turnover and production of undercarboxylated osteocalcin, which in endocrine fashion regulates insulin release from β-cells in pancreas and production of adiponectin in fat tissue (14, 24, 27, 55). It is of interest whether this regulatory circuit is affected in insulin-independent Type 2 diabetes. There is limited information on the status of bone turnover in diabetes, however longitudinal histomorphometric studies by Krakauer et al. suggest that progression of diabetic disease attenuates bone turnover (47). This observation together with the role of insulin in regulation of this process poses an interesting question whether decreased bone turnover is a manifestation of insulin resistance in bone. In general, patients with Type 2 diabetes have normal or higher bone mass as compared to age-matched non-diabetic controls, however they also have higher fracture risk indicating decrease in bone quality, which may in part result from decreased bone turnover (60). More studies need to be done in this respect.
Glucocorticoids may suppress BAT-like phenotype in marrow adipocytes
A decrease in BAT-like phenotype of marrow adipocytes with aging and diabetes (48) may account for unfavorable changes in the marrow environment affecting bone remodeling. BAT-like metabolism is controlled by β adrenergic signaling through fat-specific β3 adrenoceptor (15), which expression decreases in the bone marrow during aging and in diabetes (48). Two other adrenoceptors, β1 and β2, are expressed in osteoblasts with β2 receptor implicated in upregulation of RANKL production and increased bone resorption (59). Glucocorticoids, including endogenous cortisol, the levels of which increases with aging and diabetes (3), and which have a negative effect on bone homeostasis (84) are known to regulate adrenergic response by regulating the expression of all three forms of β adrenoceptors. Glucocorticoids inhibit the transcriptional response of the UCP1 gene to adrenergic stimulation in brown adipocytes by inhibiting the expression of β1 and β3 adrenoceptors (77). In epidydimal adipose tissue, in response to food stress, increased levels of endogeneous cortisol correlate with down-regulation of β1 and β3 adrenoceptors expression and upregulation of β2 adrenoceptor expression (23). Taking together, glucocorticoids have a dual effect on adrenergic signaling. They decrease adrenoceptor-mediated metabolic activity of brown fat and increase catabolic activity in bone. Thus, activation of glucocorticoid signaling in marrow adipocytes may lead to decrease in β3 adrenergic receptor expression and loss of BAT-like potential of marrow adipocytes, which is seen with aging and diabetes. More studies are needed to support this hypothesis.
Concluding remarks
There is an association between diseases of energy metabolism, fat content in bone and bone mass. Marrow fat is metabolically active and may acquire phenotypic characteristics of either energy producing BAT or energy storing WAT. Most importantly, it responds to changes in systemic energy metabolism. There is an increasing interest in the function of marrow fat including its capabilities to modulate marrow micro-environment to support bone remodeling and contribute to insulin-dependent fatty acid metabolism. More studies need to be done to unravel metabolic properties of this fat depot and perhaps to harness bone fat function for improving skeletal status in metabolic diseases including diabetes and osteoporosis.
Highlights.
This review summarizes available information on metabolic status of marrow fat.
Marrow fat responds to to physiologic and pathologic changes in systemic energy metabolism.
Marrow fat possesses mixed brown and white phenotype.
Marrow fat may acquire function in maintenance energy metabolism in bone.
