Abstract
COL-3 is a chemically modified tetracycline that targets multiple aspects of matrix metalloproteinase regulation. This phase I clinical trial was conducted to determine the maximum tolerated dose (MTD) of COL-3 in adults with recurrent high-grade glioma, to describe the effects of enzyme-inducing antiseizure drugs (EIADs) on its pharmacokinetics, and to obtain preliminary evidence of activity. Adults with recurrent high-grade glioma were stratified by EIAD use. COL-3 was given orally daily without interruption until disease progression or treatment-related dose-limiting toxicity (DLT). Three patients in each EIAD group were evaluated at each dose level beginning with 25 mg/m2/day and escalated by 25 mg/m2/day. Toxicity, response, and pharmacokinetics were assessed. Thirty-three patients were evaluated. The MTD was 75 mg/m2/day in the −EIAD patients while one was not determined in +EIAD patients. The common toxicities observed were anemia, ataxia, diarrhea, hypokalemia, CNS hemorrhage, and myalgia. One partial response was observed. −EIAD patients tended to have a higher steady-state trough concentration that was apparent only at the 100 mg/m2/day dose level (P = 0.01). This study suggests that: (a) EIAD use does affect the pharmacokinetics of COL-3 at higher doses; and (b) there was not enough suggestion of single-agent activity to warrant further study in recurrent high-grade gliomas.
Keywords: COL-3, Anticonvulsants, Pharmacokinetics, Gliomas
Introduction
Standard therapy for patients with high-grade gliomas is limited to four agents (carmustine, lomustine, procarbazine, and temozolomide) with limited response rates of 15–40% [1, 2]. A survival advantage has only been demonstrated with temozolomide with only 10% of patients being alive at 5 years. Therefore, other therapeutic modalities are sought for the treatment of recurrent high-grade glioma.
Matrix metalloproteinases (MMPs) are a family of approximately 30 zinc-dependent enzymes that play an important role in tumor invasion, metastasis, tumor-induced angiogenesis, and vascular invasion. MMP-2 and MMP-9 are upregulated in recurrent high-grade gliomas and MMP-2 mRNA expression correlated with higher grade gliomas [3–8]. In vitro, the synthetic MMP inhibitors batimastat, marimastat, and MMI-166 exhibited cytostatic effects with reduced glioma invasion in in vitro invasion assays [9, 10].
COL-3, 6-demethyl-6-deoxy-4-dedimethylaminotetracycline, is the first non-antimicrobial chemically modified tetracycline to be assessed for anticancer effects in humans [11, 12]. COL-3 targets multiple aspects of MMP regulation including direct MMP inhibition, downregulation of the production of the proenzyme, inhibition of the oxidative activation of the proenzyme, and an increase in the degradation of the proenzyme. A phase I clinical trial of continuous oral daily dosing of COL-3 in patients with refractory solid tumors demonstrated that this agent is safe, cutaneous phototoxicity was dose-limiting, and that doses of 70 mg/m2/day with the use of prophylactic sunblock and 36 mg/m2/day without the use of sunblock are well tolerated [13]. Disease stabilization for over 6 months was the best response observed in 3 patients with hemangioendothelioma, Sertoli-Leydig cell tumor, and fibrosarcoma. A second phase I trial of continuous oral daily dosing of COL-3 in patients with solid tumors also revealed phototoxicity was dose-limiting with a recommended dose of 50 mg/m2/day with the use of prophylactic sunblock [14]. Another phase I trial in 18 patients with AIDS-related Kaposi’s sarcoma was also completed and showed that this agent was well tolerated with a 44% response rate [15]. A phase II study was conducted in AIDS-related Kaposi’s sarcoma at a fixed dose of 50 and 100 mg/day with a 33% response rate including one complete and eleven partial responses. The results of a phase II study conducted in soft tissue sarcoma were negative with no patients responding to a dose of 50 mg/m2/day [16].
This phase I clinical trial was conducted to determine the maximum tolerated dose (MTD) of COL-3 in adults with recurrent high-grade glioma, to describe the effects of enzyme-inducing antiseizure drugs (EIADs) on its pharmacokinetics, and to obtain preliminary evidence of activity.
