Abstract
Objective
There is a need to integrate the various theoretical frameworks and formalisms for modeling clinical guidelines, workflows, and pathways, in order to move beyond providing support for individual clinical decisions and toward the provision of process-oriented, patient-centered, health information systems (HIS). In this review, we analyze the challenges in developing process-oriented HIS that formally model guidelines, workflows, and care pathways.
Methods
A qualitative meta-synthesis was performed on studies published in English between 1995 and 2010 that addressed the modeling process and reported the exposition of a new methodology, model, system implementation, or system architecture. Thematic analysis, principal component analysis (PCA) and data visualisation techniques were used to identify and cluster the underlying implementation ‘challenge’ themes.
Results
One hundred and eight relevant studies were selected for review. Twenty-five underlying ‘challenge’ themes were identified. These were clustered into 10 distinct groups, from which a conceptual model of the implementation process was developed.
Discussion and conclusion
We found that the development of systems supporting individual clinical decisions is evolving toward the implementation of adaptable care pathways on the semantic web, incorporating formal, clinical, and organizational ontologies, and the use of workflow management systems. These architectures now need to be implemented and evaluated on a wider scale within clinical settings.
Keywords: Decision support, care pathways, clinical workflow, modeling, collaborative technologies, intelligent tutoring and tailored information representation, statistical analysis of large datasets, knowledge acquisition and knowledge management, supporting practice at a distance (telehealth), measuring/improving patient safety and reducing medical errors, simulation of complex systems (at all levels: molecules to work groups to organizations), modeling physiologic and disease processes
Introduction
Computer-based workflow is primarily concerned with the automation of business processes, in which documents, information, or tasks are passed from one participant or application to another for enactment, according to a set of procedural rules. Workflow activities and procedural rules used to manage the flow activities are identified by a workflow process definition. A workflow management system (WfMS) consists of software components to store and interpret process definitions, create and manage workflow instances as they are executed, and control their interaction with workflow participants and applications.1
Clinical workflow has been defined as ‘the flow of care-related tasks as seen in the management of a patient trajectory: the allocation of multiple tasks of a provider or of coworking providers in the processes of care and the way they collaborate.’2 The application of WfMS to managing clinical workflow was first proposed by Dadam et al,3 who noted the need to formally model clinical activities while not restricting the clinician's natural work processes, allowing flexibility and ad hoc variation in execution of clinical tasks. Quaglini et al4 defined a methodology and architecture for integrating computer-interpretable clinical guidelines (CIGs)i with a commercial workflow engine for the management of acute stroke. The combination of a Petri net-based formalism for modeling clinical tasks, with a WfMS for managing the organizational process, was dubbed a ‘careflow’ system, in which the careflow process definition describes the tasks and defines their order of execution, while the execution engine provided some flexibility by allowing tasks to be skipped or substituted with other tasks outside those defined by the clinical guideline.
Schadow et al8 also suggested that WfMS can be used to implement a standardized and defined route through evidence-based clinical processes. Such processes are known as care pathways, defined as ‘structured multidisciplinary care plans that detail essential steps in the care of patients with a specific clinical problem [and] offer a structured means of developing and implementing local protocols of care based on clinical guidelines … [They] describe the tasks to be carried out together with the timing and sequence of these tasks and the discipline involved in completing the task.’9
Care pathways originated in nursing practice in the 1980s when the application of a business process management approach to the organization of clinical practice was used to improve the quality and efficiency of patient care.10 Despite a long history, the care pathway concept remains unclear.11 The term is often used interchangeably with clinical guidelines and protocols,12 although each may be considered to be a different type of workflow with a different scope13:
A clinical guideline provides recommendations for best practice for the clinical domain addressed by the guideline, but does not provide implementation details
A clinical protocol provides a local, consensus view of a guideline with explicit steps for implementation
A care pathway is a versioned document of a process, and includes actions recommended by one or more protocols and guidelines, activity role constraints, and sequencing constraints; it has goals and it provides a record of care and information about the patient state and a ‘variance record,’ that is, a method for documenting and recording where deviations from the planned pathway have occurred.13
Criticisms of care pathways may arise from the limitations of paper-based care pathway documents. It is difficult to tailor care pathway forms to the needs of the individual patient, and interdependencies between different pathways are not made explicit: multiple paths tend to be merged into a simple list of tasks,14 leading to the claim that care pathways simply provide time-based ‘cookbook’ care.15 In parallel with the development of CIG models, the ‘computerization’ of care pathways has been proposed as a way to overcome these limitations, to allow pathways to be integrated with guideline-based decision support and the electronic health record (EHR). Electronic care pathways (‘e-pathways’)16 are defined as systematically developed, computerized care pathways that describe: (1) the clinical data sets used (representation of declarative knowledge); (2) the on-screen forms and user interface elements required; (3) the formal model of the roles, tasks, sequencing, and business rules of clinical workflow (representation of procedural knowledge); and (4) the messages to be exchanged between the systems that invoke the pathway.16 Wakamiya and Yamauchi proposed five core requirements for electronic care pathway implementations: recording notes in the EHR, statistics and variance recording, provision of computerized physician order entry (CPOE), activity checklists, and editable pathway templates.17
Concerns about the duplication of effort, the lack of consistent standards, and the existence of numerous models have been raised by the care pathway and clinical guideline research communities.16 18 At the same time, it has been suggested that computerized decision support systems (CDSS), CIGs, and WfMS are individually inadequate for providing support for longitudinal care processes. The current research challenge is to integrate the various theoretical frameworks and formalisms, in order to move beyond providing support for individual clinical decisions and toward the provision of process-oriented, patient-centered, health information systems (HIS).19
While previous systematic reviews have individually considered the effectiveness of computerized guideline20–23 and care pathway implementations,24 the question of how to integrate guidelines, care pathways, EHR, and clinical workflow has rarely been addressed.19 Song et al25 identified a number of challenges to implementing ‘computer-aided healthcare workflows,’ defined as the integration of guidelines and protocols with a HIS. Following Song et al, we define a process-oriented health information system as a HIS that formally models guidelines, workflows, or care pathways and provides support for clinical decisions that extend over time.
The aims of this review are (1) to identify the cross-cutting themes that describe the theoretical and practical challenges involved in developing process-oriented HIS; (2) to summarize approaches to developing such systems and integrating them with the EHR and clinical workflow; and finally (3) to develop a conceptual implementation model from the themes and approaches.
Methodology
When one wants to explore a phenomenon about which little is known, in order to gain greater understanding and develop hypotheses to explain the phenomenon, qualitative methods are an appropriate choice.26 Therefore we reviewed the literature from this perspective, by treating each paper as a textual narrative from which to extract and categorize the underlying themes that describe the studies as a whole.
Qualitative meta-synthesis involves the interpretative analysis of the themes and categories from a representative sample of studies.27 Within the qualitative research field, study heterogeneity is accepted,27 so differences were compared and contrasted, and areas of commonality identified through a process of iterative, comparative analysis.
Search strategy and inclusion criteria
Searches were performed using ScienceDirect, Web of Science, PubMed, and the specialist health informatics OpenClinical web resource. Articles in English published since 1995 were considered in order to analyze how implementation processes have evolved over time. The broad search concepts of HIS, computerization, modeling, workflow, pathways, and guidelines were combined into search statements specific to each database queried (see appendix).
An initial screening of titles and abstracts excluded opinion pieces, editorials, letters, posters, studies related to non-computerized care pathways, and studies about other types of pathway, for example, biochemical, neural, or motor pathways. Papers on ‘patient flows,’ ‘pathways to care,’ and ‘commissioning pathways’ were also excluded at this stage as these focus on the larger goal of strategic planning rather than clinical workflow and decision making at the individual patient level. Reviews of CIG and workflow models were selected as background material, and were used as a source of additional citations.
Full text articles were screened and included if they met our three inclusion criteria: (1) the study addressed the modeling process for the computerization of clinical workflow, clinical guidelines, or care pathways within the context of a HIS; (2) the outcome was the exposition of a new methodology, knowledge model, framework, system implementation, or system architecture that instantiated the process under study; and (3) there was an evaluation, even if this was only formative and descriptive.
Data collection and quality assessment
Following Evans and Pearson,27 we created a data collection form in Microsoft Excel to identify papers for review. The quality of each was judged using criteria from Burns28 and Greenhalgh and Taylor,26 such as a clearly formulated question, rationale for and description of setting and participants, methodological, theoretical, and analytical rigor, data audit trail, and justification of conclusions.
