Abstract
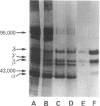
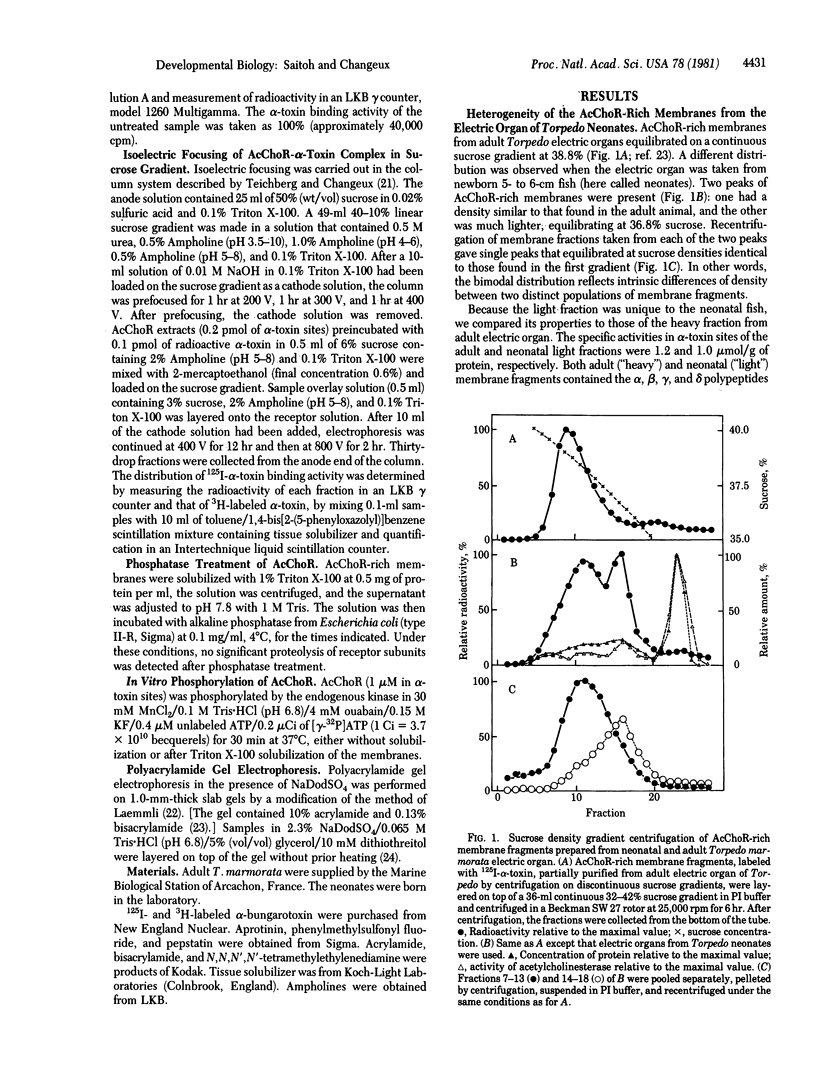
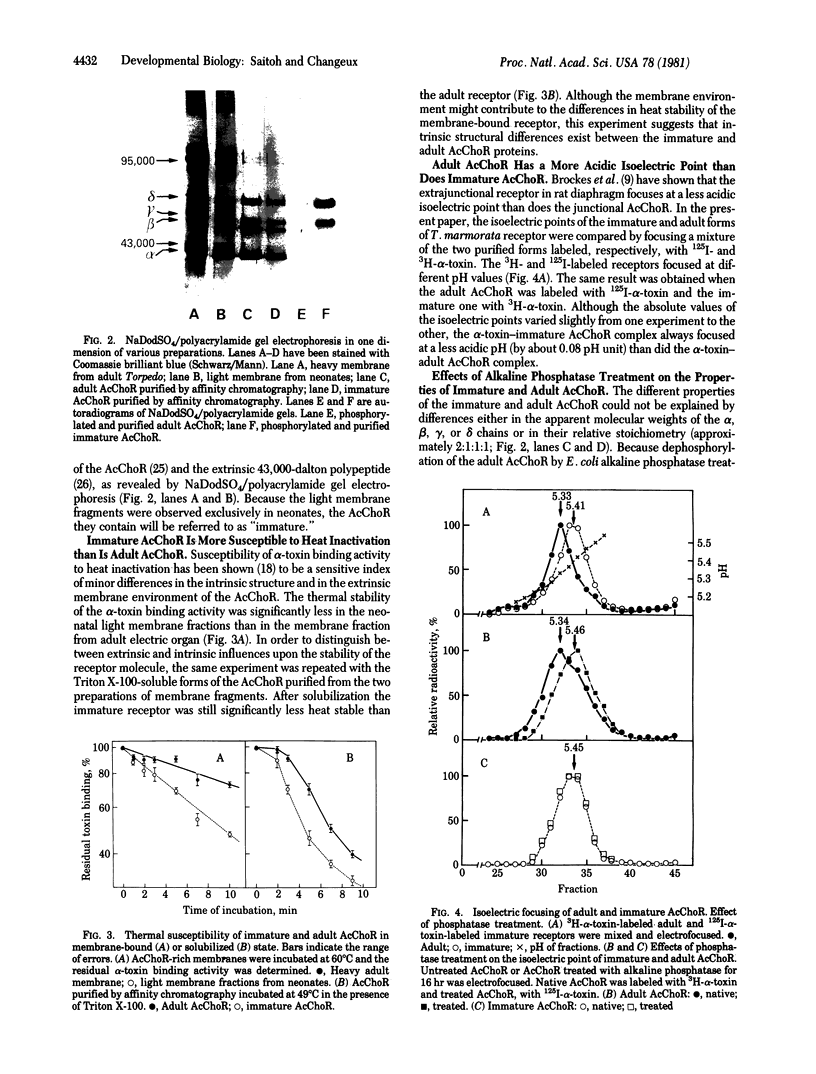
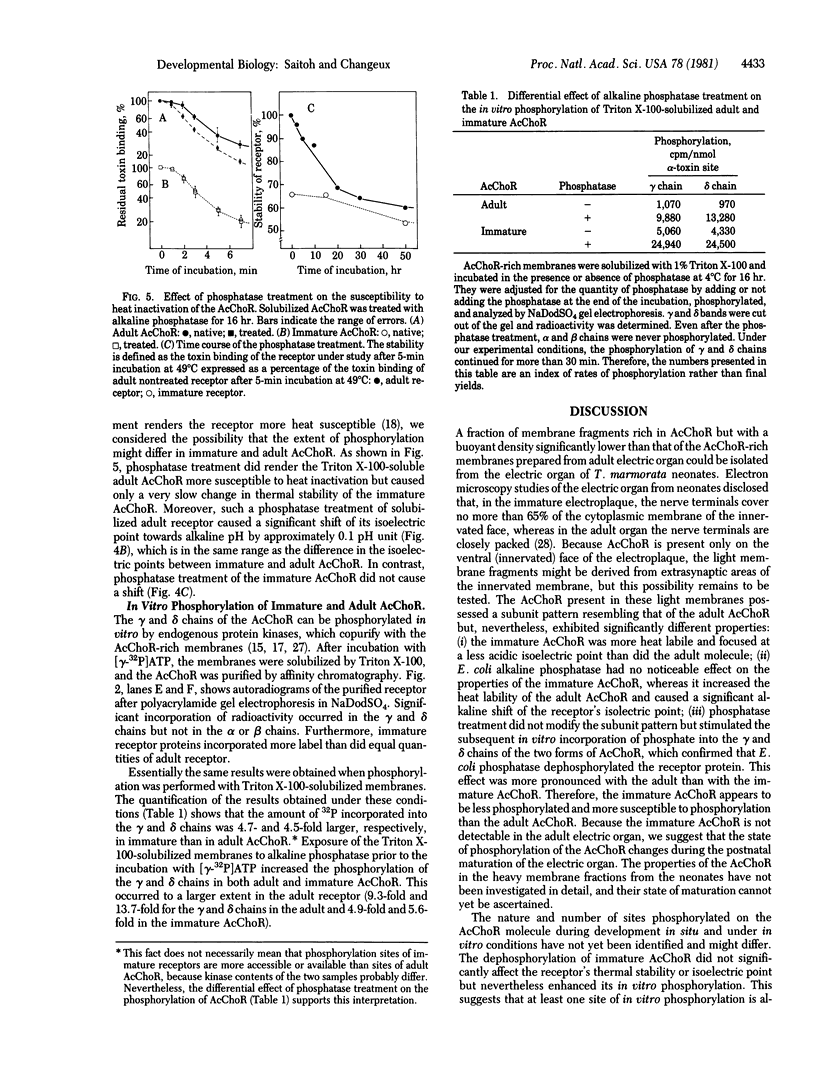
Two populations of membrane fragments, both rich in acetylcholine receptor (AcChoR), appeared during subcellular fractionation by ultracentrifugation of neonatal Torpedo marmorata electric organs. One of these equilibrated at 38.5% (wt/wt) sucrose, as did AcChoR-rich membranes from adult fish; the other equilibrated at 36.8% sucrose. AcChoR purified from these light membrane fractions gave the same subunit profile as adult AcChoR (after polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate) but was more susceptible to heat inactivation and focused at an isoelectric point more alkaline by 0.1 pH unit. Treatment of adult AcChoR with Escherichia coli alkaline phosphatase decreased its thermal stability and shifted its isoelectric point towards alkaline pH. However, identical treatment did not affect AcChoR purified from neonatal light membrane fractions. The gamma and delta chains of AcChoR can be phosphorylated in vitro by endogenous protein kinases, which copurify with AcChoR-rich membranes. Treatment of AcChoR from neonatal light membranes by E. coli alkaline phosphatase enhanced the phosphorylation of the gamma and delta chains but did so to a smaller extent than in the case of adult AcChoR. In conclusion, adult AcChoR appears to be more phosphorylated than AcChoR from neonatal light membranes, indicating that its state of phosphorylation changes during development.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almon R. R., Appel S. H. Interaction of myasthenic serum globulin with the acetylcholine receptor. Biochim Biophys Acta. 1975 May 30;393(1):66–77. doi: 10.1016/0005-2795(75)90217-2. [DOI] [PubMed] [Google Scholar]
