Abstract
AIM: To evaluate the treatment of pediatric functional chronic intestinal constipation (FCIC) with a probiotic goat yogurt.
METHODS: A crossover double-blind formula-controlled trial was carried out on 59 students (age range: 5-15 years) of a public school in Belo Horizonte, MG, Brazil, presenting a FCIC diagnostic, according to Roma III criteria. The students were randomized in two groups to receive a goat yogurt supplemented with 109 colony forming unit/mL Bifidobacterium longum (B. longum) (probiotic) daily or only the yogurt for a period of 5 wk (formula). Afterwards, the groups were intercrossed for another 5 wk. Defecation frequency, stool consistency and abdominal and defecation pain were assessed.
RESULTS: Both treatment groups demonstrated improvement in defecation frequency compared to baseline. However, the group treated with probiotic showed most significant improvement in the first phase of the study. An inversion was observed after crossing over, resulting in a reduction in stool frequency when this group was treated by formula. Probiotic and formula improved stool consistency in the first phase of treatment, but the improvement obtained with probiotic was significantly higher (P = 0.03). In the second phase of treatment, the group initially treated with probiotic showed worseningstool consistency when using formula. However, the difference was not significant. A significant improvement in abdominal pain and defecation pain was observed with both probiotic and formula in the first phase of treatment, but again the improvement was more significant for the group treated with B. longum during phase I (P < 0.05). When all data of the crossover study were analyzed, significant differences were observed between probiotic yogurt and yogurt only for defecation frequency (P = 0.012), defecation pain (P = 0.046) and abdominal pain (P = 0.015).
CONCLUSION: An improvement in defecation frequency and abdominal pain was observed using both supplemented and non-supplemented yogurt, but an additional improvement with B. longum supplementation was obtained.
Keywords: Functional chronic constipation, Probiotic, Bifidobacterium longum, Yogurt, Adolescents, Children
INTRODUCTION
The worldwide prevalence of childhood constipation in the general population ranges from 0.7% to 29.6%[1], and the wide range indicates differences in definition and selection of patients. This functional defecation disorder is characterized by infrequent defecation less than three times per week, frequent episodes of fecal incontinence, the periodic passage of large and painful stools which clog the toilet, and retentive posturing. Upon physical examination a palpable fecal mass is often found in the abdomen and the rectum. Accompanying symptoms may include irritability, decreased appetite, and/or early satiety. In the vast majority of cases (90% to 95%), no underlying organic cause is found and functional constipation is diagnosed[2].
The standard treatment consists of disimpaction and the administration of laxatives to achieve a normal bowel habit of passing a soft stool without pain. Even though the traditional treatment is well established and safe, for many patients it does not provide a satisfying improvement, prompting interest in other therapeutic strategies[3].
Probiotics are increasingly being used as an alternative in the management of constipation. Probiotics are defined as live microorganisms which when administered in adequate amounts confer a benefit on the host health[4]. In a recent review, the efficacy and safety of probiotic supplementation for the treatment of constipation was evaluated[5]. Studying 5 randomized controlled trials, with a total of 377 subjects (three trials with adults and two trials with children), the data suggests a favorable effect of some strains of Lactobacillus, Bifidobacterium and Escherichia coli (E. coli). Only one of the randomized controlled trials described the ineffectiveness of Lactobacillus rhamnosus Goldin and Gorbach as an adjunct to lactulose for the treatment of constipation in children[6]. The authors of the review concluded that until more data are available, the use of probiotics for the treatment of constipation should be considered investigational. More recently, Lactobacillus reuteri administered in infants with chronic constipation had a positive effect on frequency of bowel movements, but not on stool consistency[7] and the intake of mixed probiotic strains [Lactobacillus plantarum, Bifidobacterium breve, Bifidobacterium animalis subspecies lactis (B. animalis var. lactis)] was able to relieve evacuation disorders and hard stools in healthy adults[8].
In the present study, the ingestion of goat yogurt containing a Bifidobacterium longum (B. longum) strain was evaluated for the treatment of functional chronic intestinal constipation (FCIC) in children and adolescents.