Information for each of these criteria from each study was entered into the data collection spreadsheet. Not all criteria were relevant for each paper (eg, model formulations and system architectures may not have any participants or data audit trail). Papers that could not meet the criteria were discarded.
Data abstraction and thematic analysis
Thematic analysis was carried out using an approach informed by qualitative concept analysis, in which research aims are defined in advance, and categories are brought to the material and continually refined against it, with the goal of reducing the material.29 This was guided by the three-stage approach discussed in Miles and Huberman30: (1) initial, descriptive coding, developing toward (2) more interpretative coding (high-level concepts that encompass the descriptive coding performed in step 1) as knowledge of the phenomenon under study increases; and (3) pattern coding (emerging themes) toward the end of the analysis in which themes are developed that seek to explain and make causal links in the phenomenon. Researchers met weekly to discuss the emerging themes before agreeing on the final set.
Challenges identified by Song et al25 and Wakamiya and Yamauchi17 were used to help develop the initial working list of descriptive codes with which to annotate the data (step 1 described above). The list of codes was refined and enhanced as new themes emerged from the literature during analysis (step 2). The final set of pattern codes was used to thematically annotate each paper in the review (step 3). Up to five variables that reflected the study's key concerns, results, and conclusions, were assigned to each study—these were the ‘challenge theme’ variables, that is, factors that need to be addressed when developing a system.
RefViz31 is a tool for clustering bibliographic references for visualization and analysis. We created a custom reference file in ISI ResearchSoft RIS format,32 containing title, year, author, and challenge theme variables for each paper and imported it into RefViz. RefViz applies standard mathematical clustering algorithms to partition the data set into concept-based groups of similar papers based on the co-occurrence of themes between papers. RefViz's Galaxy view performs principal component analysis (PCA) in which a larger set of possibly correlated variables are transformed into a smaller, more fundamental set of independent variables.33
The co-occurrence and clustering of the challenge theme variables arising from the thematic analysis were explored using PCA in RefViz, in order to see if the set of variables could be transformed into a smaller number of principal components that further summarize the studies and from which an integrative, conceptual model of the implementation process could be developed.
Review findings
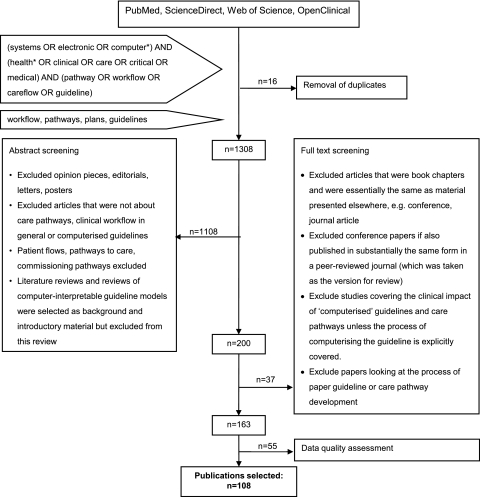
From 1308 screened citations, we retrieved 200 full text articles, and 108 met the inclusion and quality criteria for detailed review. The selection process is shown in figure 1.
Figure 1.
Screening flow-chart.
Characteristics of selected publications
The review identified 79 journal articles,4 8 12 14 17 34–107 and 29 conference proceedings papers.3 108–135 Fifty-seven (53%) studies were conducted within an academic or commercial R&D, non-clinical environment. The remainder took place within university teaching hospitals and medical centers (n=16, 15%), outpatient clinics (n=8, 7%), and general hospitals, stroke units, or emergency or ICU departments (n=27, 25%).
Methods used by selected studies ranged from qualitative research involving usability evaluations (n=1) or questionnaires, interviews, and observational studies (n=20), to formal methods papers (n=26), model formulations (n=26), system case studies (n=20), prototype implementations (n=33), and system architectures (n=26). These categories were not mutually exclusive; a number of studies had multiple objectives: for example combining model formulation, prototype implementation, and system architecture.
Eight distinct knowledge model types were identified in the publications. Fifty-four publications (50%) focused on providing details of system architecture or system prototype implementation. Forty-four (41%) studies had evaluation results reported in the form of interviews, questionnaires, and observational case studies where the study size was quantified. The remaining studies reported informal evaluation in terms of the features of the model or method, or overall benefits of the system implemented.
Challenges in implementing process-oriented systems
The final set of the 25 challenge theme variables and their descriptions, derived from thematic analysis of the 108 papers, are shown in table 1.
Table 1.
Challenge themes: 25 variables identified from initial thematic analysis
| Variable | Description |
| Clinical implementation | Implementing the model into a usable system that is congruent with individual and collaborative clinical workflow in a live, clinical environment |
| Clinician attitude | Beliefs in own self-efficacy, and relevance and quality of guidelines and pathways to clinical practice |
| Complexity | Ability to evaluate and check the model with reasonable run-time behavior (eg, polynomial time) in real-world scenarios |
| Data mapping | Mapping electronic health record (EHR) data to procedural tasks in the guideline or pathway; mapping guideline concepts to terminologies |
| Discrepancy | Potential for inconsistencies between the pathway documentation and the actual treatment process (as a result of staff miscommunication, misunderstanding, or model/implementation constraints) |
| Exception handling | Ability to handle unplanned deviations from the pathway or guideline (variance) |
| Execution | Executing the guideline or pathway model within the EHR; semantic interoperability |
| Expressivity | The need to adequately represent complex clinical information, rules, and exceptions in a formal model |
| Flexibility and adaptability | Adapting the pathway at run-time to individual patient (variance); handling incomplete or ambiguous patient data |
| Goal modeling | Modeling clinical and organizational processes is insufficient: the intention for each task needs to be explicit |
| Guideline translation | Guidelines are ambiguous and cannot easily be translated into logic rules; contain implicit knowledge that is incompletely specified |
| Information/rule extraction | Ability to automatically extract clinical knowledge and rules from guideline text |
| Localization | Adapting the pathway to local needs (consensus and collaboration). Domain experts creating shareable guidelines must agree on meaning and interpretation of the guideline |
| Maintenance | Need to keep guideline, pathway, and workflow model up to date with latest evidence or changes in clinical workflow |
| Model validation | Validation of encoded model against clinical relevance and expected results for the specific patient; explanation of reasoning |
| Model verification | Internal consistency of the model, well formedness, proofs of properties |
| Organizational change | Existing clinical workflow may need to be adapted in order to successfully implement the system. Staff buy-in, training, and workflow needs; changes of role (eg, increased data entry at point of care) |
| Organizational modeling | Need to model organizational workflow as well as medical knowledge; includes role-based access and security |
| Process modeling | Creating a computer-interpretable model of clinical processes from guidelines and local clinical knowledge |
| Reporting, querying, and visualization | Getting access to the data held in the system for reporting, statistics, visualization |
| Separation of concerns | Separation of medical knowledge from workflow knowledge that can be integrated into a combined clinical and organizational process model at run-time |
| System architecture | Selection of a suitable system architecture congruent with clinical workflow and organizational needs: for example, client-server, service-oriented architecture (SOA), semantic web, transport layer security, authentication, role-based access |
| Temporal abstraction | How to model temporal constraints and periodicity in guidelines and pathways |
| Tooling | Creation of easy to use tools to model guidelines, workflows, and pathways |
| User interface and usability | Accessing the data and guideline/pathway in an easy to use, easy to navigate way; data entry |
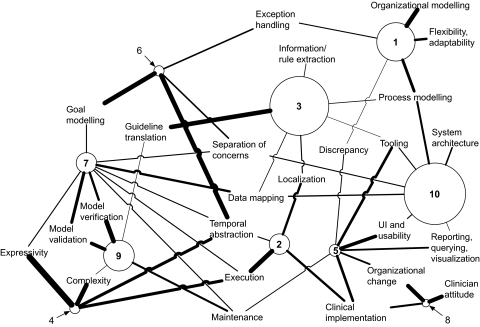
The association between themes was explored using the Galaxy and Matrix views within RefViz. The weight of each theme within each cluster is calculated by RefViz's implementation of PCA and indicates the strength of association between the theme and the cluster, on a scale from −1 (strongest negative association) through 0 (no association) to +1 (strongest positive association). For space reasons, the complete matrix of association scores is not reproduced here. From this, 10 clusters were identified, from which we developed a concept map (figure 2).
Figure 2.