Acknowledgments
Special thanks to Dr. P. Czernik for preparation of mCT renderings of bone and fat in bone. This work was supported by funds from NIH/NIA AG028935 and American Diabetes Association’s Amaranth Diabetes Fund 1-09-RA-95.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahn JD, Dubern B, Lubrano-Berthelier C, Clement K, Karsenty G. Cart overexpression is the only identifiable cause of high bone mass in melanocortin 4 receptor deficiency. Endocrinology. 2006;147:3196–202. doi: 10.1210/en.2006-0281. [DOI] [PubMed] [Google Scholar]
- 2.Almeida M, Ambrogini E, Han L, Manolagas SC, Jilka RL. Increased lipid oxidation causes oxidative stress, increased peroxisome proliferator-activated receptor-gamma expression, and diminished pro-osteogenic Wnt signaling in the skeleton. J Biol Chem. 2009;284:27438–48. doi: 10.1074/jbc.M109.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. Clinical review: The pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J Clin Endocrinol Metab. 2009;94:2692–701. doi: 10.1210/jc.2009-0370. [DOI] [PubMed] [Google Scholar]
- 4.Araneta MR, von Muhlen D, Barrett-Connor E. Sex differences in the association between adiponectin and BMD, bone loss, and fractures: the Rancho Bernardo study. J Bone Miner Res. 2009;24:2016–22. doi: 10.1359/JBMR.090519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbour KE, Zmuda JM, Boudreau R, Strotmeyer ES, Horwitz MJ, Evans RW, Kanaya AM, Harris TB, Bauer DC, Cauley JA. Adipokines and the risk of fracture in older adults. J Bone Miner Res. 2011 doi: 10.1002/jbmr.361. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berner HS, Lyngstadaas SP, Spahr A, Monjo M, Thommesen L, Drevon CA, Syversen U, Reseland JE. Adiponectin and its receptors are expressed in bone-forming cells. Bone. 2004;35:842–9. doi: 10.1016/j.bone.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet N, Pierroz DD, Ferrari SL. Adrenergic control of bone remodeling and its implications for the treatment of osteoporosis. J Musculoskelet Neuronal Interact. 2008;8:94–104. [PubMed] [Google Scholar]
- 8.Botolin S, McCabe LR. Bone loss and increased bone adiposity in spontaneous and pharmacologically induced diabetic mice. Endocrinology. 2007;148:198–205. doi: 10.1210/en.2006-1006. [DOI] [PubMed] [Google Scholar]
- 9.Bouxsein ML, Devlin MJ, Glatt V, Dhillon H, Pierroz DD, Ferrari SL. Mice lacking beta-adrenergic receptors have increased bone mass but are not protected from deleterious skeletal effects of ovariectomy. Endocrinology. 2009;150:144–52. doi: 10.1210/en.2008-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, Ghomi RH, Rosen CJ, Klibanski A. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94:2129–36. doi: 10.1210/jc.2008-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, Rosen CJ, Klibanski A, Miller KK. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring) 2011;19:49–53. doi: 10.1038/oby.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cartwright MJ, Tchkonia T, Kirkland JL. Aging in adipocytes: potential impact of inherent, depot-specific mechanisms. Exp Gerontol. 2007;42:463–71. doi: 10.1016/j.exger.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cederberg A, Gronning LM, Ahren B, Tasken K, Carlsson P, Enerback S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell. 2001;106:563–73. doi: 10.1016/s0092-8674(01)00474-3. [DOI] [PubMed] [Google Scholar]
- 14.Clemens TL, Karsenty G. The osteoblast: an insulin target cell controlling glucose homeostasis. J Bone Miner Res. 2011;26:677–80. doi: 10.1002/jbmr.321. [DOI] [PubMed] [Google Scholar]
