Abstract
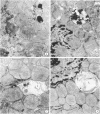
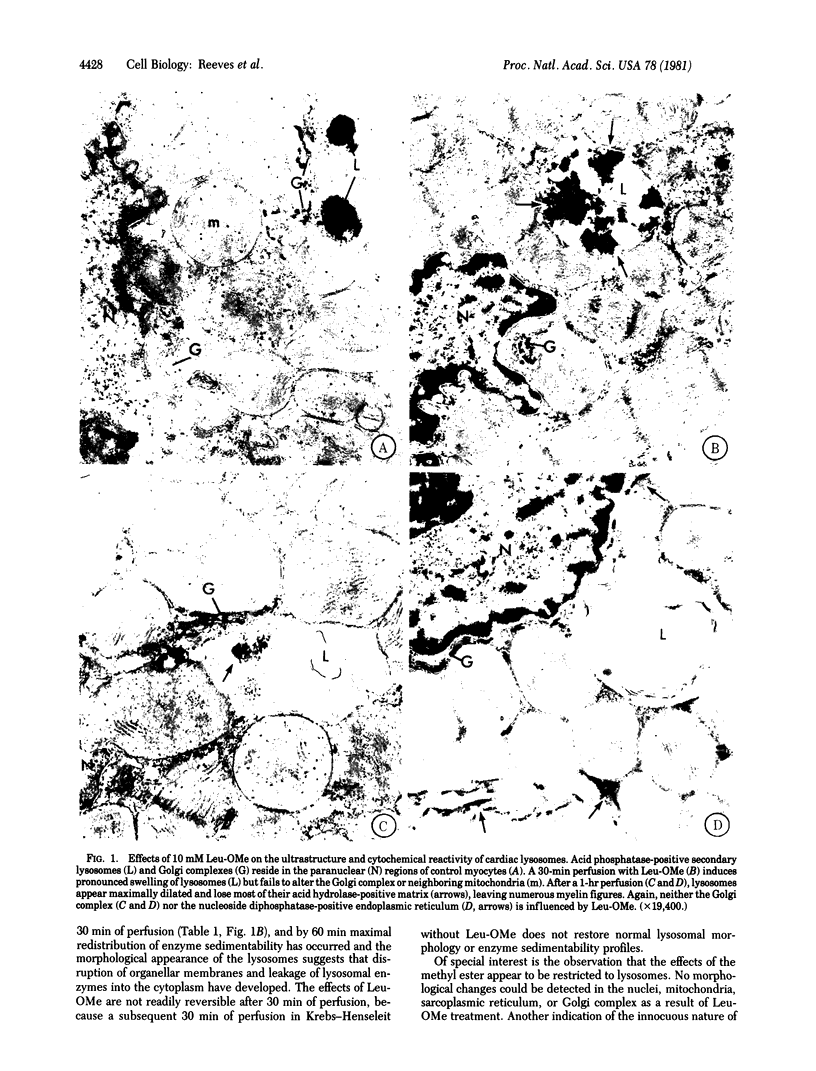
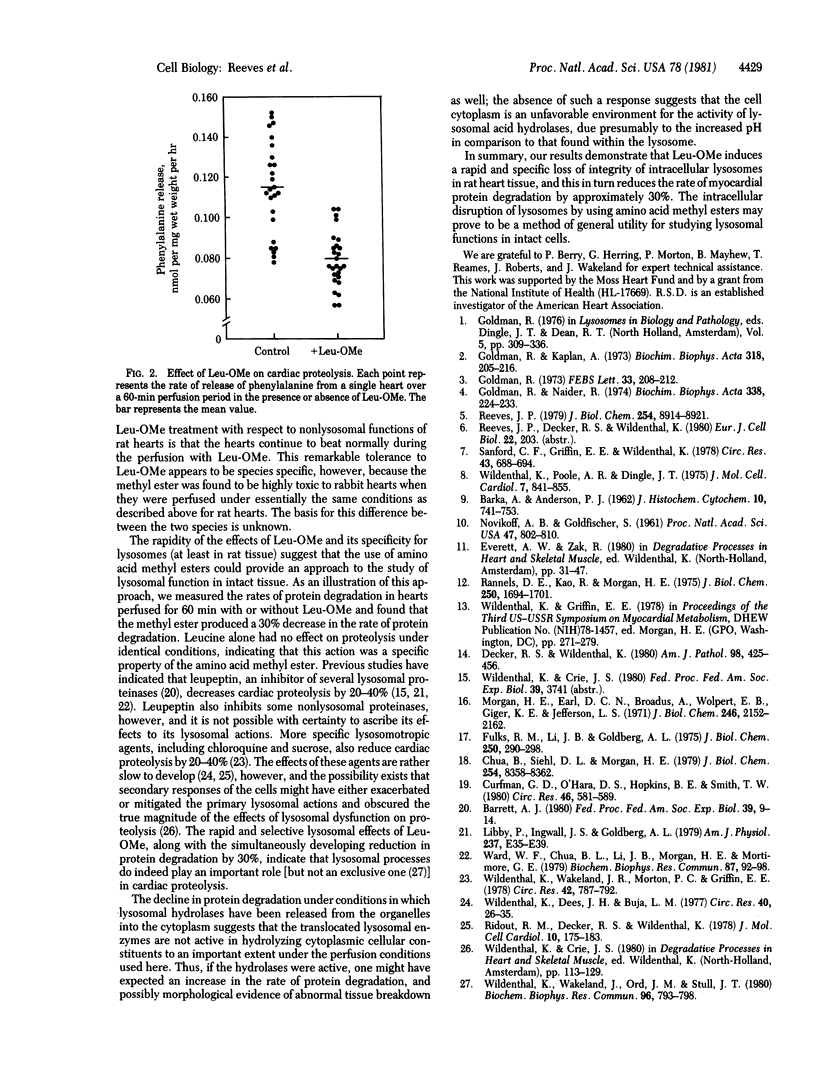
Perfusion of rat hearts with Krebs--Henseleit medium containing 10 mM L-leucine methyl ester leads to swelling of lysosomes and loss of lysosomal integrity within 30-60 min. No morphological changes can be detected in the nuclei, mitochondria, sarcoplasmic reticulum, or Golgi complex as a result of the treatment with leucine methyl ester, and the hearts continue to beat normally during the treatment period. Homogenates of rat hearts perfused with the methyl ester exhibit a decrease in the sedimentability of cathepsin D activity compared to controls, thus providing additional evidence for a loss of lysosomal integrity. Swelling and disruption of the lysosomes presumably occurs because of the extensive accumulation of leucine within the organelles resulting from the intralysosomal hydrolysis of the freely permeating methyl ester. The lysosomal dysfunction that occurs with exposure to leucine methyl ester produces a 30% decrease in cardiac protein degradation. These results provide an estimate of the contribution of lysosomes to total protein degradation in the rat heart, and they also suggest that the enzymes released as a result of lysosomal disruption are relatively inactive in hydrolyzing cellular constituents under the perfusion conditions used here. The use of amino acid methyl esters to produce rapid, specific loss of lysosomal integrity in situ provides an approach to the study of lysosomal function in intact cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J. The many forms and functions of cellular proteinases. Fed Proc. 1980 Jan;39(1):9–14. [PubMed] [Google Scholar]
- Chua B., Siehl D. L., Morgan H. E. Effect of leucine and metabolites of branched chain amino acids on protein turnover in heart. J Biol Chem. 1979 Sep 10;254(17):8358–8362. [PubMed] [Google Scholar]
