Abstract
In recent years several evidence demonstrated that some features of hippocampal biology, like neurogenesis, synaptic transmission, learning, and memory performances are deeply modulated by social, motor, and sensorial experiences. Fractalkine/CX3CL1 is a transmembrane chemokine abundantly expressed in the brain by neurons, where it modulates glutamatergic transmission and long-term plasticity processes regulating the intercellular communication between glia and neurons, being its specific receptor CX3CR1 expressed by microglia. In this paper we investigated the role of CX3CL1/CX3CR1 signaling on experience-dependent hippocampal plasticity processes. At this aim wt and CX3CR1GFP/GFP mice were exposed to long-lasting-enriched environment (EE) and the effects on hippocampal functions were studied by electrophysiological recordings of long-term potentiation of synaptic activity, behavioral tests of learning and memory in the Morris water maze paradigm and analysis of neurogenesis in the subgranular zone of the dentate gyrus (DG). We found that CX3CR1 deficiency increases hippocampal plasticity and spatial memory, blunting the potentiating effects of EE. In contrast, exposure to EE increased the number and migration of neural progenitors in the DG of both wt and CX3CR1GFP/GFP mice. These data indicate that CX3CL1/CX3CR1-mediated signaling is crucial for a normal experience-dependent modulation of hippocampal functions.
Keywords: CX3CL1, long-term potentiation, learning and memory, neurogenesis, experience
Introduction
The importance of the environment in the regulation of brain physiology and behavior has long been recognized in biological, social, and medical science. The chance to live in conditions where social interactions, but also sensory and motor inputs are potentiated (enriched environment, EE), increases brain plasticity and could be relevant to prevent or reduce the alterations in cognitive performances occurring upon aging and in several neurodegenerative diseases (van Praag et al., 1999; Nithianantharajah and Hannan, 2006). The brain of animals maintained under EE conditions has been shown to undergo molecular and morphological changes leading to: (i) modification in neuronal structure, connections, and efficacy (Globus et al., 1973; Green and Greenough, 1986; Kempermann et al., 1997); (ii) reduction of anxiety, fear, stress, and excitability (Engellenner et al., 1982); (iii) enhanced learning and memory (Escorihuela et al., 1995; Kempermann et al., 1997; van Praag et al., 1999; Rampon and Tsien, 2000). The crucial ability of the CNS to encode and retain memories is based on activity-dependent forms of synaptic plasticity and, in the mammalian brain, hippocampal long-term potentiation (LTP) is believed to be related to the storage of declarative memory (Squire and Zola-Morgan, 1991). In particular, the CA1 area of the hippocampus is critical for information processing linked to the acquisition and consolidation of spatial memories (Tsien et al., 1996). Exploration of spatially complex or EE elicits, in the CA1 hippocampal neurons, patterns of electrical activity similar to those of electrical stimulation used to experimentally induce LTP in hippocampal slices (Otto et al., 1991). In addition, hippocampal slices from the brain of “enriched” mice show enhanced LTP in the Schaffer collateral pathway of area CA1 (Duffy et al., 2001), suggesting that exposure to EE may modify synaptic physiology in hippocampal neurons (Foster et al., 1996; van Praag et al., 1999). It has been reported that exposure of mice to EE for several weeks modifies learning and memory abilities in the hippocampus. In particular, mice exposed to a prolonged period of EE, perform better in the Morris water maze test compared with age-matched mice reared in standard cages (van Praag et al., 1999). However, the factors mediating the effect of enrichment on behavior have been only partially identified. Among these factors, cytokines appear to exert an important function (Goshen et al., 2009) however, the role of CHEMOtactic cytoKINES (chemokines) has not been explored, yet. Beside their role in the immune system, chemokines play neuromodulatory roles on brain functions being constitutively expressed in the brain both in glial cells and neurons (Asensio and Campbell, 1999). In particular, chemokines contribute to cell-to-cell communication (Tran and Miller, 2003), modulating neurotransmitter release and plasticity (Giovannelli et al., 1998; Meucci et al., 1998; Limatola et al., 2000; Bezzi et al., 2001; Ragozzino et al., 2006; Rostène et al., 2007; Maggi et al., 2009), and modifying the functional properties of ionic channels (Meucci et al., 1998; Lax et al., 2002; Oh et al., 2002).
Although the mouse brain is completely formed few weeks after birth, it maintains some degrees of plasticity throughout life, including axonal remodeling, synaptogenesis, neurogenesis, migration, and integration in pre-existing circuits. The dentate gyrus (DG) of the hippocampus is one of the main neurogenic niches in the adult brain. The progenitor cells in the subgranular zone (SGZ) divide, generating neuronal precursors that express doublecortin (DCX, a microtubule-associated protein required for immature neural cells migration, Brown et al., 2003) and migrate for a short distance before they differentiate and integrate into the pre-existing neuronal networks (van Praag et al., 2002). A large number of studies suggests that EE and physical exercise stimulate the appearance of new neurons in the hippocampal DG (Kempermann and Gage, 1999; van Praag et al., 1999). The neurogenesis in the DG has been correlated with the function of pattern separation, an essential step in information processing to avoid memory interferences (Deng et al., 2010). The correct migration of neural precursors generated in DG requires, among the other factors, the timely expression of the chemokine CXCL12 and its specific receptors CXCR4 and CXCR7 (Sánchez-Alcañiz et al., 2011). Much less is known on the role of CX3CL1/CX3CR1 signaling in DG neurogenesis. CX3CL1 is constitutively expressed in several brain area while its specific receptor CX3CR1 is expressed by microglia and, in the hippocampus, it has been shown to modulate synaptic transmission between Schaffer collaterals and pyramidal neurons in the CA1 region, inducing AMPA-mediated excitatory synaptic depression and negatively modulating LTP (Bertollini et al., 2006; Ragozzino et al., 2006; Maggi et al., 2009). A recent study demonstrates reduced neurogenesis in the SGZ of CX3CR1−/− mice and potentiating effects of exogenous CX3CL1 on DG neurogenesis in aged mice, which also have lower level of hippocampal CX3CL1 expression (Bachstetter et al., 2011). In the present study we characterized the role of CX3CR1/CX3CL1 signaling in modulating neural plasticity, learning abilities and DG neurogenesis in a mouse model of EE.
Materials and Methods
Animals
Three-week-old female mice (wt: C57BL/6J and CX3CR1GFP/GFP on the C57BL/6J background from the Jackson Laboratory, Charles River) were either housed in standard environment (SE) or EE. In CX3CR1GFP/GFP mice, CX3CR1 gene was replaced by a green fluorescent protein (GFP) reporter gene (Jung et al., 2000). Littermates wt and CX3CR1GFP/GFP, obtained from CX3CR1+/GFP × CX3CR1+/GFP heterozygotes, were genotyped by PCR following the protocol and primers described by the mice supplier.