- 15.Collins S, Surwit RS. The beta-adrenergic receptors and the control of adipose tissue metabolism and thermogenesis. Recent Prog Horm Res. 2001;56:309–28. doi: 10.1210/rp.56.1.309. [DOI] [PubMed] [Google Scholar]
- 16.Devlin MJ, Cloutier AM, Thomas NA, Panus DA, Lotinun S, Pinz I, Baron R, Rosen CJ, Bouxsein ML. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res. 2010;25:2078–88. doi: 10.1002/jbmr.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Iorgi N, Mo AO, Grimm K, Wren TA, Dorey F, Gilsanz V. Bone acquisition in healthy young females is reciprocally related to marrow adiposity. J Clin Endocrinol Metab. 2010;95:2977–82. doi: 10.1210/jc.2009-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Driessler F, Baldock PA. Hypothalamic regulation of bone. J Mol Endocrinol. 2010;45:175–81. doi: 10.1677/JME-10-0015. [DOI] [PubMed] [Google Scholar]
- 19.Ducy P. The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetologia. 2011;54:1291–7. doi: 10.1007/s00125-011-2155-z. [DOI] [PubMed] [Google Scholar]
- 20.Elefteriou F. Regulation of bone remodeling by the central and peripheral nervous system. Arch Biochem Biophys. 2008;473:231–6. doi: 10.1016/j.abb.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–20. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 22.Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–4. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- 23.Farias-Silva E, dos Santos IN, Corezola do Amaral ME, Grassi-Kassisse DM, Spadari-Bratfisch RC. Glucocorticoid receptor and Beta-adrenoceptor expression in epididymal adipose tissue from stressed rats. Ann N Y Acad Sci. 2004;1018:328–32. doi: 10.1196/annals.1296.040. [DOI] [PubMed] [Google Scholar]
- 24.Ferron M, Wei J, Yoshizawa T, Del Fattore A, Depinho RA, Teti A, Ducy P, Karsenty G. Insulin Signaling in Osteoblasts Integrates Bone Remodeling and Energy Metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fonseca TL, Jorgetti V, Costa CC, Capelo LP, Covarrubias AE, Moulatlet AC, Teixeira MB, Hesse E, Morethson P, Beber EH, Freitas FR, Wang CC, Nonaka KO, Oliveira R, Casarini DE, Zorn TM, Brum PC, Gouveia CH. Double disruption of alpha2A- and alpha2C-adrenoceptors results in sympathetic hyperactivity and high-bone-mass phenotype. J Bone Miner Res. 2011;26:591–603. doi: 10.1002/jbmr.243. [DOI] [PubMed] [Google Scholar]
- 26.Fretz JA, Nelson T, Xi Y, Adams DJ, Rosen CJ, Horowitz MC. Altered metabolism and lipodystrophy in the early B-cell factor 1-deficient mouse. Endocrinology. 2010;151:1611–21. doi: 10.1210/en.2009-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fulzele K, Riddle RC, Digirolamo DJ, Cao X, Wan C, Chen D, Faugere MC, Aja S, Hussain MA, Bruning JC, Clemens TL. Insulin Receptor Signaling in Osteoblasts Regulates Postnatal Bone Acquisition and Body Composition. Cell. 2010;142:309–319. doi: 10.1016/j.cell.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gasparrini M, Rivas D, Elbaz A, Duque G. Differential expression of cytokines in subcutaneous and marrow fat of aging C57BL/6J mice. Exp Gerontol. 2009;44:613–8. doi: 10.1016/j.exger.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–56. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Gimble JM, Nuttall ME. Bone and fat: old questions, new insights. Endocrine. 2004;23:183–8. doi: 10.1385/ENDO:23:2-3:183. [DOI] [PubMed] [Google Scholar]
