Abstract
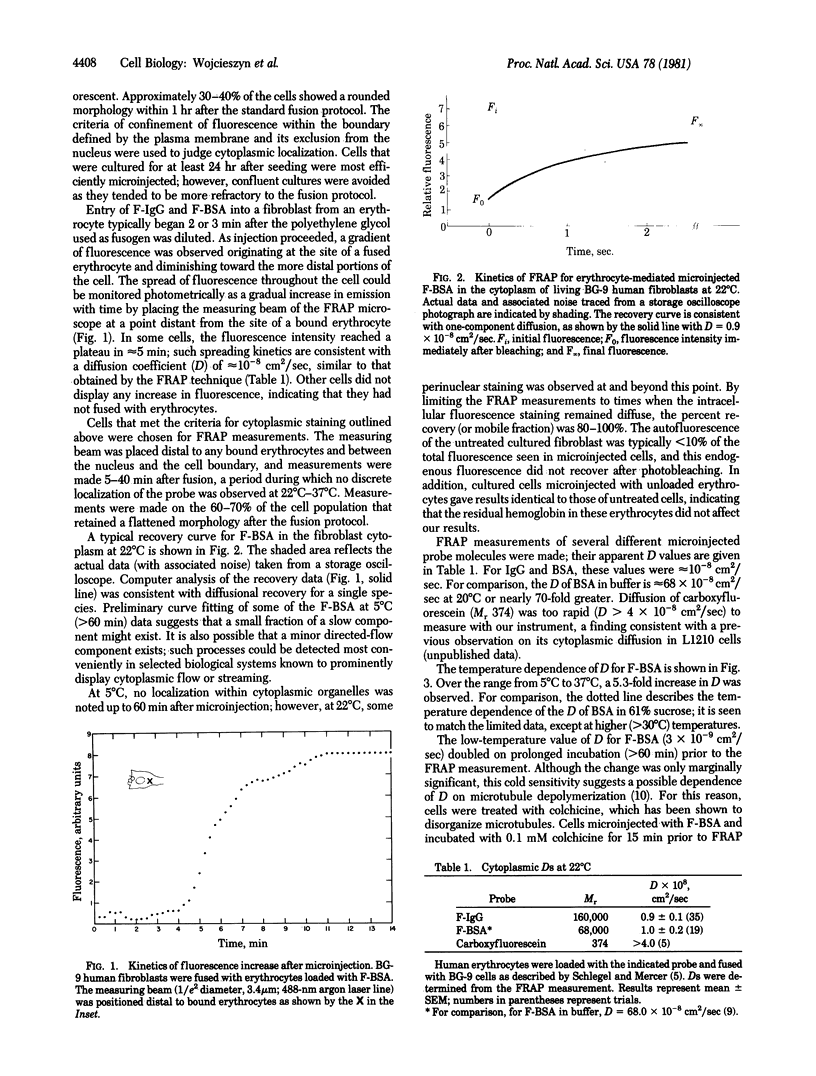
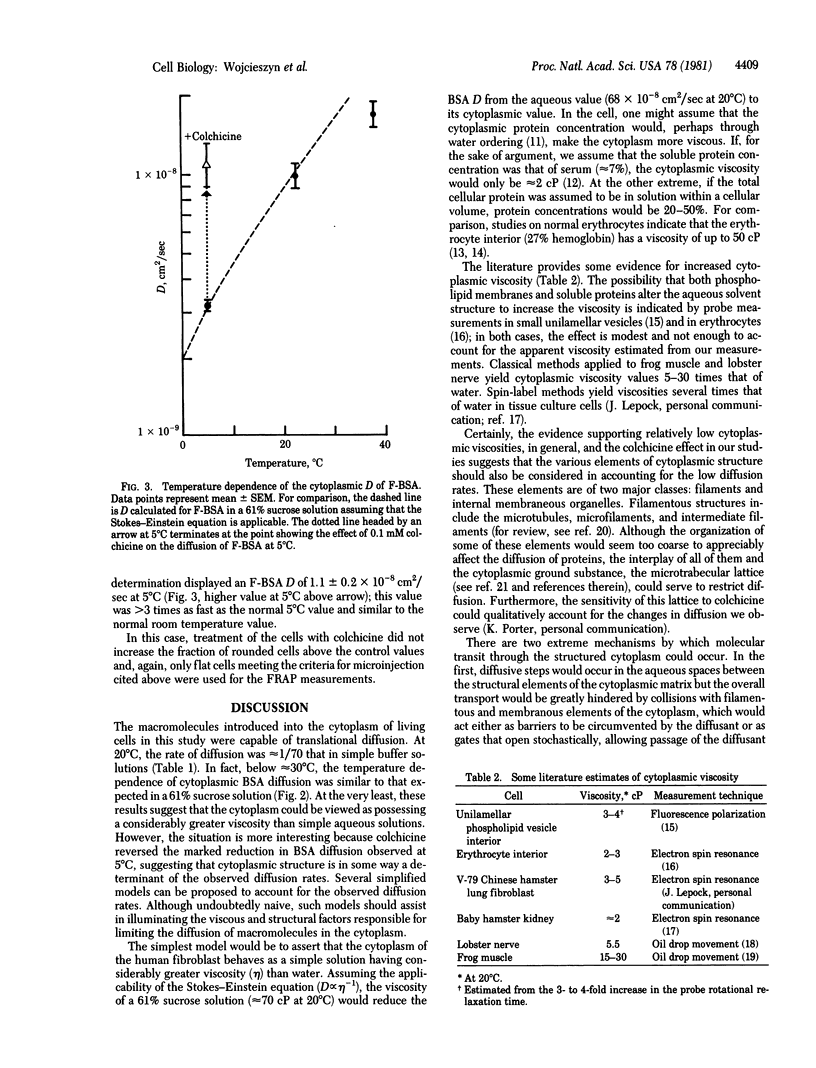
The diffusion of macromolecules introduced into the cytoplasm of human fibroblasts by erythrocyte-mediated microinjection was measured by the fluorescence recovery after photobleaching technique. The apparent diffusion coefficients for fluorescein-labeled IgG and fluorescein-labeled bovine serum albumin were approximately 10(-8) cm2/sec at 22 degrees C, consistent with the kinetics of spreading of the fluorescent probe following microinjection and approximately 1/70 the values in aqueous buffer. The diffusion of labeled bovine serum albumin was shown to be strongly dependent on temperature and, in fact, similar to that expected in a 61% aqueous sucrose solution. However, the marked reduction in diffusion at 5 degrees C could be fully reversed by incubation with 0.1 mM colchicine. These findings suggest that cytoplasmic diffusion rates are reduced relative to rates in aqueous media as a result of increased aqueous phase viscosity or the impedence provided by structural elements. Several simple models to account for the data are presented.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERS G. K. MOLECULAR EXCLUSION AND RESTRICTED DIFFUSION PROCESSES IN MOLECULAR-SIEVE CHROMATOGRAPHY. Biochemistry. 1964 May;3:723–730. doi: 10.1021/bi00893a021. [DOI] [PubMed] [Google Scholar]
- ALLISON A. C. Properties of sickle-cell haemoglobin. Biochem J. 1957 Feb;65(2):212–219. doi: 10.1042/bj0650212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barisas B. G., Leuther M. D. Fluorescence photobleaching recovery measurement of protein absolute diffusion constants. Biophys Chem. 1979 Sep;10(2):221–229. doi: 10.1016/0301-4622(79)85044-9. [DOI] [PubMed] [Google Scholar]
- Cercek L., Cercek B. Involvement of cyclic-AMP in changes of the structuredness of cytoplasmic matrix (SCM). Radiat Environ Biophys. 1974;11(3):209–212. doi: 10.1007/BF01323189. [DOI] [PubMed] [Google Scholar]