Environmental enrichment
Mice exposed to SE were housed in pairs, in standard cages (30 cm × 16 cm × 11 cm). Mice exposed to EE were housed 10 for cage (36 cm × 54 cm × 19 cm or 45 cm × 25 cm × 22 cm with a labyrinth), in the presence of an assortment of objects, including climbing ladders, running wheel, balls, plastic, and wood objects suspended from the ceiling, paper, cardboard boxes, and nesting material. Toys were changed every 2–3 days, while the bedding was changed every week. Both EE and SE groups received identical type of rodent chow and water ad libitum and were kept on a 12-h light/dark cycle. Mice were kept in EE or SE for 2.5 months before electrophysiological, behavioral, and immunofluorescence analyses. In particular, electrophysiological and immunofluorescence experiments were performed on two different sets of trained animals (for both wt and CX3CR1GFP/GFP). EE experiments were not performed on littermates due to technical limitations to obtain age- and gender-matched mice of different genotypes in sufficient number.
Hippocampal slice preparation
Electrophysiological experiments were performed from 1 to 5 days after enrichment. The experiments were performed in agreement with international guidelines on the ethical use of animals from the European Communities Council Directive of 24 November 1986 (86/609 EEC). Hippocampal slices were routinely obtained from 13 to 14-week-old C57BL/6J or CX3CR1GFP/GFP mice. Briefly, the animals were decapitated after being anesthetized with halothane. Whole brains were rapidly removed from the skull and immersed for 10 min in ice-cold artificial cerebrospinal fluid (ACSF) solution containing (in millimolar): NaCl 125, KCl 4.4, CaCl2 2.5, MgSO4 1.5, NaHPO4 1, NaHCO3 26, and glucose 10. The ACSF was continuously oxygenated with 95% O2, 5% CO2 to maintain the proper pH (7.4). Transverse 350 μm thick slices were cut at 4°C with a vibratome (DSK, Japan) and the appropriate slices were placed in a chamber containing oxygenated ACSF. After their preparation, slices were allowed to recover for 2 h. Individual slices were then transferred to the interface slice-recording chamber (BSC1, Scientific System Design Inc.) with a total fluid dead space of approximately 3 ml. Slices were maintained at 30–32°C and constantly superfused at the rate of 1.5 ml/min. Solutions were applied to the slices by a peristaltic pump.
Electrophysiological recordings
At the beginning of each recording, a concentric bipolar stimulating electrode (SNE-100 × 50 mm long Elektronik–Harvard Apparatus GmbH) was positioned in the stratum radiatum for stimulation of Schaffer collateral pathway projections to CA1. An ACSF-filled glass micropipette (0.5–1 MΩ) was positioned 200–600 μm from the stimulating electrode for recording orthodromically evoked fEPSPs. Stimuli consisted of 100 μs constant current square pulses, applied at 0.05 Hz. The intensity of the stimulus was adjusted in each experiment to evoke ∼50% of the maximal field potential amplitude without appreciable population spike contamination. Evoked responses were monitored online and stable baseline responses were recorded for at least 10 min. Only the slices that showed stable fEPSP amplitudes were included in the experiments. To analyze the time course of fEPSP slope, the recorded fEPSP was routinely averaged over 1 min (n = 3). LTP experiments were performed in ACSF and the averaged fEPSP (35–45 min post-induction) was normalized to the baseline values (0–10 min) before LTP induction (HFS, 1 train, 100 Hz, 1-s duration, test strength). LTP experiments in SE were replicated in CX3CR1GFP/GFP, wt and CX3CR1GFP/+ littermates (Figure A1 in Appendix).
For the paired-pulse ratio (PPR) test, closely spaced consecutive stimuli (50 ms interval) were used, and PPR was calculated as the ratio between the fEPSP amplitude evoked by the second stimulus (A2) over the first (A1; A2/A1). Input–output (I–O) curves were measured at the beginning of recordings.
Data acquisition and analysis
Slices were visualized with a Wild M3B (Heerbrugg, Switzerland). fEPSPs were recorded and filtered (1 kHz) with an Axopatch 200 A amplifier (Axon Instruments, CA, USA) and digitized at 10 kHz with an A/D converter (Digidata 1322A, Axon Instruments). Data were stored on a computer using pClamp 9 software (Axon Instruments) and analyzed off-line with Clamp-fit 9 program (Axon Instruments).
Morris water maze
The water maze apparatus consisted of a black Plexiglas circular pool 88 cm in diameter and 33 cm in height, and was placed in the middle of an experimental room (dimension 4 m × 4 m × 3 m). The pool was filled with water kept at a temperature of 26 ± 1°C. A plastic transparent platform (8 cm in diameter) was placed 0.5 cm below the water surface and 10 cm from the edge of the pool. All tests were carried out between 9:00 and 14:00 h. The entire procedure took 8 days. Mice were individually transferred from the home-cage to pool. To avoid visual orientation prior to release, mice were transferred from their cages into the pool in a non-transparent plastic cup, from which they glided into the water facing the pool wall. Release points were balanced across four symmetrical positions on the pool perimeter.
The ability of experimental subjects to identify and reach a visible platform was tested in the visual cued version of the task, lasting 2 days, which preceded the 6-days of the acquisition phase. Each testing day, mice underwent three trials during which they were allowed to freely swim either for 60 s (cut-off time) or until they found and climbed onto the platform; the inter-trial interval was at least 40 min. Platform finding was defined as staying for at least 3 s on it. Once the platform was found, the mice were given the opportunity to climb on a wire-mesh grid and placed back to a cage kept in a warm environment. During the acquisition phase, mice that did not find the platform were trained in locating it by placing them on the platform for 10 s at the end of the trial. On the fourth and sixth day of the acquisition phase, the platform was removed from the pool and each mouse was tested for memory retention in a 30-s probe trial. During the probe trial, the platform was removed from the pool and the time spent in the target quadrant of the maze (where the platform was located during the acquisition phase) was scored as a reliable measure of memory retention. The swim path of the mice was recorded by means of a computer-based video-tracking system Ethovision (Noldus, The Netherlands). For the acquisition phase, the variables recorded were: latency to reach the platform, mean swimming speed, path length and turned angle, and thigmotaxis. For the probe trial, the variables recorded were: time spent in each quadrant, number of platform crossing, mean swimming speed, and thigmotaxis.
Animals and tissues preparation
Four mice from each different set of trained animals (both wt and CX3CR1GFP/GFP) were anesthetized using chloral-hydrate (400 mg/kg), transcardially perfused with phosphate-buffered saline (PBS 0.1 M), followed by 4% paraformaldehyde (PFA) in PBS. The brains were removed and post-fixed in 4% PFA overnight at 4°C and then cryoprotected by 24 h immersion in PBS containing 30% (w/v) sucrose at 4°C. Brains were cut into 10 μm sagittal sections using a cryostat.