- 31.Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME. Playing with bone and fat. J Cell Biochem. 2006;98:251–66. doi: 10.1002/jcb.20777. [DOI] [PubMed] [Google Scholar]
- 32.Griffith JF, Yeung DK, Antonio GE, Lee FK, Hong AW, Wong SY, Lau EM, Leung PC. Vertebral bone mineral density, marrow perfusion, and fat content in healthy men and men with osteoporosis: dynamic contrast-enhanced MR imaging and MR spectroscopy. Radiology. 2005;236:945–51. doi: 10.1148/radiol.2363041425. [DOI] [PubMed] [Google Scholar]
- 33.Hamrick MW. Leptin, bone mass, and the thrifty phenotype. J Bone Miner Res. 2004;19:1607–11. doi: 10.1359/JBMR.040712. [DOI] [PubMed] [Google Scholar]
- 34.Hamrick MW, Della-Fera MA, Choi YH, Pennington C, Hartzell D, Baile CA. Leptin treatment induces loss of bone marrow adipocytes and increases bone formation in leptin-deficient ob/ob mice. J Bone Miner Res. 2005;20:994–1001. doi: 10.1359/JBMR.050103. [DOI] [PubMed] [Google Scholar]
- 35.Huang CY, Lee CY, Chen MY, Tsai HC, Hsu HC, Tang CH. Adiponectin increases BMP-2 expression in osteoblasts via AdipoR receptor signaling pathway. J Cell Physiol. 2010;224:475–83. doi: 10.1002/jcp.22145. [DOI] [PubMed] [Google Scholar]
- 36.Huang S, Kaw M, Harris MT, Ebraheim N, McInerney MF, Najjar SM, Lecka-Czernik B. Decreased osteoclastogenesis and high bone mass in mice with impaired insulin clearance due to liver-specific inactivation to CEACAM1. Bone. 2010;46:1138–45. doi: 10.1016/j.bone.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hwang S, Panicek DM. Magnetic resonance imaging of bone marrow in oncology, Part 1. Skeletal Radiol. 2007;36:913–20. doi: 10.1007/s00256-007-0309-3. [DOI] [PubMed] [Google Scholar]
- 38.Jiang X, Song D, Ye B, Wang X, Song G, Yang S, Hu J. Effect of intermittent administration of adiponectin on bone regeneration following mandibular osteodistraction in rabbits. J Orthop Res. 2011;29:1081–5. doi: 10.1002/jor.21355. [DOI] [PubMed] [Google Scholar]
- 39.Jurimae J, Jurimae T, Leppik A, Kums T. The influence of ghrelin, adiponectin, and leptin on bone mineral density in healthy postmenopausal women. J Bone Miner Metab. 2008;26:618–23. doi: 10.1007/s00774-008-0861-5. [DOI] [PubMed] [Google Scholar]
- 40.Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2:165–71. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- 41.Karsenty G, Oury F. The central regulation of bone mass, the first link between bone remodeling and energy metabolism. J Clin Endocrinol Metab. 2010;95:4795–801. doi: 10.1210/jc.2010-1030. [DOI] [PubMed] [Google Scholar]
- 42.Kawai M, Devlin MJ, Rosen CJ. Fat targets for skeletal health. Nat Rev Rheumatol. 2009;5:365–72. doi: 10.1038/nrrheum.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirkland JL, Tchkonia T, Pirtskhalava T, Han J, Karagiannides I. Adipogenesis and aging: does aging make fat go MAD? Exp Gerontol. 2002;37:757–67. doi: 10.1016/s0531-5565(02)00014-1. [DOI] [PubMed] [Google Scholar]
- 44.Kontani Y, Wang Y, Kimura K, Inokuma KI, Saito M, Suzuki-Miura T, Wang Z, Sato Y, Mori N, Yamashita H. UCP1 deficiency increases susceptibility to diet-induced obesity with age. Aging Cell. 2005;4:147–55. doi: 10.1111/j.1474-9726.2005.00157.x. [DOI] [PubMed] [Google Scholar]
- 45.Kopecky J, Clarke G, Enerback S, Spiegelman B, Kozak LP. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Invest. 1995;96:2914–23. doi: 10.1172/JCI118363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kozak LP, Koza RA, Anunciado-Koza R. Brown fat thermogenesis and body weight regulation in mice: relevance to humans. Int J Obes (Lond) 2010;34(Suppl 1):S23–7. doi: 10.1038/ijo.2010.179. [DOI] [PubMed] [Google Scholar]