- Axelrod D., Ravdin P., Koppel D. E., Schlessinger J., Webb W. W., Elson E. L., Podleski T. R. Lateral motion of fluorescently labeled acetylcholine receptors in membranes of developing muscle fibers. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4594–4598. doi: 10.1073/pnas.73.12.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockes J. P., Berg D. K., Hall Z. W. The biochemical properties and regulation of acetylcholine receptors in normal and denervated muscle. Cold Spring Harb Symp Quant Biol. 1976;40:253–262. doi: 10.1101/sqb.1976.040.01.026. [DOI] [PubMed] [Google Scholar]
- Cartaud J., Benedetti E. L. A morphological study of the cholinergic receptor protein from Torpedo marmorata in its membrane environment and in its detergent-extracted purified form. J Cell Sci. 1978 Feb;29:313–337. doi: 10.1242/jcs.29.1.313. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Danchin A. Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature. 1976 Dec 23;264(5588):705–712. doi: 10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M. Control of acetylcholine receptors in skeletal muscle. Physiol Rev. 1979 Jan;59(1):165–227. doi: 10.1152/physrev.1979.59.1.165. [DOI] [PubMed] [Google Scholar]
- Fischbach G. D., Schuetze S. M. A post-natal decrease in acetylcholine channel open time at rat end-plates. J Physiol. 1980 Jun;303:125–137. doi: 10.1113/jphysiol.1980.sp013275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. S., Davis C. G., Diamond I. Phosphorylation of membrane proteins at a cholinergic synapse. Proc Natl Acad Sci U S A. 1977 Jan;74(1):263–267. doi: 10.1073/pnas.74.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. S., Davis C. G., Milfay D., Diamond I. Phosphorylation of acetylcholine receptor by endogenous membrane protein kinase in receptor-enriched membranes of Torpedo californica. Nature. 1977 Jun 9;267(5611):539–540. doi: 10.1038/267539a0. [DOI] [PubMed] [Google Scholar]
- Gordon A. S., Davis C. G., Milfay D., Kaur J., Diamond I. Membrane-bound protein kinase activity in acetylcholine receptor-enriched membranes. Biochim Biophys Acta. 1980 Aug 4;600(2):421–431. doi: 10.1016/0005-2736(80)90445-9. [DOI] [PubMed] [Google Scholar]