- Curfman G. D., O'Hara D. S., Hopkins B. E., Smith T. W. Suppression of myocardial protein degradation in the rat during fasting. Effects of insulin, glucose, and leucine. Circ Res. 1980 Apr;46(4):581–589. doi: 10.1161/01.res.46.4.581. [DOI] [PubMed] [Google Scholar]
- Decker R. S., Poole A. R., Crie J. S., Dingle J. T., Wildenthal K. Lysosomal alterations in hypoxic and reoxygenated hearts. II. Immunohistochemical and biochemical changes in cathepsin D. Am J Pathol. 1980 Feb;98(2):445–456. [PMC free article] [PubMed] [Google Scholar]
- Fulks R. M., Li J. B., Goldberg A. L. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975 Jan 10;250(1):290–298. [PubMed] [Google Scholar]
- Goldman R. Dipeptide hydrolysis within intact lysosomes in vitro. FEBS Lett. 1973 Jul 1;33(2):208–212. doi: 10.1016/0014-5793(73)80194-2. [DOI] [PubMed] [Google Scholar]
- Goldman R., Kaplan A. Rupture of rat liver lysosomes mediated by L-amino acid esters. Biochim Biophys Acta. 1973 Aug 22;318(2):205–216. doi: 10.1016/0005-2736(73)90114-4. [DOI] [PubMed] [Google Scholar]
- Libby P., Ingwall J. S., Goldberg A. L. Reduction of protein degradation and atrophy in cultured fetal mouse hearts by leupeptin. Am J Physiol. 1979 Jul;237(1):E35–E39. doi: 10.1152/ajpendo.1979.237.1.E35. [DOI] [PubMed] [Google Scholar]
- Morgan H. E., Earl D. C., Broadus A., Wolpert E. B., Giger K. E., Jefferson L. S. Regulation of protein synthesis in heart muscle. I. Effect of amino acid levels on protein synthesis. J Biol Chem. 1971 Apr 10;246(7):2152–2162. [PubMed] [Google Scholar]
- NOVIKOFF A. B., GOLDFISCHER S. Nucleosidediphosphatase activity in the Golgi apparatus and its usefulness for cytological studies. Proc Natl Acad Sci U S A. 1961 Jun 15;47:802–810. doi: 10.1073/pnas.47.6.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rannels D. E., Kao R., Morgan H. E. Effect of insulin on protein turnover in heart muscle. J Biol Chem. 1975 Mar 10;250(5):1694–1701. [PubMed] [Google Scholar]