- 47.Krakauer JC, McKenna MJ, Buderer NF, Rao DS, Whitehouse FW, Parfitt AM. Bone loss and bone turnover in diabetes. Diabetes. 1995;44:775–82. doi: 10.2337/diab.44.7.775. [DOI] [PubMed] [Google Scholar]
- 48.Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone (accepted) 2011 doi: 10.1016/j.bone.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kugel H, Jung C, Schulte O, Heindel W. Age- and sex-specific differences in the 1H-spectrum of vertebral bone marrow. J Magn Reson Imaging. 2001;13:263–8. doi: 10.1002/1522-2586(200102)13:2<263::aid-jmri1038>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 50.Lazarenko OP, Rzonca SO, Hogue WR, Swain FL, Suva LJ, Lecka-Czernik B. Rosiglitazone induces decreases in bone mass and strength that are reminiscent of aged bone. Endocrinology. 2007;148:2669–2680. doi: 10.1210/en.2006-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lecka-Czernik B. PPARs in bone: the role in bone cell differentiation and regulation of energy metabolism. Curr Osteoporos Rep. 2010;8:84–90. doi: 10.1007/s11914-010-0016-1. [DOI] [PubMed] [Google Scholar]
- 52.Lecka-Czernik B, Ackert-Bicknell C, Adamo ML, Marmolejos V, Churchill GA, Shockley KR, Reid IR, Grey A, Rosen CJ. Activation of peroxisome proliferator-activated receptor gamma (PPARgamma) by rosiglitazone suppresses components of the insulin-like growth factor regulatory system in vitro and in vivo. Endocrinology. 2007;148:903–11. doi: 10.1210/en.2006-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lecka-Czernik B, Gubrij I, Moerman EA, Kajkenova O, Lipschitz DA, Manolagas SC, Jilka RL. Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPAR-gamma 2. J Cell Biochem. 1999;74:357–371. [PubMed] [Google Scholar]
- 54.Lecka-Czernik B, Suva LJ. Resolving the Two “Bony”Faces of PPAR-gamma. PPAR Res. 2006;2006:27489. doi: 10.1155/PPAR/2006/27489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–69. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lenchik L, Register TC, Hsu FC, Lohman K, Nicklas BJ, Freedman BI, Langefeld CD, Carr JJ, Bowden DW. Adiponectin as a novel determinant of bone mineral density and visceral fat. Bone. 2003;33:646–51. doi: 10.1016/s8756-3282(03)00237-0. [DOI] [PubMed] [Google Scholar]
- 57.Lian JB, Stein GS, Javed A, van Wijnen AJ, Stein JL, Montecino M, Hassan MQ, Gaur T, Lengner CJ, Young DW. Networks and hubs for the transcriptional control of osteoblastogenesis. Rev Endocr Metab Disord. 2006;7:1–16. doi: 10.1007/s11154-006-9001-5. [DOI] [PubMed] [Google Scholar]
- 58.Liney GP, Bernard CP, Manton DJ, Turnbull LW, Langton CM. Age, gender, and skeletal variation in bone marrow composition: a preliminary study at 3.0 Tesla. J Magn Reson Imaging. 2007;26:787–93. doi: 10.1002/jmri.21072. [DOI] [PubMed] [Google Scholar]
- 59.Ma Y, Nyman JS, Tao H, Moss HH, Yang X, Elefteriou F. beta2-Adrenergic receptor signaling in osteoblasts contributes to the catabolic effect of glucocorticoids on bone. Endocrinology. 2011;152:1412–22. doi: 10.1210/en.2010-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Melton LJ, 3rd, Riggs BL, Leibson CL, Achenbach SJ, Camp JJ, Bouxsein ML, Atkinson EJ, Robb RA, Khosla S. A bone structural basis for fracture risk in diabetes. J Clin Endocrinol Metab. 2008;93:4804–9. doi: 10.1210/jc.2008-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res. 1971;80:147–54. doi: 10.1097/00003086-197110000-00021. [DOI] [PubMed] [Google Scholar]