Patients and methods
Patient selection
Adult patients (age ≥18 years) with histologically proven recurrent high-grade gliomas (anaplastic astrocytoma (AA), anaplastic oligodendroglioma (AO), glioblastoma multiforme (GBM)) that were progressive or recurrent after radiation therapy were eligible for the study. Patients were required to meet the following eligibility criteria: progressive measurable disease on CT or MRI scans; Karnofsky performance status (KPS) of ≥60%, life expectancy of at least 3 months, and sufficient time for toxicities of previous therapies to have resolved (>3 months since radiation, >6 weeks since last nitrosourea, or >4 weeks since other chemotherapy). Eligibility also required demonstrating acceptable hematologic parameters (absolute neutrophil count 1,500/µl and platelet count 100,000/µl), renal function (serum creatinine <1.5 mg/dl or creatinine clearance of >60 ml/min), and hepatic function (normal total bilirubin and serum levels of aspartate and alanine aminotransferase ≤2.5X the upper limit of normal). Exclusion criteria included: >2 prior chemotherapy regimens, a prior malignancy other than curatively treated basal or squamous cell carcinoma of the skin or carcinoma in situ of the cervix or breast, malabsorption, an active infectious process (including HIV), major cardiovascular events within 3 months, females who were pregnant or nursing, or known allergies to tetracycline or its derivatives. The protocol for this study was reviewed and approved by the Cancer Therapy and Evaluation Program of the National Cancer Institute (Bethesda, MD, USA) and the institutional review board of each participating institution. All patients signed a written informed consent document, satisfying all federal and institutional policies and regulations, as a condition of registering for participation in the study.
Drug administration and dose escalation
Patients were assigned to one of two treatment groups based on preexisting use of antiseizure drugs. Patients taking carbamazepine, phenobarbital, phenytoin, primidone, or oxcarbazepine were assigned to the +EIAD group. Patients assigned to the −EIAD group were either not being treated with an antiseizure drug or taking one that does not significantly induce hepatic enzymes, such as felbamate, gabapentin, lamotrigine, levetiracetam, tiagabine, topiramate, valproic acid, and zonisamide. Inclusion in the −EIAD group also required discontinuation of any EIADs for at least 10 days. Dexamethasone was administered as clinically indicated to control peri-tumoral brain edema. Efforts were made to maintain the same dose until the radiographic tumor measurement was performed after completing the second cycle.
COL-3 (CMT-3; Metastat) was provided by CollaGenex Pharmaceuticals (Newtown, PA) and was formulated as 10 and 50-mg hard gelatin capsules. COL-3 was administered orally once daily on an uninterrupted schedule. One cycle of treatment was considered to be 28 days. Patients were instructed to fast for 1 h before and 2 h after each dose, and were not routinely premedicated. Patients were required to wear sunscreen with a sun protection factor (SPF) 30, to minimize sun exposure, and to wear protective clothing.
The initial dose level for both groups was 25 mg/m2 administered orally once daily on an uninterrupted schedule. Subsequent dose escalation increments were by 25 mg/m2, up to a maximum of 100 mg/m2. Doses were rounded down to the nearest 10-mg. Cohorts of three patients were accrued to each dose level and group.
All patients who received any drug were assessable for toxicity. All patients who received 21 days of therapy were assessable for disease response. Toxicity was graded by the National Cancer Institute’s Common Toxicity Criteria, version 2.0. The MTD was defined as one dose level below that dose level at which at least two of six patients experienced a DLT. Patients continued on the same dose of COL-3 until disease progression or toxicity required the drug to be discontinued as long as all eligibility requirements continued to be satisfied. Retreatment in the event of a DLT during any course was permitted with a 25% dose reduction after all toxicities recovered to baseline values or less than grade 2. A maximum of three dose reductions was permitted before the patient was removed from the study.
Patient evaluations
Evaluations performed within 14 days of initiating therapy included a medical history, physical and neurologic examinations, Mini-Mental Status Exam, Karnofsky Performance Status (KPS) determination, tumor measurements using MRI/CT scans with volumetric analysis, electrocardiogram, chest X-ray, vital signs, complete blood count with differential and platelet counts, blood coagulation parameters, serum chemistry profile, antinuclear antibody (ANA) titer, urinalysis, and pregnancy test for women of childbearing potential. After initiating treatment, a complete blood count with differentials and platelet count were performed weekly, or more often if significant myelosuppression was observed. A history and physical examination, KPS determination, complete blood count with differentials and platelet count, serum electrolyte profile and ANA titer were performed before beginning each cycle of therapy due to previously reported drug-induced lupus associated with COL-3 [13, 17]. In addition to these tests, a Mini-Mental Status Exam and serum chemistry profile were repeated before every odd-numbered cycle of therapy. Toxicities were evaluated during each monthly cycle and graded according to the National Cancer Institute’s Common Toxicity Criteria, version 2.0. DLT was defined as any of the following treatment-related adverse events: (1) grade 4 hematologic toxicity of any duration except anemia, (2) any grade 3 or 4 nonhematologic toxicity, and (3) a delay in starting a subsequent course of treatment for more than 7 days because of incomplete recovery from toxicity. Photosensitivity of grade 3 or less was not considered dose limiting, but all phototoxic reactions of greater than or equal to a grade 2 were reported to CTEP due to the high incidence of phototoxicity previously observed with COL-3 [13, 14].