MATERIALS AND METHODS
Subjects and eligibility criteria
Children aged 5-15 years and with FCIC, referred to a public school in the central area of the city of Belo Horizonte, Minas Gerais, Brazil, were eligible for study entry. Constipation was characterized according to Rome III criteria as presenting at least two out of six of the following symptoms for two or more months: two or fewer defecations per week; at least one episode of fecal incontinence per week; history of retentive posturing or excessive volitional stool retention; history of painful or hard bowel movements; presence of a large fecal mass in the rectum; history of wide diameter stools that may obstruct the toilet[2]. Exclusion criteria were the use of any oral laxative < 4 wk before intake, metabolic disease, a history of gastrointestinal surgery and fecal incontinence. Patients with fecal incontinence were excluded in order to make the sample more homogeneous in relation to disease severity. The follow-up protocol included defecation frequency, stool consistency, and abdominal and defecation pains recorded daily by the adolescents or parents. All children older than 12 years and/or parents gave informed consent. The study was approved by the Ethical Committee in Research of the Universidade Federal de Minas Gerais (COEP/UFMG, number ETIC0506/08).
Yogurt and bacterium
The B. longum strain used in the trial was isolated from the feces of a healthy child and identified by Multiplex Polymerase Chain Reaction. This strain was selected as a candidate for probiotic use based on technological (aerotolerance and high growth rate) and beneficial (wide antagonistic spectrum against pathogenic indicators, few antimicrobial resistance) criteria. The bacterium was grown in de Man, Rogosa and Sharp broth (Difco, Sparks, United States) for 48 h at 37 °C in an anaerobic chamber (Forma Scientific Company, Marietta, United States) containing N2 85%, H2 10% and CO2 5%. After growth, the culture was concentrated by centrifugation and resuspended in peptone sterile water. An aliquot of 1 mL of the concentrated bacterium suspension was added to 9 mL of a commercial goat yogurt (Capril Jacomé, Contagem, Brazil) to obtain a final concentration of 109 colony forming unit (CFU)/mL. The control formula was prepared by addition of 1 mL of peptoned water to 9 mL of goat yogurt. The goat yogurt contained the two classical yogurt starters, Lactobacillus delbrueckii subspecies bulgaricus and Streptococcus thermophilus from the YF-L812 commercial culture (DVS - Christian Hansen Laboratory, Horsholm, Denmark). Both yogurts were maintained at 4 °C until use and for a maximum of one week. During this period, the Bifidobacterium cells remained viable at 109 CFU/mL levels.
Study intervention
This study was carried out using a crossover double-blind, formula-controlled design with two parallel groups. In order to determine the sample size, a preliminary trial was done with 15 children to evaluate the average and variance of the difference in defecation frequency between formula and probiotic groups. To guarantee that the sample size calculation considered the crossover design, an equation for the comparison between averages of difference[9] was used considering a significance level set at 0.05, a power of 80%, a difference average of 0.75 and a variance difference of 3.15. Under the assumptions made here, the smallest sample size was 22 for each group. The students were randomized in two groups to receive 1 mL of goat yogurt supplemented with 109 CFU/mL B. longum (probiotic) daily or the same dose of goat yogurt daily for a period of 5 wk (formula). Afterwards, the groups were crossed over to alternate intervention for another 5 wk. Defecation frequency, stool consistency and abdominal or defecation pain were assessed at the first (A1), third (A2) and fifth week (A3) before crossing over, and the first (B1), third (B2) and fifth week (B3) after crossing over. The stool consistency was characterized using the Bristol Stool Scale[10]. To describe feces consistency, the subjects and/or their parents received instructions with a stool illustration and explanation in advance for the purpose of objectively selecting the stool form. The two products, goat yogurt with or without B. longum were identical in weight, color, smell, taste and package. All doctors and children involved were unaware of the treatment administered. Children were instructed to maintain their ordinary dietary habits, but were asked to avoid consuming other fermented dairy products or yogurts during the study. The allocation sequence and randomization list were computer-generated using the Epi Info Program.