Immunofluorescence
Immunofluorescence staining procedures were conducted on free-floating sections containing the hippocampal DG starting at −1.46 mm and ending at −2.80 mm from the bregma. The whole region of interest was covered by 4 sequential 40× microscopic fields. The same number of sections for mouse (25) was incubated for 1 h in 5% non-fat dry milk 5 and 0.5% Triton X-100, in PBS 0.1M. Sections were then incubated in goat anti DCX (Santa Cruz Biotechnology, CA, USA) diluted in 1% non-fat dry milk and 0.2% Triton X-100, in PBS 0.1 M, for 48 h at 4°C. After washing in PBS, the sections were incubated in secondary antibody (donkey anti goat, Alexafluor, Invitrogen) for 1 h, washed in PBS and then stained with Hoechst (Invitrogen) for 5 min, washed again and mounted on a microscope slide for the analysis of fluorescence (Axioscope 2 Zeiss). DCX+ cells in the SGZ and the granule cell layer of the DG were counted exhaustively (at 40× magnification). For analysis of cell migration, DCX+ cells were scored as migrating if their cell body was, in the granular layer, fully detached from the SGZ of at least 5 μm. In the same slices analyzed for neurogenesis, DG hippocampal area was calculated with Image J software and the counting of DCX+ cells were normalized to DG area.
Statistical analysis
The values were reported as mean ± SEM. If not specify, n refers to the number of mice analyzed. For comparisons among different groups, a two-way analysis of variance (ANOVA) was used with genotype and enrichment as between-subjects factors. ANOVA analyses were followed by Tukey (electrophysiology) or Holm–Sidak (neurogenesis analysis) post hoc analyses (Sigma Plot, 11.0 software). Mixed-model ANOVA with genotype and enrichment as between-subject factor and repeated measure as within-subject factor (e.g., day, quadrant) followed by Tukey post hoc comparisons was performed to analyze water maze data.
Results
The absence of CX3CR1 increases hippocampal LTP but abolishes the effect of EE
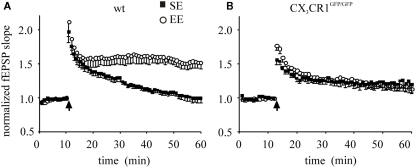
It has been reported that hippocampal LTP, induced by a weak stimulation (1 s burst of 100 Hz, HFS) in the CA1 region of the hippocampus, is enhanced by enrichment (Duffy et al., 2001). Following the same protocol of induction, we measured fEPSP potentiation in hippocampal slices in wt and CX3CR1GFP/GFP mice following exposure to either SE or EE (Figure 1A,B). In wt mice, the mean fEPSP slope potentiation, measured 35–45 min after LTP induction, was 1.05 ± 0.04 (17 slices/8 mice) and 1.56 ± 0.08 of baseline (17 slices/9 mice) in SE and EE, respectively. In CX3CR1GFP/GFP mice, LTP induction produced fEPSP slope potentiation of 1.19 ± 0.06 (17 slices/7 mice) and 1.26 ± 0.07 (27 slices/9 mice) in SE and EE, respectively.
Figure 1.
CA1 plasticity of wt and CX3CR1GFP/GFP mice exposed either to SE or EE conditions. Points represent mean ± SEM. Of fEPSP slopes evoked every 20 s and normalized as detailed in the Section “Materials and Methods.” Arrows indicate time of application of HFS. (A) Enhancement of LTP in wt mice raised in EE: 45 min after HFS, EE wt mice (open circle) developed a robust LTP of fEPSP slope compared to that of mice raised in SE (dark square, p < 0.001). (B) In CX3CR1GFP/GFP mice LTP is enhanced and EE produced no effect: after HFS, the amplitude of fEPSP potentiation of EE CX3CR1GFP/GFP mice (open circle) is not different from that obtained in SE mice (dark square). Note that that basal LTP evoked in SE is increased in CX3CR1GFP/GFP mice compared to wt [in (A), p = 0.006].
A main effect of housing condition was found [F1,27 = 10.232, p = 0.002). However a significant housing × genotype interaction [F(1,27) = 20.035, p < 0.001] showed that the housing condition affected only mice of the wt group. In particular, the post hoc analysis revealed that (i) the fEPSP potentiation was significantly higher in CX3CR1GFP/GFP SE compared to wt SE (p = 0.042), indicating that in control conditions CX3CR1GFP/GFP mice showed an enhanced plasticity, also confirmed in littermates (Figure A1 in Appendix); (ii) the wt EE showed a significant enhancement compared to wt SE (p < 0.001), indicating that EE affects only wt mice.
Short-term plasticity and basal fEPSP responses to Schaffer collateral stimulation are not affected in CX3CR1GFP/GFP mice
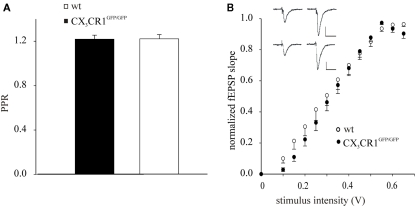
To analyze if the lack of effect of EE on LTP in CX3CR1GFP/GFP mice could be due to altered responses to basal synaptic transmission, we performed studies of PPR and fEPSP I–O curves of synaptic transmission in wt and CX3CR1GFP/GFP mice. PPR, that is the ratio between the fEPSP amplitude evoked by the second stimulus over the first, represents a form of pre-synaptic short-term plasticity, whose variation is generally associated with changes in transmitter release probability (Zucker, 1989). To evaluate PPR values in wt and CX3CR1GFP/GFP mice, we stimulated Schaffer collateral pathway projections to CA1 at 50 ms intervals. As shown in Figure 2A, PPR was 1.22 ± 0.03 (15 slices/7mice) and 1.22 ± 0.04 (9 slices/5 mice), in CX3CR1GFP/GFP and wt mice, respectively. These findings indicate that the transmitter release probability at Schaffer collateral input is comparable between the two genotypes. We then investigated synaptic strength in CX3CR1GFP/GFP mice recording fEPSP I–O curves of synaptic transmission in term of slope of the evoked potentials. Sample traces of fEPSP recorded at different intensities are illustrated in Figure 2B: the I–O relationships of the fEPSP slope CA1 curves of the wt (7 slices/5 mice) were indistinguishable from those of CX3CR1GFP/GFP mice (12 slices/7 mice), indicating similar basal fEPSP responses to Schaffer collateral stimulation over a range of stimulus intensities.
Figure 2.
Schaffer collateral-hippocampal CA1 basal responses in wt and CX3CR1GFP/GFP mice. (A) Histogram of fEPSP amplitude showing the facilitation of the fEPSP evoked by the second pulse, in wt and CX3CR1GFP/GFP mice, expressed as the ratio of the amplitude of the second response to that of the first (PPR). Error bars: ±SEM. (B) Stimulus intensity response curves (I–O) in wt and CX3CR1GFP/GFP mice. fEPSP in the CA1 area was increased in slope in an intensity-dependent manner. Inset: sample recordings at 0.3 (left) and 0.6 Volt (right) stimulation intensity in either wt (upper) or CX3CR1GFP/GFP mice (lower); scale vertical bar: 0.5 mV; scale horizontal bar: 50 ms.
CX3CR1GFP/GFP mice learned the water maze task faster than wt mice and their performance was not affected by the EE
In order to assess the role of CX3CL1/CX3CR1 signaling on learning and memory abilities, we investigated the effects of 2.5 months exposure to either SE or EE on wt and CX3CR1GFP/GFP mice in the Morris water maze test. The four experimental groups, wt SE (n = 6), wt EE (n = 6), CX3CR1GFP/GFP SE (n = 8), and CX3CR1GFP/GFP EE (n = 8) mice, showed no differences in the learning performance in the visual phase, indicating that unexpected drawbacks due to enrichment and/or to genetic manipulation did not interfere with the ability to solve the maze.