Response to therapy was determined by MRI or CT imaging and neurologic examinations. The use of CT was restricted to patients who were unable to undergo MRI for physical or medical reasons. Imaging studies to provide tumor measurements were performed within 14 days of beginning treatment (baseline) and repeated before every odd-numbered cycle of therapy. A confirmatory scan was obtained 1 month after the initial detection of a complete or partial response. Standard response criteria of the New Approaches to Brain Tumor Therapy CNS Consortium (NABTT) were used as described previously [18]. The pathology and scans for all patients responding to therapy were to be centrally reviewed.
Pharmacokinetics
Sampling to determine the pre-treatment plasma concentrations (Cmin) of COL-3 during the continuous oral administration was performed for the duration a patient was on-study. Blood samples were collected in heparin-containing tubes before drug administration and at 24 ± 3, 48 ± 3, 72 ± 3, and 96 ± 3 h, then weekly through cycle 2 on day 1 (cycle 1, week 2, 3, and 4, and cycle 2 week 1, 2, 3, and 4), and monthly on subsequent cycles on week 1 day 1 until the patient was removed from study. Blood samples were placed immediately in a water/ice bath and centrifuged at 1000 g for 10 min at 4°C. The plasma was removed and frozen at −20°C. The samples were then transferred to the Analytical Pharmacology Core laboratory at the Sidney Kimmel Comprehensive Center at Johns Hopkins and stored at −70°C until analysis. Concentrations of COL-3 in plasma were determined by a validated reversed-phase high-performance liquid chromatography assay with UV detection, as described previously [19]. COL-3 was quantitated in plasma over the concentration range of 75–10,000 ng/ml. A dilutional QC was validated at 30,000 ng/ml.
COL-3 pharmacokinetic parameters were not calculated by standard noncompartmental methods due to insufficient sampling. All of the data was initially graphed as a concentration–time curve for both individual patients and at a dosage level split into the 2 different arms of the study (i.e., −EIAD versus +EIAD). Any sample that was documented to not be a pre-treatment sample (i.e., within 3 h prior to the next dose or after a dose) was not utilized in subsequent analysis. Individual and mean concentration–time plots were constructed using only documented Cmin and the actual time of the sample. Cmin at steady state (Css,min) was calculated for each patient by taking the average of the Cmin after the cycle 1 week 2 sample when steady-state was achieved in each patient. Dose-normalized Css,min was calculated by dividing Css,min by the nominal dose expressed in mg.
A two-sided Student’s t-test was used to compare dose-normalized Css,min as a function of arm of the study (−EIAD versus +EIAD). One-way ANOVA was used to compare the differences in dose-normalized Css,min as a function of drug administration schedule (a combination of arm of the study and dose level), followed by a Tukey–Kramer’s multiple-comparison test. Statistical tests were performed using JMP Statistical Discovery software, version 4.0.4 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
Thirty-three patients were accrued to this Phase I trial from February 2001 to December 2002. Three patients did not complete the first cycle due to progressive disease. These patients were eligible for toxicity and pharmacology evaluations, but were excluded from the efficacy analyses. Patient characteristics are listed in Table 1. There were 24 patients with glioblastoma, 6 with anaplastic astrocytoma, and 3 with anaplastic oligodendroglioma. The median KPS was 70%. 75% of the 16 patients in the −EIAD arm were not taking an anticonvulsant. Phenytoin was used by 53% of the 17 patients in the +EIAD arm. A stable daily dose of dexamethasone was used by 38% of the patients in the −EIAD arm and 53% of the patients in the +EIAD arm.
Table 1.