Statistical analysis
Pearson exact test and Wilcoxon test were used to compare the variables (defecation frequency, stool consistency and abdominal and defecation pain) between the two groups. Differences in the variable distributions at each moment of each sequence of intervention were used for these analyses. The data were analyzed using the software Statistical Package for Social Sciences (SPSS 15.0). Normality was evaluated by Shapiro Wilk test. All tests were two sided, and P < 0.05 was considered statistically significant. All analyses were performed on an intention-to-treat basis.
RESULTS
The participant flow diagram (Figure 1) shows that among 286 students interviewed, 67 (23.4%) were diagnosed with FCIC following the Roma III criteria. Seven of them were excluded based on the exclusion criteria, and the remaining students were randomized to receive the probiotic or formula treatment. After the beginning of the trial only one parental withdrawal occurred in the formula group. There was no adverse effect due to the interventions in the present study protocol.
Figure 1.
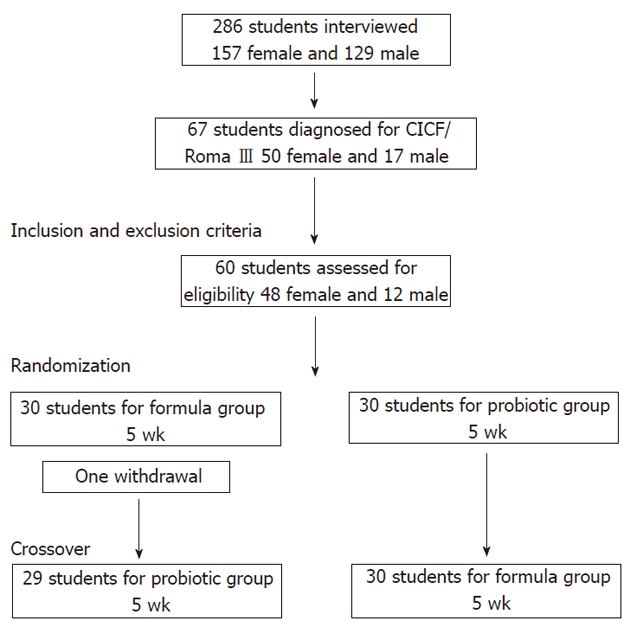
Participant flow diagram.
Table 1 summarizes the subjects’ baseline demographic and clinical characteristics. The two groups were comparable in regard to age, sex, and baseline features of constipation. More female subjects than male were present in the two groups and at a similar frequency (79.3% and 80.0% in formula and probiotic groups, respectively)
Table 1.
Characteristics of subjects at baseline
| Formula (n = 29) | Probiotic (n = 30) | |
| Female/Male | 23/6 | 24/6 |
| Age (yr) | ||
| 5 to 7 | 6 | 12 |
| 8 to 9 | 7 | 5 |
| 10 to 12 | 12 | 11 |
| 13 to 15 | 4 | 2 |
| Previous treatment for intestinal constipation | ||
| Yes | 5 | 3 |
| No | 24 | 27 |
| Defecation frequency | ||
| ≤ 2 times/wk | 19 | 17 |
| 3-6 times/wk | 7 | 13 |
| 7 or more times/wk | 3 | 0 |
| Stool consistency Bristol scale | ||
| 1 | 9 | 3 |
| 2 | 7 | 13 |
| 3 | 11 | 13 |
| 4 | 2 | 1 |
| 5 | 0 | 0 |
| Defecation pain (> 1/wk) | ||
| Yes | 26 | 20 |
| No | 3 | 10 |
| Abdominal pain (> 1/wk) | ||
| Yes | 25 | 26 |
| No | 4 | 4 |
Figure 2A shows and compares the evolution of the two groups for hard stool consistency (Bristol scale < 4) during the trial. An improvement was observed with both treatments as compared to baseline. This improvement was greater in the probiotic group during the first part of the intervention when a significant difference (P = 0.03) was observed between the two groups after 5 wk of treatment (A3 phase). After crossing over, an inversion was observed, but this was not statistically significant.
Figure 2.