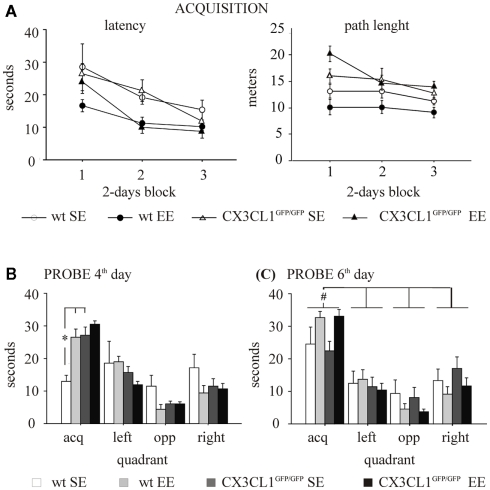
In the acquisition phase, all mice significantly reduced the latency to find the platform day after day [F(2,48) = 22.379, p < 0.0001]. However, the experimental subjects exposed to EE condition displayed better learning and memory abilities than those exposed to SE, as shown by the significant main effect of the environment [F(1,24) = 9.001, p = 0.0062; Figure 3A, left]. In line with the literature (Tremml et al., 2002; Harris et al., 2009), a reduced thigmotactic behavior in EE compared to SE mice was found [F(1,24) = 6.864, p = 0.0150; data not shown]. This may, at least in part, explain the difference in learning performances among groups shown during acquisition (Wolfer et al., 1998).
Figure 3.
Morris water maze in wt and CX3CR1GFP/GFP mice exposed to either SE or EE condition. (A) Acquisition. Left: latency to find the platform. All subjects learnt to find the platform. However, EE mice, independently from their genotype, showed a significant reduction in escape latency compared with SE mice. Right: path length to find the platform. A main effect of genotype was found for path length, CX3CR1GFP/GFP swimming longer paths than wt during the acquisition phase. (B) Probe on the fourth day. SE CX3CR1GFP/GFP mice spent an amount of time similar to that spent by EE CX3CR1GFP/GFP mice and significantly longer than that spent by SE wt mice. (C) Probe on the sixth day. All experimental groups spent significantly more time in the quadrant where the platform had been located during training than in the other quadrants. The EE mice showed a better learning performance than SE mice, independently from their genotype. *p < 0.05 vs. both EE wt and SE CX3CR1GFP/GFP mice. #p < 0.05 vs. the other quadrants for each experimental group. Data are mean ± SEM.
The effect of the environment was also evident during the probe phase on the fourth and the sixth day [respectively, F(3,72) = 4.650, p = 0.0050 and F(3,72) = 3.726, p = 0.0150; Figures 3B,C]. In particular, in both probe phases, EE mice spent significantly longer time in the quadrant where the platform was located during the acquisition phase (acquisition quadrant) compared to SE mice. By contrast, the effect of genotype was evident only during the probe phase on the fourth day [F(3,72) = 4.201, p = 0.0085] even though the analysis of genotype × environment interaction did not reach statistical significance [F(3,72) = 2.179, p = 0.0979]. However, post hoc analysis revealed that: (i) CX3CR1GFP/GFP mice learned the water maze task faster than wt mice, the time spent by SE CX3CR1GFP/GFP mice in the acquisition quadrant being significantly longer than that spent by SE wt (p < 0.05); (ii) EE did not improve the water maze task performance in CX3CR1GFP/GFP mice, since EE mice spent an amount of time similar to SE mice in the acquisition quadrant. On the probe phase of the sixth day, a main effect of quadrant was found [F(3,72) = 30.069, p < 0.0001]. In addition, after pooling data of the two genotypes within each house condition because of no genotype effect, the interaction house condition × quadrant is statistically significant [F(3,78) = 4.146, p = 0.0088] and post hoc analysis revealed that all groups spent more time in the platform quadrant (p < 0.05; Figure 3C).
With regard to the other parameters considered in the analysis, a main effect of the genotype was found for speed measured during the acquisition phase and the day 4 probe [respectively, Fs(1,24) = 29.284 and 4.576, p < 0.001 and p = 0.0428], CX3CR1GFP/GFP mice swimming faster than wt, but not in the day 6 probe. A main effect of genotype was found also for path length, CX3CR1GFP/GFP mice swimming longer paths than wt during the acquisition phase (Figure 3A, right), the day 4 and the day 6 probe (data not shown). However, since the genotype did not affect learning performance during the acquisition phase and speed and path length are not relevant for evaluating quadrant preference during the probe phase, the results obtained have not likely been influenced by these factors. Both during the acquisition and the probe phases, no difference in thigmotactic behavior among the experimental groups was found.
EE enhances neurogenesis in the DG of both wt and CX3CR1GFP/GFP mice.
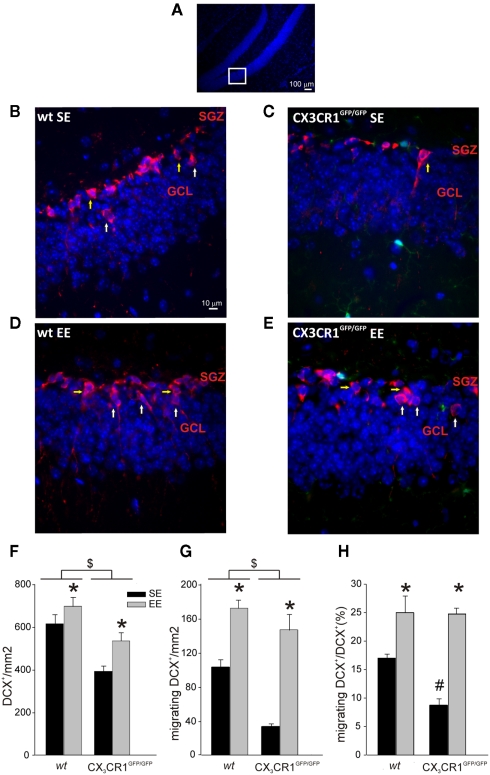
CX3CR1GFP/GFP mice do not have evident alterations in brain development (Jung et al., 2000) but show decreased proliferation and neurogenesis in the SGZ and granular layer of the DG (Bachstetter et al., 2011). In order to investigate if the differences in learning and memory abilities observed in CX3CR1GFP/GFP mice could be correlated with alterations in DG neurogenesis, we analyzed the number of neuronal precursors (DCX positive cells) present in the DG of wt and CX3CR1GFP/GFP mice grown in SE or EE (n = 4 for both housing conditions and genotypes) (representative image shown in Figure 4A). We reported that the number of neuronal precursors was affected not only by the lack of CX3CR1, as shown by the significant effect of genotype [F(1,13) = 28.327, p < 0.001], but also by the housing conditions [F(1,13) = 19,238, p < 0.001] without any significant interaction between the two factors (p = 0.760). The mean area values of the DG analyzed for wt and CX3CR1GFP/GFP mice were not significantly different, indicating that the effect of genotype was not dependent on different DG size (wt: 0.143 mm ± 0.004 mm, CX3CR1GFP/GFP: 0.141 mm ± 0.004 mm). Post hoc analysis revealed that the number of DCX positive cells was: (i) significantly higher in wt compared to CX3CR1GFP/GFP (p < 0.001; Figure 4B–F , as previously described (Bachstetter et al., 2011); (ii) increased by exposure to EE in both genotypes (p < 0.001; Figures 4D–F).
