Abstract
Over the past several decades, there has been increasing interest in understanding the roles of the immune system in the development and progression of cancer. The importance of the immune system in human skin cancer has been long recognised based primarily upon the increased incidence of skin cancers in organ transplant recipients and mechanisms of ultraviolet light-mediated immunomodulation. In this review, we integrate multiple lines of evidence highlighting the roles of the immune system in skin cancer. First, we discuss the concepts of cancer immunosurveillance and immunoediting as they might relate to human skin cancers. We then describe the clinical and molecular mechanisms of skin cancer development and progression in the contexts of therapeutic immunosuppression in organ transplant recipients, viral oncogenesis, and ultraviolet light-induced immunomodulation with a primary focus on basal cell carcinoma and squamous cell carcinoma. The clinical evidence supporting expanding roles for immunotherapy is also described. Finally, we discuss recent research examining the functions of particular immune cell subsets in skin cancer and how they might contribute to both anti-tumour and pro-tumour effects. A better understanding of the biological mechanisms of cancer immunosurveillance holds the promise of enabling better therapies.
INTRODUCTION
Skin cancer is the most common class of human malignancies. The most common types are the two major non-melanoma skin cancers (NMSC) of keratinocytic origin: basal cell (BCC) and squamous cell carcinoma (SCC). In the United States alone, more than 3 million cases of BCC and SCC are estimated to occur annually1,2 with the total direct and indirect costs exceed $2 billion dollars annually3. In this review, we discuss theoretical frameworks and clinical and molecular evidence for how the immune system is involved in the pathogenesis, progression, and persistence of skin cancers, particularly NMSC.
CANCER IMMUNOSURVEILLANCE
In 1909, Paul Ehrlich first suggested that the immune system could be protective against cancer4. Around the same time, William Coley laid the foundation for cancer immunotherapy by describing patients who experienced spontaneous tumour regression after developing post-surgical infections, notably erysipelas5. Subsequently, Coley administered Serratia marrascens and Streptococcus pyogenes to elicit tumour regression; however, studies were not well-controlled and interest faded with the rise of chemotherapy and radiation6.
In the 1950s, the original immunosurveillance theory of Burnet and Thomas was bolstered by the finding that syngeneic mice rejected tumours upon secondary challenge and could be vaccinated7. This suggested that the immune system could recognise tumour-specific antigens and acquire anti-tumour immunity. The term “cancer immunoediting” as advanced by Robert Schreiber and colleagues has been used to emphasise that the immune system continuously sculpts tumours8 (Fig. 1). Immunodeficient mice lacking lymphocytes and components of interferon signaling such as IFN-γ and Stat1 are more susceptible to carcinogen-induced and spontaneous primary tumours9,10. These findings, combined with the identification of tumour antigens in experimental cancer models and in humans, provide a basis for the activation of innate and adaptive immune systems in anti-tumour responses11. To test the immunogenicity of tumours arising in immunocompetent versus immunodeficient mice, they were isolated and re-introduced into naïve wild-type mice, whereupon it was found that those tumours arising in immunodeficient mice were more immunogenic (more likely to be rejected) than those arising in immunocompetent mice9,12. Thus it was postulated that tumours are continuously “edited” by the intact immune system in three stages such that immunogenic tumour cells are progressively eliminated, effectively selecting for cells more likely to be able to evade the immune response (Fig. 1).
Figure 1. A Paradigm of Cancer Immunosurveillance.
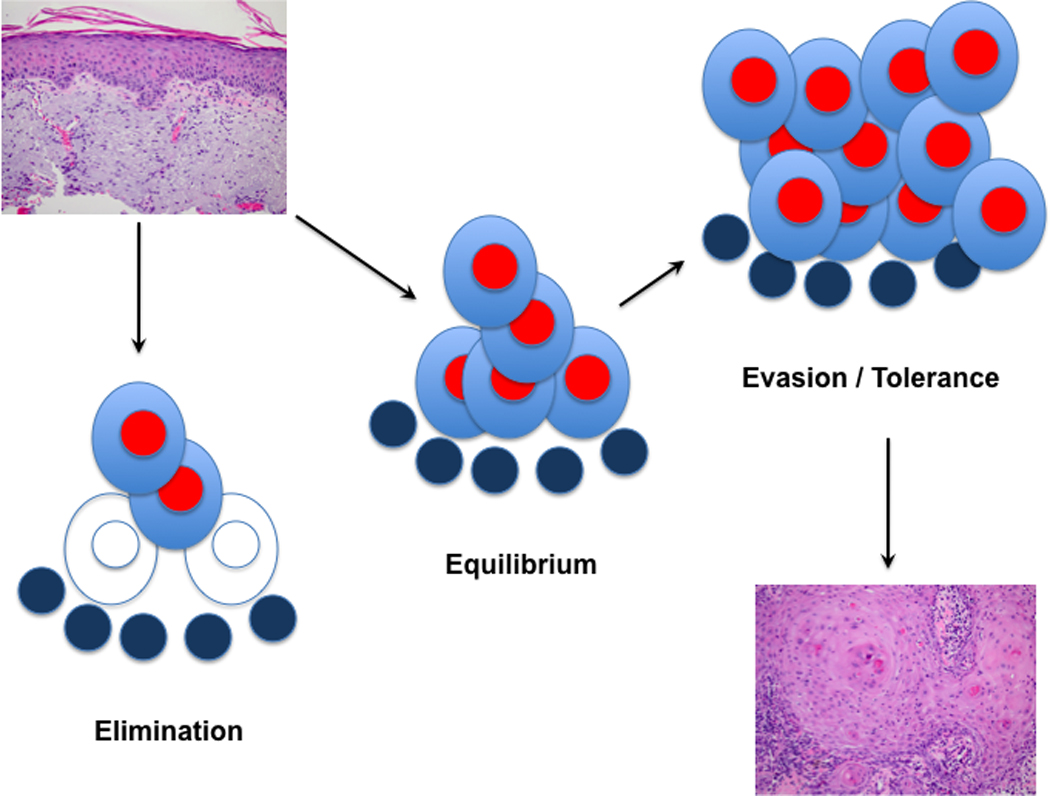
Initially, there are pre-cancerous lesions, such as the illustrated actinic keratosis (upper left), in which elimination may occur due to the killing of altered cells by elements of the immune system. Alternatively, a stage of equilibrium may result where tumour cells and immune cells interact during a period of stable tumour size. During this period, immune cells may select to more aggressive and/or less immunogenic tumour variants. Eventually, perhaps as a result of this process, the tumour expands and continues to grow despite the presence of an immune response. This continued growth can be observed in invasive squamous cell carcinoma which typically does not spontaneously regress (lower right).
In the elimination phase, innate and adaptive arms of the immune system are able to destroy cancer cells, and has yet to be definitively demonstrated. If the tumour is not completely eliminated, an equilibrium can develop in which the immune system and tumour interact to yield alterations in both cell populations. Evidence for tumour dormancy is largely indirect from reports of decades-long latencies in recurrent primary cancers or metastases in patients (discussed below), but it has also been reported in carcinogen-induced sarcomas in mice in which acute antibody-mediated depletion of immune function resulted in outgrowth of previously occult tumour cells13. In the escape phase, tumour cells become less immunogenic, adapt to evade the immune response, and/or actively immunosuppress the host8. This likely results from a combination of factors including the presence of immunosuppressive leukocyte subsets and the suppression of tumour antigen presentation through downregulation of antigen processing or MHC class I expression14.
Though definitive evidence for these stages is still emerging in humans, there are some lines of evidence suggesting that this a useful conceptual framework for cancer immunosurveillance. The composition and quantity of intratumoural immune infiltrates has prognostic value in multiple tumour types15–17. Spontaneous anti-tumour responses occur in patients sometimes resulting in paraneoplastic neurologic disorders, and there is an increased risk of multiple tumour types including skin cancer in immunosuppressed individuals. The immunoediting framework may also apply to the development of skin cancer, because of the typically long latencies and the apparent spontaneous resolution of pre-neoplastic lesions such as actinic keratoses, but these specific phases have yet to be delineated in-vivo.
CLINICAL STUDIES LINKING IMMUNOSUPPRESSION WITH SKIN CANCER
Pharmacological Immunosuppression
The impact of immunosuppressive therapy on cutaneous cancer development has been studied most comprehensively in solid organ transplant recipients (OTRs). Although immunosuppression is necessary for preventing transplant rejection, these lifelong treatments promote carcinogenesis. Skin cancer is the most common, comprising 40% of post-transplant malignancies18, 90% of which are BCC and SCC19. In kidney recipients, 15 to 40% will develop skin cancers within the first 10 years, and as many as 82% do so after 20 years20,21. The most common skin cancers among OTRs are (in decreasing order of frequency) SCC, BCC, Kaposi’s sarcoma (KS), melanoma, and Merkel cell carcinoma (MCC) Table I)22. In large series, there is an estimated 65 to 250 fold increased incidence of cutaneous SCC and a 10 fold increased incidence of BCC in renal transplant recipients (RTRs) versus immunocompetent populations, and the risk is higher in men23–26. Skin cancers are also more aggressive in OTRs27–29, with a 3-year disease specific survival of 56%30,31 and a metastasis rate of 7% compared to 0.25% in the general population32. Cutaneous cancer incidence may correspond to the degree of immunosuppression20,25,33, correlating with lower CD4 counts34, and the reduction of immunosuppression reduces the rate of initial and subsequent skin cancers33,35.
Table I.
Population-based standardised incidence ratios in cutaneous malignancies in organ transplant recipients
A modest risk of skin cancer in haematopoietic cell transplantation (HCT) recipients may be attributed not only to radiation, but also to altered immune function and immunosuppression. Aberrant cutaneous immune responses in lymphoid malignancies are probably best understood in the context of chronic lymphocytic leukaemia (CLL), the most common leukaemia, in which pseudolymphomatous reactions to otherwise innocuous stimuli such as insect bites are well described36. CLL patients have an 8-fold increased risk for NMSC37,38, are 7 to 14 times more likely to suffer recurrences from SCC and BCC, respectively, following Mohs surgery39,40, and are more likely to develop and die of metastatic SCC41. NMSCs can be densely infiltrated by neoplastic B-cells, and this is proposed to compromise anti-tumour T-cell responses42,43.
There are other contexts in which HCT patients are predisposed to skin cancers. The positive correlation between chronic graft versus host disease (GVHD) and NMSC, especially SCC44–48, may be due to GVHD prophylaxis as well as to chronic inflammation44–48. Retrospective analyses have also found an increased risk of SCC in this patient population with azathioprine therapy, especially when combined with cyclosporine and steroids49,50.
The degree of risk conferred by particular drugs and their mechanisms of action remains an active area of research. Calcineurin inhibitors (CNIs), such as cyclosporine and tacrolimus, are among the drugs most strongly linked to skin cancer development. The risk of SCC is increased with cyclosporine use in psoriasis patients exposed to UVA radiation51. In RTRs, cyclosporine promotes cutaneous carcinogenesis in a dose-dependent manner33,52. CNIs inhibit Langerhans cells,53,54 dermal dendritic cells (DC)55,56, and T-cell signaling and proliferation, and cyclosporine directly promotes tumour development57–59. Inhibition of calcineurin enhances carcinoma development in mouse models, and correlative evidence in human SCC implicates induction of ATF3 and bypass of senescence59,60. Tacrolimus is increasingly used for OTRs due to a favourable side effect profile as compared to cyclosporine in some settings.61 With respect to NMSC, studies have either shown a trend towards reduction in risk with tacrolimus as compared to cyclosporine particularly at early time points62–64, or no significant difference65.
