Abstract
Objective
The aim of this study was to measure the effect of an electronic heparin-induced thrombocytopenia (HIT) alert on provider ordering behaviors and on patient outcomes.
Materials and Methods
A pop-up alert was created for providers when an individual's platelet values had decreased by 50% or to <100 000/mm3 in the setting of recent heparin exposure. The authors retrospectively compared inpatients admitted between January 24, 2008 and August 24, 2008 to a control group admitted 1 year prior to the HIT alert. The primary outcome was a change in HIT antibody testing. Secondary outcomes included an assessment of incidence of HIT antibody positivity, percentage of patients started on a direct thrombin inhibitor (DTI), length of stay and overall mortality.
Results
There were 1006 and 1081 patients in the control and intervention groups, respectively. There was a 33% relative increase in HIT antibody test orders (p=0.01), and 33% more of these tests were ordered the first day after the criteria were met when a pop-up alert was given (p=0.03). Heparin was discontinued in 25% more patients in the alerted group (p=0.01), and more direct thrombin inhibitors were ordered for them (p=0.03). The number who tested HIT antibody-positive did not differ, however, between the two groups (p=0.99). The length of stay and mortality were similar in both groups.
Conclusions
The HIT alert significantly impacted provider behaviors. However, the alert did not result in more cases of HIT being detected or an improvement in overall mortality. Our findings do not support implementation of a computerized HIT alert.
Keywords: Computer alert, heparin induced thrombocytopenia (HIT), decision-support systems, clinical, medication errors, hospital information systems
Background and significance
Heparin-induced thrombocytopenia (HIT) is a known adverse event in patients exposed to heparin, an anticoagulant medication commonly used in hospitals to prevent and treat blood clots.1–3 While the incidence of HIT is relatively low (0.5–6.5%) depending on the at-risk population,4–6 this immunologic phenomenon can result in severe morbidity and mortality for patients, with over 50% of those affected developing new arterial or venous clots within 90 days.7 Successful treatment and prevention of complications necessitate early identification, cessation of heparin, and the prompt initiation of another type of alternative anticoagulant.6 8–12 Failure to diagnose HIT in a timely manner can result in increased patient morbidity and mortality, and has been the basis for numerous lawsuits.13–15
Unfortunately, the presentation of HIT can be a subtle pattern of decreasing platelet counts to levels considered otherwise normal over a relatively long period of time (5–14 days).16 This trend can be difficult to recognize, given the abundance of information health providers must process in making clinical decisions. Given the potential importance of timely interventions in preventing complications, therapy for HIT must be initiated presumptively, typically before the results of confirmatory tests, such as HIT antibody assays, are known.
Since HIT is both difficult to recognize in its early stages and carries substantial morbidity and mortality, a Montefiore Medical Center (MMC) task force was charged with creating a system-wide intervention to assist providers. Automated clinical alerts can be successful at notifying healthcare providers of potentially dangerous patient situations and have been shown to improve patient care in some instances.17 18 Consequently, the task force created the clinical specifications for an alert integrated into the MMC Clinical Information System (CIS) to automatically detect patients with features consistent with HIT. The computer programmers encoded these specifications into an algorithm, referred to herein as the ‘HIT alert’ that was implemented throughout MMC on January 24, 2008. We hypothesized that our alert would improve providers' awareness of patients with features consistent with HIT and that the awareness could be observed as an increase in provider diagnostic and treatment orders. These behavioral changes would then result in a shorter delay to appropriate treatment, increased detection of HIT, and ultimately reduced mortality and reduced length of stay.