Concept map derived from RefViz Galaxy and Matrix analysis, showing association between study clusters and the ‘challenge theme’ variables. The radius of each circle is proportional to the number of studies in the cluster; the thickness of the line between cluster and theme is proportional to the strength of association between the cluster and the theme.
In figure 2, each cluster is shown as a circle, where the radius of the circle is proportional to the number of papers in the cluster. Only the positively associated themes (ie, with non-zero or non-negative weights) are shown, and the thickness of the line is proportional to the strength of association between the cluster and the theme.
Table 2 provides a description of each challenge theme cluster, where the numeric group identifier relates to each cluster in figure 2.
Table 2.
Description of the challenge theme clusters shown in the concept map of figure 2
| ID | Studies in the cluster | Cluster description |
| 10 | 24 Studies8 14 46 61 67 68 71 73 78 83 85 90 91 96 108 111 112 116 118 121 126 129 134 | Creating a procedural, clinical process model aided by knowledge acquisition tools and supported by the system architecture; mapping declarative concepts between a local electronic health record (EHR) or ‘virtual medical record’ model and the process model; user interface (UI) and usability design congruent with the model; separation of organizational, medical, and UI models |
| 3 | 23 Studies34–36 42 43 45 51 52 59 81 84 87 97 100 105–107 110 113 123 124 133 | Collaborative process between informaticians and domain experts of translating implicit, procedural knowledge into computable rules; extracting declarative and procedural knowledge into a process model; localization of the guideline/pathway for a specific institution and mapping to the local EHR |
| 1 | 15 Studies3 4 39 41 60 74 86 92–94 103 104 115 125 128 | Integration of clinical and organizational processes with regard to institution-specific clinical workflow and preferences; handling workflow exceptions (adaptive organizational workflow); bindings/congruence of enacted workflow with documented clinical processes |
| 9 | 12 Studies49 62 65 66 72 76 88 98 99 101 102 122 | Verification and validity of the clinical process model; formal proofs; model-driven update and maintenance of the knowledge base |
| 7 | 8 Studies35 44 47 48 57 58 70 79 | Clinical validity of EHR—guideline concept mappings; verification of rule-set completeness and consistency; verification and validation of temporal constraints and run-time execution |
| 2 | 8 Studies50 54 75 89 95 114 119 135 | Enactment of the model within local EHR/health information systems (HIS); handling clinician judgment, task sequencing, and temporal constraints, exceptions, variance (adaptive clinical workflow) |
| 5 | 7 Studies17 36 40 53 55 56 109 | Addressing usability barriers to implementation of a computerized guideline or pathway; integration with clinical and organizational workflow; development of new tools to support clinical workflow; modification of existing workflow to fit computerized workflow; reporting workflow/pathway statistics, and exceptions |
| 6 | 4 Studies12 77 82 132 | Formal modeling of clinical goals and their temporal constraints; separation of clinical and organizational knowledge; allowance for unplanned run-time deviations in the model |
| 4 | 4 Studies63 69 130 131 | Handling of complex temporal expressions within the pathway that provides adequate abstraction while remaining computable (trade-off between expressivity and complexity) |
| 8 | 3 Studies38 80 117 | Overcoming the organizational and individual barriers to implementation of a computerized workflow, guideline, or pathway; need for both computerized and real workflow to adapt to each other |
Approaches to implementing process-oriented systems
Electronic health record integration
Twenty-six studies considered the problem of how to integrate a clinical process model with data in the EHR. Of these 26 studies, only three68 79 121 were part of a system implementation within a clinical environment; the remainder were data modeling and/or integration studies within an academic institution. In terms of approach, the studies can be split into three categories:
Studies that advocated the use of the same underlying data model for both the guideline or pathway knowledge model and the EHR, using models such as the HL7 Reference Information Model (RIM), Unified Service Action Model (USAM), or openEHR8 111 116 120
Studies that attempted to map guideline or pathway knowledge model concepts to data items within the EHR via guideline expression languages (eg, GELLO),67 the use of a ‘virtual medical record’ (VMR),52 89 112 121 123 standardized vocabulary resources such as UMLS and SNOMED CT,52 67 85 90 133 or a ‘middleware’ mapping ontology layer,35 64 or manually, on a system-specific basis,68 127 or via a translation table54
Studies that recognized the need for EHR integration, but did not implement it.45 57 108 110 114
Clinical workflow integration and point-of-care use
Studies that considered the use of guidelines and pathways at the point of care can be divided into model formulations and practical implementations of systems.
A number of the model formulation studies suggest that the barrier to the accessibility of guidelines or care pathways might be addressed by developing an ontology that integrates organizational and clinical workflow with EHR data requirements45 67 111 119; however, these papers do not suggest how such point-of-care execution should be implemented in practice.
We found that implementations of workflow integration with point-of-care use tended to be one of three types:
Use of an integrated device for data collection, display, and guideline-based decision support. Examples included the use of ICU bedside monitoring workstations providing real-time data trending, and care plan and test result information,62 the use of mobile devices providing access to clinical guidelines,97 and an emergency triage pathway implemented as a rules-based expert system in a mobile device.96 Evaluation details for each of these, however, were brief, tending to focus on the hardware/software infrastructure and non-quantified statements about system accuracy.
Use of electronic patient encounter forms that mirror the structure of existing paper forms. Examples included a guideline-based system for reminders and order recommendations,84 and a care pathway for proximal femoral fracture91 where guideline-based recommendations were presented as default selections on the form (eg, automatically ticked checkboxes). Neither appeared to offer pathways tailored to the specific needs of the patient, nor made it clear how computer access would be available at all points of the clinical workflow.
Augmented use of paper forms for system input and/or output. Examples included a rules-based system using guidelines encoded in Arden Syntax that used optical character recognition (OCR) to scan paper forms, completed at the bedside, to provide patient-specific, point-of-care recommendations and reminders,109 and a system that provided a print-out of daily workflow tasks according to the care pathway modeled. The printed task lists could be used at the point of care as a clinical reminder, but patient-specific recommendations or decision support were not provided.40
System implementations: knowledge models, software, and architecture
Table 3 defines the eight distinct knowledge model types that were identified. In the studies retrieved, formal task-network models, which support the representation of both guideline concepts and workflow patterns, were the most commonly described and implemented.
Table 3.
Frequency and description of knowledge model types used by studies
| Knowledge model | Description |
| Document model (5 studies, 1 system implemented93) | Human readable document with concepts represented in situ, usually preserving the original structure of the source document (Guideline Elements Mode (GEM) or other document-centric extensible mark-up language (XML) schema) |
| Semantic web (9 studies, 6 systems implemented64 69 74 89 108 119) | Models proposed by the world wide web consortium (W3C) for representing information on the web (web ontology language (OWL) ontologies, Semantic Web Rule Language (SWRL) rules, OWL-S web services) |
| Formal workflow model (8 studies, 3 systems implemented4 71 92) | Formalized workflow constructs underpinned by a formal mathematical model (Petri Nets, Yet Another Workflow Language (YAWL)) |
| Object model (8 studies, 2 systems implemented36 110) | Object-oriented techniques to model collection of hierarchical, interacting classes that represent guideline, workflow, or pathway concepts (Unified Modeling Language (UML), HL7 Reference Information Model (RIM), openEHR) |
| General task-network model (14 studies, 4 systems implemented14 50 103 104) | Flowcharts or process maps without formal semantics (Program Evaluation Review Technique/Critical Path Method (PERT/CPM), activity-on-node) |
| General workflow model (14 studies, 11 systems implemented3 39 74 91 93 103 104 114 118 129 134) | General workflow semantics (Business Process Modeling Notation (BPMN), Business Process Execution Language (BPEL)) |
| Block-structured, procedural, logic rules (20 studies, 11 systems implemented3 43 51 65 72 84 96–98 101 109) | Block-structured, procedural programming languages, and IF…THEN rules (Arden Syntax; decision tables) |
| Formal task-network model (48 studies, 23 systems implemented4 36 40 45 54 57 59 61 62 71 75 78 79 87 89 95 99 111 118 121 126 131 135) | Guideline-based clinical tasks—actions, decisions, queries—that unfold over time, with a formal syntax and semantics (Guideline Interchange Format (GLIF), PROforma, Asbru) |
These models were instantiated in the 54 studies that described a system architecture and prototype implementation (see table 4, available as an online data supplement at www.jamia.org). Eighteen of these (33%) explicitly implemented clinical workflow support via a defined workflow process and/or workflow engine; and 26 (48%) described integration with the EHR, but this appears to be largely limited to conceptual integration—few studies have implemented this in a live, clinical setting.95 Eleven (20%) described both workflow and EHR integration.