Azathioprine is associated with increased skin cancer risk, particularly for SCC, which may partly be due to increased photosensitization and UVA-mediated mutagenesis through direct incorporation of the metabolite 6-thioguanine into DNA52,66,67. Increased numbers of p53-mutant foci were found in the skin of azathioprine-treated RTRs, and corresponded to decreased DNA repair activity in treated keratinocytes68. Inflammatory bowel disease patients have a modest increase in NMSC that is especially pronounced with chronic thiopurine (azathioprine/6-mercaptopurine) use69. Many trials in OTRs evaluate combination regimens making it difficult to discern the individual contributions of azathioprine70; however there is data suggesting that the dose-dependent risk of SCC associated with azathioprine use is significantly higher than that associated with cyclosporine or corticosteroid use71. Many trials trials show a significantly elevated risk of NMSC with azathioprine and a relative protective effect of mycophenolate mofetil (MMF)72–75 with up to 27% relative risk reduction74, though at least one trial shows no significant difference65. MMF has also been associated with an elevated risk of BCC in heart transplant recipients76. MMF is also an antimetabolite, inhibiting de novo purine biosynthesis through inhibition of inosine monophosphate dehydrogenase77. However, it does not appear to have the photosensitizing or mutagenic properties of azathioprine, perhaps explaining the lower relative risk of malignancy with this drug78.
Recently, the mammalian target of rapamycin (mTOR) inhibitors, which include sirolimus (rapamycin), temsirolimus, and everolimus, have elicited significant interest because they have direct anti-tumour properties. Temsirolimus and everolimus are used to treat renal cell carcinoma79. Originally, sirolimus was found to be immunosuppressive by inhibiting the ability of T-cells to proliferate in response to interleukin (IL)-280,81. The target of this group of drugs, mTOR, is a multifunctional protein kinase that regulates protein translation82–84, survival, cell growth, and cell proliferation85–87 in response to growth factors through its two main targets 4E-BP1 and S6K179,88. mTOR activity can promote cell motility through regulation of actin reorganization and adhesion88, and promote secretion of angiogenic factors such as VEGF-A/C by tumour cells thus accounting for the ability of mTOR inhibitors to suppress metastasis89–91. In retrospective analyses, RTRs92 who received sirolimus/everolimus without cyclosporine, or sirolimus maintenance therapy after early cyclosporine withdrawal, had reduced numbers of de novo skin malignancies, and some patients experienced regression of skin cancers such as KS, sebaceous carcinomas, and SCC that were present prior to initiation of mTOR inhibitor therapy93–98. De Fijter and colleagues described 53 RTRs from 8 European centres who developed NMSC, but after conversion from a CNI to an mTOR inhibitor, 37 of them underwent remission. Although 15 relapsed, there was no correlation with drug levels and some continued to receive low-dose CNIs98. Most studies suggest that mTOR inhibitors are an important alternative to CNIs and that the intrinsic anti-tumour activities of these drugs may be particularly useful in the transplant setting.
Biologic therapies are increasingly used for autoimmune conditions including rheumatoid arthritis, psoriasis, and Crohn’s disease because they are effective and steroid-sparing. Infliximab, adalimumab, and golimumab are monoclonal antibodies that target tumour necrosis factor (TNF)-α, and ustekinumab is one that targets the p40 subunit of IL12 and IL2399,100. Etanercept functions as a decoy receptor for TNF-α, and alefacept binds CD2 on T-cells, preventing their interaction with antigen presenting cells and promoting apoptosis. The majority of these drugs directly target pro-inflammatory mediators whereas alefacept targets a critically important cellular interaction necessary for promotion of local inflammation101. Several case and cohort studies suggest that infliximab, etanercept, and adalimumab are associated with rapid development of NMSC, especially SCC69,102–107, and that etanercept and adalimumab are associated with resurgence of latent metastatic melanoma108. This connection may be related to disruption of cancer immunosurveillance and polarization of the immune response towards a Th2 cytokine profile, which is less able to control tumour growth109,110. A recent meta-analysis of randomised controlled trials of anti-TNF-α biologics for psoriasis indicated that there is no statistically significant increased risk of malignancy; however it is likely that the collective experience with these drugs in large trials is insufficient to assess this risk accurately, and there are likely to be different risk profiles in other patient populations such as those with rheumatoid arthritis111.
Other commonly used immunosuppressants also affect skin cancer risk though they are much less extensively studied. Oral glucocorticoid use is correlated with an up to 2 to 3 fold increase in NMSC112–114. Buchbinder and colleagues identified a 3 fold increase in melanoma among rheumatoid arthritis patients treated with methotrexate115.
Inadvertent transplantation of occult melanoma to OTRs is the most common cause of donor-derived malignancy to result in metastasis116,117. It makes up 28% of metastatic donor-transmitted cancers,118 occurs 6 months to 16 years after the surgery, and is associated with a less than 5% overall five-year survival rate118–121. This extended dormancy may be explained by a prolonged equilibrium phase. In one study of donor-derived metastatic melanoma, 11 of 16 patients died but 4 showed complete remission after nephrectomy and cessation of immunosuppression118. Similarly, a deceleration of NMSC was observed in RTRs who discontinued immunosuppressants122. This suggests that the host immune system can enforce long-term tumour dormancy and destroy an immunogenic cancer once competence has been restored.
Ultraviolet (UV)-Induced Immunosuppression
UV radiation, particularly UVB (290–320nm), is considered the most important environmental risk factor for skin cancer (Fig. 2). Chronic exposure causes actinic keratoses and SCC, while intermittent high-dose exposure correlates more with risk for BCC and melanoma123. In addition to facilitating mutagenesis through induction of DNA photoproducts, UV exposure has dramatic effects on immune function. In the foundational experiments of photoimmunology, Margaret Kripke and colleagues demonstrated the antigenicity and immunogenicity of UV-induced skin cancers by showing that these tumors are rejected by syngeneic hosts but not by syngeneic hosts exposed to UVB radiation124,125. This exposure induced “suppressor T cells” that could specifically inhibit the ability of syngeneic mice to reject UV-induced but not other types of transplanted tumours126. It is now known that these “suppressor T cells” are either NKT cells 127 or regulatory T-cells (Tregs; discussed below) depending on the context, and these cells dampen immune responses in-vivo 128. In UV-induced skin cancer models, chronic irradiation at one site could accelerate UV-induced tumour development at distinct, previously-shielded sites consistent with the systemic immunosuppression demonstrated by Kripke and colleagues129.
Figure 2. Mechanisms of Ultraviolet (UV)-Induced Immunomodulation and Carcinogenesis.
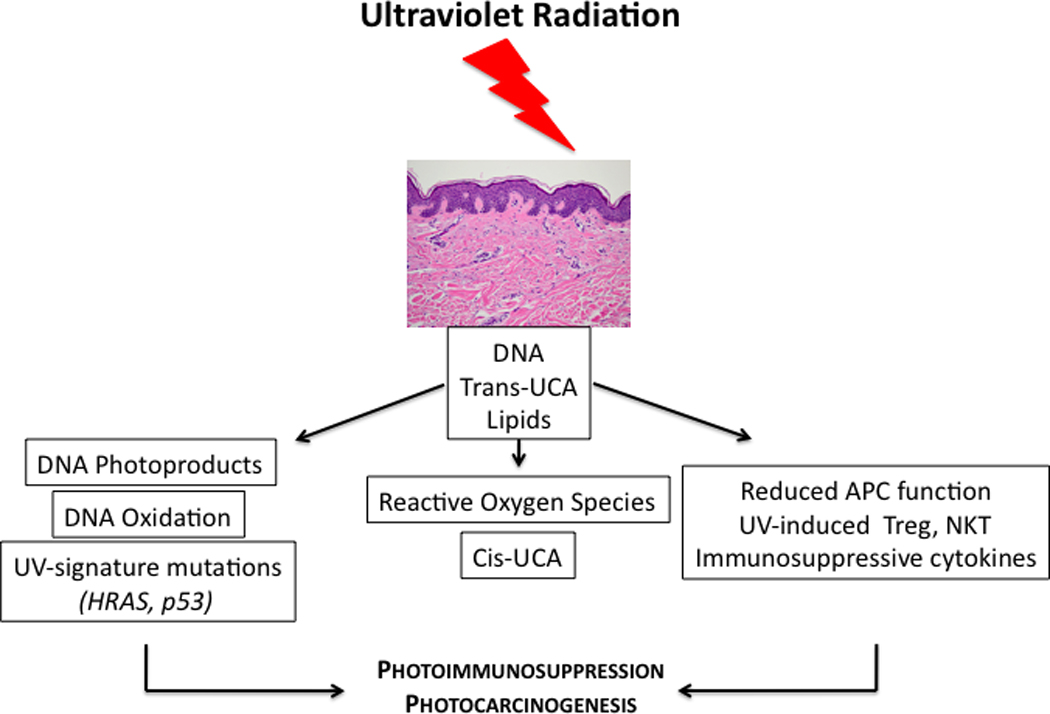
UV radiation is absorbed by DNA, trans-UCA, and membrane lipids. This results in multiple effects on DNA (left column), generation of various chemical species (middle column), and changes in various cellular compartments (right column). The production of DNA photoproducts, cis-UCA, reactive oxygen species, and active vitamin D produces a range of additional biological mediators that ultimately result in enhanced DNA damage, DNA mutagenesis, decreased DNA repair, and immunosuppression. These factors conspire to induce carcinogenesis.
Though most experiments detailing UV-mediated immunosuppression have been performed in animal models,130 exposure in humans has been found to inhibit delayed-type (DTH)131–133 and contact hypersensitivity reactions (CHS)134–137. In both contexts, UV-mediated immunosuppression can be local and systemic. For example, CHS can be suppressed if the sensitiser is applied to a previously irradiated site; however at 3 to 4 mean erythemal doses of UVB, elicitation can be significantly suppressed even if sensitisers are applied to distant non-irradiated sites134,138. DTH measure antigen-specific responses following vaccination. In reported human studies, UV exposure was given after exposure or infection139, and like CHS, suppression of elicitation reactions occurs at both local and distant unirradiated sites133,140. Interestingly, UVB-mediated suppression of CHS positively correlates with a prior history of skin cancer141, and UV-induced immunosuppression is enhanced in males which may explain the increased incidence and risk of mortality in skin cancers in men142.
The mechanistic basis for UV-induced immunosuppression is likely to overlap significantly for local and systemic effects. Local effects include the depletion and downregulation of Langerhans cell antigen-presenting capacity143,144. UV exposure also stimulates keratinocytes and macrophages to produce cytokines such as IL-10, which is important for systemic immunosuppression144,145. The chromophores that are thought to be most important for UV radiation are DNA, membrane lipids, and trans-urocanic acid (trans-UCA) which is isomerised to cis-UCA. The resultant combination of DNA damage, generation of reactive oxygen species, and cytokines conspires to mutagenise epidermal cells and create an immunosuppressive environment that favours tumour formation and progression (Fig. 2)146–148.
Viral Etiologies
Studies have demonstrated an increased incidence of certain viral-associated cancers in immunosuppressed populations secondary to virus reactivation.149 Viral infection is important in the pathogenesis of certain variants of SCC150,151, KS152, and MCC.
Human papillomavirus (HPV) can be found in almost all skin samples tested, including in neonates153. High-risk α-HPVs are known to cause cervical, periungual, and anogenital carcinomas151,154. However, high-risk HPV serotypes that express the oncogenic E6 and E7 variants that abrogate p53 and retinoblastoma (pRB) protein family function, respectively, are not reproducibly found in cutaneous SCC155,156, and, in contrast to anogenital SCC or cervical carcinoma due to high-risk α-HPV, HPV gene expression is not present in human cutaneous SCC157 indicating that HPV is not required for maintenance of SCC. Although this does not formally exclude a role for HPV in the initiation of sporadic SCC, for example by synergizing with UV-induced DNA damage or apoptosis156,158–161, the high incidence of INK4A and p53 inactivation in SCC suggests that the best established oncogenic activities of HPV proteins may not be relevant155.