- Reeves J. P. Accumulation of amino acids by lysosomes incubated with amino acid methyl esters. J Biol Chem. 1979 Sep 25;254(18):8914–8921. [PubMed] [Google Scholar]
- Ridout R. M., Decker R. S., Wildenthal K. Chloroquine-induced lysosomal abnormalities in cultured foetal mouse hearts. J Mol Cell Cardiol. 1978 Feb;10(2):175–183. doi: 10.1016/0022-2828(78)90041-x. [DOI] [PubMed] [Google Scholar]
- Sanford C. F., Griffin E. E., Wildenthal K. Synthesis and degradation of myocardial protein during the development and regression of thyroxine-induced cardiac hypertrophy in rats. Circ Res. 1978 Nov;43(5):688–694. doi: 10.1161/01.res.43.5.688. [DOI] [PubMed] [Google Scholar]
- Ward W. F., Chua B. L., Li J. B., Morgan H. E., Mortimore G. E. Inhibition of basal and deprivation-induced proteolysis by leupeptin and pepstatin in perfused rat liver and heart. Biochem Biophys Res Commun. 1979 Mar 15;87(1):92–98. doi: 10.1016/0006-291x(79)91651-6. [DOI] [PubMed] [Google Scholar]
- Wildenthal K., Dees J. H., Buja L. M. Cardiac lysosomal derangements in mouse heart after long-term exposure to nonmetabolizable sugars. Circ Res. 1977 Jan;40(1):26–35. doi: 10.1161/01.res.40.1.26. [DOI] [PubMed] [Google Scholar]
- Wildenthal K., Poole A. R., Dingle J. T. Influence of starvation on the activities and localization of cathepsin D and other lysosomal enzymes in hearts of rabbits and mice. J Mol Cell Cardiol. 1975 Nov;7(11):841–855. doi: 10.1016/0022-2828(75)90135-2. [DOI] [PubMed] [Google Scholar]
- Wildenthal K., Wakeland J. R., Morton P. C., Griffin E. E. Inhibition of protein degradation in mouse hearts by agents that cause lysosomal dysfunction. Circ Res. 1978 Jun;42(6):787–792. doi: 10.1161/01.res.42.6.787. [DOI] [PubMed] [Google Scholar]
- Wildenthal K., Wakeland J. R., Ord J. M., Stull J. T. Interference with lysosomal proteolysis fails to reduce cardiac myosin degradation. Biochem Biophys Res Commun. 1980 Sep 30;96(2):793–798. doi: 10.1016/0006-291x(80)91424-2. [DOI] [PubMed] [Google Scholar]