System architectures ranged from standalone desktop14 36 51 54 61 65 78 83 87 97–99 117 and web browser applications43 72 119 126 to client-server systems4 40 55 57 62 79 96 103 109 110 135 and distributed, web service applications.3 39 45 59 69 71 74 89 92 93 111 118 121
Systems (not mutually exclusive) included computerized guideline implementations36 40 43 45 50 51 54 57 59 61 62 64 65 69 72 75 78 79 84 89 95–98 101 109–111 118 121 131 (n=31), computerized care pathway systems14 55 83 91 108 114 117 119 126 134 135 (n=11), integrated guideline and WfMSs4 71 89 103 104 111 118 129 (n=8), computerized clinical workflow systems3 39 74 92 93 (n=5), and automated guideline formalization and verification applications87 98 99 (n=3). For the pure guideline-based systems, for the clinical knowledge component there was a general trend from the use of ad hoc, procedural code toward the use of more formal, task-network models. For the care pathway systems, the trend was from the use of informal or unspecified models toward the use of a general workflow model with a task-network or semantic web formalism. Only two of these91 117 appeared to meet all the requirements proposed by Wakamiya and Yamauchi.17
A number of studies suggested that integration of the care pathway or guideline with an organization's clinical workflow and EHR requires a tightly coupled architecture,52 61 62 92 96 109 129 which arguably reduces system portability and interoperability but has the benefit of greater efficiency.79 Others proposed a modular approach to reduce coupling between systems. These still tended to be database-centric, tied to specific mapping tables, database engines, or commercial workflow tools.40 50 103 104 Those that integrated a guideline-based system with an existing EHR typically implemented an ‘event listener’ that monitors the EHR for new clinical events or data from which opportunities for decision support are identified and invoked,4 62 75 89 95 104 135 although this can be inefficient in the use of network and database resources.79
Some recent approaches utilize a service-oriented architecture (SOA), where standard messaging interfaces (such as hypertext transfer protocol (HTTP) and simple object access protocol (SOAP)) enable loose coupling between applications.39 59 64 69 71 74 89 93 111 118 Semantic web-based care pathway architectures64 69 74 89 108 augment the SOA approach by allowing dynamic, context-aware composition of workflows from individual web services. These use W3C standards such as OWL-S and SWRL for defining classes of services and resources, and the rules that relate them.
Toward a conceptual implementation model
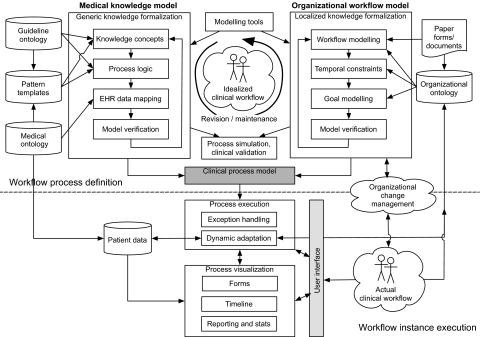
A conceptual model of the implementation process was developed from the theme clusters shown in figure 2 and table 2, and by referencing each cluster back to the studies from which they were derived. The model is shown in figure 3 and described below.
Figure 3.
Conceptual model for implementing process-oriented health information systems.
Development of a process-oriented HIS is an iterative, collaborative process34 43 45 46 52 68 70 81 83 106 115 121 127 that involves defining a clinical process model (shaded in figure) comprising formalized medical knowledge (usually from guidelines) (top-left of figure) and organizational workflow (top-right of figure). A graphical knowledge acquisition tool is typically used to assist in this task.3 4 41 45 48 50 65 71 72 78 91 95 111 118 135 The model (typically derived from one or more of the types presented in table 3) represents an idealized view of the knowledge concepts, processes, and rules of clinical workflow required to enact the guideline or pathway, and tailored to local intervention strategies.
Medical knowledge formalization typically involves the use of an ontology for the guideline concepts and process logic,4 12 42 44 45 50 52 64 66 67 69 70 74 75 96 103 108 119 123 126 and a standard medical terminology to map guideline concepts to terms in the EHR data model or VMR.4 35 52 54 64 67–69 89 108 112 121 123 127 133 Extraction and formalization of rules from guideline statements can be automated, sometimes with a high degree of recall and precision,42 87 124 via the use of linguistic phrase pattern templates37 42 and information extraction pipelines.87 113 Such techniques may be useful for facilitating automatic updates to the knowledge base.88
This generic model needs to be localized to the setting/institution.4 52 89 101 127 This task can be commenced prior to modeling, to create a ‘consensus’ version of the guideline,45 46 51 59 95 ready for formalization, or the encoded generic model can be shared among institutions, each adapting it according to local needs and data items available in the institution's EHR.52 57 71 101 119 127 Localization also involves creation of an organizational workflow model, or addition of workflow concepts to the formalized medical knowledge model. Workflow modeling may make use of an organizational ontology4 73 74 92 103 to formalize tasks, roles, and treatment goals.4 12 44 82 90 132 Definition of temporal constraints, often not present in the guidelines themselves,77 is required for activity sequencing and scheduling.50 63 73 77 102 124 130–132
Model checking techniques and tools provide formal means of verifying that encoded models are correct and consistent,4 48 49 66 76 77 99 102 particularly when maintaining and updating them.102 Simulated runs of the model are used to ensure that the output is clinically valid.4 43 52 54 57 59 63 64 66 114 122 135
To execute the clinical process model within a HIS, architecture, user-interface design, and mode of delivery need careful consideration in order to be congruent with actual clinical workflow.14 17 36 56 61 85 91 96 109 This includes visualization of the run-time pathway,61 design of on-screen forms based on the paper forms of a manual care pathway,83 84 91 or automatic generation of forms directly from the pathway ontology or process model.69 The enacted process should allow dynamic adaptation at run-time: this may be manual and clinician-led, where tasks can be skipped, repeated, or new tasks added,3 41 57 93 or may be system-led via reasoning over new knowledge added to the ontology at run-time.74 108
Implementation in a live, clinical environment requires strategies for organizational change management to overcome inertia and allay concerns over lack of support and perceived threats to professional autonomy that workflow automation may bring.38 80 117
Discussion
The conceptual model for the implementation of process-oriented systems comprises a distillation of the cross-cutting challenge themes that have been abstracted from 15 years of published research. It attempts to provide a concise synthesis for practitioners and implementers, by summarizing the various approaches that have been proposed and implemented to date, while remaining neutral in terms of software, hardware, and knowledge/information model. The use of thematic analysis and PCA to summarize the findings of a large corpus of publications may be useful in future reviews, although further work is needed on applying and validating this technique.
In the system implementations that we reviewed, there was the assumption that real-world clinical processes are best represented by a formal model in which discrete events occur, performed by users with pre-defined roles. However, the application of computerized workflow systems to the complex, contextual nature of clinical workflow has recently been questioned.2 It may not always be practical to decompose care-related tasks into a sequence of discrete workflow steps. Some tasks may be partially, or provisionally, completed while other tasks are carried out in parallel. New knowledge gained from downstream or parallel clinical processes may allow provisionally undertaken tasks to be completed, or may require them to be canceled.
The ‘semantic web’ approaches to solving this ‘adaptive workflow’ problem (which is a concern also discussed in the general literature on workflow systems136 137) have, in addition to the implementations described here, so far yielded a care pathway ontology138 139 which appears to share many features of older task-network models. However, the crucial distinction is that the semantic web approaches represent an ‘open world’ view140 that allows new facts and relationships to be expressed without the constraint of a pre-defined schema,108 whereas earlier approaches only permit knowledge statements that are explicitly permitted by the schema. Full realization of these approaches would require a knowledge backbone of best practice on the semantic web,138 and semi-automatic methods for transforming guideline text into a standard formalism, although recent work in this area has achieved some useful results.42 87 124
We have noted the transition from the reporting of standalone systems to the reporting of complete enterprise integration architectures.89 Whether these architectures, in combination with semantic web approaches, can solve the problem of clinical workflow integration and adaptation, is an area of current research.141 The implementation of adaptive, multi-agent, semantically aware, service-oriented workflows, incorporating formal models of clinical guidelines, appears to be a major challenge.142
By focusing on descriptive studies to provide a rich picture of a process, we have not considered any measures of the effect of these systems on clinical practice, nor which parts of the process are associated with successful outcomes. However, a recent systematic review of the effectiveness of clinical pathways noted that the poor quality of reporting of the pathway implementation process prevented analysis of factors that might be critical to success.24 In the system implementation studies we selected, the implementation process was generally well described, but evaluations tended to be formative and weak. Future reporting of implementations should contain a richer evaluation of both the process and the outcome, to enable future systematic reviews to consider both aspects, and to determine the relative importance of the challenge themes identified.