Methods
Study intervention
To facilitate communication and classification of the HIT alert in the broader schema of clinical decision-support (CDS) interventions, we incorporated the taxonomy classification suggested by Wright et al.19 To trigger an alert, the patient had to satisfy two conditions: a significant platelet count drop and a recent heparin exposure. The alert algorithm was initiated by a new platelet result in the CIS. The alert algorithm compared each platelet count to the patient's baseline platelet count. If the most recently stored platelet count represented a 50% decrease from the baseline platelet count or dropped below 100 000/mm3 (where the baseline platelet count was greater than 100 000/mm3), the first part of the algorithm was satisfied. These criteria were based on those described in Harrison's Principles of Internal Medicine (17th edition).20
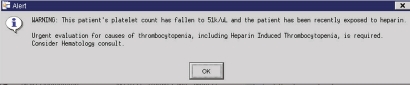
Median LOS was calculated from the distribution of the lengths of stay of all the participants. Each LOS was then categorized as above or below that median. The clinical task force specified the baseline platelet count as the first platelet count recorded after the patient's admission to the hospital. However, because of performance constraints that threatened to slow the entire CIS, programmers modified the definition of the baseline platelet count. Consequently, in the finalized algorithm, the platelet count immediately preceding the first time a patient's inpatient account was accessed by a provider was identified as the baseline platelet count. As an example, if Patient A had two complete blood counts performed as an inpatient before any provider looked up their record in the CIS, only the later count would serve as the baseline count in the HIT alert algorithm. While this was an uncommon event, it shaped the construction of the HIT alert within the CDS framework. The second part of the alert algorithm then inquired whether a patient received either a heparin or low-molecular-weight heparin as an inpatient or had an outpatient prescription active in the MMC CIS in the 14 days prior to this platelet count. If a patient was so ‘exposed’, a HIT alert appeared as a synchronous ‘pop-up’ notification once to every clinician who subsequently entered that patient's computerized record until that patient no longer met the HIT alert criteria (figure 1). Any provider who accessed that patient's electronic medical record was required to acknowledge receipt of the alert before any further use of the CIS was permitted.
Figure 1.
Heparin-induced thrombocytopenia alert.
Study design, setting, and patient population
This IRB-approved retrospective cohort study was carried out at MMC, a large, diverse, urban academic medical center in the Bronx, New York, which uses the CIS Carecast 5.1.6 (GE Healthcare). All laboratory results are stored electronically, and all orders are entered through the CIS. A log of when providers received the HIT alert is not typically stored.
The intervention group consisted of all patients aged 21 or older admitted to MMC between January 24, 2008 and August 24, 2008 who met the HIT alert specifications. A historical control group consisted of patients who were admitted in the preceding year (January 24, 2007–August 24, 2007) who would have met the HIT alert specifications had the alert algorithm been implemented 1 year earlier. If a patient met HIT alert specifications on multiple admissions during the time period, only the first admission was included in this study. Both cohorts were identified through electronic queries of the MMC CIS using the SqlDbx database environment (ACS Technologies, New York).
Patient demographics and Charlson comorbidity scores were determined using Clinical Looking Glass (CLG), an interactive software application developed at MMC that integrates clinical and administrative datasets, and allows them to be reproduced in a programable format for statistical access. The index date was considered to be the date of the platelet result that fulfilled the HIT alert specifications.
Outcome measures
The primary outcome was provider behavior as measured by the percentage of patients who were tested for the HIT antibody within 14 days following the index date described above. The Heparin-PF4 antibody assay used was a commercial assay (PF4 Enhanced, GTI Diagnostics, Waukesha, Wisconsin) where antibodies against PF4 are complexed to polyvinyl sulfonate.21 OD values of ≥400 units were regarded as positive. All positive results were confirmed by demonstration of >50% inhibition of a positive reaction with the addition of heparin.22 Secondary outcomes included the proportion of HIT antibody tests ordered within 24 h following the index date, the number of alternative anticoagulation orders intended to treat HIT within 14 days following the index date, the proportion of heparin orders active at the time of the index date but discontinued within 24 h, the incidence of HIT antibody positivity, the 90-day mortality, and the greater than median length of stay post-index date. We assumed that alternative anticoagulants were intended to treat HIT if either a HIT antibody test was ordered in the previous 14 days or a clinician documented in the medical record that the patient was being treated for possible HIT.