Baseline demographic and clinical characteristics
| Characteristics | N (%) or Median (range) |
|---|---|
| Age (years) | 51 (27–73) |
| Gender | |
| Male | 18 (55) |
| Female | 15 (45) |
| KPS | |
| 60% | 2 (6) |
| 70 or 80% | 20 (61) |
| 90 or 100% | 11 (33) |
| Histological diagnosis | |
| Anaplastic astrocytoma | 6 (18) |
| Anaplastic oligodendroglioma | 2 (6) |
| Glioblastoma multiforme | 25 (76) |
| Concomitant antiseizure drugs | |
| Enzyme inducing | 17 (52) |
| Carbemazepine | 6 (35) |
| Phenobarbital | 1 (6) |
| Phenytoin | 9 (53) |
| Primidone | 1 (6) |
| None or not enzyme inducing | 16 (48) |
| Lamotrigine | 1 (6) |
| Levetiracetam | 2 (13) |
| Valproic Acid | 1 (6) |
| Glucocorticoid use | |
| +EIAD cohort | 6 (35) |
| −EIAD cohort | 9 (56) |
Dose escalation and toxicities
A total of 61 complete courses of COL-3 were administered to 33 patients enrolled in the study. The dose levels evaluated in both treatment arms and the dose limiting toxicities experienced by patients during the first cycle of therapy are listed in Table 2. Severe toxicities (grade 4 hematologic or grade 3 or greater nonhematologic) that did not occur during the first cycle are listed in Table 3. All four dose levels were evaluated in both the −EIAD and +EIAD arms. Toxicities associated with COL-3 that were not dose-limiting were anemia, ataxia, diarrhea, and photosensitivity. In the −EIAD arm, no dose limiting toxicities were observed in the first 3 cohorts (25, 50, or 75 mg/m2/day). At the 100 mg/m2/day level one patient experienced grade 3 myalgia and the cohort was expanded to 6 patients, one of who developed grade 3 fatigue. In the +EIAD arm, no dose limiting toxicities were observed in the first cohort (25 mg/m2/day). At the 50 mg/m2/day level, one patient experienced grade 5 central nervous system hemorrhage combined with grade 3 hypokalemia and the cohort was expanded to 6 patients with no further toxicities. Both events were assessed as unlikely related to COL-3 administration, dose escalation continued with no further dose limiting toxicities observed through the 100 mg/m2/day dose level. There were no delays in treatment or dose reductions due to toxicity on either arm. The maximum tolerated dose (MTD) was 75 mg/m2/day in the −EIAD arm and 100 mg/m2/day in the +EIAD arm.
Table 2.
Dose levels evaluated and summary of dose-limiting toxicities during the first cycle of therapy
| COL-3 treatment group (daily dose, mg/m2) | ||||||||
|---|---|---|---|---|---|---|---|---|
| −EIAD | +EIAD | |||||||
| 25 | 50 | 75 | 100 | 25 | 50 | 75 | 100 | |
| No. of patients evaluateda | 3 | 3 | 3 | 8 | 3 | 6 | 4 | 4 |
| Dose-limiting toxicity | 0 | 0 | 0 | 2 | 0 | 1b | 0 | 0 |
| Hematologic toxicity (grade 4) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nonhematologic toxicity (grade 3/4/5) | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 |
| Ataxia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CNS hemorrhage | 0 | 0 | 0 | 0 | 0 | 1b | 0 | 0 |
| Diarrhea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fatigue | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Hypokalemia | 0 | 0 | 0 | 0 | 0 | 1b | 0 | 0 |
| Myalgia | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Photosensitivity | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
−EIAD patients not taking enzyme-inducing antiseizure drugs; +EIAD patients taking enzyme-inducing antiseizure drugs
Total number of patients enrolled at each dose level
These two events occurred in the same patient, and were unlikely to be related to study drug but were considered dose-limiting and counted as one toxicity
Table 3.