Figure 4.
Neuronal precursors in the DG of wt and CX3CR1GFP/GFP mice. (A) Representative low-scale magnification of mouse hippocampal dentate gyrus (DG) coronal section stained for Hoechst (blue). The highlight square illustrates the DG regions amplified in (B–E) where DCX+ cells are shown. Scale bar, 100 μm. (B–E) Representative images of DCX+ cells (red) in the DG of wt and CX3CR1GFP/GFP mice, in SE and EE as indicated. Migrating DCX+ cells are marked with white arrows, non-migrating cells with yellow arrows. Green cells in CX3CR1GFP/GFP slice are microglia. Scale bar, 10 μm. (F) Histograms showing the number of DCX+ cells square millimeter in the DG of wt (left) and CX3CR1GFP/GFP mice (right) in SE (top) and EE (bottom). *Is EE vs. SE and $ is CX3CR1GFP/GFP vs. wt. (p < 0.001). (G) Migrating DCX+ cells square millimeter in the DG of wt and CX3CR1GFP/GFP mice in SE and EE. *Is EE vs. SE and $ is CX3CR1GFP/GFP vs. wt. (p < 0.001). (H) Histograms showing the ratio between migrating vs. total DCX+ in wt and CX3CR1GFP/GFP mice in SE and EE. *Is EE vs. SE (p < 0.001) and # is within SE CX3CR1GFP/GFP vs. wt. (p < 0.05). Data are presented as mean ± SEM.
We then evaluated the rate of radial migration of neuronal precursors in the DG, the step that precedes their differentiation into granule neurons and the establishment of synaptic contacts in the pre-existing hippocampal circuitries (Cameron et al., 1993; Markakis and Gage, 1999). For this reason we measured the number of migrating neuronal precursors, defined as the DCX+ cells whose cell body was at least 5 μm distant from the SGZ. We found that the number of DCX+ migrating cells was affect by both housing [F(1,12) = 85.165, p < 0.001] and genotype [F(1,12) = 27.570, p < 0.001] conditions but no significant interaction of these two factors was found (p = 0.287). Post hoc analysis revealed that: (i) migrating cells were increased in both genotypes in EE compared to SE condition (p < 0.001; Figures 4B–E,G; (ii) wt mice showed a higher number of migrating cells compared to CX3CR1GFP/GFP (p < 0.001; Figures 4B–E,G).
When we measured the ratio of migrating DCX+/total DCX+ cells, we found a main effect of housing [F1,12 = 51,011, p < 0.001] and genotype condition [F(1,12) = 6,399, p = 0.026] with a significant factors interaction [F(1,12) = 5.668, p = 0.035]. Post hoc analysis revealed that the ratio between migrating DCX+/total DCX+ cells was significantly different in the two genotypes only in SE, CX3CR1GFP/GFP mice showing reduced levels compared to wt (p < 0.05), but not in EE condition (Figures 4B–E,H).
Discussion
The main aim of the present work was to investigate the role of CX3CR1 in mediating the effects of prolonged EE on hippocampal function and neurogenesis. As expected according to the literature (van Praag et al., 2000; Duffy et al., 2001), EE enhanced neuronal plasticity in wt but, surprisingly, not in CX3CR1GFP/GFP mice. In particular, following exposure to long-term EE, CX3CR1GFP/GFP mice showed no improvement in both CA1 hippocampal LTP and in water maze performance on day 4 probe. In contrast, EE enhanced hippocampal neurogenesis and migration of adult new born neuronal precursors in the DG of both genotypes.
We also report that the lack of CX3CR1 has several effects by itself. In particular, in CX3CR1GFP/GFP mice grown under SE conditions, hippocampal LTP, and spatial memory are improved whereas DG neuronal precursor generation and migration are reduced when compared with wt mice (see also Bachstetter et al., 2011). Specifically, we reported that, although in SE CX3CR1GFP/GFP mice showed similar responses to basal synaptic transmission (measured as I–O functional curves and PPF), upon a weak stimulation (100 Hz train) they had a more robust CA1 LTP compared to wt mice, demonstrating microglia-driven alterations of plasticity phenomena. This is in line with our previous results, demonstrating that treatment with exogenous CX3CL1 inhibits CA1 LTP through the activity of adenosine system, specifically of the adenosine receptor type 3 (AR3; Maggi et al., 2009). The role of microglia in modulating neuronal circuitry functions and hippocampal plasticity is also suggested by recent findings demonstrating that, during development, CX3CR1GFP/GFP mice had a decreased density in microglia cells that were compromised in their capacity to engulf synaptic material leading to immature connectivity, augmented LTD, and a global increase in excitatory synapses with immature functional characteristics (Paolicelli et al., 2011).
It has been proposed that exposure to EE may modify synaptic physiology and plasticity in hippocampal neurons (Green and Greenough, 1986; Foster et al., 1996; van Praag et al., 1999) being able to produce an enhancement of LTP in the area CA1 (Duffy et al., 2001). In plasticity processes, signaling between peri-synaptic astrocytes, microglia and neurons plays an important role. Astrocytes and microglia secrete an array of cytokines and factors which contribute to synaptic modulation, learning and memory processes in healthy and diseased brain (Volterra and Meldolesi, 2005; Hanisch and Kettenmann, 2007). Nevertheless, the exact mechanisms as to how cytokines and in particular chemokines, participate in the molecular and cellular processes thought to subserve memory formation and responsiveness to environmental stimuli remain to be clarified. Interestingly, it has been recently reported that mice lacking type 1 receptor for interleukin 1 (IL-1) display impaired hippocampal memory and LTP that are restored by EE (Goshen et al., 2009).
Here we showed that, in the absence of CX3CR1, EE failed to affect hippocampal CA1 LTP, whereas it produced an increased plasticity in wt animals. These evidence suggest that the absence of microglia-driven CX3CR1 signaling occludes the enrichment effects (although we are not at saturating LTP level) possibly because of some overlapping mechanisms that need further investigation. We speculate that this lack of response to environmental stimulation might be explained with altered microglia–neurons cross-talk, affecting the circuitry underlying synaptic plasticity. These changes could reduce the responsiveness of hippocampal CA1 region to EE stimuli that, in wt animals, have been shown to increase the number of synaptic contacts and the density of non-perforated synapses in this area (Rampon and Tsien, 2000).