MCC, a highly aggressive cancer, was recently found to express Merkel cell polyomavirus (MCPyV) gene products162. The risk of MCC is increased in the immunosuppressed (Table I)163, and MCPyV genes are present more often in NMSC of OTRs as compared to immunocompetent patients, particularly those with Bowen’s disease and BCC.164 In immunocompetent hosts, the virus is present in over 60% of normal skin samples165,166, 30% of BCC, and 15% of SCC164,167. MCPyV DNA is integrated into MCC tumour cell genomes, and expression of a mutated viral large T-antigen that is capable of inhibiting pRB is found in MCC tumour tissues168,169. In addition, this expression is required for the survival and proliferation of MCC cell lines170. This evidence collectively suggests that MCPyV is critically important in the pathogenesis of MCC, although the precise molecular mechanisms remain to be elucidated. Recently, two articles show that high-titer virus-specific antibody responses and denser intratumoural CD8+_T-cell correlate with improved prognosis15,171, suggesting that MCPyV is also immunogenic and that MCC may be amenable to immunotherapy.
IMMUNOTHERAPY
Immunotherapy for skin cancer has been most extensively studied in melanoma. Immune agonists such as systemic IFN-α-2b and high-dose IL-2 have both been used as adjuvants for late-stage melanoma with modestly increased disease-free survival in a small proportion of patients172,173. Because melanomas express specific antigens such as Mart1 and gp100 that elicit T-cell responses, there has been sustained interest in vaccine-based and adoptive T-cell immunotherapies. Many systemic immunotherapies now combine vaccination against these antigens or chemotherapy with adoptive T-cell immunotherapy for which at least 19 trials are currently open around the world174. In these methods, lymphocytes are extracted from melanomas or peripheral blood, stimulated and expanded ex-vivo, and then re-introduced back into the patient. In some methods, T-cells are genetically engineered to have specificity for particular antigens175,176. Though some long-lasting remissions have been reported and initial response rates have been as high as 72%177, it has been difficult to predict responses in patients.
Recently, ipilimumab, a humanised antibody against the inhibitory T-cell receptor CTLA-4, was shown to extend overall survival in unresectable Stage III/IV melanoma from an average of 6.4 months with gp100 vaccine alone to 10 months with ipilimumab alone or in combination with gp100 vaccine178. CTLA-4 normally suppresses the activity of T-cells after initial T-cell receptor engagement in order to limit immune responses and to prevent autoimmunity179–181. It was soon shown that CTLA-4 blockade could enhance anti-tumour immune responses in combination with vaccines, resection, and chemotherapy182–186. In accordance with this function, multiple biomarker studies indicate that ipilimumab enhances T-cell responses as measured by increased numbers of activated effector CD4 and CD8 T-cells187,188. In the near future there will be multiple studies examining combinations of ipilimumab with chemotherapy, radiation, and other immunotherapies189.
For other skin cancers such as NMSC, topical or local immunomodulatory has been used. Imiquimod is clinically effective against superficial primary skin tumours and cutaneous metastases190, including BCC, Bowen’s disease, erythroplasia de Queyrat, and lentigo maligna191. Imiquimod activates Toll-like receptor (TLR) 7 on plasmacytoid DCs, causing the NF-κβ-dependent secretion of pro-inflammatory cytokines such as IFN-γ and the chemokines CXCR3, CXCL10, CXCL11, and CCL8 that collectively regulate lymphocyte trafficking192–194. This results in further activation of antigen-presenting cells and enhancement of Th1 and cytotoxic CD8+ T-cell responses194–196. Imiquimod also antagonises adenosine receptor signaling pathways, thereby suppressing the regulatory arm of the immune response, which normally functions to limit inflammation197. In SCC, imiquimod decreases the number of peritumoural regulatory T cells (Tregs) recruited, the amount of IL-10 and transforming growth factor (TGF)-β produced, and it restores vascular E-selectin expression198. E-selectin is a cell adhesion protein important for the extravasation of inflammatory cells, and may be important for enabling anti-tumour responses. Although these mechanisms have not been as extensively studied in actinic keratosis and superficial BCC, short-term clearance following imiquimod treatment has been demonstrated199–201 suggesting that in some circumstances, recruitment of an appropriate inflammatory response can result in tumour clearance. Intralesional IFN-α-2b, another immune agonist, also has activity against BCC and SCC202,203.
WOUND HEALING AND CHRONIC INFLAMMATION
Though we have emphasised how the immune system may control tumour progression, a link between inflammation and carcinogenesis has also been established. Virchow posited a connection between wound healing and cancer when he demonstrated the resemblance between tumour stroma and granulation tissue204. The microenvironments of healing tissue and invasive tumours are very similar: both are characterised by inflammation and are rich in growth factors, chemotactic and angiogenic factors, and migrating cells205. Chronic inflammation may also promote cancers, as suggested by associations between inflammatory bowel disease and colorectal carcinoma, gastroesophageal reflux disease and esophageal adenocarcinoma, and chronic skin wounds and Marjolin’s ulcers. In small series and reviews of case reports, Marjolin’s ulcers, carcinomas that arise in sites of chronic wounds are more aggressive with rates of metastasis over 20%206. This is true in series describing carcinomas in leg ulcers207–209, ulcerated leprosy lesions210, scar carcinomas211,212, and burns (the most common presentation of Marjolin’s ulcers)206, though rates of SCC in burn sites are not significantly increased213,214. These associations could result from increased mutagenesis, facilitated by elevated free radicals and continuous cell proliferation in the setting of chronic inflammatory stress215. It has been suggested that aggressive management to close wounds quickly may largely forestall development of carcinomas in these areas206. Importantly, the correlation between chronic inflammation and cancer is not always seen. The prominent chronic inflammation seen in psoriasis has not been consistently linked to increased skin cancer rates, even after immunosuppression and elevated UV exposure secondary to treatment216.
THE ROLES OF IMMUNE CELL SUBSETS IN NON-MELANOMA SKIN CANCER
Initial characterisation of peritumoural infiltrates in cutaneous SCC showed that there are polyclonal CD3+ αβ T-cells present, only a minority of which are CD8+217. Multiple series have indicated that peritumoural inflammatory infiltrates are more cellular in immunocompetent individuals as compared to immunosuppressed ones218,219. Increased tumour-related immune responses are associated with improved prognosis in MCC15,171, and increased density of tumoural T-cell infiltrates is associated with improved prognosis in melanoma16,17. Mechanistic studies aimed at understanding how these cells function in the tumour microenvironment have been reported primarily in NMSC.
A subset of the “suppressor T-cells” identified by Kripke and colleagues that suppress the rejection of UV-induced tumours are now considered to be Tregs. Tregs are now known to be critical for maintaining tolerance to self-antigens and are ideal candidate culprits in enforcing tolerance to tumours by perhaps suppressing otherwise effective anti-tumour immune responses.220–222. The lineage-specific transcription factor and marker FOXP3 is required for the maintenance and function of Tregs, which are believed to suppress immune function by elaborating IL10 and TGF-β, competing with effector T-cells for IL2, and inhibiting antigen presentation by DC223. Increased numbers of Tregs are linked to a worse prognosis in breast, ovarian, pancreatic, and hepatocellular carcinomas224–228, and have been identified in BCC229, SCC198,230, and primary and metastatic melanomas231–233. In addition, vitamin D3 and immunosuppressants such as dexamethasone and sirolimus can induce differentiation of naive T-helper cells into Tregs234,235. Up to 50% of T cells in SCC isolates are reported to be FOXP3+ Tregs198, and one study found that Tregs increase in number with the progression of pre-cancerous actinic keratoses to established SCC236. A rise in the percentage of Tregs was also seen in the progression of benign nevi to atypical nevi to radial growth phase melanomas231. The risk of SCC in RTRs is modified by peripheral blood immune phenotype, as those with elevated FOXP3+CD4+:CD8+ T-cell ratios appear to be at greater risk237. However, others have reported that perineoplastic inflammatory infiltrates in SCC of OTRs have a significant reduction in Tregs and TGF-β when compared to those of non-transplant populations219,238. However, important caveats to these sometimes contradictory studies are that Treg numbers are not always compared to those of other T-cell subsets such as cytotoxic killer cells (their relative abundance might be more relevant), that FOXP3 can be transiently expressed by activated non-Treg T-cells, and that the actual function of Tregs in the tumour microenvironment is not known.
Before T-cells can be activated, they must be presented their cognate antigen. DCs fulfill this function as professional antigen-presenting cells and induce lymphocyte activation and proliferation; however, they can also inhibit immune responses depending on their degree of differentiation239. BCC infiltrates contain elevated numbers of immature CD11c+ myeloid DCs, suggesting a suppressive immune environment, since immature DCs are thought to cause T-cell anergy instead of activation229. In SCC, tumoural myeloid DCs were less able to stimulate allogeneic T-cell proliferation in a mixed-lymphocyte reaction than those isolated from normal skin, also suggesting functional compromise. Increased expression of IL-10 and TGF-β around the tumour tissue was proposed as the mechanism for this deficiency240. Finally, plasmacytoid DCs are potent inducers of IFN-α and are present in cutaneous peritumoural tissue as well as tumours treated with imiquimod194,241,242. These cells are reduced in SCC from OTRs as compared to those from immunocompetent individuals219. However, their exact role in tumour modulation is still unknown.
As with dendritic cells, tumour-associated macrophages (TAM) may play significant roles in both tumour progression and suppression. The presence of TAMs in BCC correlates with increased invasion, microvessel density, and COX-2 expression, properties that are linked to more aggressive cancers243. In SCC, TAMs are heterogeneously activated with markers that suggest both pro-tumour and anti-tumour activities244, and also appear responsible for vascular endothelial growth factor-C-induced lymphangiogenesis245.
This recent work characterising the often complex and contradictory roles of specific immune cells in NMSC has started to shed light on how immune cells can both control and promote tumourigenesis and progression (Fig. 3). It is only through advances in cell culturing and isolation techniques that such work is even possible now. The directed manipulation of the immune system to control cancer will require this type of detailed knowledge so that interventions may be rationally designed based upon our understanding of how immune cells interact with cancer cells.
Figure 3. Multiple Cancer-Immune Interactions Occur in Skin Cancer.
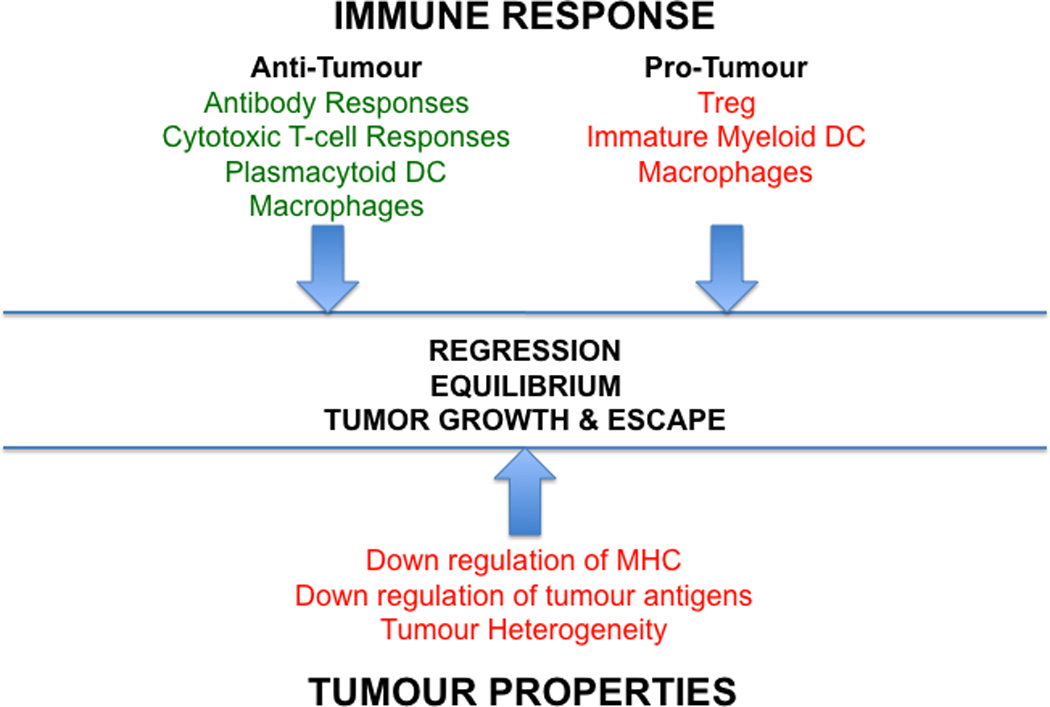
Antigen-specific and non-specific interactions characterise the immune response to cancer as well as tumour-intrinsic adaptations. Those thought to favour tumour development are highlighted in red and those that favour tumour elimination are highlighted in green. The combination of these interactions ultimately dictate the outcome in terms of tumour regression, equilibrium or progression. Lymphocyte responses can be antigen-specific or non-specific. T-cells, macrophages, and DCs can have pro- and anti-tumour effects. Tumour cells may adapt as well by downregulating MHC class I and processing and expression of tumour antigens, and may acquire new mutations or exhibit heterogeneous immunogenicity, enabling immune escape.