Dose levels evaluated and summary of severe clinical toxicities during all cycles of therapy
| COL-3 treatment group (daily dose, mg/m2) | ||||||||
|---|---|---|---|---|---|---|---|---|
| −EIAD | +EIAD | |||||||
| 25 | 50 | 75 | 100 | 25 | 50 | 75 | 100 | |
| No. of patients evaluateda | 3 | 3 | 3 | 8 | 3 | 6 | 4 | 4 |
| Hematologic toxicity (grade 4) | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Anemia | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Nonhematologic toxicity (grade 3/4) | 0 | 0 | 1 | 6 | 0 | 1 | 0 | 0 |
| Ataxia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CNS hemorrhage | 0 | 0 | 0 | 0 | 0 | 1b | 0 | 0 |
| Diarrhea | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Fatigue | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Hypokalemia | 0 | 0 | 0 | 1 | 0 | 1b | 0 | 0 |
| Myalgia | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Photosensitivity | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 |
−EIAD, patients not taking enzyme-inducing antiseizure drugs; +EIAD, patients taking enzyme-inducing antiseizure drugs
Total number of patients enrolled at each dose level
These two events occurred in the same patient, and were unlikely to be related to study drug but were considered dose-limiting and counted as one toxicity
Responses
Of the 33 patients enrolled, 27 patients were eligible for tumor response. No complete responses were observed. One partial response was observed at 75 mg/m2/day on the +EIAD arm for an overall response rate of 4% (1/27). The partial response was observed in a patient with anaplastic oligodendroglioma, who had failed one prior chemotherapy regimen and survived 302 days after initiating treatment with COL-3 at 75 mg/m2/day in the +EIAD arm. In addition, on this non-cytotoxic agent, three patients with glioblastoma achieved stable disease and survived from 216 to 244 days. One of the 3 patients failed one prior regimen and 2 had failed 2 prior regimens. Two of these patients were treated at the 100 mg/m2 level and one at the 50 mg/m2 level. All three of these patients were in the +EIAD arm of the study. Twenty-six of the 27 (96%) patients enrolled have died. With the exception of the patients listed above, 23 patients received one to three courses before developing progressive disease.
Pharmacokinetics
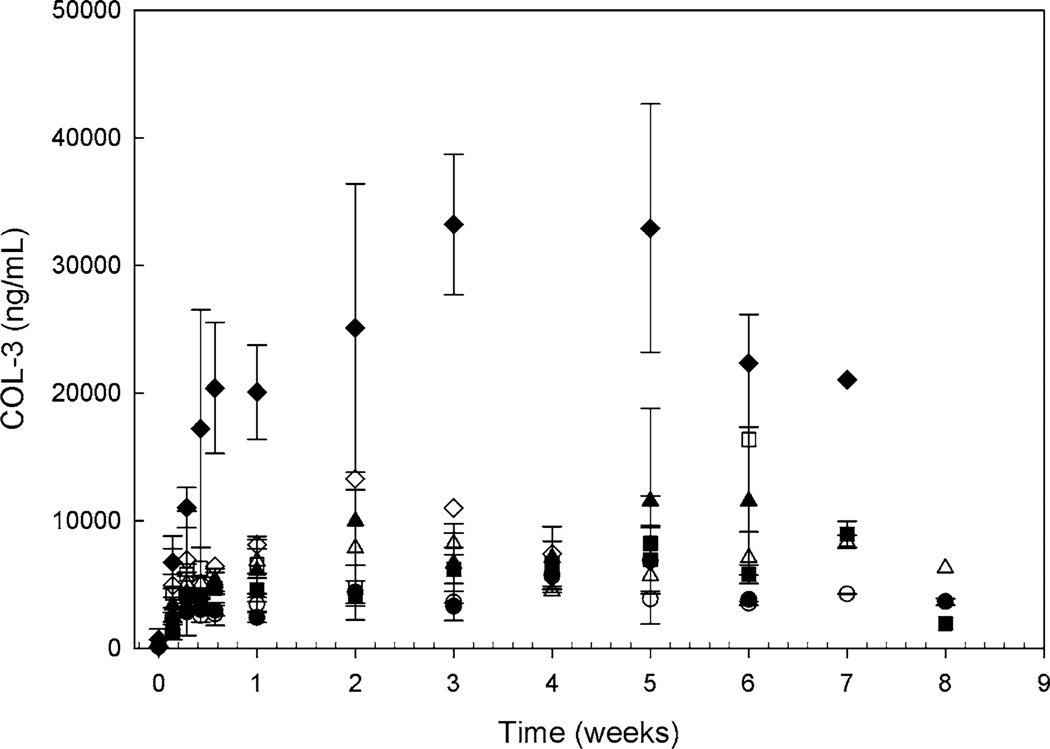
Plasma pharmacokinetic studies were completed in 30 of the 33 patients enrolled on the trial and trough values were obtained 66% of the time. Subsequent analysis was performed utilizing only the trough values. Mean plasma concentration–time profiles are presented in Fig. 1. It appears that steady-state is achieved after 1 week of continuous daily dosing of COL-3 which would be consistent with a compound with a documented average half-life of 39.3–56.7 h [13, 15]. There was a trend for the −EIAD patients to have higher COL-3 dose-normalized Css,min (P = 0.07). However, there was substantial intra-patient variability in Css,min. There is a statistically significant increase in Css,min at the 100 mg/m2 dosage level on the −EIAD arm (P = 0.01).