HIT antibody positivity was defined as the proportion of HIT antibody tests found to be positive. For each cohort, we calculated two separate HIT antibody positivity rates: (1) the number of patients who tested positive for HIT antibody of those who had HIT antibody studies ordered, and (2) the number of patients who tested positive of all patients who met the HIT alert criteria, regardless of whether or not a test for HIT antibody had been sent. Mortality and date of death within 90 days of the index date were obtained from the Social Security Administration death registry. Patients who did not have a Social Security number were excluded from mortality analysis.
Data analysis
A χ2 test (or Fisher exact test when appropriate) was used to compare categorical characteristics and outcomes expressed as proportions between control and intervention groups. An independent-samples t test was used to compare the mean age of the two cohorts. For continuous variables not meeting normality assumptions, the non-parametric Mann–Whitney test was used to test for a significant difference between mean ranks of the groups. Binary logistic regression was used to compare 90-day mortality and separately LOS greater than the median, while adjusting for the Charlson score. HRs for mortality by group were also assessed with Cox models while adjusting for age, sex, and Charlson score. A two-tailed α of 0.05 was used to denote statistical significance. All analyses were performed using Intercooled Stata 8.2 for Windows.
Results
There were 24 951 unique inpatients admitted during the control time period, of which 10 986 were exposed to heparin. Of those patients, 1006 (9.2%) met the criteria for the HIT alert and constituted the control cohort. During the intervention period, there were 26 359 inpatient admissions, of which 11 983 were exposed to heparin, and 1081 (9.0%) met the criteria for the HIT alert and constituted the intervention cohort. Demographic information is shown in table 1. The two groups were similar in age, gender, race, and ethnicity scores. The median length of stay was 6.3 days. The intervention group had a mean Charlson comorbidity score that was only modestly, albeit statistically significantly, higher.
Table 1.
Characteristics of study sample
| Control (n=1006) | Intervention (n=1081) | p Value | |
| Female (%) | 56.2 | 53.4 | 0.20 |
| White (%) | 29.3 | 26.2 | 0.11 |
| Hispanic (%) | 31.6 | 34.5 h | 0.16 |
| Mean age, years (SD) | 65.2 (16.1) | 64.7 (16.2) | 0.52 |
| Charlson score (SD) | 3.48 (2.8) | 3.91 (3.1) | <0.01 |
As detailed in table 2, of the 1006 inpatients in the control cohort, 172 (17.1%) were tested for the HIT antibody within 14 days following the index date. In contrast, 266 (24.6%) patients were ordered for HIT antibodies in the intervention group (p<0.01). In addition to this 43.9% increase in request for HIT antibody studies, 25% more patients in the intervention group who were on heparin at the time of crossing the platelet threshold had their heparin order discontinued within the next 24 h as compared to the control group (26.5% vs 21.2%, p=0.01).
Table 2.
Impact on physician behavior
| Control N=1006 | Intervention N=1081 | p Value | |
| 1. HIT antibody testing sent (n,%) | 172 (17.1%) | 266 (24.6%) | <0.01 |
| 2. Heparin discontinued <24 h + | 213 (21.2%) | 286 (26.5%) | <0.01 |
| 3. Alternative anticoagulation ordered | 26 (2.6%) | 47 (4.4%) | 0.03 |
| 3a. No HIT antibody test ordered post-index date* | 3 (12%) | 4 (9%) | |
| 3b. Before HIT antibody test results reported | 14 (54%) | 36 (77%) | 0.12 |
| 3c. After HIT antibody test results reported | 9 (35%) | 7 (15%) |
5/7 of these patients had heparin-induced thrombocytopenia (HIT) antibody tests ordered before the threshold date (three in control and two in intervention). The remaining two had direct thrombin inhibitor therapy ordered because the provider documented a high enough suspicion of HIT that sending the HIT antibody would not change management.