CONCLUSIONS
Significant clinical evidence implicates the immune system in the development, progression, and destruction of skin cancers. However, active cancer immunosurveillance, particularly by adaptive immune cells, is not universally accepted. In clinical experience, established tumours very rarely spontaneously regress, though it is impossible to know how many of these events occur undetected. Because tumours possess both genetic and non-genetic heterogeneity, there may be differential outgrowth of particular subclones but it is unknown how this might relate to immunoediting14. Although it has been argued that many tumours do not exhibit inflammation14, infiltrates are frequently observed in skin cancers16,198,246, even those in which there is no secondary ulceration or infection.
The elevated risk of skin cancers in therapeutically immunosuppressed individuals is well-established, but recent discoveries indicate direct tumour-promoting effects of certain drugs, such as cyclosporine on SCC development. UV radiation appears to have dual effects in mutagenesis and immune suppression as well. Although the natural course of tumour-immune interactions remains incompletely understood, ongoing studies will surely aid in improving cancer immunotherapy.
What’s known
The importance of the immune system in human skin cancer has been recognised based upon the increased incidence of skin cancers in organ transplant recipients and ultraviolet (UV) light-mediated immunomodulation.
Studies primarily in mice have demonstrated roles for specific immune cell subsets such as lymphocytes and macrophages in promoting and inhibiting tumour formation.
Studies in mice have identified mediators of UV-mediated immunomodulation.
What’s new
Recent research identifies specific mediators of immune modulation following exposure to ultraviolet light, immunosuppressants, and immune activators in humans.
Calcineurin inhibitors are now known to directly affect human keratinocytes likely synergizing with immunosuppressive effects to promote cancer.
Functions of specific immune cell subsets such as tumoural macrophages and regulatory T-cells in human skin cancers have been shown to both promote and inhibit tumour progression.
Acknowledgements
K.Y.T gratefully acknowledges support from the American Skin Association (Research Scholar Grant), NIH (1R03AR059246), the American Cancer Society IRG (MD Anderson Cancer Center), and institutional funding.
Footnotes
Disclosures: None declared.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 3.Group TL. The Burden of Skin Diseases 2005. 2005 [Google Scholar]
- 4.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- 5.Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin Orthop Relat Res. 1991;262:3–11. [PubMed] [Google Scholar]
- 6.Starnes CO. Coley's toxins in perspective. Nature. 1992;357:11–12. doi: 10.1038/357011a0. [DOI] [PubMed] [Google Scholar]
- 7.Thomas L. On immunosurveillance in human cancer. Yale J Biol Med. 1982;55:329–333. [PMC free article] [PubMed] [Google Scholar]
- 8.Innocence without virtue--graft-versus-host disease in man. N Engl J Med. 1969;281:732–733. doi: 10.1056/NEJM196909252811311. [DOI] [PubMed] [Google Scholar]
- 9.Shankaran V, Ikeda H, Bruce AT, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan DH, Shankaran V, Dighe AS, et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U S A. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheever MA, Allison JP, Ferris AS, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 13.Koebel CM, Vermi W, Swann JB, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 14.Khong HT, Restifo NP. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat Immunol. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paulson KG, Iyer JG, Tegeder AR, et al. Transcriptome-Wide Studies of Merkel Cell Carcinoma and Validation of Intratumoral CD8+ Lymphocyte Invasion As an Independent Predictor of Survival. J Clin Oncol. 2011;29:1539–1546. doi: 10.1200/JCO.2010.30.6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clemente CG, Mihm MC, Jr, Bufalino R, et al. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–1310. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Rao UN, Lee SJ, Luo W, et al. Presence of tumor-infiltrating lymphocytes and a dominant nodule within primary melanoma are prognostic factors for relapse-free survival of patients with thick (t4) primary melanoma: pathologic analysis of the e1690 and e1694 intergroup trials. Am J Clin Pathol. 2010;133:646–653. doi: 10.1309/AJCPTXMEFOVYWDA6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penn I. Post-transplant malignancy: the role of immunosuppression. Drug Saf. 2000;23:101–113. doi: 10.2165/00002018-200023020-00002. [DOI] [PubMed] [Google Scholar]
- 19.Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. 2003;348:1681–1691. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- 20.Bavinck JNB, Hardie DR, Green A, et al. The risk of skin cancer in renal transplant recipients in Queensland, Australia: A follow-up study. Transplantation. 1996;61:715–721. doi: 10.1097/00007890-199603150-00008. [DOI] [PubMed] [Google Scholar]
- 21.Ramsay HM, Fryer AA, Hawley CM, et al. Non-melanoma skin cancer risk in the Queensland renal transplant population. Br J Dermatol. 2002;147:950–956. doi: 10.1046/j.1365-2133.2002.04976.x. [DOI] [PubMed] [Google Scholar]
- 22.Berg D, Otley CC. Skin cancer in organ transplant recipients: Epidemiology, pathogenesis, and management. J Am Acad Dermatol. 2002;47:1–17. doi: 10.1067/mjd.2002.125579. [DOI] [PubMed] [Google Scholar]
- 23.Birkeland SA, Storm HH, Lamm LU, et al. Cancer risk after renal transplantation in the Nordic countries, 1964–1986. Int J Cancer. 1995;60:183–189. doi: 10.1002/ijc.2910600209. [DOI] [PubMed] [Google Scholar]
- 24.Hartevelt MM, Bavinck JN, Kootte AM, et al. Incidence of skin cancer after renal transplantation in The Netherlands. Transplantation. 1990;49:506–509. doi: 10.1097/00007890-199003000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Jensen P, Hansen S, Moller B, et al. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J Am Acad Dermatol. 1999;40:177–186. doi: 10.1016/s0190-9622(99)70185-4. [DOI] [PubMed] [Google Scholar]
- 26.Lindelof B, Sigurgeirsson B, Gabel H, et al. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol. 2000;143:513–519. [PubMed] [Google Scholar]
- 27.Veness MJ, Quinn DI, Ong CS, et al. Aggressive cutaneous malignancies following cardiothoracic transplantation: the Australian experience. Cancer. 1999;85:1758–1764. [PubMed] [Google Scholar]
- 28.Euvrard S, Kanitakis J, Pouteil-Noble C, et al. Aggressive squamous cell carcinomas in organ transplant recipients. Transplant Proc. 1995;27:1767–1768. [PubMed] [Google Scholar]
- 29.Lott DG, Manz R, Koch C, et al. Aggressive behavior of nonmelanotic skin cancers in solid organ transplant recipients. Transplantation. 2010;90:683–687. doi: 10.1097/TP.0b013e3181ec7228. [DOI] [PubMed] [Google Scholar]
- 30.Carucci JA, Martinez JC, Zeitouni NC, et al. In-transit metastasis from primary cutaneous squamous cell carcinoma in organ transplant recipients and nonimmunosuppressed patients: clinical characteristics, management, and outcome in a series of 21 patients. Dermatol Surg. 2004;30:651–655. doi: 10.1111/j.1524-4725.2004.30151.x. [DOI] [PubMed] [Google Scholar]
- 31.Martinez JC, Otley CC, Stasko T, et al. Defining the clinical course of metastatic skin cancer in organ transplant recipients: a multicenter collaborative study. Arch Dermatol. 2003;139:301–306. doi: 10.1001/archderm.139.3.301. [DOI] [PubMed] [Google Scholar]
- 32.Wells JL, Shirai K. Systemic therapy for squamous cell carcinoma of the skin in organ transplant recipients. Am J Clin Oncol. 2011;3:3. doi: 10.1097/COC.0b013e318201a3ef. [DOI] [PubMed] [Google Scholar]
- 33.Dantal J, Hourmant M, Cantarovich D, et al. Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. Lancet. 1998;351:623–628. doi: 10.1016/S0140-6736(97)08496-1. [DOI] [PubMed] [Google Scholar]
- 34.Ducloux D, Carron PL, Rebibou JM, et al. CD4 lymphocytopenia as a risk factor for skin cancers in renal transplant recipients. Transplantation. 1998;65:1270–1272. doi: 10.1097/00007890-199805150-00022. [DOI] [PubMed] [Google Scholar]
- 35.Otley CC, Maragh SL. Reduction of immunosuppression for transplant-associated skin cancer: rationale and evidence of efficacy. Dermatol Surg. 2005;31:163–168. doi: 10.1111/j.1524-4725.2005.31038. [DOI] [PubMed] [Google Scholar]
- 36.Davis MD, Perniciaro C, Dahl PR, et al. Exaggerated arthropod-bite lesions in patients with chronic lymphocytic leukemia: a clinical, histopathologic, and immunopathologic study of eight patients. J Am Acad Dermatol. 1998;39:27–35. doi: 10.1016/s0190-9622(98)70398-6. [DOI] [PubMed] [Google Scholar]
- 37.Adami J, Frisch M, Yuen J, et al. Evidence of an association between non-Hodgkin's lymphoma and skin cancer. BMJ. 1995;310:1491–1495. doi: 10.1136/bmj.310.6993.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manusow D, Weinerman BH. Subsequent neoplasia in chronic lymphocytic leukemia. JAMA. 1975;232:267–269. [PubMed] [Google Scholar]
- 39.Mehrany K, Weenig RH, Pittelkow MR, et al. High recurrence rates of squamous cell carcinoma after Mohs' surgery in patients with chronic lymphocytic leukemia. Dermatol Surg. 2005;31:38–42. doi: 10.1111/j.1524-4725.2005.31006. discussion. [DOI] [PubMed] [Google Scholar]
- 40.Mehrany K, Weenig RH, Pittelkow MR, et al. High recurrence rates of Basal cell carcinoma after mohs surgery in patients with chronic lymphocytic leukemia. Arch Dermatol. 2004;140:985–988. doi: 10.1001/archderm.140.8.985. [DOI] [PubMed] [Google Scholar]
- 41.Mehrany K, Weenig RH, Lee KK, et al. Increased metastasis and mortality from cutaneous squamous cell carcinoma in patients with chronic lymphocytic leukemia. J Am Acad Dermatol. 2005;53:1067–1071. doi: 10.1016/j.jaad.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 42.Smoller BR, Warnke RA. Cutaneous infiltrate of chronic lymphocytic leukemia and relationship to primary cutaneous epithelial neoplasms. J Cutan Pathol. 1998;25:160–164. doi: 10.1111/j.1600-0560.1998.tb01709.x. [DOI] [PubMed] [Google Scholar]
- 43.Dargent JL, Kornreich A, Andre L, et al. Cutaneous infiltrate of chronic lymphocytic leukemia surrounding a primary squamous cell carcinoma of the skin. Report of an additional case and reflection on its pathogenesis. J Cutan Pathol. 1998;25:479–480. doi: 10.1111/j.1600-0560.1998.tb01779.x. [DOI] [PubMed] [Google Scholar]
- 44.Curtis RE, Rowlings PA, Deeg HJ, et al. Solid cancers after bone marrow transplantation. N Engl J Med. 1997;336:897–904. doi: 10.1056/NEJM199703273361301. [DOI] [PubMed] [Google Scholar]
- 45.Leisenring W, Friedman DL, Flowers MED, et al. Nonmelanoma skin and mucosal cancers after hematopoietic cell transplantation. J Clin Oncol. 2006;24:1119–1126. doi: 10.1200/JCO.2005.02.7052. [DOI] [PubMed] [Google Scholar]