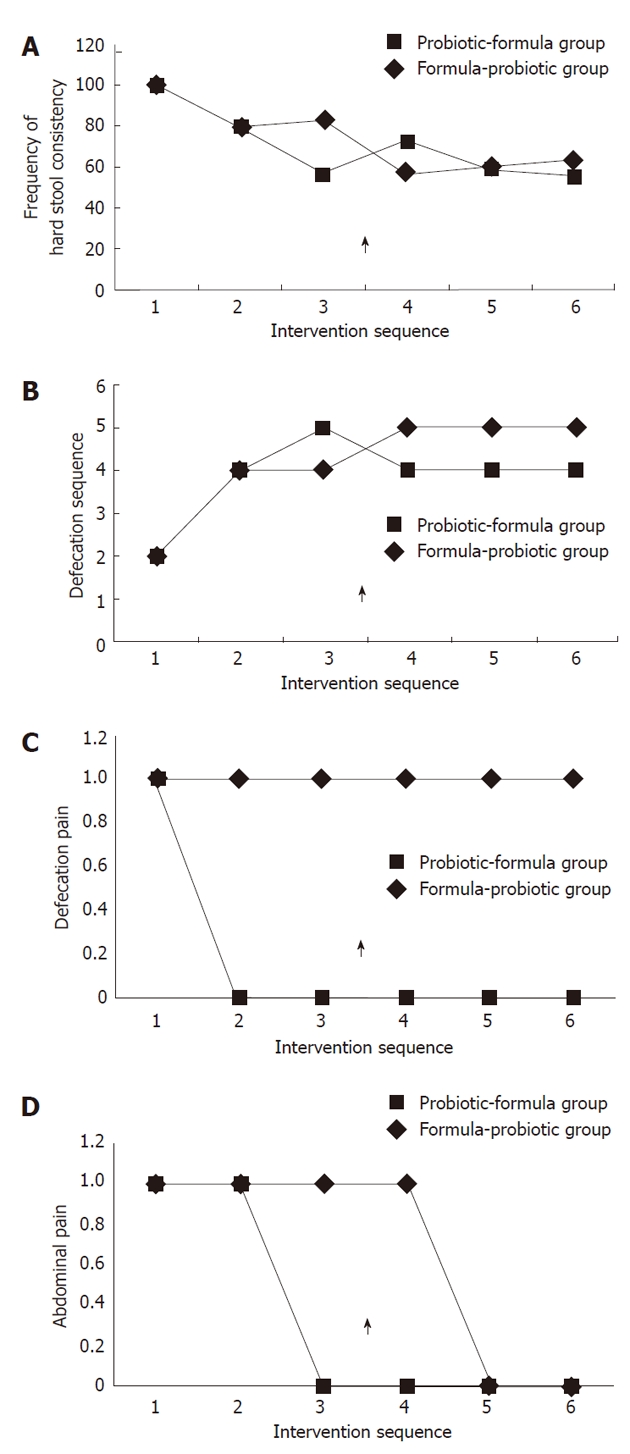
Evolution of hard stool consistency (Bristol scale < 4) (A), defecation frequency (B), defecation pain (C) and abdominal pain (D)during the intervention sequence. 1: A1; 2: A2; 3: A3; 4: B1; 5: B2; 6: B3. Arrow shows the moment of inversion in the intervention sequence.
Figure 2B shows and compares the evolution of the two groups for defecation frequency during the trial. A significant improvement in defecation frequency was also noted for both groups when compared to baseline with a tendency to a slight additional improvement at the end of the first intervention when B. longum was supplemented, and an inversion after crossing over.
Figure 2C shows and compares the evolution of defecation pain in the two groups during the trial. An improvement was observed for both treatments in relation to the baseline, but with a better evolution for the probiotic group. However, a significant difference (P = 0.009) between formula and probiotic was observed only for phase B1, and contrarily to Figures 1 and 2 an inversion was not observed after crossing over.
Figure 2D shows and compares the evolution of abdominal pain in the two groups during the trial. When the symptomatology was compared before and after the intervention, a significant improvement was noted for both groups as compared to baseline, but again with better results for the probiotic group. However, at the end of the second intervention after crossing over, the symptomatology was similar for the two groups.
When all data of the crossover study were analyzed, significant differences were observed between probiotic yogurt and yogurt only for defecation frequency (P = 0.012), defecation pain (P = 0.046) and abdominal pain (P = 0.015).