Patients in the intervention group received more orders for alternative forms of anticoagulation than in the control group (4.4% vs 2.6 %, p<0.04). These alternative anticoagulation orders were further categorized depending on whether the order was placed on suspicion of HIT (ie, before a HIT antibody result was received) or whether the order was placed after a HIT antibody result was reported. This analysis revealed that patients in the intervention group had a non-significant trend (p=0.12) toward receiving a greater proportion of their anticoagulation orders before a HIT antibody result was received.
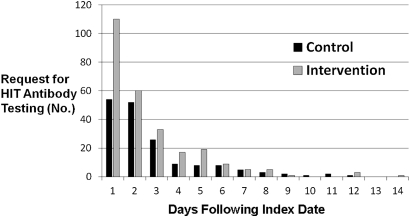
Requests for HIT antibody testing within the first day after the index date as a proportion of all HIT antibody testing orders were also higher for intervention than for the control (41.9% vs 31.5% p=0.03). This translates to an increase of 33% in first-day response testing after the pop-up was introduced (figure 2).
Figure 2.
Timing of heparin-induced thrombocytopenia (HIT) antibody testing requests. Testing on the first day following the index date as a proportion of all tests ordered was higher for intervention than for the control (41.9% vs 31.5%, p=0.03).
While patients in the intervention group received more HIT antibody orders, this increase in orders did not translate into higher HIT antibody positivity rates (table 3). The incidence of HIT positivity in the control and intervention groups was 16.3 and 10.9%, respectively (p=0.10). Serotonin release assay testing as a confirmatory test was infrequently carried out, and when these were separately assessed, the results did not significantly alter these data.
Table 3.
Heparin-induced thrombocytopenia (HIT) antibody positivity and detection rates
| HIT antibody positivity rate | Control | Intervention | p Value |
| HIT antibody positive/HIT antibody tested | 16.3% (n=28/172) | 10.9% (n=29/266) | 0.10 |
| HIT antibody positive/entire cohort | 2.8% (n=28/1006 | 2.7% (n=29/1081) | 0.99 |
Despite receiving more HIT antibody and alternative anticoagulation orders as well as more prompt heparin discontinuation orders, the two groups had a similar 90-day mortality (30.8% vs 29.0% for control and intervention), with an OR (adjusted for Charlson score) of 1.0 (95% CI 0.8 to 1.2, p=0.98). The adjusted HR for mortality for being in the intervention cohort was 1.03 (95% CI 0.88 to 1.21, p=0.71). Similarly, the intervention group did not have a smaller proportion above the median LOS following the platelet threshold date when adjusted for Charlson score (p=0.91, table 4).
Table 4.
Mortality and length of stay by group*
| Control, n=1006 | Intervention, n=1081 | OR (95% CI) | p Value | |
| 90-day mortality | 29.0 | 34.2 | 1.0 (0.8 to 1.2) | 0.98 |
| Exceeded median length of stay | 49.7 | 50.3 | 1.0 (0.8 to 1.2) | 0.94 |
After adjusting for Charlson score.
Since the time a provider in the historical control cohort first viewed a patient's electronic chart was not stored, we could not retrospectively implement the exact alert algorithm for this group. In order to make the two groups exactly comparable, instead of using the actual HIT alert, we used the same HIT specification algorithm for both groups. We hypothesized that our methods for deriving the intervention and control cohorts using the original alert specifications would accurately capture the patients who also triggered the alert algorithm. To assess this hypothesis, we analyzed 5562 inpatient admissions that took place from February 14 to March 17, 2008 in the intervention period, during which time we had also programmed the CIS to log every patient who triggered an alert. One hundred thirty-one patients triggered a HIT alert. When we retrospectively queried the same time period using the alert specification method that we used for the main study analysis, we captured those 131 patients (100% sensitivity). However, the query also identified 23 additional patients who met the criteria but did not trigger an alert. Seventeen of these patients did not trigger the alert because the platelet count identified by the algorithm as the ‘baseline’ platelet count was lower than the actual first platelet count of the admission. As detailed in the Methods section, this potential discrepancy was not incorporated into the algorithm. In six cases, the alert did not fire because the patients' clinical status changed before a provider could log onto the account and receive the alert. Of the six, three patients' platelet counts recovered to above the threshold by the observed count, one patient died, one patient was discharged, and one patient's providers did not view the labs before the endpoint of this substudy. The positive predictive value of the query was 85%; 15% of patients who met the clinical specifications designed by the HIT alert task force did not trigger an actual alert to providers due to differing definitions of the baseline platelet count and could not, therefore, be analyzed in this study.