- 46.Hasegawa W, Pond GR, Rifkind JT, et al. Long-term follow-up of secondary malignancies in adults after allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004;35:51–55. doi: 10.1038/sj.bmt.1704706. [DOI] [PubMed] [Google Scholar]
- 47.Lishner M, Patterson B, Kandel R, et al. Cutaneous and mucosal neoplasms in bone marrow transplant recipients. Cancer. 1990;65:473–476. doi: 10.1002/1097-0142(19900201)65:3<473::aid-cncr2820650316>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 48.Rizzo JD, Curtis RE, Socie G, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113:1175–1183. doi: 10.1182/blood-2008-05-158782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deeg HJ, Socie G, Schoch G, et al. Malignancies after marrow transplantation for aplastic anemia and fanconi anemia: a joint Seattle and Paris analysis of results in 700 patients. Blood. 1996;87:386–392. [PubMed] [Google Scholar]
- 50.Curtis RE, Metayer C, Rizzo JD, et al. Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: an international case-control study. Blood. 2005;105:3802–3811. doi: 10.1182/blood-2004-09-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marcil I, Stern RS. Squamous-cell cancer of the skin in patients given PUVA and ciclosporin: nested cohort crossover study. Lancet. 2001;358:1042–1045. doi: 10.1016/S0140-6736(01)06179-7. [DOI] [PubMed] [Google Scholar]
- 52.Guba M, Graeb C, Jauch K-W, et al. Pro- and anti-cancer effects of immunosuppressive agents used in organ transplantation. Transplantation. 2004;77:1777–1782. doi: 10.1097/01.tp.0000120181.89206.54. [DOI] [PubMed] [Google Scholar]
- 53.Dupuy P, Bagot M, Michel L, et al. Cyclosporin A inhibits the antigen-presenting functions of freshly isolated human Langerhans cells in vitro. J Invest Dermatol. 1991;96:408–413. doi: 10.1111/1523-1747.ep12469772. [DOI] [PubMed] [Google Scholar]
- 54.Borghi-Cirri MB, Riccardi-Arbi R, Bacci S, et al. Inhibited differentiation of Langerhans cells in the rat epidermis upon systemic treatment with cyclosporin A. Histol Histopathol. 2001;16:107–112. doi: 10.14670/HH-16.107. [DOI] [PubMed] [Google Scholar]
- 55.Sauma D, Fierro A, Mora JR, et al. Cyclosporine preconditions dendritic cells during differentiation and reduces IL-2 and IL-12 production following activation: a potential tolerogenic effect. Transplant Proc. 2003;35:2515–2517. doi: 10.1016/j.transproceed.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 56.Abdul M, Charron D, Haziot A. Selective effects of cyclosporine a on Th2-skewed dendritic cells matured with viral-like stimulus by means of toll-like receptors. Transplantation. 2008;86:880–884. doi: 10.1097/TP.0b013e3181861f1d. [DOI] [PubMed] [Google Scholar]
- 57.Hojo M, Morimoto T, Maluccio M, et al. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999;397:530–534. doi: 10.1038/17401. [DOI] [PubMed] [Google Scholar]
- 58.Han W, Ming M, He TC, et al. Immunosuppressive cyclosporin A activates AKT in keratinocytes through PTEN suppression: implications in skin carcinogenesis. J Biol Chem. 2010;285:11369–11377. doi: 10.1074/jbc.M109.028142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu X, Nguyen BC, Dziunycz P, et al. Opposing roles for calcineurin and ATF3 in squamous skin cancer. Nature. 2010;465:368–372. doi: 10.1038/nature08996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walsh SB, Xu J, Xu H, et al. Cyclosporine a mediates pathogenesis of aggressive cutaneous squamous cell carcinoma by augmenting epithelial-mesenchymal transition: Role of TGFbeta signaling pathway. Mol Carcinog. 2011;9:20744. doi: 10.1002/mc.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scott LJ, McKeage K, Keam SJ, et al. Tacrolimus: a further update of its use in the management of organ transplantation. Drugs. 2003;63:1247–1297. doi: 10.2165/00003495-200363120-00006. [DOI] [PubMed] [Google Scholar]
- 62.Kasiske BL, Snyder JJ, Gilbertson DT, et al. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4:905–913. doi: 10.1111/j.1600-6143.2004.00450.x. [DOI] [PubMed] [Google Scholar]
- 63.Marcen R, Galeano C, Fernandez-Rodriguez A, et al. Effects of the new immunosuppressive agents on the occurrence of malignancies after renal transplantation. Transplant Proc. 2010;42:3055–3057. doi: 10.1016/j.transproceed.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 64.Marcen R, Pascual J, Tato AM, et al. Influence of immunosuppression on the prevalence of cancer after kidney transplantation. Transplant Proc. 2003;35:1714–1716. doi: 10.1016/s0041-1345(03)00669-9. [DOI] [PubMed] [Google Scholar]
- 65.Bichari W, Bartiromo M, Mohey H, et al. Significant risk factors for occurrence of cancer after renal transplantation: a single center cohort study of 1265 cases. Transplant Proc. 2009;41:672–673. doi: 10.1016/j.transproceed.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 66.Bottomley WW, Ford G, Cunliffe WJ, et al. Aggressive squamous cell carcinomas developing in patients receiving long-term azathioprine. Br J Dermatol. 1995;133:460–462. doi: 10.1111/j.1365-2133.1995.tb02678.x. [DOI] [PubMed] [Google Scholar]
- 67.O'Donovan P, Perrett CM, Zhang X, et al. Azathioprine and UVA light generate mutagenic oxidative DNA damage. Science. 2005;309:1871–1874. doi: 10.1126/science.1114233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Graaf YG, Rebel H, Elghalbzouri A, et al. More epidermal p53 patches adjacent to skin carcinomas in renal transplant recipients than in immunocompetent patients: the role of azathioprine. Exp Dermatol. 2008;17:349–355. doi: 10.1111/j.1600-0625.2007.00651.x. [DOI] [PubMed] [Google Scholar]
- 69.Long MD, Herfarth HH, Pipkin CA, et al. Increased risk for mon-melanoma skin cancer in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2010;8:268–274. doi: 10.1016/j.cgh.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gallagher MP, Kelly PJ, Jardine M, et al. Long-term cancer risk of immunosuppressive regimens after kidney transplantation. J Am Soc Nephrol. 2010;21:852–858. doi: 10.1681/ASN.2009101043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ingvar A, Smedby KE, Lindelof B, et al. Immunosuppressive treatment after solid organ transplantation and risk of post-transplant cutaneous squamous cell carcinoma. Nephrol Dial Transplant. 2010;25:2764–2771. doi: 10.1093/ndt/gfp425. [DOI] [PubMed] [Google Scholar]
- 72.Molina BD, Leiro MG, Pulpon LA, et al. Incidence and risk factors for nonmelanoma skin cancer after heart transplantation. Transplant Proc. 2010;42:3001–3005. doi: 10.1016/j.transproceed.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 73.Einollahi B, Nemati E, Lessan-Pezeshki M, et al. Skin cancer after renal transplantation: Results of a multicenter study in Iran. Ann Transplant. 2010;15:44–50. [PubMed] [Google Scholar]
- 74.O'Neill JO, Edwards LB, Taylor DO. Mycophenolate Mofetil and Risk of Developing Malignancy After Orthotopic Heart Transplantation: Analysis of the Transplant Registry of the International Society for Heart and Lung Transplantation. The Journal of Heart and Lung Transplantation. 2006;25:1186–1191. doi: 10.1016/j.healun.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 75.Mabrouk D, Gurcan HM, Keskin DB, et al. Association between cancer and immunosuppressive therapy--analysis of selected studies in pemphigus and pemphigoid. Ann Pharmacother. 2010;44:1770–1776. doi: 10.1345/aph.1P309. [DOI] [PubMed] [Google Scholar]
- 76.Brewer JD, Colegio OR, Phillips PK, et al. Incidence of and risk factors for skin cancer after heart transplant. Arch Dermatol. 2009;145:1391–1396. doi: 10.1001/archdermatol.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ransom JT. Mechanism of action of mycophenolate mofetil. Ther Drug Monit. 1995;17:681–684. doi: 10.1097/00007691-199512000-00023. [DOI] [PubMed] [Google Scholar]
- 78.Hofbauer GF, Straub G, Meyer R, et al. World Congress of Nephrology. Italy: Milan; 2009. Switching azathiorine to mycophenolate mofetil reduces skin photosensitivity in kidney transplant recipients. [Google Scholar]
- 79.Klumpen HJ, Beijnen JH, Gurney H, et al. Inhibitors of mTOR. Oncologist. 2010;15:1262–1269. doi: 10.1634/theoncologist.2010-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sehgal SN. Rapamune (RAPA, rapamycin, sirolimus): mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin Biochem. 1998;31:335–340. doi: 10.1016/s0009-9120(98)00045-9. [DOI] [PubMed] [Google Scholar]
- 81.Kuo CJ, Chung J, Fiorentino DF, et al. Rapamycin selectively inhibits interleukin-2 activation of p70 S6 kinase. Nature. 1992;358:70–73. doi: 10.1038/358070a0. [DOI] [PubMed] [Google Scholar]
- 82.von Manteuffel SR, Dennis PB, Pullen N, et al. The insulin-induced signalling pathway leading to S6 and initiation factor 4E binding protein 1 phosphorylation bifurcates at a rapamycin-sensitive point immediately upstream of p70s6k. Mol Cell Biol. 1997;17:5426–5436. doi: 10.1128/mcb.17.9.5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brunn GJ, Hudson CC, Sekulic A, et al. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science. 1997;277:99–101. doi: 10.1126/science.277.5322.99. [DOI] [PubMed] [Google Scholar]
- 84.Hara K, Yonezawa K, Kozlowski MT, et al. Regulation of eIF-4E BP1 phosphorylation by mTOR. J Biol Chem. 1997;272:26457–26463. doi: 10.1074/jbc.272.42.26457. [DOI] [PubMed] [Google Scholar]
- 85.Hara K, Maruki Y, Long X, et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 86.Kim DH, Sarbassov DD, Ali SM, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 87.Kim DH, Sarbassov DD, Ali SM, et al. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell. 2003;11:895–904. doi: 10.1016/s1097-2765(03)00114-x. [DOI] [PubMed] [Google Scholar]
- 88.Zhou H, Huang S. mTOR signaling in cancer cell motility and tumor metastasis. Crit Rev Eukaryot Gene Expr. 2010;20:1–16. doi: 10.1615/critreveukargeneexpr.v20.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Luan FL, Ding R, Sharma VK, et al. Rapamycin is an effective inhibitor of human renal cancer metastasis. Kidney Int. 2003;63:917–926. doi: 10.1046/j.1523-1755.2003.00805.x. [DOI] [PubMed] [Google Scholar]
- 90.Huber S, Bruns CJ, Schmid G, et al. Inhibition of the mammalian target of rapamycin impedes lymphangiogenesis. Kidney Int. 2007;71:771–777. doi: 10.1038/sj.ki.5002112. [DOI] [PubMed] [Google Scholar]
- 91.Kobayashi S, Kishimoto T, Kamata S, et al. Rapamycin, a specific inhibitor of the mammalian target of rapamycin, suppresses lymphangiogenesis and lymphatic metastasis. Cancer Sci. 2007;98:726–733. doi: 10.1111/j.1349-7006.2007.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Monaco AP. The role of mTOR inhibitors in the management of posttransplant malignancy. Transplantation. 2009;87:157–163. doi: 10.1097/TP.0b013e318193886e. [DOI] [PubMed] [Google Scholar]
- 93.Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant. 2004;18:446–449. doi: 10.1111/j.1399-0012.2004.00188.x. [DOI] [PubMed] [Google Scholar]