DISCUSSION
The prevalence of FCIC observed in the present study (23.4%) was similar to the data cited in the literature[1]. The predominance of FCIC in female subjects (about 80%) was also described in the literature[1].
Within the first week of intervention, a significant improvement in all constipation symptoms was observed in both treatment groups (yogurt or yogurt plus B. longum) when compared to the baseline. However, when the yogurt was supplemented with the probiotic, further improvement was obtained when compared to the yogurt only. Yogurt is generally considered to alleviate gastrointestinal conditions such as constipation and diarrhea[11]. However, regarding the effect of yogurt alone on constipation, few reports are available in the literature, and the results reported are contradictory. Additionally, in most of the clinical trials comparing the effect of probiotic yogurt with control yogurt, the starter lactic acid bacteria are heat-killed in the second situation, which does not correspond to the reality. In the few studies where viability of the starter strains was maintained in the control yogurt, improvement of constipation symptoms was observed in both probiotic and control groups with an increment in the first one[12].
There are several hypotheses to explain how probiotics might have therapeutic potential for the treatment of constipation. Firstly, quite old and well known observations showed that the absence of gut microbiota in germ-free animals result in abnormal characteristics of the intestinal morphology and function such as increased transit time of contents, altered myenteric neurons, impaired intestinal muscle function and decreased intestinal mass[13,14]. Interestingly, the mono-association of germ-free animals with Lactobacillus acidophilus or Bifidobacterium bifidum reduced the migrating myoelectric complex period and accelerated the small intestinal transit. Inversely, some E. coli strains presented an inverse effect when mono-associated in gnotobiotic animals[14]. Short-chain fatty acids (SCFAs), main metabolic products derived from the fermentative activity of the gut microbiota, have a direct influence on intestinal motility through the Gpr41 receptor[15]. In colonized Gpr41 knockout mice, an increased intestinal transit rate was associated with a reduced expression of peptide YY, an enteroendocrine cell-derived hormone that normally inhibits gut motility[16]. Secondly, there are some data suggesting differences in the intestinal microbiota of healthy individuals and patients with chronic constipation[17,18]. The main features were an increased number of clostridia and enterobacteria, and a decrease in bifidobacteria and lactobacilli. These differences have an influence on the metabolic profile of the gut environment, and particularly on SCFA pattern[19]. However, a key question is if this dysbiosis is a secondary manifestation of constipation, or is a factor contributing to constipation. Another set of data favoring the microbiota influence describes the higher defecation frequency and softer stool consistency in breast-fed than in formula-fed infants in the first four months of life, which can be due to the higher fecal levels of bifidobacteria in breast-fed infants[20]. Thirdly, studies involving the administration of Bifidobacterium animalis subspecies lactis DN-173010 have shown improved colonic transit times, both in a healthy population[21] and in constipated patients[22]. Another study showed that the intake of probiotic (Lactobacillus helveticus and B. longum) can modify the gut microbial ecology and metabolic profiles[23]. Finally, in a study using a guinea-pig isolated tissue model, results showed that cytoplasmatic fraction of probiotic bacteria (Lactobacillus, Bifidobacterium) stimulated the contraction of the ileum segment and induced proximal colon relaxation[24].
In conclusion, an improvement in constipation symptoms was observed using both supplemented and non-supplemented yogurt. An additional improvement with B. longum supplementation was suggested in the present intercrossed double-blind formula-controlled study.
ACKNOWLEDGMENTS
The authors acknowledge the staff of the Instituto de Educação, Belo Horizonte, Brazil, and all of the families and children who participated in the present study.
COMMENTS
Background
The worldwide prevalence of childhood constipation in the general population ranges from 0.7% to 29.6%, and the standard treatment that consists of disimpaction and administration of laxatives does not provide satisfying improvement in 40% of the children. Development of new therapeutic strategies is necessary to treat these patients more effectively, and probiotics are increasingly being used as one of such alternatives in the management of constipation.