Fig. 1.
Mean COL-3 plasma concentration–time profile following administration of COL-3 25 mg/m2 (circle), 50 mg/m2 (square), 75 mg/m2 (triangle), and 100 mg/m2 (diamond) on the −EIAD or +EIAD arm plotted on a log-linear scale. The closed symbols are −EIAD, the open symbols are +EIAD
Discussion
The study reported here was designed to determine the MTD of COL-3 in adults with recurrent high-grade glioma, to describe the effects of concurrent EIAD administration on its pharmacokinetics, and to obtain preliminary evidence of activity. All three goals were met.
COL-3 administered once a day orally was relatively well-tolerated in patients with recurrent high-grade glioma. The most commonly reported adverse effect was phototoxicity. Fatigue and myalgia were dose limiting in the −EIAD arm, while dose-limiting toxicities were not observed in the +EIAD arm. The MTD in the −EIAD arm is 75 mg/m2, which is consistent with previous Phase I studies with COL-3 [13, 14]. A true MTD was not defined in the +EIAD arm due to stopping at the pre-determined maximum dose of 100 mg/m2.
The difference in the MTD between both arms can be potentially explained by the differences noted in the pharmacology. Following administration of COL-3 on an oral once daily regimen, there appears to be higher COL-3 concentrations on the −EIAD arm of the study with the difference only being significant at the 100 mg/m2 dose level. This suggests that there is an induction of COL-3 clearance with the +EIAD. Since COL-3 is not metabolized by cytochrome P450 pathways, the induction must occur via glucuronidation [20–22]. Phenytoin, phenobarbital, and carbamazepine have also been documented to induce glucuronidation via UGT enzymes [23, 24]. Therefore, the induction in clearance may be due to the induction of UGTs that are noted both in vivo and in vitro to be a documented pathway of COL-3 metabolism [20–22].
One of the objectives was to assess response to COL-3 when administered alone. Only one patient at 75 mg/m2/day in the +EIAD arm experienced a partial response for an overall response rate of 4%, which was below the maximum dose explored in the +EIAD arm (100 mg/m2/day). This does not warrant further exploration of COL-3 as monotherapy in patients with recurrent high-grade gliomas. There have been two trials with another MMP inhibitor, marimastat, in combination with temozolomide for both anaplastic gliomas and recurrent GBM [25, 26]. In the GBM trial, the combination was recommended for further testing since the progression-free survival was 39% at 6 months with a median overall survival of 45 weeks [26]. While in the anaplastic gliomas trial, the combination was deemed too toxic with minimal benefit compared to temozolomide alone [25]. The main toxicity observed in both trials was the joint and tendon pain frequently seen with the other MMP inhibitors. Based on the results of this exploratory trial in recurrent GBM and the results from the marimastat trials, further studies of COL-3 were not pursued. This study suggests that: (a) EIAD use does affect the pharmacokinetics of COL-3 at higher doses; and (b) as a single agent, COL-3 has little activity in recurrent high-grade gliomas.
Acknowledgments
This study is conducted for the New Approaches to Brain Tumor Therapy CNS Consortium
National Cancer Institute; National Institutes of Health, Department of Health and Human Services, Bethesda, Maryland [U01-CA62475 to S.G; P30 CA006973].
Footnotes
Conflict of interest statement None.
Contributor Information
Michelle A. Rudek, Department of Oncology, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD 21231, USA
Pamela New, Department of Neurosurgery, Baylor College of Medicine, Houston, TX 77030, USA.
Tom Mikkelsen, Department of Neurosurgery, Henry Ford Hospital, Detroit, MI 48202, USA.
Surasak Phuphanich, Department of Hematology-Oncology, Winship Cancer Institute, Emory University, Atlanta, GA 30322, USA.
Jane B. Alavi, Department of Hematology/Oncology, Hospital of the University of Pennsylvania, Philadelphia, PA, USA
Louis B. Nabors, Department of Neurology, University of Alabama, Birmingham, AL 35294, USA
Steven Piantadosi, Department of Oncology, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD 21231, USA.
Joy D. Fisher, Email: jfisher@jhmi.edu, Department of Oncology, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD 21231, USA.
