Abstract
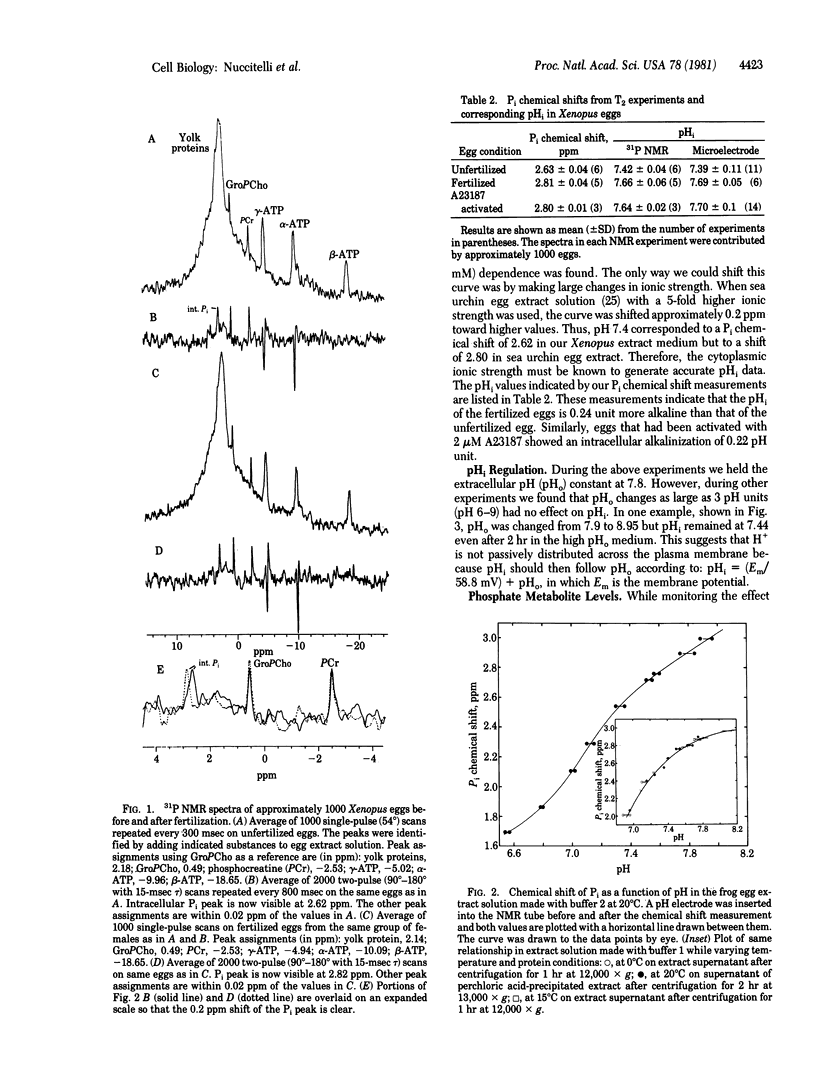
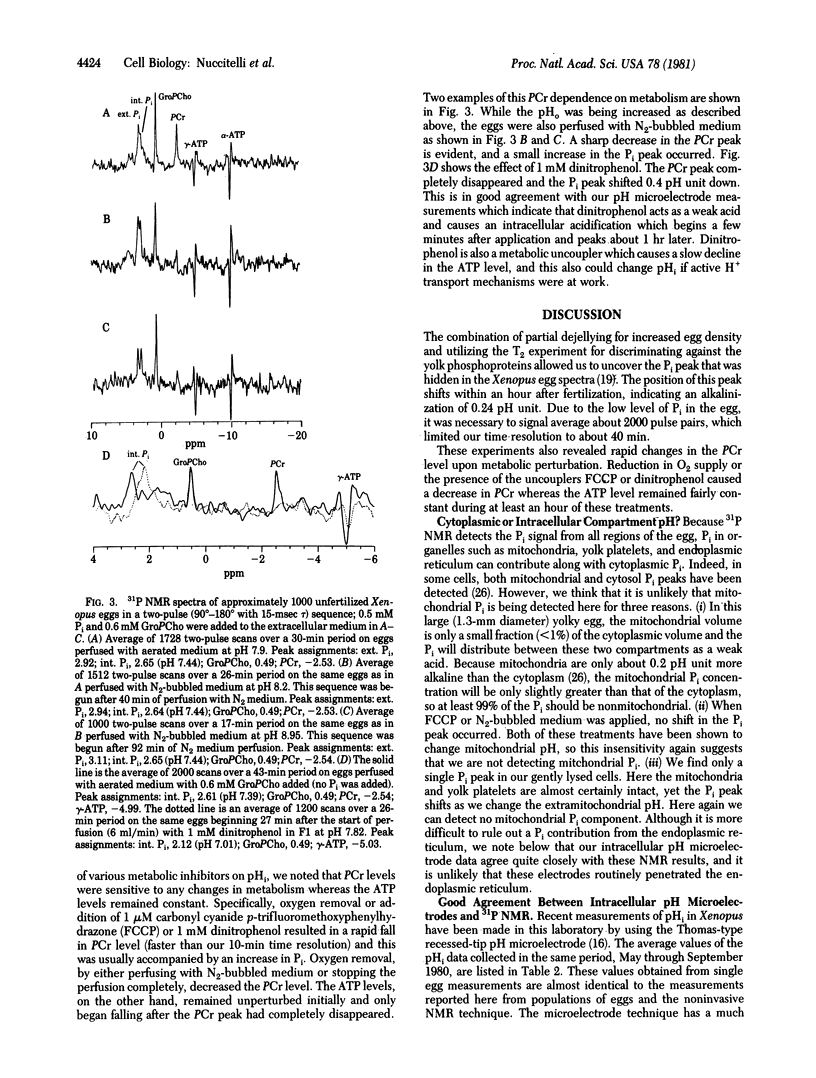
31P NMR spectra of mature eggs of the frog (Xenopus laevis) were taken prior to and after both fertilization and activation by a Ca2+/H+ ionophore (A23187). The eggs were constantly perfused with fresh well-buffered solution during the experiments, and the intracellular pH (pHi) was determined from the pH-dependent chemical shift of the internal Pi peak. The detection of this Pi peak in the presence of overlapping yolk phosphoprotein signals was accomplished by a T2 experiment which discriminated against the broader yolk phosphoprotein peak. The average pHi of the unfertilized, fertilized, and activated eggs was 7.42, 7.66, and 7.64, respectively. Thus, a cytoplasmic alkalinization of 0.24 pH unit occurs within 90 min 90 min after fertilization. These values are practically identical to pHi measurements made in this laboratory on Xenopus eggs by using pH-sensitive glass microelectrodes. These 31P NMR studies also indicate that extracellular pH changes as large as 3 pH units had no effect on pHi. We also found that phosphocreatine levels are very sensitive to metabolic perturbations such as oxygen depletion or metabolic inhibitor application. These treatments resulted in a rapid decrease in the phosphocreatine concentration; the ATP concentration declined only slowly after the phosphocreatine peak had disappeared.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton J. K., den Hollander J. A., Lee T. M., MacLaughlin A., Shulman R. G. Measurement of the internal pH of yeast spores by 31P nuclear magnetic resonance. Proc Natl Acad Sci U S A. 1980 May;77(5):2470–2473. doi: 10.1073/pnas.77.5.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt C. T., Glonek T., Bárány M. Analysis of phosphate metabolites, the intracellular pH, and the state of adenosine triphosphate in intact muscle by phosphorus nuclear magnetic resonance. J Biol Chem. 1976 May 10;251(9):2584–2591. [PubMed] [Google Scholar]
- Busby S. J., Gadian D. G., Radda G. K., Richards R. E., Seeley P. J. Phosphorus nuclear-magnetic-resonance studies of compartmentation in muscle. Biochem J. 1978 Jan 15;170(1):103–114. doi: 10.1042/bj1700103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. M., Ogawa S., Rottenberg H., Glynn P., Yamane T., Brown T. R., Shulman R. G. P nuclear magnetic resonance studies of isolated rat liver cells. Nature. 1978 Jun 15;273(5663):554–556. doi: 10.1038/273554a0. [DOI] [PubMed] [Google Scholar]