A strong association between LTP and cognition has been widely described in previous studies illustrating LTP as prime candidate for the cellular mechanisms of learning and memory (Tsien et al., 1996; Malenka, 2003). EE has been shown to enhance learning and memory functions in various tasks (van Praag et al., 1999). In particular, enriched mice display a better performance in the Morris water maze compared to controls (Pacteau et al., 1989; Kempermann et al., 1997). Here, we confirm these findings, showing that EE improved the performance of both genotypes with regard to latency to reach the platform during the acquisition phase and the preference for the platform quadrant during both probe phases. Indeed, the preference for the platform quadrant emerged earlier in the EE group, appearing after 4 days of acquisition, while it was evident only after 6 days in the SE group. Post hoc analysis revealed that the effect of the housing condition at day 4 probe is mainly due to a lack of preference shown by SE wt while CX3CR1GFP/GFP mice, independently from the housing conditions, showed a significant preference, indicating enhanced cognitive abilities. Interestingly, improved cognitive functions have been described in mice lacking interleukin 6 (IL-6, Braida and Sacerdote, 2004) whereas increased levels of IL-6 were associated with decline in learning and memory abilities (Monje and Toda, 2003).
The lack of differences in learning performance at day 4 probe is in accordance with the LTP data, corroborating a reduced sensitivity to environmental stimulation associated with CX3CR1 deficiency. However, we cannot exclude the presence of a ceiling effect, being the preference for the platform quadrant already at high levels after 4 days of acquisition, no further improvement due to EE might be possible. At day 6 probe, the housing condition affected the preference for the platform quadrant also in CX3CR1GFP/GFP mice, possibly because the prolonged water maze training allowed differences associated to housing condition to clearly emerge. It is worth noticing that the procedure followed in the present work consisting in exposing mice to a probe trial (i.e., day 4 probe), which is an extinction trial, during the acquisition phase may have interfered with the learning process. However, the preference for the acquisition quadrant shown by all experimental groups in the day 6 probe trial suggests that such interference, if any, was limited. Further investigations are warranted to better characterize the effect of CX3CR1 deficiency on learning and memory.
Considerable evidence suggest that hippocampal neurogenesis plays a role in learning and memory functions (Shors et al., 2001; Snyder et al., 2005) and that EE stimulates hippocampal neurogenesis in the DG (Kempermann et al., 1997; Kempermann and Gage, 1999; van Praag et al., 1999) but the precise mechanisms by which neurogenesis facilitates memory formation remain to be fully understood. Recent data suggest that neurogenesis may be associated with some forms of hippocampal-dependent memory but not others (Shors and Townsend, 2002; Saxe et al., 2007; Garthe et al., 2009) and that reducing neurogenesis does not affect LTP in CA1 area (Garthe et al., 2009).
CX3CL1 has been recently shown to be involved in the regulation of DG neurogenesis, since CX3CR1GFP/GFP mice have a reduced number of neuronal precursors in the DG (Bachstetter et al., 2011). We investigated whether EE was able to affect hippocampal neurogenesis in the absence of a normal CX3CL1-CX3CR1 signaling. Our results confirm that mice lacking CX3CR1 have, in SE, a reduced neurogenesis in the SGZ and granular layer of the DG, as measured by DCX staining (Bachstetter et al., 2011). We report that exposure to EE induced a significant increase of neurogenesis and migration of new born cells in the DG of both wt and CX3CR1GFP/GFP mice and that the differences in the percentage of new born migrating cells observed in CX3CR1 deficient mice are lost upon EE exposure. This observation indicates that EE may modulate specific signals in the neurogenic niche, likely modulating survival and differentiation of new born cells, that are functional also in the absence of CX3CR1 but probably do not overlap with those involved in basal regulation. Possible candidates are factors involved in new born cell survival, local network activity (Deng et al., 2010), and epigenetic factors (Covic et al., 2010; Lopez-Atalaya et al., 2011). Interestingly, hippocampal neurogenesis induced by EE was associated with the activation of microglia (Ziv et al., 2006). It has been proposed that disruption of microglia-driven CX3CR1 signaling decreases survival and proliferation of neuronal progenitors via mechanisms that involve indirect modification of niche environment, in particular through IL-1β (Bachstetter et al., 2011).
Our results also suggest that learning-induced regulation of adult neurogenesis is not specifically associated with modification in CA1 hippocampal plasticity or Morris water maze performance, substantiating the current idea that these forms of hippocampal-dependent plasticity and learning processes are not directly related with DG neurogenesis. Future studies are warranted to discern if specific plasticity processes in DG area could be altered in CX3CR1GFP/GFP mice, in particular those related to adult-generated granule cells that have a potential role in spatially precise, place specific strategy (Garthe et al., 2009).
In conclusion, we provide the first experimental evidence that the absence of a constitutive CX3CL1/CX3CR1 signaling leads to an increase in hippocampal plasticity and learning performance and to a decrease in hippocampal-dependent responses to environmental stimulation, demonstrating microglia-driven alterations of plasticity phenomena.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors acknowledge the technical assistance of Giuseppina Chece and Fabrizio Cecchini for mice genotyping, Alessandro Felici for animal care and Cristina Bertollini for initial EE experiments. Maria Scianni was supported by Ph.D. program in Neurophysiology, Sapienza University, Rome, Italy. Grants: Istituto Pasteur, Fondazione Cenci Bolognetti to Cristina Limatola; Ministero della Salute (Ricerca corrente, cinque per mille, Antidoping) to Cristina Limatola; Mariani Foundation of Milan (Grant n. R-09-76) to Cristina Limatola; Fondazione Viva la Vita to Cristina Limatola.
Appendix
Figure A1.
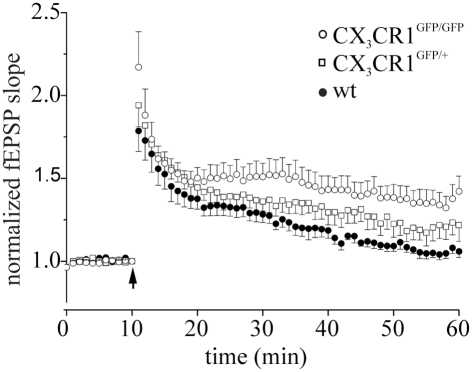
LTP in littermates wt, CX3CR1GFP/GFP and CX3CR1+/GFP mice. Points represent mean ± SEM. Of fEPSP slopes. Arrows indicate time of application of HFS. Note that after HFS, CX3CR1GFP/GFP mice (open circle) developed a robust LTP of fEPSP slope (45 min after induction: 1.34 ± 0.28 respect to baseline, 8 slices/3 mice), whereas wt mice displayed no LTP (dark circle, 1.04 ± 0.07, 6 slices/2 mice, p < 0.05 to CX3CR1GFP/GFP) and heterozygotes (open square, 1.22 ± 0.19, 6 slices/2 mice) an intermediate phenotype, respectively.