Research frontiers
There is growing interest in the use of probiotics in organic and functional gastrointestinal disorders. A limited number of studies have been published about the effects of probiotics on constipation in children, and encouraging results have been obtained. But so far there is no hard evidence to recommend this use in children, and a general consensus in the scientific literature recommends more clinical trials. In the present intercrossed double-blind formula-controlled study, an additional improvement in constipation symptoms was observed using yogurt supplemented with Bifidobacterium longum when compared to non-supplemented yogurt.
Innovations and breakthroughs
Recent reports have highlighted that the administration of probiotics could be recommended as an adjunctive therapy of chronic constipation. It is well known that the indigenous digestive microbiota affects intestinal motility and accelerates small intestinal transit. Among the components of this microbial ecosystem, bacteria of the Bifidobacterium genus seems to be involved in the phenomenon and therefore are microorganisms of choice for use as probiotics.
Applications
The results of the present clinical trial suggest that the ingestion of a goat yogurt supplement with a Bifidobacterium strain may represent an alternative strategy in the treatment of pediatric functional constipation.
Peer review
A clearcut, well conducted trial showing how the addition of a specific probiotic agent can improve intestinal symptoms in children and adolescent with constipation.
Footnotes
Supported by Grants from Conselho Nacional de Desenvolvimento Cientifico e Tecnológico and Fundação de Amparo à Pesquisa do Estado de Minas Gerais
Peer reviewer: Mario Guslandi, Professor, Department of Gastroenterology, S: Raffaele University Hospital, S: Raffaele University Hospitalvia Olgettina 60, Milano 20132, Italy
S- Editor Tian L L- Editor O’Neill M E- Editor Xiong L
References
- 1.van den Berg MM, Benninga MA, Di Lorenzo C. Epidemiology of childhood constipation: a systematic review. Am J Gastroenterol. 2006;101:2401–2409. doi: 10.1111/j.1572-0241.2006.00771.x. [DOI] [PubMed] [Google Scholar]
- 2.Rasquin A, Di Lorenzo C, Forbes D, Guiraldes E, Hyams JS, Staiano A, Walker LS. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130:1527–1537. doi: 10.1053/j.gastro.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bongers ME, Benninga MA, Maurice-Stam H, Grootenhuis MA. Health-related quality of life in young adults with symptoms of constipation continuing from childhood into adulthood. Health Qual Life Outcomes. 2009;7:20. doi: 10.1186/1477-7525-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joint FAO; WHO. Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food. London, Canada: 2002. [Google Scholar]
- 5.Chmielewska A, Szajewska H. Systematic review of randomised controlled trials: probiotics for functional constipation. World J Gastroenterol. 2010;16:69–75. doi: 10.3748/wjg.v16.i1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banaszkiewicz A, Szajewska H. Ineffectiveness of Lactobacillus GG as an adjunct to lactulose for the treatment of constipation in children: a double-blind, placebo-controlled randomized trial. J Pediatr. 2005;146:364–369. doi: 10.1016/j.jpeds.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 7.Coccorullo P, Quitadamo P, Martinelli M, Staiano A. Novel and alternative therapies for childhood constipation. J Pediatr Gastroenterol Nutr. 2009;48 Suppl 2:S104–S106. doi: 10.1097/MPG.0b013e3181a15fd7. [DOI] [PubMed] [Google Scholar]
- 8.Del Piano M, Carmagnola S, Anderloni A, Andorno S, Ballarè M, Balzarini M, Montino F, Orsello M, Pagliarulo M, Sartori M, et al. The use of probiotics in healthy volunteers with evacuation disorders and hard stools: a double-blind, randomized, placebo-controlled study. J Clin Gastroenterol. 2010;44 Suppl 1:S30–S34. doi: 10.1097/MCG.0b013e3181ee31c3. [DOI] [PubMed] [Google Scholar]