- Colman A., Gadian D. G. 31P nuclear-magnetic-resonance studies on the developing embryos of Xenopus laevis. Eur J Biochem. 1976 Jan 15;61(2):387–396. doi: 10.1111/j.1432-1033.1976.tb10032.x. [DOI] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Contraction and recovery of living muscles studies by 31P nuclear magnetic resonance. J Physiol. 1977 Jun;267(3):703–735. doi: 10.1113/jphysiol.1977.sp011835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel D., Steinhardt R., Humphreys T., Mazia D. An analysis of the partial metabolic derepression of sea urchin eggs by ammonia: the existence of independent pathways. Dev Biol. 1974 Oct;40(2):245–255. doi: 10.1016/0012-1606(74)90127-4. [DOI] [PubMed] [Google Scholar]
- Gerson D. F., Burton A. C. The relation of cycling of intracellular pH to mitosis in the acellular slime mould Physarum polycephalum. J Cell Physiol. 1977 May;91(2):297–303. doi: 10.1002/jcp.1040910214. [DOI] [PubMed] [Google Scholar]
- Gillies R. J., Deamer D. W. Intracellular pH changes during the cell cycle in Tetrahymena. J Cell Physiol. 1979 Jul;100(1):23–31. doi: 10.1002/jcp.1041000103. [DOI] [PubMed] [Google Scholar]
- Gillies R. J., Ugurbil K., den Hollander J. A., Shulman R. G. 31P NMR studies of intracellular pH and phosphate metabolism during cell division cycle of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2125–2129. doi: 10.1073/pnas.78.4.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. D., Epel D. Intracellular pH and activation of sea urchin eggs after fertilisation. Nature. 1976 Aug 19;262(5570):661–664. doi: 10.1038/262661a0. [DOI] [PubMed] [Google Scholar]
- Mahler M. The relationship between initial creatine phosphate breakdown and recovery oxygen consumption for a single isometric tetanus of the frog sartorius muscle at 20 degrees C. J Gen Physiol. 1979 Feb;73(2):159–174. doi: 10.1085/jgp.73.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar-Gentina V., Passonneau J. V., Vergara J. L., Rapoport S. I. Metabolic correlates of fatigue and of recovery from fatigue in single frog muscle fibers. J Gen Physiol. 