Discussion
Our results suggest that the HIT alert does significantly impact provider behavior leading to increased and more rapid confirmatory HIT testing, treatment with alternative anticoagulation, and cessation of heparin. However, the alert did not increase the detection rate of HIT, or reduce the 90-day mortality or length of stay postindex date. Increasing HIT antibody testing, alternative anticoagulation use, and heparin discontinuation orders in the intervention group all further support the general consensus that CDS interventions can be powerful tools in directing provider behavior. There was a non-significant trend for providers in the intervention group to order alternative anticoagulation prior to the results of any HIT antibody testing more frequently than those in the control cohort. If indeed this does reflect a real change in practice behavior, a possible explanation could be that the alert by itself created a sense of urgency among the providers to be more aggressive in responding to the clinical scenario.
A greater proportion of total HIT orders occurred within the first 24 h after the triggering platelet count in the alerted cohort, suggesting that the alert also potentially decreased delays in ordering HIT antibody tests. That these behavioral changes were not even more robust could be partially explained by alert fatigue, a commonly described phenomenon in which providers are less likely to respond to similar alerts over time.23
Despite a significant increase in HIT testing, however, there was no subsequent meaningful improvement in the detection rate of HIT. The additional testing associated with the intervention group only resulted in more HIT antibody-negative patients. This important result ran contrary to our hypothesis that increasing the number of HIT antibody tests in patients with features consistent with HIT would increase the detection rate of patients with HIT. The most likely explanation for this finding is that changes in platelet count and timing of heparin exposure by themselves do not reliably predict which patients had HIT. This possibility is reflected by algorithms such as the 4T score, a tool created to help physicians diagnose HIT that incorporates not only platelet count changes and heparin exposure but also factors such as evidence of thrombosis and whether clinicians believe there are other, more likely explanations for the patients' findings.24 25 The 4T score requires clinical judgment as one of its ‘T’s and is therefore not amenable to an automated alert algorithm. Evaluation of the tool26 has demonstrated that the positive predictive value in patients referred for HIT testing with an intermediate or high risk 4T score ranges widely, from a dismal 11% to a still unsatisfactory 56%, demonstrating the difficulty in making this diagnosis.
The substudy comparing patients who actually triggered the HIT alert to those who met the original alert specifications demonstrated that the database query did an excellent job in identifying all patients who actually triggered the alert. However, 15% of patients included in the intervention group because they met the alert specifications did not actually trigger an alert. As noted in the Results section, these patients did not actually trigger alerts due to technical reasons associated with implementing the algorithm rather than not meeting the clinical criteria. Since the same method was applied to both intervention and control groups, it is unlikely that the small difference between the specifications of the study and the actual algorithm would have a differential impact on results.
This paper builds on research performed by Riggio et al on a prior HIT decision-support intervention.27 These researchers found that, paradoxically, their HIT alert delayed initiation of diagnostic and treatment modalities and had no impact on clinical outcomes. Our study improved on a specific limitation described by these investigators, which was their ability to only identify patients who had HIT rather than those who met the HIT alert criteria in the control group. Consequently, their primary outcome related to timeliness of diagnosis and treatment for patients found later to have HIT, while our outcomes centered on improving provider response to patients with platelet count decreases typical of HIT. Our ability to closely approximate this control group allowed more direct measurement of the alert's impact on HIT antibody orders placed, detection rates, and mortality.