- 94.Campistol JM, Eris J, Oberbauer R, et al. Sirolimus therapy after early cyclosporine withdrawal reduces the risk for cancer in adult renal transplantation. J Am Soc Nephrol. 2006;17:581–589. doi: 10.1681/ASN.2005090993. [DOI] [PubMed] [Google Scholar]
- 95.Kahan BD, Yakupoglu YK, Schoenberg L, et al. Low incidence of malignancy among sirolimus/cyclosporine-treated renal transplant recipients. Transplantation. 2005;80:749–758. doi: 10.1097/01.tp.0000173770.42403.f7. [DOI] [PubMed] [Google Scholar]
- 96.Tessmer CS, Magalhaes LV, Keitel E, et al. Conversion to sirolimus in renal transplant recipients with skin cancer. Transplantation. 2006;82:1792–1793. doi: 10.1097/01.tp.0000250767.67472.58. [DOI] [PubMed] [Google Scholar]
- 97.Fernandez A, Marcen R, Pascual J, et al. Conversion from calcineurin inhibitors to everolimus in kidney transplant recipients with malignant neoplasia. Transplant Proc. 2006;38:2453–2455. doi: 10.1016/j.transproceed.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 98.de Fijter JW. Use of proliferation signal inhibitors in non-melanoma skin cancer following renal transplantation. Nephrol Dial Transplant. 2007;22 Suppl 1:i23–i26. doi: 10.1093/ndt/gfm086. [DOI] [PubMed] [Google Scholar]
- 99.Garcia-Valladares I, Cuchacovich R, Espinoza LR. Comparative assessment of biologics in treatment of psoriasis: drug design and clinical effectiveness of ustekinumab. Drug Des Devel Ther. 2011;5:41–49. doi: 10.2147/DDDT.S10494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tak PP, Kalden JR. Advances in rheumatology: new targeted therapeutics. Arthritis Res Ther. 2011;13 Suppl 1:S5. doi: 10.1186/1478-6354-13-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Webber A, Hirose R, Vincenti F. Novel strategies in immunosuppression: issues in perspective. Transplantation. 2011;91:1057–1064. doi: 10.1097/TP.0b013e3182145306. [DOI] [PubMed] [Google Scholar]
- 102.Esser AC, Abril A, Fayne S, et al. Acute development of multiple keratoacanthomas and squamous cell carcinomas after treatment with infliximab. J Am Acad Dermatol. 2004;50:S75–S77. doi: 10.1016/j.jaad.2003.11.044. [DOI] [PubMed] [Google Scholar]
- 103.Smith KJ, Skelton HG. Rapid onset of cutaneous squamous cell carcinoma in patients with rheumatoid arthritis after starting tumor necrosis factor [alpha] receptor IgG1-Fc fusion complex therapy. J Am Acad Dermatol. 2001;45:953–956. doi: 10.1067/mjd.2001.117725. [DOI] [PubMed] [Google Scholar]
- 104.Chakravarty EF, Michaud K, Wolfe F. Skin cancer, rheumatoid arthritis, and tumor necrosis factor inhibitors. J Rheumatol. 2005;32:2130–2135. [PubMed] [Google Scholar]
- 105.Fryrear RS, 2nd, Wiggins AK, Sangueza O, et al. Rapid onset of cutaneous squamous cell carcinoma of the penis in a patient with psoriasis on etanercept therapy. J Am Acad Dermatol. 2004;51:1026. doi: 10.1016/j.jaad.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 106.Ly L, Czarnecki D. The rapid onset of multiple squamous cell carcinomas during etanercept treatment for psoriasis. Br J Dermatol. 2007;157:1076–1078. doi: 10.1111/j.1365-2133.2007.08182.x. [DOI] [PubMed] [Google Scholar]
- 107.Comte C, Guilhou JJ, Guillot B, et al. Rapid onset and fatal outcome of two squamous cell carcinomas of the genitalia in a patient treated with etanercept for cutaneous psoriasis. Dermatology. 2008;217:284–285. doi: 10.1159/000150603. [DOI] [PubMed] [Google Scholar]
- 108.Fulchiero GJ, Jr, Salvaggio H, Drabick JJ, et al. Eruptive latent metastatic melanomas after initiation of antitumor necrosis factor therapies. J Am Acad Dermatol. 2007;56:S65–S67. doi: 10.1016/j.jaad.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 109.Becher B, Blain M, Giacomini PS, et al. Inhibition of Th1 polarization by soluble TNF receptor is dependent on antigen-presenting cell-derived IL-12. J Immunol. 1999;162:684–688. [PubMed] [Google Scholar]
- 110.Smith KJ, Skelton H. Reply. J Am Acad Dermatol. 2003;49:359. doi: 10.1016/s0190-9622(02)61779-7. [DOI] [PubMed] [Google Scholar]
- 111.Dommasch ED, Abuabara K, Shin DB, et al. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: A systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64:1035–1050. doi: 10.1016/j.jaad.2010.09.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Karagas MR, Cushing GL, Jr, Greenberg ER, et al. Non-melanoma skin cancers and glucocorticoid therapy. Br J Cancer. 2001;85:683–686. doi: 10.1054/bjoc.2001.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jensen AO, Thomsen HF, Engebjerg MC, et al. Use of oral glucocorticoids and risk of skin cancer and non-Hodgkin's lymphoma: a population-based case-control study. Br J Cancer. 2009;100:200–205. doi: 10.1038/sj.bjc.6604796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sorensen HT, Mellemkjaer L, Nielsen GL, et al. Skin cancers and non-hodgkin lymphoma among users of systemic glucocorticoids: a population-based cohort study. J Natl Cancer Inst. 2004;96:709–711. doi: 10.1093/jnci/djh118. [DOI] [PubMed] [Google Scholar]
- 115.Buchbinder R, Barber M, Heuzenroeder L, et al. Incidence of melanoma and other malignancies among rheumatoid arthritis patients treated with methotrexate. Arthritis Rheum. 2008;59:794–799. doi: 10.1002/art.23716. [DOI] [PubMed] [Google Scholar]
- 116.Strauss DC, Thomas JM. Transmission of donor melanoma by organ transplantation. Lancet Oncol. 2010;11:790–796. doi: 10.1016/S1470-2045(10)70024-3. [DOI] [PubMed] [Google Scholar]
- 117.Zwald FO, Christenson LJ, Billingsley EM, et al. Melanoma in solid organ transplant recipients. Am J Transplant. 2010;10:1297–1304. doi: 10.1111/j.1600-6143.2010.03078.x. [DOI] [PubMed] [Google Scholar]
- 118.Penn I. Malignant melanoma in organ allograft recipients. Transplantation. 1996;61:274–278. doi: 10.1097/00007890-199601270-00019. [DOI] [PubMed] [Google Scholar]
- 119.MacKie RM, Reid R, Junor B. Fatal melanoma transferred in a donated kidney 16 years after melanoma surgery. N Engl J Med. 2003;348:567–568. doi: 10.1056/NEJM200302063480620. [DOI] [PubMed] [Google Scholar]
- 120.Kim JK, Carmody IC, Cohen AJ, et al. Donor transmission of malignant melanoma to a liver graft recipient: case report and literature review. Clini Transplant. 2009;23:571–574. doi: 10.1111/j.1399-0012.2008.00928.x. [DOI] [PubMed] [Google Scholar]
- 121.Morris-Stiff G, Steel A, Savage P, et al. Transmission of donor melanoma to multiple organ transplant recipients. Am J Transplant. 2004;4:444–446. doi: 10.1111/j.1600-6143.2004.00335.x. [DOI] [PubMed] [Google Scholar]
- 122.Otley CC, Coldiron BM, Stasko T, et al. Decreased skin cancer after cessation of therapy with transplant-associated immunosuppressants. Arch Dermatol. 2001;137:459–463. [PubMed] [Google Scholar]
- 123.Kutting B, Drexler H. UV-induced skin cancer at workplace and evidence-based prevention. Int Arch Occup Environ Health. 2010;23:23. doi: 10.1007/s00420-010-0532-4. [DOI] [PubMed] [Google Scholar]
- 124.Kripke ML. Antigenicity of murine skin tumors induced by ultraviolet light. J Natl Cancer Inst. 1974;53:1333–1336. doi: 10.1093/jnci/53.5.1333. [DOI] [PubMed] [Google Scholar]
- 125.Kripke ML. Latency, histology, and antigenicity of tumors induced by ultraviolet light in three inbred mouse strains. Cancer Res. 1977;37:1395–1400. [PubMed] [Google Scholar]
- 126.Fisher MS, Kripke ML. Suppressor T lymphocytes control the development of primary skin cancers in ultraviolet-irradiated mice. Science. 1982;216:1133–1134. doi: 10.1126/science.6210958. [DOI] [PubMed] [Google Scholar]
- 127.Moodycliffe AM, Nghiem D, Clydesdale G, et al. Immune suppression and skin cancer development: regulation by NKT cells. Nat Immunol. 2000;1:521–525. doi: 10.1038/82782. [DOI] [PubMed] [Google Scholar]
- 128.Schwarz T. 25 years of UV-induced immunosuppression mediated by T cells-from disregarded T suppressor cells to highly respected regulatory T cells. Photochem Photobiol. 2008;84:10–18. doi: 10.1111/j.1751-1097.2007.00223.x. [DOI] [PubMed] [Google Scholar]
- 129.de Gruijl FR. UV-induced immunosuppression in the balance. Photochem Photobiol. 2008;84:2–9. doi: 10.1111/j.1751-1097.2007.00211.x. [DOI] [PubMed] [Google Scholar]
- 130.Ullrich SE. Mechanisms underlying UV-induced immune suppression. Mutat Res. 2005;571:185–205. doi: 10.1016/j.mrfmmm.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 131.Cestari TF, Kripke ML, Baptista PL, et al. Ultraviolet radiation decreases the granulomatous response to lepromin in humans. J Invest Dermatol. 1995;105:8–13. doi: 10.1111/1523-1747.ep12312309. [DOI] [PubMed] [Google Scholar]
- 132.Damian DL, Halliday GM, Taylor CA, et al. Ultraviolet radiation induced suppression of Mantoux reactions in humans. J Invest Dermatol. 1998;110:824–827. doi: 10.1046/j.1523-1747.1998.00176.x. [DOI] [PubMed] [Google Scholar]
- 133.Moyal D. Immunosuppression induced by chronic ultraviolet irradiation in humans and its prevention by sunscreens. Eur J Dermatol. 1998;8:209–211. [PubMed] [Google Scholar]
- 134.Cooper KD, Oberhelman L, Hamilton TA, et al. UV exposure reduces immunization rates and promotes tolerance to epicutaneous antigens in humans: relationship to dose, CD1a-DR+ epidermal macrophage induction, and Langerhans cell depletion. Proc Natl Acad Sci U S A. 1992;89:8497–8501. doi: 10.1073/pnas.89.18.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Damian DL, Halliday GM, Barnetson RS. Broad-spectrum sunscreens provide greater protection against ultraviolet-radiation-induced suppression of contact hypersensitivity to a recall antigen in humans. J Invest Dermatol. 1997;109:146–151. doi: 10.1111/1523-1747.ep12319200. [DOI] [PubMed] [Google Scholar]
- 136.Kelly DA, Seed PT, Young AR, et al. A commercial sunscreen's protection against ultraviolet radiation-induced immunosuppression is more than 50% lower than protection against sunburn in humans. J Invest Dermatol. 2003;120:65–71. doi: 10.1046/j.1523-1747.2003.12005.x. [DOI] [PubMed] [Google Scholar]
- 137.Matthews YJ, Halliday GM, Phan TA, et al. Wavelength dependency for UVA-induced suppression of recall immunity in humans. J Dermatol Sci. 2010;59:192–197. doi: 10.1016/j.jdermsci.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 138.Kelly DA, Walker SL, McGregor JM, et al. A single exposure of solar simulated radiation suppresses contact hypersensitivity responses both locally and systemically in humans: quantitative studies with high-frequency ultrasound. J Photochem Photobiol B. 1998;44:130–142. doi: 10.1016/S1011-1344(98)00136-5. [DOI] [PubMed] [Google Scholar]
- 139.Norval M. Effects of solar radiation on the human immune system. J Photochem Photobiol B. 2001;63:28–40. doi: 10.1016/s1011-1344(01)00200-7. [DOI] [PubMed] [Google Scholar]
- 140.O'Dell BL, Jessen RT, Becker LE, et al. Diminished immune response in sun-damaged skin. Arch Dermatol. 1980;116:559–561. [PubMed] [Google Scholar]
- 141.Yoshikawa T, Rae V, Bruins-Slot W, et al. Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans. J Invest Dermatol. 1990;95:530–536. doi: 10.1111/1523-1747.ep12504877. [DOI] [PubMed] [Google Scholar]