- 62.Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004;3:379–89. doi: 10.1111/j.1474-9728.2004.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moore SG, Dawson KL. Red and yellow marrow in the femur: age-related changes in appearance at MR imaging. Radiology. 1990;175:219–23. doi: 10.1148/radiology.175.1.2315484. [DOI] [PubMed] [Google Scholar]
- 64.Moyer-Mileur LJ, Slater H, Jordan KC, Murray MA. IGF-1 and IGF-binding proteins and bone mass, geometry, and strength: relation to metabolic control in adolescent girls with type 1 diabetes. J Bone Miner Res. 2008;23:1884–91. doi: 10.1359/jbmr.080713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muruganandan S, Roman AA, Sinal CJ. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program. Cell Mol Life Sci. 2009;66:236–53. doi: 10.1007/s00018-008-8429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–63. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Olmsted-Davis E, Gannon FH, Ozen M, Ittmann MM, Gugala Z, Hipp JA, Moran KM, Fouletier-Dilling CM, Schumara-Martin S, Lindsey RW, Heggeness MH, Brenner MK, Davis AR. Hypoxic adipocytes pattern early heterotopic bone formation. Am J Pathol. 2007;170:620–32. doi: 10.2353/ajpath.2007.060692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, Nagasawa T. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33:387–99. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 69.Oshima K, Nampei A, Matsuda M, Iwaki M, Fukuhara A, Hashimoto J, Yoshikawa H, Shimomura I. Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem Biophys Res Commun. 2005;331:520–6. doi: 10.1016/j.bbrc.2005.03.210. [DOI] [PubMed] [Google Scholar]
- 70.Rosen CJ, Bouxsein ML. Mechanism of disease: is osteoporosis the obesity of bone? Nature Clinical Practice Rheumatology. 2006;2:35–43. doi: 10.1038/ncprheum0070. [DOI] [PubMed] [Google Scholar]
- 71.Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev. 2002;16:22–6. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sage AP, Lu J, Atti E, Tetradis S, Ascenzi MG, Adams DJ, Demer LL, Tintut Y. Hyperlipidemia induces resistance to PTH bone anabolism in mice via oxidized lipids. J Bone Miner Res. 2010 doi: 10.1002/jbmr.312. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB. MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos Int. 2007;18:641–7. doi: 10.1007/s00198-006-0285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shinoda Y, Yamaguchi M, Ogata N, Akune T, Kubota N, Yamauchi T, Terauchi Y, Kadowaki T, Takeuchi Y, Fukumoto S, Ikeda T, Hoshi K, Chung UI, Nakamura K, Kawaguchi H. Regulation of bone formation by adiponectin through autocrine/paracrine and endocrine pathways. J Cell Biochem. 2006;99:196–208. doi: 10.1002/jcb.20890. [DOI] [PubMed] [Google Scholar]
- 75.Shockley KR, Lazarenko OP, Czernik PJ, Rosen CJ, Churchill GA, Lecka-Czernik B. PPARg2 nuclear receptor controls multiple regulatory pathways of osteoblast differentiation from marrow mesenchymal stem cells. J Cell Biochem. 2009;106:232–246. doi: 10.1002/jcb.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shockley KR, Rosen CJ, Churchill GA, Lecka-Czernik B. PPARγ2 regulates a molecular signature of marrow mesenchymal stem cells. PPAR Research. 2007;2007:81219. doi: 10.1155/2007/81219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soumano K, Desbiens S, Rabelo R, Bakopanos E, Camirand A, Silva JE. Glucocorticoids inhibit the transcriptional response of the uncoupling protein-1 gene to adrenergic stimulation in a brown adipose cell line. Mol Cell Endocrinol. 2000;165:7–15. doi: 10.1016/s0303-7207(00)00276-8. [DOI] [PubMed] [Google Scholar]