Stuart A. Grossman, Department of Oncology, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD 21231, USA
References
- 1.Grossman SA, Levin V, Sawaya R, Maor M, Loeffler J. National comprehensive cancer network adult brain tumor practice guidelines. Oncology. 1997;11:237–277. [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Costello PC, Del Maestro RF, Stetler-Stevenson WG. Gelatinase A expression in human malignant gliomas. Ann N Y Acad Sci. 1994;732:450–452. doi: 10.1111/j.1749-6632.1994.tb24782.x. [DOI] [PubMed] [Google Scholar]
- 4.Friedberg MH, Glantz MJ, Klempner MS, Cole BF, Perides G. Specific matrix metalloproteinase profiles in the cerebrospinal fluid correlated with the presence of malignant astrocytomas, brain metastases, and carcinomatous meningitis. Cancer. 1998;82:923–930. doi: 10.1002/(sici)1097-0142(19980301)82:5<923::aid-cncr18>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Kachra Z, Beaulieu E, Delbecchi L, Mousseau N, Berthelet F, Moumdjian R, Del Maestro R, Beliveau R. Expression of matrix metalloproteinases and their inhibitors in human brain tumors. Clin Exp Metastasis. 1999;17:555–566. doi: 10.1023/a:1006760632766. [DOI] [PubMed] [Google Scholar]
- 6.Sawaya RE, Yamamoto M, Gokaslan ZL, Wang SW, Mohanam S, Fuller GN, McCutcheon IE, Stetler-Stevenson WG, Nicolson GL, Rao JS. Expression and localization of 72 kDa type IV collagenase (MMP-2) in human malignant gliomas in vivo. Clin Exp Metastasis. 1996;14:35–42. doi: 10.1007/BF00157684. [DOI] [PubMed] [Google Scholar]
- 7.Wang M, Wang T, Liu S, Yoshida D, Teramoto A. The expression of matrix metalloproteinase-2 and -9 in human gliomas of different pathological grades. Brain Tumor Pathol. 2003;20:65–72. doi: 10.1007/BF02483449. [DOI] [PubMed] [Google Scholar]
- 8.Lu KV, Jong KA, Rajasekaran AK, Cloughesy TF, Mischel PS. Upregulation of tissue inhibitor of metalloproteinases (TIMP)-2 promotes matrix metalloproteinase (MMP)-2 activation and cell invasion in a human glioblastoma cell line. Lab Invest. 2004;84:8–20. doi: 10.1038/sj.labinvest.3700003. [DOI] [PubMed] [Google Scholar]
- 9.Tonn JC, Kerkau S, Hanke A, Bouterfa H, Mueller JG, Wagner S, Vince GH, Roosen K. Effect of synthetic matrix-metalloproteinase inhibitors on invasive capacity and proliferation of human malignant gliomas in vitro. Int J Cancer. 1999;80:764–772. doi: 10.1002/(sici)1097-0215(19990301)80:5<764::aid-ijc22>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 10.Nakabayashi H, Yawata T, Shimizu K. Anti-invasive and antiangiogenic effects of MMI-166 on malignant glioma cells. BMC Cancer. 2010;10:339. doi: 10.1186/1471-2407-10-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golub LM, Ramamurthy NS, McNamara TF, Greenwald RA, Rifkin BR. Tetracyclines inhibit connective tissue breakdown: new therapeutic implications for an old family of drugs. Crit Rev Oral Biol Med. 1991;2:297–321. doi: 10.1177/10454411910020030201. [DOI] [PubMed] [Google Scholar]
- 12.Hidalgo M, Eckhardt SG. Development of matrix metalloproteinase inhibitors in cancer therapy. J Natl Cancer Inst. 2001;93:178–193. doi: 10.1093/jnci/93.3.178. [DOI] [PubMed] [Google Scholar]
- 13.Rudek MA, Figg WD, Dyer V, Dahut W, Turner ML, Steinberg SM, Liewehr DJ, Kohler DR, Pluda JM, Reed E. Phase I clinical trial of oral COL-3, a matrix metalloproteinase inhibitor, in patients with refractory metastatic cancer. J Clin Oncol. 2001;19:584–592. doi: 10.1200/JCO.2001.19.2.584. [DOI] [PubMed] [Google Scholar]