- 142.Damian DL, Patterson CR, Stapelberg M, et al. UV radiation-induced immunosuppression is greater in men and prevented by topical nicotinamide. J Invest Dermatol. 2008;128:447–454. doi: 10.1038/sj.jid.5701058. [DOI] [PubMed] [Google Scholar]
- 143.Schwarz A, Noordegraaf M, Maeda A, et al. Langerhans cells are required for UVR-induced immunosuppression. J Invest Dermatol. 2010;130:1419–1427. doi: 10.1038/jid.2009.429. [DOI] [PubMed] [Google Scholar]
- 144.Loser K, Beissert S. Regulation of cutaneous immunity by the environment: an important role for UV irradiation and vitamin D. Int Immunopharmacol. 2009;9:587–589. doi: 10.1016/j.intimp.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 145.Aubin F. Mechanisms involved in ultraviolet light-induced immunosuppression. Eur J Dermatol. 2003;13:515–523. [PubMed] [Google Scholar]
- 146.Stege H, Roza L, Vink AA, et al. Enzyme plus light therapy to repair DNA damage in ultraviolet-B-irradiated human skin. Proc Natl Acad Sci U S A. 2000;97:1790–1795. doi: 10.1073/pnas.030528897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sreevidya CS, Fukunaga A, Khaskhely NM, et al. Agents that reverse UV-Induced immune suppression and photocarcinogenesis affect DNA repair. J Invest Dermatol. 2010;130:1428–1437. doi: 10.1038/jid.2009.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Walterscheid JP, Ullrich SE, Nghiem DX. Platelet-activating factor, a molecular sensor for cellular damage, activates systemic immune suppression. J Exp Med. 2002;195:171–179. doi: 10.1084/jem.20011450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nindl I, Rosl F. Molecular concepts of virus infections causing skin cancer in organ transplant recipients. Am J Transplant. 2008;8:2199–2204. doi: 10.1111/j.1600-6143.2008.02392.x. [DOI] [PubMed] [Google Scholar]
- 150.Dubina M, Goldenberg G. Viral-associated nonmelanoma skin cancers: a review. Am J Dermatopathol. 2009;31:561–573. doi: 10.1097/DAD.0b013e3181a58234. [DOI] [PubMed] [Google Scholar]
- 151.Moy RL, Eliezri YD, Nuovo GJ, et al. Human papillomavirus type 16 DNA in periungual squamous cell carcinomas. JAMA. 1989;261:2669–2673. [PubMed] [Google Scholar]
- 152.Mendez JC, Procop GW, Espy MJ, et al. Relationship of HHV8 replication and Kaposi's sarcoma after solid organ transplantation. Transplantation. 1999;67:1200–1201. doi: 10.1097/00007890-199904270-00022. [DOI] [PubMed] [Google Scholar]
- 153.Hsu JY, Chen AC, Keleher A, et al. Shared and persistent asymptomatic cutaneous human papillomavirus infections in healthy skin. J Med Virol. 2009;81:1444–1449. doi: 10.1002/jmv.21529. [DOI] [PubMed] [Google Scholar]
- 154.Bouvard V, Baan R, Straif K, et al. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10:321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 155.Boukamp P. Non-melanoma skin cancer: what drives tumor development and progression? Carcinogenesis. 2005;26:1657–1667. doi: 10.1093/carcin/bgi123. [DOI] [PubMed] [Google Scholar]
- 156.Tuttleton Arron S, Jennings L, Nindl I, et al. Viral oncogenesis and its role in nonmelanoma skin cancer. Br J Dermatol. 2011;164:1201–1213. doi: 10.1111/j.1365-2133.2011.10322.x. [DOI] [PubMed] [Google Scholar]
- 157.Arron ST, Ruby JG, Dybbro E, et al. Transcriptome Sequencing Demonstrates that Human Papillomavirus Is Not Active in Cutaneous Squamous Cell Carcinoma. J Invest Dermatol. 2011 doi: 10.1038/jid.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Weissenborn SJ, Nindl I, Purdie K, et al. Human papillomavirus-DNA loads in actinic keratoses exceed those in non-melanoma skin cancers. J Invest Dermatol. 2005;125:93–97. doi: 10.1111/j.0022-202X.2005.23733.x. [DOI] [PubMed] [Google Scholar]
- 159.Jackson S, Storey A. E6 proteins from diverse cutaneous HPV types inhibit apoptosis in response to UV damage. Oncogene. 2000;19:592–598. doi: 10.1038/sj.onc.1203339. [DOI] [PubMed] [Google Scholar]
- 160.Jackson S, Harwood C, Thomas M, et al. Role of Bak in UV-induced apoptosis in skin cancer and abrogation by HPV E6 proteins. Genes Dev. 2000;14:3065–3073. doi: 10.1101/gad.182100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Iftner T, Elbel M, Schopp B, et al. Interference of papillomavirus E6 protein with single-strand break repair by interaction with XRCC1. EMBO J. 2002;21:4741–4748. doi: 10.1093/emboj/cdf443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Koljonen V, Kukko H, Tukiainen E, et al. Incidence of Merkel cell carcinoma in renal transplant recipients. Nephrol Dial Transplant. 2009;24:3231–3235. doi: 10.1093/ndt/gfp334. [DOI] [PubMed] [Google Scholar]
- 164.Kassem A, Technau K, Kurz AK, et al. Merkel cell polyomavirus sequences are frequently detected in nonmelanoma skin cancer of immunosuppressed patients. Int J Cancer. 2009;125:356–361. doi: 10.1002/ijc.24323. [DOI] [PubMed] [Google Scholar]
- 165.Wieland U, Mauch C, Kreuter A, et al. Merkel cell polyomavirus DNA in persons without merkel cell carcinoma. Emerg Infect Dis. 2009;15:1496–1498. doi: 10.3201/eid1509.081575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Foulongne V, Dereure O, Kluger N, et al. Merkel cell polyomavirus DNA detection in lesional and nonlesional skin from patients with Merkel cell carcinoma or other skin diseases. Br J Dermatol. 2010;162:59–63. doi: 10.1111/j.1365-2133.2009.09381.x. [DOI] [PubMed] [Google Scholar]
- 167.Dworkin AM, Tseng SY, Allain DC, et al. Merkel cell polyomavirus in cutaneous squamous cell carcinoma of immunocompetent individuals. J Invest Dermatol. 2009;129:2868–2874. doi: 10.1038/jid.2009.183. [DOI] [PubMed] [Google Scholar]
- 168.Shuda M, Arora R, Kwun HJ, et al. Human Merkel cell polyomavirus infection. I MCV T antigen expression in Merkel cell carcinoma, lymphoid tissues and lymphoid tumors. Int J Cancer. 2009;125:1243–1249. doi: 10.1002/ijc.24510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shuda M, Feng H, Kwun HJ, et al. T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus. Proc Natl Acad Sci U S A. 2008;105:16272–16277. doi: 10.1073/pnas.0806526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Houben R, Shuda M, Weinkam R, et al. Merkel cell polyomavirus-infected Merkel cell carcinoma cells require expression of viral T antigens. J Virol. 2010;84:7064–7072. doi: 10.1128/JVI.02400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Touze A, Le Bidre E, Laude H, et al. High levels of antibodies against merkel cell polyomavirus identify a subset of patients with merkel cell carcinoma with better clinical outcome. J Clin Oncol. 2011;29:1612–1619. doi: 10.1200/JCO.2010.31.1704. [DOI] [PubMed] [Google Scholar]
- 172.Mocellin S, Pasquali S, Rossi CR, et al. Interferon alpha adjuvant therapy in patients with high-risk melanoma: a systematic review and meta-analysis. J Natl Cancer Inst. 2010;102:493–501. doi: 10.1093/jnci/djq009. [DOI] [PubMed] [Google Scholar]
- 173.Tsai KY. Systemic adjuvant therapy for patients with high-risk melanoma. Arch Dermatol. 2007;143:779–782. doi: 10.1001/archderm.143.6.779. [DOI] [PubMed] [Google Scholar]
- 174.Hershkovitz L, Schachter J, Treves AJ, et al. Focus on adoptive T cell transfer trials in melanoma. Clin Dev Immunol. 2011;2010 doi: 10.1155/2010/260267. 260267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Johnson LA, Morgan RA, Dudley ME, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tivol EA, Borriello F, Schweitzer AN, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 180.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 181.Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. [PubMed] [Google Scholar]
- 182.Hurwitz AA, Yu TF, Leach DR, et al. CTLA-4 blockade synergizes with tumor-derived granulocyte-macrophage colony-stimulating factor for treatment of an experimental mammary carcinoma. Proc Natl Acad Sci U S A. 1998;95:10067–10071. doi: 10.1073/pnas.95.17.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hurwitz AA, Foster BA, Kwon ED, et al. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60:2444–2448. [PubMed] [Google Scholar]
- 184.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kwon ED, Foster BA, Hurwitz AA, et al. Elimination of residual metastatic prostate cancer after surgery and adjunctive cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) blockade immunotherapy. Proc Natl Acad Sci U S A. 1999;96:15074–15079. doi: 10.1073/pnas.96.26.15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mokyr MB, Kalinichenko T, Gorelik L, et al. Realization of the therapeutic potential of CTLA-4 blockade in low-dose chemotherapy-treated tumor-bearing mice. Cancer Res. 1998;58:5301–5304. [PubMed] [Google Scholar]
- 187.Hodi FS, Mihm MC, Soiffer RJ, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Liakou CI, Kamat A, Tang DN, et al. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci U S A. 2008;105:14987–14992. doi: 10.1073/pnas.0806075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hoos A, Ibrahim R, Korman A, et al. Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy. Semin Oncol. 2010;37:533–546. doi: 10.1053/j.seminoncol.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 190.Wagstaff AJ, Perry CM. Topical imiquimod: a review of its use in the management of anogenital warts, actinic keratoses, basal cell carcinoma and other skin lesions. Drugs. 2007;67:2187–2210. doi: 10.2165/00003495-200767150-00006. [DOI] [PubMed] [Google Scholar]
- 191.Imiquimod for superficial and in situ skin malignancy. Drug Ther Bull. 2009;47:113–116. doi: 10.1136/dtb.2009.09.0040. [DOI] [PubMed] [Google Scholar]
- 192.Torres A, Storey L, Anders M, et al. Immune-mediated changes in actinic keratosis following topical treatment with imiquimod 5% cream. J Transl Med. 2007;5:7. doi: 10.1186/1479-5876-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wenzel J, Uerlich M, Haller O, et al. Enhanced type I interferon signaling and recruitment of chemokine receptor CXCR3-expressing lymphocytes into the skin following treatment with the TLR7-agonist imiquimod. J Cutan Pathol. 2005;32:257–262. doi: 10.1111/j.0303-6987.2005.00297.x. [DOI] [PubMed] [Google Scholar]
- 194.Wolf IH, Kodama K, Cerroni L, et al. Nature of inflammatory infiltrate in superficial cutaneous malignancies during topical imiquimod treatment. Am J Dermatopathol. 2007;29:237–241. doi: 10.1097/01.dad.0000211531.33670.94. [DOI] [PubMed] [Google Scholar]
- 195.Huang SJ, Hijnen D, Murphy GF, et al. Imiquimod enhances IFN-gamma production and effector function of T cells infiltrating human squamous cell carcinomas of the skin. J Invest Dermatol. 2009;129:2676–2685. doi: 10.1038/jid.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.De Giorgi V, Salvini C, Chiarugi A, et al. In vivo characterization of the inflammatory infiltrate and apoptotic status in imiquimod-treated basal cell carcinoma. Int J Dermatol. 2009;48:312–321. doi: 10.1111/j.1365-4632.2009.03916.x. [DOI] [PubMed] [Google Scholar]
- 197.Schon MP, Schon M, Klotz KN. The small antitumoral immune response modifier imiquimod interacts with adenosine receptor signaling in a TLR7- and TLR8-independent fashion. J Invest Dermatol. 2006;126:1338–1347. doi: 10.1038/sj.jid.5700286. [DOI] [PubMed] [Google Scholar]