- 78.Steiner RM, Mitchell DG, Rao VM, Schweitzer ME. Magnetic resonance imaging of diffuse bone marrow disease. Radiol Clin North Am. 1993;31:383–409. [PubMed] [Google Scholar]
- 79.Tavassoli M. Fatty involution of marrow and the role of adipose tissue in hematopoiesis. In: TM, editor. Handbook of the Hematopoietic Microenvironment. Clifton, NJ, U.S.A: Humana Press; 1989. pp. 157–187. [Google Scholar]
- 80.Thrailkill KM, Jo CH, Cockrell GE, Moreau CS, Fowlkes JL. Enhanced excretion of vitamin D binding protein in type 1 diabetes: a role in vitamin D deficiency? J Clin Endocrinol Metab. 2011;96:142–9. doi: 10.1210/jc.2010-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 82.Uemura T, Ohta Y, Nakao Y, Manaka T, Nakamura H, Takaoka K. Epinephrine accelerates osteoblastic differentiation by enhancing bone morphogenetic protein signaling through a cAMP/protein kinase A signaling pathway. Bone. 2010;47:756–65. doi: 10.1016/j.bone.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 83.Vernochet C, Peres SB, Davis KE, McDonald ME, Qiang L, Wang H, Scherer PE, Farmer SR. C/EBPalpha and the corepressors CtBP1 and CtBP2 regulate repression of select visceral white adipose genes during induction of the brown phenotype in white adipocytes by peroxisome proliferator-activated receptor gamma agonists. Mol Cell Biol. 2009;29:4714–28. doi: 10.1128/MCB.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weinstein RS, Wan C, Liu Q, Wang Y, Almeida M, O’Brien CA, Thostenson J, Roberson PK, Boskey AL, Clemens TL, Manolagas SC. Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in aged mice. Aging Cell. 2010;9:147–61. doi: 10.1111/j.1474-9726.2009.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Williams GA, Callon KE, Watson M, Costa JL, Ding Y, Dickinson M, Wang Y, Naot D, Reid IR, Cornish J. Skeletal phenotype of the leptin receptor-deficient db/db mouse. J Bone Miner Res. 2011 doi: 10.1002/jbmr.367. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 86.Williams GA, Wang Y, Callon KE, Watson M, Lin JM, Lam JB, Costa JL, Orpe A, Broom N, Naot D, Reid IR, Cornish J. In vitro and in vivo effects of adiponectin on bone. Endocrinology. 2009;150:3603–10. doi: 10.1210/en.2008-1639. [DOI] [PubMed] [Google Scholar]
- 87.Wren TA, Chung SA, Dorey FJ, Bluml S, Adams GB, Gilsanz V. Bone Marrow Fat Is Inversely Related to Cortical Bone in Young and Old Subjects. J Clin Endocrinol Metab. 2011 doi: 10.1210/jc.2010-1922. [DOI] [PubMed] [Google Scholar]
- 88.Yang S, Nguyen ND, Center JR, Eisman JA, Nguyen TV. Association between beta-blocker use and fracture risk: the Dubbo Osteoporosis Epidemiology Study. Bone. 2011;48:451–5. doi: 10.1016/j.bone.2010.10.170. [DOI] [PubMed] [Google Scholar]
- 89.Yeung DK, Griffith JF, Antonio GE, Lee FK, Woo J, Leung PC. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: a proton MR spectroscopy study. J Magn Reson Imaging. 2005;22:279–85. doi: 10.1002/jmri.20367. [DOI] [PubMed] [Google Scholar]
- 90.Yokota T, Meka CS, Medina KL, Igarashi H, Comp PC, Takahashi M, Nishida M, Oritani K, Miyagawa J, Funahashi T, Tomiyama Y, Matsuzawa Y, Kincade PW. Paracrine regulation of fat cell formation in bone marrow cultures via adiponectin and prostaglandins. J Clin Invest. 2002;109:1303–10. doi: 10.1172/JCI14506. [DOI] [PMC free article] [PubMed] [Google Scholar]