Review limitations
Our review has only considered studies that were published in English in peer-reviewed journals or conference proceedings published between 1995 and 2010. Consideration of information from additional sources, for example, public- and privately-funded research consortia, technical reports, and professional textbooks, might lead to additional insights.
One criticism of attempting to carry out a meta-synthesis of qualitative research is that the results may have little validity, as they are based on a third level of interpretation, far removed from the original event.27 Although development of the challenge themes was based on those identified in an earlier expert opinion paper,25 these would need to be validated by other researchers to improve the reliability and validity of our findings.
Conclusion
We have surveyed the literature on the computerization of clinical workflow, guidelines, and pathways and have extracted the underlying, cross-cutting themes that describe the challenges to implementing process-oriented HIS using thematic analysis techniques. We have used PCA to cluster these themes into 10 distinct groups, from which a conceptual model of the implementation process was developed.
The development of systems supporting individual clinical decisions is evolving toward the implementation of adaptive care pathways on the semantic web, incorporating formal, clinical, and organizational ontologies, and the use of WfMS. Such architectures now need to be implemented and evaluated on a wider scale within clinical settings.
Acknowledgments
We thank the two anonymous reviewers and Professor Francis Lau for their valuable comments on an earlier version of the manuscript.
Appendix. Database search strategy
The following broad search concepts were used to query ScienceDirect and Web of Science:
-
Concept 1: computer systems
(systems OR electronic OR computer*) AND
-
Concept 2: healthcare
(health* OR clinical OR care OR medical) AND
-
Concept 3: guidelines and workflows
(pathway OR workflow OR careflow OR guideline)
These three concepts were combined to perform a title search on ScienceDirect and Web of Science:
TITLE ((systems OR electronic OR computer*) AND (health* OR clinical OR care OR medical) AND (pathway OR workflow OR careflow OR guideline))
The following all-fields search statement was performed in ScienceDirect:
ALL (workflow pathways plans guidelines)
The following search statements were executed on PubMed and the results combined:
(electronic OR computer-interpretable OR computerized OR computerised) AND ((care OR clinical) pathway)
modelling AND ((clinical guideline) OR ((care OR clinical) pathway) OR workflow)
workflow AND ((care OR clinical) pathway)
(clinical guideline) AND ((care OR clinical) pathway)
Footnotes
Funding: Phil Gooch acknowledges funding and support from the Engineering and Physical Sciences Research Council (EPSRC) in carrying out this review as part of his PhD studentship (EP/P504872/1).
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
In an effort to remove some of the barriers to the adoption and use of clinical guidelines at the point of care, several formalisms for encoding guideline content into a computer-interpretable format have been proposed. A number of comparative analyses of the most developed formalisms have been published.5–7
References
- 1.Workflow Management Coalition WFMC-TC-1011 Ver 3 Terminology and Glossary English. Winchester, UK: Workflow Management Coalition, 1999 [Google Scholar]
- 2.Niazkhani Z, Pirnejad H, Berg M, et al. The impact of computerized provider order entry systems on inpatient clinical workflow: a literature review. J Am Med Inform Assoc 2009;16:539–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dadam P, Reichert M, Kuhn K, eds. Clinical workflows—the killer application for process-oriented information systems? Proc 4th Int'l Conf on Business Information Systems (BIS '00). Poznan, Poland: Ulmer Informatik-Berichte, 2000 [Google Scholar]
- 4.Quaglini S, Stefanelli M, Cavallini A, et al. Guideline-based careflow systems. Artif Intell Med 2000;20:5–22 [DOI] [PubMed] [Google Scholar]
- 5.Isern D, Moreno A. Computer-based execution of clinical guidelines: a review. Int J Med Inform 2008;77:787–808 [DOI] [PubMed] [Google Scholar]
- 6.Peleg M, Tu S, Bury J, et al. Comparing computer-interpretable guideline models: a case-study approach. J Am Med Inform Assoc 2003;10:52–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Clercq P, Kaiser K, Hasman A. Computer-interpretable guideline formalisms. Stud Health Technol Inform 2008;139:22–43 [PMC free article] [PubMed] [Google Scholar]
- 8.Schadow G, Russler DC, McDonald CJ. Conceptual alignment of electronic health record data with guideline and workflow knowledge. Int J Med Inform 2001;64:259–74 [DOI] [PubMed] [Google Scholar]
- 9.Campbell H, Hotchkiss R, Bradshaw N, et al. Integrated care pathways. BMJ 1998;316:133–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zander K. Nursing case management: strategic management of cost and quality outcomes. J Nurs Adm 1988;18:23–30 [PubMed] [Google Scholar]
- 11.European Pathways Association Clinical/Care Pathways. 2007. http://www.e-p-a.org/000000979b08f9803/index.html (accessed 28 Sep 2010). [Google Scholar]
- 12.Fox J, Alabassi A, Patkar V, et al. An ontological approach to modelling tasks and goals. Comput Biol Med 2006;36:837–56 [DOI] [PubMed] [Google Scholar]
- 13.Page R, Herbert I. Developing e-pathway standards. In: de Luc K, Todd J, eds. E-Pathways: Computers and the Patient's Journey Through Care. Oxford: Radcliffe Medical Press, 2003:155–82 [Google Scholar]
- 14.Chu S, Cesnik B. Improving clinical pathway design: lessons learned from a computerised prototype. Int J Med Inform 1998;51:1–11 [DOI] [PubMed] [Google Scholar]
- 15.Morris AH. Developing and implementing computerized protocols for standardization of clinical decisions. Ann Intern Med 2000;132:373–83 [DOI] [PubMed] [Google Scholar]
- 16.de Luc K, Todd J. Introduction. In: de Luc K, Todd J, eds. E-Pathways: Computers and the Patient's Journey Through Care. Oxford: Radcliffe Medical Press, 2003:1–14 [Google Scholar]
- 17.Wakamiya S, Yamauchi K. What are the standard functions of electronic clinical pathways? Int J Med Inform 2009;78:543–50 [DOI] [PubMed] [Google Scholar]
- 18.Kawamoto K. Integration of knowledge resources into applications to enable clinical decision support: architectural considerations. In: Greenes RA, ed. Clinical Decision Support: The Road Ahead. Burlington, VT: Academic Press, 2007:502–37 [Google Scholar]
- 19.Fox J, Black E, Chronakis I, et al. From guidelines to careflows: modelling and supporting complex clinical processes. Stud Health Technol Inform 2008;139:44–62 [PubMed] [Google Scholar]
- 20.Shiffman RN, Liaw Y, Brandt CA, et al. Computer-based guideline implementation systems: a systematic review of functionality and effectiveness. J Am Med Inform Assoc 1999;6:104–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latoszek-Berendsen A, Tange H, van den Herik HJ, et al. From clinical practice guidelines to computer-interpretable guidelines. A literature overview. Methods Inf Med 2010;49:550–70 [DOI] [PubMed] [Google Scholar]
- 22.Kawamoto K, Houlihan CA, Balas EA, et al. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ 2005;330:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garg AX, Adhikari NK, McDonald H, et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA 2005;293:1223–38 [DOI] [PubMed] [Google Scholar]
- 24.Rotter T, Kinsman L, James E, et al. Clinical Pathways: Effects on Professional Practice, Patient Outcomes, Length of Stay and Hospital Costs. Dresden, Germany: Department of Public Health, Dresden Medical School, University of Dresden, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Song X, Hwong B, Matos G, et al. Understanding requirements for computer-aided healthcare workflows: experiences and challenges. ICSE '06: Proceedings of the 28th International Conference on Software Engineering. New York, NY, USA: ACM, 2006:930–4 [Google Scholar]