Our research agreed with some of their findings about HIT CDS alerts. The alert triggered further HIT testing in only 19% of patients in their population and 22% in ours. In both studies, despite increases in testing for HIT, there was no improvement in the detection of HIT. On the other hand, the HIT alert in the Riggio study did not improve the time to therapeutic intervention in patients who had HIT. A possible explanation might be that Jefferson had employed multiple other alerts for other clinical conditions, resulting in alert fatigue. While our study was not powered to compare timeliness of interventions in patients who ultimately had HIT, we do demonstrate that the HIT alert reduces delays in treatment for patients with criteria consistent with HIT.
Our study is limited inherently by its retrospective cohort design. Furthermore, our control and intervention groups were determined by a retrospective database query rather than a run-in period where patients who met the threshold were identified but their providers were not alerted. However, based on our validation study, we are confident that almost all of the patients who were supposed to receive the alert did in fact trigger the alert.
While there were patients included in both groups who likely did not trigger the alert, this finding is similar to what one would observe in an intention-to-treat analysis and should not differentially bias the results for the following reasons: first, the same query was employed to identify the control and intervention; second, the additional patients in the intervention group who did not trigger an alert would, if anything, likely have diluted the effect of the intervention on provider behavior. The fact that we still observe important, statistically significant differences in our two groups makes those results even more compelling.
Another limitation was that, at baseline, the two groups differed in their mean Charlson comorbidity score. However, although the difference was statistically significant, it was small enough in magnitude that it was likely not to be clinically meaningful. Furthermore, when we adjusted for this variable in the mortality and length of stay analyses, the results were consistent.
Unfortunately, resources were not available to manually review all patient records in the control and intervention groups to determine (1) whether the alert impacted important clinical outcomes such as thrombosis and hemorrhage or (2) whether there were any patterns among those patients who did not receive further testing. The length of inpatient hospital stay post-HIT alert was incorporated into our study to reflect such morbidity, and this did not demonstrate a significant difference. Given that the alert did not require providers to list their over-ride reason, and we did not conduct a qualitative exploration of providers' perceptions, we can only speculate why providers did not act upon the alert.
We could not measure whether the alert resulted in increased utilization of resources, that is, more hematology consults, increased drug costs, or other increases in resource allocation and financial costs. We also recognize that HIT antibody positivity is not the gold standard for the diagnosis of HIT. Nonetheless, by using this parameter for both cohorts, it functioned as a sensitive surrogate marker for measuring physician behavior.
Our study also has several strengths including a large sample and the ability to assess mortality and length-of-stay outcomes as well as the change in clinician behavior. Also, the overall incidence of HIT at MMC was consistent with that reported in other studies4–6 suggesting that our population is not unique. Further, the consistency of our results with the findings of others, regarding HIT alerts, adds confidence that our results were not a statistical or methodological aberration.27
Private business consortiums such as the Leapfrog group28 as well as the Federal government through the HITECH legislation are advocating for the implementation of CDS interventions in an effort to improve patient quality of care.29 While CDS interventions have the potential to lead to improved patient care, they can also lead to adverse consequences including overtreatment and increased, unnecessary costs.30 Stopping heparin and starting alternative anticoagulation in patients are not inconsequential decisions. Alternative anticoagulation is more costly and typically has an increased hemorrhagic risk.31
As CDS intervention proposals become more sophisticated in an attempt to model clinical reasoning, their implementation will inevitably place higher demands on computer information systems. To minimize this disruption, programmers must make modifications when translating these specifications into computer code. Consequently, it is important, as this research highlights, to validate a CDS intervention's specifications against its actual performance before widespread adoption.
Our research highlights the complexity of translating paper-based decision support into computerized alerts. Any algorithm, no matter how well intentioned and meaningfully developed and no matter how well designed to minimize harm to patient and increase awareness, must be evaluated prior to its widespread implementation. Such practice is not required in most institutions today.