References
- Asensio V. C., Campbell I. L. (1999). Chemokines in the CNS: plurifunctional mediators in diverse states. Trends Neurosci. 22, 504–512 10.1016/S0166-2236(99)01453-8 [DOI] [PubMed] [Google Scholar]
- Bachstetter A. D., Morganti J. M., Jernberg J., Schlunk A., Mitchell S. H., Brewster K. W., Hudson C. E., Cole M. J., Harrison J. K., Bickford P. C., Gemma C. (2011). Fractalkine and CX(3)CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol. Aging 32, 2030–2044 10.1016/j.neurobiolaging.2009.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertollini C., Ragozzino D., Gross C., Limatola C., Eusebi F. (2006). Fractalkine/CX3CL1 depresses central synaptic transmission in mouse hippocampal slices. Neuropharmacology 51, 816–821 10.1016/j.neuropharm.2006.05.027 [DOI] [PubMed] [Google Scholar]
- Bezzi P., Domercq M., Brambilla L., Galli R., Schols D., De Clercq E., Vescovi A., Bagetta G., Kollias G., Meldolesi J., Volterra A. (2001). CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat. Neurosci. 4, 702–710 10.1038/89490 [DOI] [PubMed] [Google Scholar]
- Braida D., Sacerdote P. (2004). Cognitive function in young and adult IL (interleukin)-6 deficient mice. Behav. Brain Res. 153, 423–429 10.1016/j.bbr.2003.12.018 [DOI] [PubMed] [Google Scholar]
- Brown J. P., Couillard-Després S., Cooper-Kuhn C. M., Winkler J., Aigner L., Kuhn H. G. (2003). Transient expression of doublecortin during adult neurogenesis. J. Comp. Neurol. 467, 1–10 10.1002/cne.10874 [DOI] [PubMed] [Google Scholar]
- Cameron H. A., Woolley C. S., McEwen B. S., Gould E. (1993). Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience 56, 337–344 10.1016/0306-4522(93)90335-D [DOI] [PubMed] [Google Scholar]
- Covic M., Karaca E., Lie D. C. (2010). Epigenetic regulation of neurogenesis in the adult hippocampus. Heredity 105, 122–134 10.1038/hdy.2010.27 [DOI] [PubMed] [Google Scholar]
- Deng W., Aimone J. B., Gage F. H. (2010). New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 11, 339–350 10.1038/nrn2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy S. N., Craddock K. J., Abel T., Nguyen P. V. (2001). Environmental enrichment modifies the PKA-dependence of hippocampal LTP and improves hippocampus-dependent memory. Learn. Mem. 8, 26–34 10.1101/lm.36301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engellenner W. J., Goodlett C. R., Burright R. G., Donovick P. J. (1982). Environmental enrichment and restriction: effects on reactivity, exploration and maze learning in mice with septal lesions. Physiol. Behav. 29, 885–893 10.1016/0031-9384(82)90339-0 [DOI] [PubMed] [Google Scholar]
- Escorihuela R. M., Tobena A., Fernandez-Tereul A. (1995). Environmental enrichment and postnatal handling prevent spatial learning deficits in aged hypoemotional (Roman high-avoidance) and hyperemotional (Roman low-avoidance) rats. Learn. Mem. 2, 40–48 10.1101/lm.2.1.40 [DOI] [PubMed] [Google Scholar]
- Foster T. C., Gagne J., Massicotte G. (1996). Mechanism of altered synaptic strength due to experience: relation to long-term potentiation. Brain Res. 736, 243–250 10.1016/0006-8993(96)00707-X [DOI] [PubMed] [Google Scholar]
- Garthe A., Behr J., Kempermann G. (2009). Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS ONE 4, e5464. 10.1371/journal.pone.0005464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannelli A., Limatola C., Ragozzino D., Mileo A. M., Ruggieri A., Ciotti M. T., Mercanti D., Santoni A., Eusebi F. (1998). CXC chemokines interleukin-8 (IL-8) and growth-related gene product α (GROα) modulate Purkinje neuron activity in mouse cerebellum. J. Neuroimmunol. 92, 122–132 10.1016/S0165-5728(98)00192-1 [DOI] [PubMed] [Google Scholar]
- Globus A., Rosenzweig M. R., Bennett E. L., Diamond M. C. (1973). Effects of differential experience on dendritic spine counts in rat cerebral cortex. J. Comp. Physiol. Psychol. 82, 175–181 10.1037/h0033910 [DOI] [PubMed] [Google Scholar]
- Goshen I., Avital A., Kreisel T., Licht T., Segal M., Yirmiya R. (2009). Environmental enrichment restores memory functioning in mice with impaired IL-1 signaling via reinstatement of long-term potentiation and spine size enlargement. J. Neurosci. 29, 3395–3403 10.1523/JNEUROSCI.5352-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green E. J., Greenough W. T. (1986). Altered synaptic transmission in dentate gyrus of rats reared in complex environments: evidence from hippocampal slices maintained in vitro. J. Neurophysiol. 55, 739–750 [DOI] [PubMed] [Google Scholar]
- Hanisch U. K., Kettenmann H. (2007). Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 10, 1387–1394 10.1038/nn1997 [DOI] [PubMed] [Google Scholar]
- Harris A. P., D’Eath R. B., Healy S. D. (2009). Environmental enrichment enhances spatial cognition in rats by reducing thigmotaxis (wall hugging) during testing. Anim. Behav. 77, 1459–1464 10.1016/j.anbehav.2009.02.019 [DOI] [Google Scholar]
- Jung S., Aliberti J., Graemmel P., Sunshine M. J., Kreutzberg G. W., Sher A., Littman D. R. (2000). Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106–4114 10.1128/MCB.20.11.4106-4114.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G., Gage F. H. (1999). Experience-dependent regulation of adult hippocampal neurogenesis: effects of long-term stimulation and stimulus withdrawal. Hippocampus 9, 321–332 [DOI] [PubMed] [Google Scholar]
- Kempermann G., Kuhn H. G., Gage F. H. (1997). More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493–495 10.1038/386493a0 [DOI] [PubMed] [Google Scholar]
- Lax P., Limatola C., Fucile F., Trettel F., Di Bartolomeo S., Renzi M., Ragozzino D., Eusebi F. (2002). Chemokine receptor CXCR2 regulates the functional properties of AMPA-type glutamate receptor GluR1 in HEK cells. J. Neuroimmunol. 129, 66–73 10.1016/S0165-5728(02)00178-9 [DOI] [PubMed] [Google Scholar]
- Limatola C., Giovannelli A., Maggi L., Ragozzino D., Castellani L., Ciotti M. T., Vacca F., Mercanti D., Santoni A., Eusebi F. (2000). SDF-1a-mediated modulation of synaptic transmission in rat cerebellum. Eur. J. Neurosci. 12, 2497–2504 10.1046/j.1460-9568.2000.00139.x [DOI] [PubMed] [Google Scholar]