- 26.Greenhalgh T, Taylor R. How to read a paper: papers that go beyond numbers (qualitative research). BMJ 1997;315:740–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans D, Pearson A. Systematic reviews of qualitative research. Clin Eff Nurs 2001;5:111–19 [Google Scholar]
- 28.Burns N. Standards for qualitative research. Nurs Sci Q 1989;2:4–52 [DOI] [PubMed] [Google Scholar]
- 29.Flick U. Coding and Categorizing. An Introduction to Qualitative Research. 4th edn London: Sage, 2009:305–32 [Google Scholar]
- 30.Miles MB, Huberman AM. Early steps in analysis. Qualitative Data Analysis: An Expanded Sourcebook. Thousand Oaks, CA: Sage, 1994:55–89 [Google Scholar]
- 31.Glassman NR. RefViz 1.0.1. J Med Libr Assoc 2005;93:293–4 [Google Scholar]
- 32.Thomson Reuters RIS Format Specifications. 2001. http://www.refman.com/support/risformat_intro.asp (accessed 8 Apr 2011). [Google Scholar]
- 33.Jolliffe IT. Principal Component Analysis. 2nd edn New York, NY, USA: Springer, 2002 [Google Scholar]
- 34.Peleg M, Gutnik LA, Snow V, et al. Interpreting procedures from descriptive guidelines. J Biomed Inform 2006;39:184–95 [DOI] [PubMed] [Google Scholar]
- 35.Peleg M, Keren S, Denekamp Y. Mapping computerized clinical guidelines to electronic medical records: Knowledge-data ontological mapper (KDOM). J Biomed Inform 2008;41:180–201 [DOI] [PubMed] [Google Scholar]
- 36.Peleg M, Shachak A, Wang D, et al. Using multi-perspective methodologies to study users' interactions with the prototype front end of a guideline-based decision support system for diabetic foot care. Int J Med Inform 2009;78:482–93 [DOI] [PubMed] [Google Scholar]
- 37.Peleg M, Tu SW. Design patterns for clinical guidelines. Artif Intell Med 2009;47:1–24 [DOI] [PubMed] [Google Scholar]
- 38.Phansalkar S, Weir CR, Morris AH, et al. Clinicians' perceptions about use of computerized protocols: a multicenter study. Int J Med Inform 2008;77:184–93 [DOI] [PubMed] [Google Scholar]
- 39.Poulymenopoulou M, Malamateniou F, Vassilacopoulos G. Emergency healthcare process automation using workflow technology and web services. Med Inform Internet Med 2003;28:195–207 [DOI] [PubMed] [Google Scholar]
- 40.Quaglini S, Grandi M, Baiardi P, et al. A computerized guideline for pressure ulcer prevention. Int J Med Inform 2000;58–59:207–17 [DOI] [PubMed] [Google Scholar]
- 41.Quaglini S, Stefanelli M, Lanzola G, et al. Flexible guideline-based patient careflow systems. Artif Intell Med 2001;22:65–80 [DOI] [PubMed] [Google Scholar]
- 42.Serban R, ten Teije A, van Harmelen F, et al. Extraction and use of linguistic patterns for modelling medical guidelines. Artif Intell Med 2007;39:137–49 [DOI] [PubMed] [Google Scholar]
- 43.Seroussi B, Bouaud J, Chatellier G. Guideline-based modeling of therapeutic strategies in the special case of chronic diseases. Int J Med Inform 2005;74:89–99 [DOI] [PubMed] [Google Scholar]
- 44.Shahar Y, Miksch S, Johnson P. The Asgaard project: a task-specific framework for the application and critiquing of time-oriented clinical guidelines. Artif Intell Med 1998;14:29–51 [DOI] [PubMed] [Google Scholar]
- 45.Shahar Y, Young O, Shalom E, et al. A framework for a distributed, hybrid, multiple-ontology clinical-guideline library, and automated guideline-support tools. J Biomed Inform 2004;37:325–44 [DOI] [PubMed] [Google Scholar]
- 46.Shalom E, Shahar Y, Taieb-Maimon M, et al. A quantitative assessment of a methodology for collaborative specification and evaluation of clinical guidelines. J Biomed Inform 2008;41:889–903 [DOI] [PubMed] [Google Scholar]
- 47.Shiffman RN, Michel G, Essaihi A, et al. Bridging the guideline implementation gap: a systematic, document-centered approach to guideline implementation. J Am Med Inform Assoc 2004;11:418–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stausberg J, Bilir H, Waydhas C, et al. Guideline validation in multiple trauma care through business process modeling. Int J Med Inform 2003;70:301–7 [DOI] [PubMed] [Google Scholar]
- 49.ten Teije A, Marcos M, Balser M, et al. Improving medical protocols by formal methods. Artif Intell Med 2006;36:193–209 [DOI] [PubMed] [Google Scholar]
- 50.Terenziani P, Molino G, Torchio M. A modular approach for representing and executing clinical guidelines. Artif Intell Med 2001;23:249–76 [DOI] [PubMed] [Google Scholar]
- 51.Tierney WM, Overhage JM, Takesue BY, et al. Computerizing guidelines to improve care and patient outcomes: the example of heart failure. J Am Med Inform Assoc 1995;2:316–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tu SW, Campbell JR, Glasgow J, et al. The SAGE guideline model: achievements and overview. J Am Med Inform Assoc 2007;14:589–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Unertl KM, Weinger MB, Johnson KB, et al. Describing and modeling workflow and information flow in chronic disease care. J Am Med Inform Assoc 2009;16:826–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Veselý A, Zvárová J, Peleska J, et al. Medical guidelines presentation and comparing with Electronic Health Record. Int J Med Inform 2006;75:240–5 [DOI] [PubMed] [Google Scholar]
- 55.Wakamiya S, Yamauchi K. A new approach to systematization of the management of paper-based clinical pathways. Comput Methods Programs Biomed 2006;82:169–76 [DOI] [PubMed] [Google Scholar]
- 56.Wallace CJ, Bigelow S, Xu X, et al. Collaborative practice: usability of text-based, electronic patient care guidelines. Comput Inform Nurs 2007;25:39–44 [DOI] [PubMed] [Google Scholar]
- 57.Wang D, Peleg M, Tu SW, et al. Design and implementation of the GLIF3 guideline execution engine. J Biomed Inform 2004;37:305–18 [DOI] [PubMed] [Google Scholar]
- 58.Wright A, Sittig DF. A framework and model for evaluating clinical decision support architectures. J Biomed Inform 2008;41:982–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Young O, Shahar Y, Liel Y, et al. Runtime application of Hybrid-Asbru clinical guidelines. J Biomed Inform 2007;40:507–26 [DOI] [PubMed] [Google Scholar]
- 60.Aarts J, Ash J, Berg M. Extending the understanding of computerized physician order entry: implications for professional collaboration, workflow and quality of care. Int J Med Inform 2007;76(Suppl 1):S4–13 [DOI] [PubMed] [Google Scholar]
- 61.Aigner W, Miksch S. CareVis: integrated visualization of computerized protocols and temporal patient data. Artif Intell Med 2006;37:203–18 [DOI] [PubMed] [Google Scholar]
- 62.Allart L, Vilhelm C, Mehdaoui H, et al. An architecture for online comparison and validation of processing methods and computerized guidelines in intensive care units. Comput Methods Programs Biomed 2009;93:93–103 [DOI] [PubMed] [Google Scholar]
- 63.Anselma L, Terenziani P, Montani S, et al. Towards a comprehensive treatment of repetitions, periodicity and temporal constraints in clinical guidelines. Artif Intell Med 2006;38:171–95 [DOI] [PubMed] [Google Scholar]
- 64.Argüello Casteleiro M, Des J, Prieto MJ, et al. Executing medical guidelines on the web: towards next generation healthcare. Knowl Base Syst 2009;22:545–51 [Google Scholar]
- 65.Bindels R, de Clercq PA, Winkens RA, et al. A test ordering system with automated reminders for primary care based on practice guidelines. Int J Med Inform 2000;58–59:219–33 [DOI] [PubMed] [Google Scholar]
- 66.Bottrighi A, Giordano L, Molino G, et al. Adopting model checking techniques for clinical guidelines verification. Artif Intell Med 2009;48:1–19 [DOI] [PubMed] [Google Scholar]
- 67.Boxwala AA, Peleg M, Tu S, et al. GLIF3: a representation format for sharable computer-interpretable clinical practice guidelines. J Biomed Inform 2004;37:147–61 [DOI] [PubMed] [Google Scholar]