Determining whether to implement a HIT CDS intervention depends on the objectives of the institution. If an organization's goal is to reduce delays in diagnosis and treatment in patients with features concerning for HIT, then this alert has the potential to be a useful tool. However, if the desire is to improve detection of HIT, our study offers no evidence that the intervention in its current form would be effective. Whether or not a more sophisticated algorithm could reduce the number of false-positive alerts and improve clinical outcomes warrants further study. Until such time, these data do not support implementation of a computerized HIT alert.
Acknowledgments
We gratefully acknowledge S Islam and E Bellin, for their help with Clinical Looking Glass queries.
Footnotes
Competing interests: None.
Ethics approval: Ethics approval was provided by the Montefiore/Einstien IRB.
Provenance and peer review: Not commissioned; internally IRB peer reviewed.
References
- 1.Warkentin TE, Greinacher A, Koster A, et al. Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest 2008;133:340S–80S [DOI] [PubMed] [Google Scholar]
- 2.Chong BH. Heparin-induced thrombocytopenia. J Thromb Haemost 2003;1:1471–8 [DOI] [PubMed] [Google Scholar]
- 3.Warkentin TE. Heparin-induced thrombocytopenia: pathogenesis and management. Br J Haematol 2003;121:535–55 [DOI] [PubMed] [Google Scholar]
- 4.Ahmed I, Majeed A, Poell R. Heparin-induced thrombocytopenia–diagnosis and management update. Postgrad Med J 2007;83:575–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliveira GB, Crespo EM, Becker RC, et al. ; Complications After Thrombocytopenia Caused by Heparin (CATCH) Registry Investigators Incidence and prognostic significance of thrombocytopenia in patients treated with prolonged heparin therapy. Arch Intern Med 2008;168:94–102 [DOI] [PubMed] [Google Scholar]
- 6.Creekmore FM, Oderda GM, Pendleton RC, et al. Incidence and economic implications of heparin-induced thrombocytopenia in medical patients receiving prophylaxis for venous thromboembolism. Pharmacotherapy 2006;26:1438–45 [DOI] [PubMed] [Google Scholar]
- 7.Warkentin TE, Kelton JG. A 14-year study of heparin-induced thrombocytopenia. Am J Med 1996;101:502–7 [DOI] [PubMed] [Google Scholar]
- 8.Greinacher A, Volpel H, Janssens U, et al. Recombinant hirudin (lepirudin) provides safe and effective anticoagulation in patients with heparin-induced thrombocytopenia: a prospective study. Circulation 1999;99:73–80 [DOI] [PubMed] [Google Scholar]
- 9.Lewis BE, Wallis DE, Berkowitz SD, et al. Argatroban anticoagulant therapy in patients with heparin-induced thrombocytopenia. Circulation 2001;103:1838–43 [DOI] [PubMed] [Google Scholar]
- 10.Finks SW. Bivalirudin use in carotid endarterectomy in a patient with heparin-induced thrombocytopenia. Ann Pharmacother 2006;40:340–3 [DOI] [PubMed] [Google Scholar]
- 11.Papadopoulos S, Flynn JD, Lewis DA. Fondaparinux as a treatment option for heparin-induced thrombocytopenia. Pharmacotherapy 2007;27:921–6 [DOI] [PubMed] [Google Scholar]
- 12.Wallis DE, Workman DL, Lewis BE, et al. Failure of early heparin cessation as treatment for heparin-induced thrombocytopenia. Am J Med 1999;106:629–35 [DOI] [PubMed] [Google Scholar]
- 13.Hill v. Sacred Heart Medical Center. 177 P.3d 1152 (Wash.Ct App. Div., 2008). http://www.caselaw.findlaw.com/wa-court-of-appeals/1458279.html [Google Scholar]