1978 Nov;72(5):593–606. doi: 10.1085/jgp.72.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navon G., Ogawa S., Shulman R. G., Yamane T. 31P nuclear magnetic resonance studies of Ehrlich ascites tumor cells. Proc Natl Acad Sci U S A. 1977 Jan;74(1):87–91. doi: 10.1073/pnas.74.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink T. J., Tsien R. Y., Warner A. E. Free calcium in Xenopus embryos measured with ion-selective microelectrodes. Nature. 1980 Feb 14;283(5748):658–660. doi: 10.1038/283658a0. [DOI] [PubMed] [Google Scholar]
- Setlow B., Setlow P. Measurements of the pH within dormant and germinated bacterial spores. Proc Natl Acad Sci U S A. 1980 May;77(5):2474–2476. doi: 10.1073/pnas.77.5.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S. S., Steinhardt R. A. Direct measurement of intracellular pH during metabolic derepression of the sea urchin egg. Nature. 1978 Mar 16;272(5650):253–254. doi: 10.1038/272253a0. [DOI] [PubMed] [Google Scholar]
- Slack C., Warner A. E., Warren R. L. The distribution of sodium and potassium in amphibian embryos during early development. J Physiol. 1973 Jul;232(2):297–312. doi: 10.1113/jphysiol.1973.sp010271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt R. A., Mazia D. Development of K + -conductance and membrane potentials in unfertilized sea urchin eggs after exposure to NH 4 OH. Nature. 1973 Feb 9;241(5389):400–401. doi: 10.1038/241400a0. [DOI] [PubMed] [Google Scholar]
- Tomoda A., Tsuda-Hirota S., Minakami S. Glycolysis of red cells suspended in solutions of impermeable solutes. Intracellular pH and glycolysis. J Biochem. 1977 Mar;81(3):697–701. doi: 10.1093/oxfordjournals.jbchem.a131506. [DOI] [PubMed] [Google Scholar]
- Yoshizaki K., Nishikawa H., Yamada S., Morimoto T., Watari H. Intracellular pH measurement in frog muscle by means of 31P-nuclear magnetic resonance. Jpn J Physiol. 1979;29(2):211–225. doi: 10.2170/jjphysiol.29.211. [DOI] [PubMed] [Google Scholar]
- de Laat S. W., Buwalda R. J., Habets A. M. Intracellular ionic distribution, cell membrane permeability and membrane potential of the Xenopus egg during first cleavage. Exp Cell Res. 1974 Nov;89(1):1–14. doi: 10.1016/0014-4827(74)90180-3. [DOI] [PubMed] [Google Scholar]