- 198.Clark RA, Huang SJ, Murphy GF, et al. Human squamous cell carcinomas evade the immune response by down-regulation of vascular E-selectin and recruitment of regulatory T cells. J Exp Med. 2008;205:2221–2234. doi: 10.1084/jem.20071190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Micali G, Lacarrubba F, Dinotta F, et al. Treating skin cancer with topical cream. Expert Opin Pharmacother. 2010;11:1515–1527. doi: 10.1517/14656566.2010.481284. [DOI] [PubMed] [Google Scholar]
- 200.Love WE, Bernhard JD, Bordeaux JS. Topical imiquimod or fluorouracil therapy for basal and squamous cell carcinoma: a systematic review. Arch Dermatol. 2009;145:1431–1438. doi: 10.1001/archdermatol.2009.291. [DOI] [PubMed] [Google Scholar]
- 201.Bath-Hextall FJ, Perkins W, Bong J, et al. Interventions for basal cell carcinoma of the skin. Cochrane Database Syst Rev. 2007:CD003412. doi: 10.1002/14651858.CD003412.pub2. [DOI] [PubMed] [Google Scholar]
- 202.Kim KH, Yavel RM, Gross VL, et al. Intralesional interferon alpha-2b in the treatment of basal cell carcinoma and squamous cell carcinoma: revisited. Dermatol Surg. 2004;30:116–120. doi: 10.1111/j.1524-4725.2004.30020.x. [DOI] [PubMed] [Google Scholar]
- 203.Good LM, Miller MD, High WA. Intralesional agents in the management of cutaneous malignancy: a review. J Am Acad Dermatol. 2011;64:413–422. doi: 10.1016/j.jaad.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 204.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 205.Schafer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9:628–638. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 206.Kerr-Valentic MA, Samimi K, Rohlen BH, et al. Marjolin's ulcer: modern analysis of an ancient problem. Plast Reconstr Surg. 2009;123:184–191. doi: 10.1097/PRS.0b013e3181904d86. [DOI] [PubMed] [Google Scholar]
- 207.Baldursson B, Sigurgeirsson B, Lindelof B. Leg ulcers and squamous cell carcinoma. An epidemiological study and a review of the literature. Acta Derm Venereol. 1993;73:171–174. doi: 10.2340/0001555573171174. [DOI] [PubMed] [Google Scholar]
- 208.Baldursson BT, Hedblad MA, Beitner H, et al. Squamous cell carcinoma complicating chronic venous leg ulceration: a study of the histopathology, course and survival in 25 patients. Br J Dermatol. 1999;140:1148–1152. doi: 10.1046/j.1365-2133.1999.02879.x. [DOI] [PubMed] [Google Scholar]
- 209.Asuquo M, Ugare G, Ebughe G, et al. Marjolin's ulcer: the importance of surgical management of chronic cutaneous ulcers. Int J Dermatol. 2007;46 Suppl 2:29–32. doi: 10.1111/j.1365-4632.2007.03382.x. [DOI] [PubMed] [Google Scholar]
- 210.Richardus JH, Smith TC. Squamous cell carcinoma in chronic ulcers in leprosy: a review of 38 consecutive cases. Lepr Rev. 1991;62:381–388. doi: 10.5935/0305-7518.19910044. [DOI] [PubMed] [Google Scholar]
- 211.Eroglu A, Camlibel S. Risk factors for locoregional recurrence of scar carcinoma. Br J Surg. 1997;84:1744–1746. doi: 10.1046/j.1365-2168.1997.02853.x. [DOI] [PubMed] [Google Scholar]
- 212.Mullen JT, Feng L, Xing Y, et al. Invasive squamous cell carcinoma of the skin: defining a high-risk group. Ann Surg Oncol. 2006;13:902–909. doi: 10.1245/ASO.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 213.Lindelof B, Krynitz B, Granath F, et al. Burn injuries and skin cancer: a population-based cohort study. Acta Derm Venereol. 2008;88:20–22. doi: 10.2340/00015555-0339. [DOI] [PubMed] [Google Scholar]
- 214.Mellemkjaer L, Holmich LR, Gridley G, et al. Risks for skin and other cancers up to 25 years after burn injuries. Epidemiology. 2006;17:668–673. doi: 10.1097/01.ede.0000239651.06579.a4. [DOI] [PubMed] [Google Scholar]
- 215.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 216.Nickoloff BJ, Ben-Neriah Y, Pikarsky E. Inflammation and cancer: is the link as simple as we think? J Invest Dermatol. 2005;124:x–xiv. doi: 10.1111/j.0022-202X.2005.23724.x. [DOI] [PubMed] [Google Scholar]
- 217.Haeffner AC, Zepter K, Elmets CA, et al. Analysis of tumor-infiltrating lymphocytes in cutaneous squamous cell carcinoma. Arch Dermatol. 1997;133:585–590. [PubMed] [Google Scholar]
- 218.Harwood CA, Proby CM, McGregor JM, et al. Clinicopathologic features of skin cancer in organ transplant recipients: a retrospective case-control series. J Am Acad Dermatol. 2006;54:290–300. doi: 10.1016/j.jaad.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 219.Muhleisen B, Petrov I, Gachter T, et al. Progression of cutaneous squamous cell carcinoma in immunosuppressed patients is associated with reduced CD123+ and FOXP3+ cells in the perineoplastic inflammatory infiltrate. Histopathology. 2009;55:67–76. doi: 10.1111/j.1365-2559.2009.03324.x. [DOI] [PubMed] [Google Scholar]
- 220.Filaci G, Fenoglio D, Fravega M, et al. CD8+ CD28- T regulatory lymphocytes inhibiting T cell proliferative and cytotoxic functions infiltrate human cancers. J Immunol. 2007;179:4323–4334. doi: 10.4049/jimmunol.179.7.4323. [DOI] [PubMed] [Google Scholar]
- 221.Ghiringhelli F, Menard C, Terme M, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med. 2005;202:1075–1085. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Chen ML, Pittet MJ, Gorelik L, et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci U S A. 2005;102:419–424. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sakaguchi S, Wing K, Onishi Y, et al. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009;21:1105–1111. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- 224.Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 225.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 226.Kobayashi N, Hiraoka N, Yamagami W, et al. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13:902–911. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 227.Hiraoka N, Onozato K, Kosuge T, et al. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12:5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- 228.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kaporis HG, Guttman-Yassky E, Lowes MA, et al. Human basal cell carcinoma is associated with Foxp3+ T cells in a Th2 dominant microenvironment. J Invest Dermatol. 2007;127:2391–2398. doi: 10.1038/sj.jid.5700884. [DOI] [PubMed] [Google Scholar]
- 230.Schwarz S, Butz M, Morsczeck C, et al. Increased number of CD25 FoxP3 regulatory T cells in oral squamous cell carcinomas detected by chromogenic immunohistochemical double staining. J Oral Pathol Med. 2008;37:485–489. doi: 10.1111/j.1600-0714.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- 231.Mourmouras V, Fimiani M, Rubegni P, et al. Evaluation of tumour-infiltrating CD4+CD25+FOXP3+ regulatory T cells in human cutaneous benign and atypical naevi, melanomas and melanoma metastases. Br J Dermatol. 2007;157:531–539. doi: 10.1111/j.1365-2133.2007.08057.x. [DOI] [PubMed] [Google Scholar]
- 232.Viguier M, Lemaitre F, Verola O, et al. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 233.Ahmadzadeh M, Felipe-Silva A, Heemskerk B, et al. FOXP3 expression accurately defines the population of intratumoral regulatory T cells that selectively accumulate in metastatic melanoma lesions. Blood. 2008;112:4953–4960. doi: 10.1182/blood-2008-06-163048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.O'Garra A, Barrat FJ. In vitro generation of IL-10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by Th1- and Th2-inducing cytokines. Immunol Lett. 2003;85:135–139. doi: 10.1016/s0165-2478(02)00239-0. [DOI] [PubMed] [Google Scholar]
- 235.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 236.Jang TJ. Prevalence of Foxp3 positive T regulatory cells is increased during progression of cutaneous squamous tumors. Yonsei Med J. 2008;49:942–948. doi: 10.3349/ymj.2008.49.6.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Carroll RP, Segundo DS, Hollowood K, et al. Immune phenotype predicts risk for posttransplantation squamous cell carcinoma. J Am Soc Nephrol. 2010;21:713–722. doi: 10.1681/ASN.2009060669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kosmidis M, Dziunycz P, Suarez-Farinas M, et al. Immunosuppression affects CD4+ mRNA expression and induces Th2 dominance in the microenvironment of cutaneous squamous cell carcinoma in organ transplant recipients. J Immunother. 2010;33:538–546. doi: 10.1097/CJI.0b013e3181cc2615. [DOI] [PubMed] [Google Scholar]
- 239.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–449. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 240.Bluth MJ, Zaba LC, Moussai D, et al. Myeloid dendritic cells from human cutaneous squamous cell carcinoma are poor stimulators of T-cell proliferation. J Invest Dermatol. 2009;129:2451–2462. doi: 10.1038/jid.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Charles J, Chaperot L, Salameire D, et al. Plasmacytoid dendritic cells and dermatological disorders: focus on their role in autoimmunity and cancer. Eur J Dermatol. 2010;20:16–23. doi: 10.1684/ejd.2010.0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Urosevic M, Dummer R, Conrad C, et al. Disease-independent skin recruitment and activation of plasmacytoid predendritic cells following imiquimod treatment. J Natl Cancer Inst. 2005;97:1143–1153. doi: 10.1093/jnci/dji207. [DOI] [PubMed] [Google Scholar]
- 243.Tjiu JW, Chen JS, Shun CT, et al. Tumor-associated macrophage-induced invasion and angiogenesis of human basal cell carcinoma cells by cyclooxygenase-2 induction. J Invest Dermatol. 2009;129:1016–1025. doi: 10.1038/jid.2008.310. [DOI] [PubMed] [Google Scholar]
- 244.Pettersen JS, Fuentes-Duculan J, Suarez-Farinas M, et al. Tumor-Associated Macrophages in the Cutaneous SCC Microenvironment Are Heterogeneously Activated. J Invest Dermatol. 2011;10:10. doi: 10.103/jid.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Moussai D, Mitsui H, Pettersen JS, et al. The human cutaneous squamous cell carcinoma microenvironment is characterized by increased lymphatic density and enhanced expression of macrophage-derived VEGF-C. J Invest Dermatol. 2011;131:229–236. doi: 10.1038/jid.2010.266. [DOI] [PubMed] [Google Scholar]
- 246.Bluth MJ, Zaba LC, Moussai D, et al. Myeloid Dendritic Cells from Human Cutaneous Squamous Cell Carcinoma Are Poor Stimulators of T-Cell Proliferation. J Invest Dermatol. 2009 doi: 10.1038/jid.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Vajdic CM, McDonald SP, McCredie MR, et al. Cancer incidence before and after kidney transplantation. JAMA. 2006;296:2823–2831. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- 248.Le Mire L, Hollowood K, Gray D, et al. Melanomas in renal transplant recipients. Br J Dermatol. 2006;154:472–477. doi: 10.1111/j.1365-2133.2005.07094.x. [DOI] [PubMed] [Google Scholar]
- 249.Hollenbeak CS, Todd MM, Billingsley EM, et al. Increased incidence of melanoma in renal transplantation recipients. Cancer. 2005;104:1962–1967. doi: 10.1002/cncr.21404. [DOI] [PubMed] [Google Scholar]