- Clement N. R., Gould J. M. Viscosity of the internal aqueous phase of unilamellar phospholipid vesicles. Arch Biochem Biophys. 1980 Jul;202(2):650–652. doi: 10.1016/0003-9861(80)90473-7. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Derzko Z., Wu E. S., Hou Y., Poste G. Measurement of the lateral mobility of cell surface components in single, living cells by fluorescence recovery after photobleaching. J Supramol Struct. 1976;5(4):565(417)–576(428). doi: 10.1002/jss.400050411. [DOI] [PubMed] [Google Scholar]
- Koppel D. E., Axelrod D., Schlessinger J., Elson E. L., Webb W. W. Dynamics of fluorescence marker concentration as a probe of mobility. Biophys J. 1976 Nov;16(11):1315–1329. doi: 10.1016/S0006-3495(76)85776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Lindmo T., Steen H. B. Flow cytometric measurement of the polarization of fluorescence from intracellular fluorescein in mammalian cells. Biophys J. 1977 May;18(2):173–187. doi: 10.1016/S0006-3495(77)85606-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfa R., Steinhardt J. A temperature-dependent latent-period in the aggregation of sickle-cell deoxyhemoglobin. Biochem Biophys Res Commun. 1974 Aug 5;59(3):887–893. doi: 10.1016/s0006-291x(74)80062-8. [DOI] [PubMed] [Google Scholar]
- Morse P. D., 2nd, Lusczakoski D. M., Simpson D. A. Internal microviscosity of red blood cells and hemoglobin-free resealed ghosts: a spin-label study. Biochemistry. 1979 Oct 30;18(22):5021–5029. doi: 10.1021/bi00589a033. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Microtubules. Annu Rev Biochem. 1973;42:507–540. doi: 10.1146/annurev.bi.42.070173.002451. [DOI] [PubMed] [Google Scholar]
- Paine P. L., Moore L. C., Horowitz S. B. Nuclear envelope permeability. Nature. 1975 Mar 13;254(5496):109–114. doi: 10.1038/254109a0. [DOI] [PubMed] [Google Scholar]
- RENKIN E. M. Filtration, diffusion, and molecular sieving through porous cellulose membranes. J Gen Physiol. 1954 Nov 20;38(2):225–243. [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. H. Axonal transport: components, mechanisms, and specificity. Annu Rev Neurosci. 1979;2:467–504. doi: 10.1146/annurev.ne.02.030179.002343. [DOI] [PubMed] [Google Scholar]
- Wolosewick J. J., Porter K. R. Microtrabecular lattice of the cytoplasmic ground substance. Artifact or reality. J Cell Biol. 1979 Jul;82(1):114–139. doi: 10.1083/jcb.82.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]