- 68.Brokel JM, Shaw MG, Nicholson C. Expert clinical rules automate steps in delivering evidence-based care in the electronic health record. Comput Inform Nurs 2006;24:196–205 [DOI] [PubMed] [Google Scholar]
- 69.Casteleiro MA, Des Diz JJ. Clinical practice guidelines: a case study of combining OWL-S, OWL, and SWRL. Knowl Base Syst 2008;21:247–55 [Google Scholar]
- 70.Choi J, Currie LM, Wang D, et al. Encoding a clinical practice guideline using guideline interchange format: a case study of a depression screening and management guideline. Int J Med Inform 2007;76(Suppl 2):S302–7 [DOI] [PubMed] [Google Scholar]
- 71.Ciccarese P, Caffi E, Quaglini S, et al. Architectures and tools for innovative Health Information Systems: the Guide Project. Int J Med Inform 2005;74:553–62 [DOI] [PubMed] [Google Scholar]
- 72.Colombet I, Aguirre-Junco AR, Zunino S, et al. Electronic implementation of guidelines in the EsPeR system: a knowledge specification method. Int J Med Inform 2005;74:597–604 [DOI] [PubMed] [Google Scholar]
- 73.Combi C, Gozzi M, Oliboni B, et al. Temporal similarity measures for querying clinical workflows. Artif Intell Med 2009;46:37–54 [DOI] [PubMed] [Google Scholar]
- 74.Dang J, Hedayati A, Hampel K, et al. An ontological knowledge framework for adaptive medical workflow. J Biomed Inform 2008;41:829–36 [DOI] [PubMed] [Google Scholar]
- 75.de Clercq PA, Hasman A, Blom JA, et al. Design and implementation of a framework to support the development of clinical guidelines. Int J Med Inform 2001;64:285–318 [DOI] [PubMed] [Google Scholar]
- 76.Duftschmid G, Miksch S. Knowledge-based verification of clinical guidelines by detection of anomalies. Artif Intell Med 2001;22:23–41 [DOI] [PubMed] [Google Scholar]
- 77.Duftschmid G, Miksch S, Gall W. Verification of temporal scheduling constraints in clinical practice guidelines. Artif Intell Med 2002;25:93–121 [DOI] [PubMed] [Google Scholar]
- 78.Fox J, Johns N, Lyons C, et al. PROforma: a general technology for clinical decision support systems. Comput Methods Programs Biomed 1997;54:59–67 [DOI] [PubMed] [Google Scholar]
- 79.Goud R, Hasman A, Peek N. Development of a guideline-based decision support system with explanation facilities for outpatient therapy. Comput Methods Programs Biomed 2008;91:145–53 [DOI] [PubMed] [Google Scholar]
- 80.Goud R, van Engen-Verheul M, de Keizer NF, et al. The effect of computerized decision support on barriers to guideline implementation: a qualitative study in outpatient cardiac rehabilitation. Int J Med Inform 2010;79:430–7 [DOI] [PubMed] [Google Scholar]
- 81.Green CJ, Fortin P, Maclure M, et al. Information system support as a critical success factor for chronic disease management: necessary but not sufficient. Int J Med Inform 2006;75:818–28 [DOI] [PubMed] [Google Scholar]
- 82.Grando A, Peleg M, Glasspool D. A goal-oriented framework for specifying clinical guidelines and handling medical errors. J Biomed Inform 2010;43:287–99 [DOI] [PubMed] [Google Scholar]
- 83.Hayward-Rowse L, Whittle T. A pilot project to design, implement and evaluate an electronic integrated care pathway. J Nurs Manag 2006;14:564–71 [DOI] [PubMed] [Google Scholar]
- 84.Henry SB, Douglas K, Galzagorry G, et al. A template-based approach to support utilization of clinical practice guidelines within an electronic health record. J Am Med Inform Assoc 1998;5:237–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoelzer S, Schweiger R, Dudeck J. Representation of practice guidelines with XML–modeling with XML schema. Methods Inf Med 2002;41:305–12 [PubMed] [Google Scholar]
- 86.Johnson KB, FitzHenry F. Case report: activity diagrams for integrating electronic prescribing tools into clinical workflow. J Am Med Inform Assoc 2006;13:391–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kaiser K, Akkaya C, Miksch S. How can information extraction ease formalizing treatment processes in clinical practice guidelines? A method and its evaluation. Artif Intell Med 2007;39:151–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaiser K, Miksch S. Versioning computer-interpretable guidelines: semi-automatic modeling of ‘Living Guidelines’ using an information extraction method. Artif Intell Med 2009;46:55–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Laleci GB, Dogac A. A semantically enriched clinical guideline model enabling deployment in heterogeneous healthcare environments. IEEE Trans Inf Technol Biomed 2009;13:263–73 [DOI] [PubMed] [Google Scholar]
- 90.Latoszek-Berendsen A, Talmon J, de Clercq P, et al. With good intentions. Int J Med Inform 2007;76(Suppl 3):S440–6 [DOI] [PubMed] [Google Scholar]
- 91.Lenz R, Blaser R, Beyer M, et al. IT support for clinical pathways: lessons learned. Int J Med Inform 2007;76(Suppl 3):S397–402 [DOI] [PubMed] [Google Scholar]
- 92.Leonardi G, Panzarasa S, Quaglini S, et al. Interacting agents through a web-based health serviceflow management system. J Biomed Inform 2007;40:486–99 [DOI] [PubMed] [Google Scholar]
- 93.Malamateniou F, Vassilacopoulos G. Developing a virtual patient record using XML and web-based workflow technologies. Int J Med Inform 2003;70:131–9 [DOI] [PubMed] [Google Scholar]
- 94.Malhotra S, Jordan D, Shortliffe E, et al. Workflow modeling in critical care: piecing together your own puzzle. J Biomed Inform 2007;40:81–92 [DOI] [PubMed] [Google Scholar]
- 95.Maviglia SM, Zielstorff RD, Paterno M, et al. Automating complex guidelines for chronic disease: lessons learned. J Am Med Inform Assoc 2003;10:154–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Michalowski W, Slowinski R, Wilk S, et al. Design and development of a mobile system for supporting emergency triage. Methods Inf Med 2005;44:14–24 [PubMed] [Google Scholar]
- 97.Mikulich VJ, Liu YC, Steinfeldt J, et al. Implementation of clinical guidelines through an electronic medical record: physician usage, satisfaction and assessment. Int J Med Inform 2001;63:169–78 [DOI] [PubMed] [Google Scholar]
- 98.Miller DW, Jr, Frawley SJ, Miller PL. Using semantic constraints to help verify the completeness of a computer-based clinical guideline for childhood immunization. Comput Methods Programs Biomed 1999;58:267–80 [DOI] [PubMed] [Google Scholar]
- 99.Miller PL. Domain-constrained generation of clinical condition sets to help test computer-based clinical guidelines. J Am Med Inform Assoc 2001;8:131–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Miller PL, Frawley SJ. Trade-offs in producing patient-specific recommendations from a computer-based clinical guideline: a case study. J Am Med Inform Assoc 1995;2:238–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Miller PL, Frawley SJ, Sayward FG. Informatics issues in the national dissemination of a computer-based clinical guideline: a case study in childhood immunization. Proc AMIA Symp 2000:580–4 [PMC free article] [PubMed] [Google Scholar]
- 102.Miller PL, Frawley SJ, Sayward FG. Maintaining and incrementally revalidating a computer-based clinical guideline: a case study. J Biomed Inform 2001;34:99–111 [DOI] [PubMed] [Google Scholar]
- 103.Panzarasa S, Maddè S, Quaglini S, et al. Evidence-based careflow management systems: the case of post-stroke rehabilitation. J Biomed Inform 2002;35:123–39 [DOI] [PubMed] [Google Scholar]
- 104.Panzarasa S, Stefanelli M. Workflow management systems for guideline implementation. Neurol Sci 2006;27(Suppl 3):S245–9 [DOI] [PubMed] [Google Scholar]
- 105.Patel VL, Branch T, Wang D, et al. Analysis of the process of encoding guidelines: a comparison of GLIF2 and GLIF3. Methods Inf Med 2002;41:105–13 [PubMed] [Google Scholar]