- Lopez-Atalaya J. P., Ciccarelli A., Viosca J., Valor L. M., Jimenez-Minchan M., Canals S., Giustetto M., Barco A. (2011). CBP is required for environmental enrichment-induced neurogenesis and cognitive enhancement. EMBO J. [Epub ahead of print]. 10.1038/emboj.2011.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi L., Trettel F., Scianni M., Bertollini C., Eusebi F., Fredholm B. B., Limatola C. (2009). LTP impairment by fractalkine/CX3CL1 in mouse hippocampus is mediated through the activity of adenosine receptor type 3 (A3R). J. Neuroimmunol. 215, 36–42 10.1016/j.jneuroim.2009.07.016 [DOI] [PubMed] [Google Scholar]
- Malenka R. C. (2003). The long-term potential of LTP. Nat. Rev. Neurosci. 4, 923–926 10.1038/nrn1258 [DOI] [PubMed] [Google Scholar]
- Markakis E. A., Gage F. H. (1999). Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J. Comp. Neurol. 406, 449–460 [DOI] [PubMed] [Google Scholar]
- Meucci O., Fatatis A., Simen A. A., Bushell T. J., Gray P. W., Miller R. J. (1998). Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc. Natl. Acad. Sci. U.S.A. 95, 14500–14505 10.1073/pnas.95.24.14500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje M. L., Toda H. (2003). Inflammatory blockade restores adult hippocampal neurogenesis. Science 302, 1760–1765 10.1126/science.1088417 [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J., Hannan A. J. (2006). Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat. Rev. Neurosci. 7, 697–709 10.1038/nrn1970 [DOI] [PubMed] [Google Scholar]
- Oh S. B., Endoh T., Simen A. A., Ren D., Miller R. J. (2002). Regulation of calcium currents by chemokines and their receptors. J. Neuroimmunol. 123, 66–75 10.1016/S0165-5728(01)00485-4 [DOI] [PubMed] [Google Scholar]
- Otto T., Eichenbaum H., Wiener S. I., Wible C. G. (1991). Learning-related patterns of CA1 spike trains parallel stimulation parameters optimal for inducing hippocampal long-term potentiation. Hippocampus 1, 181–192 10.1002/hipo.450010206 [DOI] [PubMed] [Google Scholar]
- Pacteau C., Einon D., Sinden J. (1989). Early rearing environment and dorsal hippocampal ibotenic acid lesions: long-term influences on spatial learning and alternation in the rat. Behav. Brain Res. 34, 79–96 10.1016/S0166-4328(89)80092-0 [DOI] [PubMed] [Google Scholar]
- Paolicelli R., Bolasco G., Pagani F., Maggi L., Scianni M., Panzanelli P., Giustetto M., Ferreira T. A., Guiducci E., Dumas L., Ragozzino R., Gross C. (2011). Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458 10.1126/science.1202529 [DOI] [PubMed] [Google Scholar]
- Ragozzino D., Di Angelantonio S., Trettel F., Bertollini C., Maggi L., Gross C., Charo I. F., Limatola C., Eusebi F. (2006). Chemokine fractalkine/CX3CL1 negatively modulates active glutamatergic synapses in rat hippocampal neurons. J. Neurosci. 26, 10488–10498 10.1523/JNEUROSCI.3192-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampon C., Tsien J. Z. (2000). Genetic analysis of learning behavior-induced structural plasticity. Hippocampus 10, 605–609 [DOI] [PubMed] [Google Scholar]
- Rostène W., Kitabgi P., Parsadaniantz S. M. (2007). Chemokines: a new class of neuromodulator? Nat. Rev. Neurosci. 8, 895–903 10.1038/nrn2255 [DOI] [PubMed] [Google Scholar]
- Sánchez-Alcañiz J. A., Haege S., Mueller W., Pla R., Mackay F., Schulz S., López-Bendito G., Stumm R., Marín O. (2011). Cxcr7 controls neuronal migration by regulating chemokine responsiveness. Neuron 69, 77–90 10.1016/j.neuron.2010.12.006 [DOI] [PubMed] [Google Scholar]
- Saxe M. D., Malleret G., Vronskaya S., Mendez I., Garcia A. D., Sofroniew M. V., Kandel E. R., Hen R. (2007). Paradoxical influence of hippocampal neurogenesis on working memory. Proc. Natl. Acad. Sci. U.S.A. 104, 4642–4646 10.1073/pnas.0611718104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors T. J., Miesegaes G., Beylin A., Zhao M., Rydel T., Gould E. (2001). Neurogenesis in the adult is involved in the formation of trace memories. Nature 410, 372–376 10.1038/35066584 [DOI] [PubMed] [Google Scholar]
- Shors T. J., Townsend D. A. (2002). Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus 12, 578–584 10.1002/hipo.10103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder J. S., Hong N. S., McDonald R. J., Wojtowicz J. M. (2005). A role for adult neurogenesis in spatial long-term memory. Neuroscience 130, 843–852 10.1016/j.neuroscience.2004.10.009 [DOI] [PubMed] [Google Scholar]
- Squire L. R., Zola-Morgan S. (1991). The medial temporal lobe memory system. Science 20, 1380–1386 10.1126/science.1896849 [DOI] [PubMed] [Google Scholar]
- Tran P. B., Miller R. J. (2003). Chemokine receptors: signposts to brain development and disease. Nat. Rev. Neurosci. 4, 444–455 10.1038/nrn1116 [DOI] [PubMed] [Google Scholar]
- Tremml P., Lipp H. P., Müller U., Wolfer D. P. (2002). Enriched early experiences of mice underexpressing the β-amyloid precursor protein restore spatial learning capabilities but not normal openfield behavior of adult animals. Genes Brain Behav. 1, 230–241 10.1034/j.1601-183X.2002.10405.x [DOI] [PubMed] [Google Scholar]
- Tsien J. Z., Huerta P. T., Tonegawa S. (1996). The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 87, 1327–1338 10.1016/S0092-8674(00)81827-9 [DOI] [PubMed] [Google Scholar]
- van Praag H., Christie B. R., Sejnowski T. J., Gage F. H. (1999). Running enhances neurogenesis, learning, and LTP in mice. Proc. Natl. Acad. Sci. U.S.A. 96, 13427–13431 10.1073/pnas.96.23.13427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H., Kempermann G., Gage F. H. (2000). Neural consequences of environmental enrichment. Nat. Rev. Neurosci. 1, 191–198 10.1038/35042057 [DOI] [PubMed] [Google Scholar]
- van Praag H., Schinder A. F., Christie B. R., Toni N., Palmer T. D., Gage F. H. (2002). Functional neurogenesis in the adult hippocampus. Nature 415, 1030–1034 10.1038/4151030a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volterra A., Meldolesi J. (2005). Astrocytes, from brain glue to communication elements: the revolution continues. Nat. Rev. Neurosci. 6, 626–640 10.1038/nrn1722 [DOI] [PubMed] [Google Scholar]
- Wolfer D. P., Stagljar-Bozicevic M., Errington M. L., Lipp H. P. (1998). Spatial memory and learning in transgenic mice: fact or artifact? News Physiol. Sci. 13, 118–123 [DOI] [PubMed] [Google Scholar]
- Ziv Y., Ron N., Butovsky O., Landa G., Sudai E., Greenberg N., Cohen H., Kipnis J., Schwartz M. (2006). Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat. Neurosci. 9, 268–275 10.1038/nn1629 [DOI] [PubMed] [Google Scholar]
- Zucker R. S. (1989). Short-term synaptic plasticity. Annu. Rev. Neurosci. 12, 13–31 10.1146/annurev.ne.12.030189.000305 [DOI] [PubMed] [Google Scholar]