- 14.Mundy J. Amputee Files Lawsuit Against Heparin Maker. Lawyers and Settlements.com, 2009. http://www.lawyersandsettlements.com/articles/13301/heparin-injection-side-effects.html (accessed 2 Jan 2011). [Google Scholar]
- 15.Bernstein Liebhard and Lifshitz ‘Heparin Induced Thrombocytopenia’. Consumer Injury Lawyers; http://www.consumerinjurylawyers.com/side-effects-and-diseases/Heparin-Induced-Thrombocytopenia/index.html (accessed 2 Jan 2011). [Google Scholar]
- 16.de Maistre E, Gruel Y, Lasne D. Diagnosis and management of heparin-induced thrombocytopenia. Can J Anaesth 2006;53:S123–4 [DOI] [PubMed] [Google Scholar]
- 17.Bates DW, Leape LL, Cullen DJ, et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA 1998;280:1311–16 [DOI] [PubMed] [Google Scholar]
- 18.Kaushal R, Shojania KG, Bates DW. Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review. Arch Intern Med 2003;163:1409–16 [DOI] [PubMed] [Google Scholar]
- 19.Wright A, Goldberg H, Hongsermeier T, et al. A description and functional taxonomy of rule-based decision support content at a large integrated delivery network. J Am Med Inform Assoc 2007;14:489–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weitz J. Antiplatelet, anticoagulant, and fibrinolytic drugs. In: Kasper DL, Braunwald E, Fauci AS, et al., eds. Harrison's Principles of Internal Medicine. 17th edn New York: McGraw-Hill, 2008:735–48 [Google Scholar]
- 21.Prechel M, Walenga JM. The laboratory diagnosis and clinical management of patients with heparin-induced thrombocytopenia: an update. Semin Thromb Hemost 2008;34:86–96 [DOI] [PubMed] [Google Scholar]
- 22.Zwicker JI, Uhl L, Huang WY, et al. Thrombosis and ELISA optical density values in hospitalized patients with heparin induced thrombocytopenia. J Thromb Haemost 2004;2:2133–7 [DOI] [PubMed] [Google Scholar]
- 23.Bates DW, Kuperman GJ, Wang J, et al. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J Am Med Inform Assoc 2003;6:523–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lo GK, Juh D, Warkentin TE, et al. Evaluation of pretest clinical score (4 T's) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost 2006;4:759–65 [DOI] [PubMed] [Google Scholar]
- 25.Pouplard C, Gueret P, Fouassier M, et al. Prospective evaluation of the ‘4Ts’ score and particle gel immunoassay specific to heparin/PF4 for the diagnosis of heparin-induced thrombocytopenia. J Thromb Haemost 2007;5:1373–9 [DOI] [PubMed] [Google Scholar]
- 26.Bryant A, Low J, Austin S, et al. Timely diagnosis and management of heparin-induced thrombocytopenia in a frequent request, low incidence single centre using clinical 4T's score and particle gel immunoassay. Br J Haematol 2008;143:721–6 [DOI] [PubMed] [Google Scholar]
- 27.Riggio JM, Cooper MK, Leiby BE, et al. Effectiveness of a clinical decision support system to identify heparin induced thrombocytopenia. J Thromb Thrombolysis 2009;28:124–31 [DOI] [PubMed] [Google Scholar]
- 28.Metzger J, Welebob E, Turisco F, et al. The Leapfrog Group's CPOE Standard and Evaluation Tool. Patient Safety and Quality Healthcare, 2008. http://www.psqh.com/julaug08/cpoe.html (accessed 20 Mar 2009). [Google Scholar]
- 29.The American Recovery and Reinvestment Act of 2009. Public Law 111-5 (17 Feb 2008). [Google Scholar]
- 30.Lo GK, Sigouin CS, Warkentin TE. What is the potential for overdiagnosis of heparin-induced thrombocytopenia? Am J Hematol 2007;82:1037–43 [DOI] [PubMed] [Google Scholar]
- 31.Product Information: Argatroban Injection. Research Triangle Park, NC: GlaxoSmithKline, 2009 [Google Scholar]