- 14.Syed S, Takimoto C, Hidalgo M, Rizzo J, Kuhn JG, Hammond LA, Schwartz G, Tolcher A, Patnaik A, Eckhardt SG, Rowinsky EK. A phase I and pharmacokinetic study of Col-3 (Metastat), an oral tetracycline derivative with potent matrix metalloproteinase and antitumor properties. Clin Cancer Res. 2004;10:6512–6521. doi: 10.1158/1078-0432.CCR-04-0804. [DOI] [PubMed] [Google Scholar]
- 15.Cianfrocca M, Cooley TP, Lee JY, Rudek MA, Scadden DT, Ratner L, Pluda JM, Figg WD, Krown SE, Dezube BJ. Matrix metalloproteinase inhibitor COL-3 in the treatment of AIDS-related Kaposi’s sarcoma: a phase I AIDS malignancy consortium study. J Clin Oncol. 2002;20:153–159. doi: 10.1200/JCO.2002.20.1.153. [DOI] [PubMed] [Google Scholar]
- 16.Chu QS, Forouzesh B, Syed S, Mita M, Schwartz G, Cooper J, Curtright J, Rowinsky EK. A phase II and pharmacological study of the matrix metalloproteinase inhibitor (MMPI) COL-3 in patients with advanced soft tissue sarcomas. Invest New Drugs. 2007;25:359–367. doi: 10.1007/s10637-006-9031-6. [DOI] [PubMed] [Google Scholar]
- 17.Ghate JV, Turner ML, Rudek MA, Figg WD, Dahut W, Dyer V, Pluda JM, Reed E. Drug-induced lupus associated with COL-3: report of 3 cases. Arch Dermatol. 2001;137:471–474. [PubMed] [Google Scholar]
- 18.Gilbert MR, Supko JG, Batchelor T, Lesser G, Fisher JD, Piantadosi S, Grossman S. Phase I clinical and pharmacokinetic study of irinotecan in adults with recurrent malignant glioma. Clin Cancer Res. 2003;9:2940–2949. [PubMed] [Google Scholar]
- 19.Rudek MA, Hartke C, Zabelina Y, Zhao M, New P, Baker SD. A sensitive method for determination of COL-3, a chemically modified tetracycline, in human plasma using high-performance liquid chromatography and ultraviolet detection. J Pharm Biomed Anal. 2005;37:751–756. doi: 10.1016/j.jpba.2004.10.052. [DOI] [PubMed] [Google Scholar]
- 20.Rudek MA, Venitz J, Ando Y, Reed E, Pluda JM, Figg WD. Factors involved in the pharmacokinetics of COL-3, a matrix metalloproteinase inhibitor, in patients with refractory metastatic cancer: clinical and experimental studies. J Clin Pharmacol. 2003;43:1124–1135. doi: 10.1177/0091270003256675. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Zhou S, Huynh H, Chan E. Significant intestinal excretion, one source of variability in pharmacokinetics of COL-3, a chemically modified tetracycline. Pharm Res. 2005;22:397–404. doi: 10.1007/s11095-004-1877-8. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Huynh H, Chan E. Evidence for dissolution rate-limited absorption of COL-3, a Matrix metalloproteinase inhibitor, leading to the irregular absorption profile in rats after oral administration. Pharm Res. 2002;19:1655–1662. doi: 10.1023/a:1020901328583. [DOI] [PubMed] [Google Scholar]
- 23.Anderson GD. A mechanistic approach to antiepileptic drug interactions. Ann Pharmacother. 1998;32:554–563. doi: 10.1345/aph.17332. [DOI] [PubMed] [Google Scholar]
- 24.Sutherland L, Ebner T, Burchell B. The expression of UDP-glucuronosyltransferases of the UGT1 family in human liver and kidney and in response to drugs. Biochem Pharmacol. 1993;45:295–301. doi: 10.1016/0006-2952(93)90064-4. [DOI] [PubMed] [Google Scholar]
- 25.Groves MD, Puduvalli VK, Conrad CA, Gilbert MR, Yung WK, Jaeckle K, Liu V, Hess KR, Aldape KD, Levin VA. Phase II trial of temozolomide plus marimastat for recurrent anaplastic gliomas: a relationship among efficacy, joint toxicity and anticonvulsant status. J Neurooncol. 2006;80:83–90. doi: 10.1007/s11060-006-9160-y. [DOI] [PubMed] [Google Scholar]
- 26.Groves MD, Puduvalli VK, Hess KR, Jaeckle KA, Peterson P, Yung WK, Levin VA. Phase II trial of temozolomide plus the matrix metalloproteinase inhibitor, marimastat, in recurrent and progressive glioblastoma multiforme. J Clin Oncol. 2002;20:1383–1388. doi: 10.1200/JCO.2002.20.5.1383. [DOI] [PubMed] [Google Scholar]