- 9.Chow SC, Shao J, Wang H. Sample Size Calculations in Clinical Research. 2nd Ed. USA: CRC;; 2008. [Google Scholar]
- 10.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 11.Adolfsson O, Meydani SN, Russell RM. Yogurt and gut function. Am J Clin Nutr. 2004;80:245–256. doi: 10.1093/ajcn/80.2.245. [DOI] [PubMed] [Google Scholar]
- 12.Guyonnet D, Chassany O, Ducrotte P, Picard C, Mouret M, Mercier CH, Matuchansky C. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: a multicentre, randomized, double-blind, controlled trial. Aliment Pharmacol Ther. 2007;26:475–486. doi: 10.1111/j.1365-2036.2007.03362.x. [DOI] [PubMed] [Google Scholar]
- 13.Abrams GD, Bishop JE. Effect of the normal microbial flora on gastrointestinal motility. Proc Soc Exp Biol Med. 1967;126:301–304. doi: 10.3181/00379727-126-32430. [DOI] [PubMed] [Google Scholar]
- 14.Husebye E, Hellström PM, Sundler F, Chen J, Midtvedt T. Influence of microbial species on small intestinal myoelectric activity and transit in germ-free rats. Am J Physiol Gastrointest Liver Physiol. 2001;280:G368–G380. doi: 10.1152/ajpgi.2001.280.3.G368. [DOI] [PubMed] [Google Scholar]
- 15.Tazoe H, Otomo Y, Kaji I, Tanaka R, Karaki SI, Kuwahara A. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J Physiol Pharmacol. 2008;59 Suppl 2:251–262. [PubMed] [Google Scholar]
- 16.Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salminen S, Salminen E. Lactulose, lactic acid bacteria, intestinal microecology and mucosal protection. Scand J Gastroenterol Suppl. 1997;222:45–48. doi: 10.1080/00365521.1997.11720717. [DOI] [PubMed] [Google Scholar]
- 18.Zoppi G, Cinquetti M, Luciano A, Benini A, Muner A, Bertazzoni Minelli E. The intestinal ecosystem in chronic functional constipation. Acta Paediatr. 1998;87:836–841. doi: 10.1080/080352598750013590. [DOI] [PubMed] [Google Scholar]
- 19.Martin FP, Dumas ME, Wang Y, Legido-Quigley C, Yap IK, Tang H, Zirah S, Murphy GM, Cloarec O, Lindon JC, et al. A top-down systems biology view of microbiome-mammalian metabolic interactions in a mouse model. Mol Syst Biol. 2007;3:112. doi: 10.1038/msb4100153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Picard C, Fioramonti J, Francois A, Robinson T, Neant F, Matuchansky C. Review article: bifidobacteria as probiotic agents -- physiological effects and clinical benefits. Aliment Pharmacol Ther. 2005;22:495–512. doi: 10.1111/j.1365-2036.2005.02615.x. [DOI] [PubMed] [Google Scholar]
- 21.Marteau P, Cuillerier E, Meance S, Gerhardt MF, Myara A, Bouvier M, Bouley C, Tondu F, Bommelaer G, Grimaud JC. Bifidobacterium animalis strain DN-173 010 shortens the colonic transit time in healthy women: a double-blind, randomized, controlled study. Aliment Pharmacol Ther. 2002;16:587–593. doi: 10.1046/j.1365-2036.2002.01188.x. [DOI] [PubMed] [Google Scholar]
- 22.Agrawal A, Houghton LA, Morris J, Reilly B, Guyonnet D, Goupil Feuillerat N, Schlumberger A, Jakob S, Whorwell PJ. Clinical trial: the effects of a fermented milk product containing Bifidobacterium lactis DN-173 010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2009;29:104–114. doi: 10.1111/j.1365-2036.2008.03853.x. [DOI] [PubMed] [Google Scholar]
- 23.Vitali B, Ndagijimana M, Cruciani F, Carnevali P, Candela M, Guerzoni ME, Brigidi P. Impact of a synbiotic food on the gut microbial ecology and metabolic profiles. BMC Microbiol. 2010;10:4. doi: 10.1186/1471-2180-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massi M, Ioan P, Budriesi R, Chiarini A, Vitali B, Lammers KM, Gionchetti P, Campieri M, Lembo A, Brigidi P. Effects of probiotic bacteria on gastrointestinal motility in guinea-pig isolated tissue. World J Gastroenterol. 2006;12:5987–5994. doi: 10.3748/wjg.v12.i37.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]