- 106.Patel VL, Allen VG, Arocha JF, et al. Representing clinical guidelines in GLIF: individual and collaborative expertise. J Am Med Inform Assoc 1998;5:467–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Patel VL, Arocha JF, Diermeier M, et al. Methods of cognitive analysis to support the design and evaluation of biomedical systems: the case of clinical practice guidelines. J Biomed Inform 2001;34:52–66 [DOI] [PubMed] [Google Scholar]
- 108.Alexandrou D, Xenikoudakis F, Mentzas G, eds. Adaptive clinical pathways with semantic web rules. Proceedings of the First International Conference on Health Informatics, HEALTHINF 2008. Funchal, Madeira, Portugal: INSTICC—Institute for Systems and Technologies of Information, Control and Communication, 2008 [Google Scholar]
- 109.Anand V, Biondich PG, Liu G, et al. Child health improvement through computer automation: the CHICA system. Stud Health Technol Inform 2004;107(Pt 1):187–91 [PubMed] [Google Scholar]
- 110.Barnes M, Barnett GO. An architecture for a distributed guideline server. Proc Annu Symp Comput Appl Med Care 1995:233–7 [PMC free article] [PubMed] [Google Scholar]
- 111.Barretto SA, Warren J, Goodchild A, et al. Linking guidelines to Electronic Health Record design for improved chronic disease management. AMIA Annu Symp Proc 2003:66–70 [PMC free article] [PubMed] [Google Scholar]
- 112.Bernstein K, Bruun-Rasmussen M, Vingtoft S. A method for specification of structured clinical content in electronic health records. Stud Health Technol Inform 2006;124:515–21 [PubMed] [Google Scholar]
- 113.Bouffier A, Poibeau T. Automatically restructuring practice guidelines using the GEM DTD. Proceedings of the Workshop on BioNLP 2007: Biological, Translational, and Clinical Language Processing (Prague, Czech Republic, June 29–29, 2007. Morristown, NJ: Association for Computational Linguistics, 2007:113–20 [Google Scholar]
- 114.Burkle T, Baur T, Hoss N. Clinical pathways development and computer support in the EPR: lessons learned. Stud Health Technol Inform 2006;124:1025–30 [PubMed] [Google Scholar]
- 115.Cabitza F, Sarini M, Simone C, eds. Providing awareness through situated process maps: the hospital care case. GROUP '07: Proceedings of the 2007 International ACM Conference on Supporting Group Work. New York, NY, USA: ACM, 2007 [Google Scholar]
- 116.Chen R, Georgii-Hemming P, Ahlfeldt H. Representing a chemotherapy guideline using openEHR and rules. Stud Health Technol Inform 2009;150:653–7 [PubMed] [Google Scholar]
- 117.Chu S. Computerised clinical pathway as process quality improvement tool. In: Patel VL, Rogers R, Haux R, eds. Medinfo 2001: Proceedings of the 10th World Congress on Medical Informatics, Pts 1 and 2. Amsterdam: IOS Press, 2001:1135–9 [PubMed] [Google Scholar]
- 118.Ciccarese P, Caffi E, Boiocchi L, et al. A guideline management system. Stud Health Technol Inform 2004;107(Pt 1):28–32 [PubMed] [Google Scholar]
- 119.Daniyal A, Abidi SR, Abidi SS. Computerizing clinical pathways: ontology-based modeling and execution. Stud Health Technol Inform 2009;150:643–7 [PubMed] [Google Scholar]
- 120.Ebrahiminia V, Duclos C, Toussi ME, et al. Representing the patient's therapeutic history in medical records and in guideline recommendations for chronic diseases using a unique model. Stud Health Technol Inform 2005;116:101–6 [PubMed] [Google Scholar]
- 121.Eccher C, Seyfang A, Ferro A, et al. Embedding oncologic protocols into the provision of care: the Oncocure project. Stud Health Technol Inform 2009;150:663–7 [PubMed] [Google Scholar]
- 122.Fox J, Bury J. A quality and safety framework for point-of-care clinical guidelines. Proc AMIA Symp 2000:245–9 [PMC free article] [PubMed] [Google Scholar]
- 123.Hrabak KM, Campbell JR, Tu SW, et al. Creating interoperable guidelines: requirements of vocabulary standards in immunization decision support. Stud Health Technol Inform 2007;129(Pt 2):930–4 [PubMed] [Google Scholar]
- 124.Lobach DF, Kerner N. A systematic process for converting text-based guidelines into a linear algorithm for electronic implementation. Proc AMIA Symp 2000:507–11 [PMC free article] [PubMed] [Google Scholar]
- 125.Mans R, Schonenberg H, Leonardi G, et al. Process mining techniques: an application to stroke care. Stud Health Technol Inform 2008;136:573–8 [PubMed] [Google Scholar]
- 126.Patkar V, Fox J. Clinical guidelines and care pathways: a case study applying PROforma decision support technology to the breast cancer care pathway. Stud Health Technol Inform 2008;139:233–42 [PubMed] [Google Scholar]
- 127.Peleg M, Wang D, Fodor A, et al. Lessons learned from adapting a generic narrative diabetic-foot guideline to an institutional decision-support system. Stud Health Technol Inform 2008;139:243–52 [PubMed] [Google Scholar]
- 128.Russello G, Dong C, Dulay N. Personalising situated workflow systems for pervasive healthcare applications. 2nd International Conference on Pervasive Computing Technologies for Healthcare. New York: IEEE, 2008:173–7 [Google Scholar]
- 129.Sartipi K, Mohammad HY, Douglas GD, eds. Mined-knowledge and decision support services in electronic health. Proceedings of the International Workshop on Systems Development in SOA Environments. Washington, DC, USA: IEEE Computer Society, 2007 [Google Scholar]
- 130.Seyfang A, Miksch S. Advanced temporal data abstraction for guideline execution. Stud Health Technol Inform 2004;139:263–72 [PubMed] [Google Scholar]
- 131.Seyfang A, Paesold M, Votruba P, et al. Improving the execution of clinical guidelines and temporal data abstraction high-frequency domains. Stud Health Technol Inform 2008;139:263–72 [PubMed] [Google Scholar]
- 132.Shahar Y, Miksch S, Johnson P. An intention-based language for representing clinical guidelines. Proc AMIA Annu Fall Symp 1996:592–6 [PMC free article] [PubMed] [Google Scholar]
- 133.Sonnenberg FA, Hagerty CG, Acharya J, et al. Vocabulary requirements for implementing clinical guidelines in an electronic medical record: a case study. AMIA Annu Symp Proc 2005:709–13 [PMC free article] [PubMed] [Google Scholar]
- 134.Tschopp M, Despond M, Grauser D, et al. Computer-based physician order entry: implementation of clinical pathways. Stud Health Technol Inform 2009;150:673–7 [PubMed] [Google Scholar]
- 135.Verlaenen K, Joosen W, Verbaeten P, eds. Arriclides: an architecture integrating clinical decision support models. 40th Annual Hawaii International Conference on System Sciences (HICSS'07). Washington, DC, USA: IEEE Computer Society, 2007 [Google Scholar]
- 136.Buhler PA, Vidal JM. Towards Adaptive Workflow Enactment Using Multiagent Systems. Inform Tech Manag 2005;6:61–87 [Google Scholar]
- 137.Guenther CW, Reichert M, van der Aalst WM, eds. Supporting flexible processes with adaptive workflow and case handling. Proceedings WETICE'08, 3rd IEEE Workshop on Agile Cooperative Process-aware Information Systems (ProGility'08). Rome, Italy: IEEE Computer Society, 2008 [Google Scholar]
- 138.Abidi SR, Chen H, eds. Adaptable personalized care planning via a semantic web framework. 20th Intl Cong European Fed for Medical Informatics Maastricht. Maastricht: IOS Press, 2006 [Google Scholar]
- 139.Hurley KF, Abidi SR, eds. Ontology engineering to model clinical pathways: towards the computerization and execution of clinical pathways. Twentieth IEEE International Symposium on Computer-Based Medical Systems (CBMS'07). Maribor, Slovenia: IEEE Computer Society, 2007 [Google Scholar]
- 140.Wang HH, Noy N, Rector A, et al., eds. Frames and OWL side by side. 10th International Protege Conference. Budapest, Hungary: Stanford Center for Biomedical Informatics Research, 2007 [Google Scholar]
- 141.Hristoskova A, Moeyersoon D, Van Hoecke S, et al. Dynamic composition of medical support services in the ICU: Platform and algorithm design details. Comput Methods Programs Biomed 2010;100:248–64 [DOI] [PubMed] [Google Scholar]
- 142.Safe and Sound Consensus on Project Objectives. 2009. http://www.clinicalfutures.org.uk/consensus (accessed 6 Oct 2010). [Google Scholar]