- Gordon A. S., Milfay D., Davis C. G., Diamond I. Protein phosphatase activity in acetylcholine receptor-enriched membranes. Biochem Biophys Res Commun. 1979 Apr 13;87(3):876–883. doi: 10.1016/0006-291x(79)92039-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Labat-Robert J., Saitoh T., Godeau G., Robert L., Changeux J. P. Distribution of macromolecules from the intercellular matrix in the electroplaque of Electrophorus electricus. FEBS Lett. 1980 Nov 3;120(2):259–263. doi: 10.1016/0014-5793(80)80311-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindstrom J., Walter B., Einarson B. Immunochemical similarities between subunits of acetylcholine receptors from Torpedo, Electrophorus, and mammalian muscle. Biochemistry. 1979 Oct 16;18(21):4470–4480. doi: 10.1021/bi00588a004. [DOI] [PubMed] [Google Scholar]
- Mellinger J., Belbenoit P., Ravaille M., Szabo T. Electric organ development in Torpedo marmorata, Chondrichthyes. Dev Biol. 1978 Nov;67(1):167–188. doi: 10.1016/0012-1606(78)90307-x. [DOI] [PubMed] [Google Scholar]
- Michler A., Sakmann B. Receptor stability and channel conversion in the subsynaptic membrane of the developing mammalian neuromuscular junction. Dev Biol. 1980 Nov;80(1):1–17. doi: 10.1016/0012-1606(80)90494-7. [DOI] [PubMed] [Google Scholar]
- Nathanson N. M., Hall Z. W. Subunit structure and peptide mapping of junctional and extrajunctional acetylcholine receptors from rat muscle. Biochemistry. 1979 Jul 24;18(15):3392–3401. doi: 10.1021/bi00582a028. [DOI] [PubMed] [Google Scholar]
- Oswald R., Sobel A., Waksman G., Roques B., Changeux J. P. Selective labelling by [3H]trimethisoquin azide of polypeptide chains present in acetylcholine receptor-rich membranes from Torpedo marmorata. FEBS Lett. 1980 Feb 25;111(1):29–34. doi: 10.1016/0014-5793(80)80754-x. [DOI] [PubMed] [Google Scholar]
- Saitoh T., Changeux J. P. Phosphorylation in vitro of membrane fragments from Torpedo marmorata electric organ. Effect on membrane solubilization by detergents. Eur J Biochem. 1980 Mar;105(1):51–62. doi: 10.1111/j.1432-1033.1980.tb04473.x. [DOI] [PubMed] [Google Scholar]
- Saitoh T., Oswald R., Wennogle L. P., Changeux J. P. Conditions for the selective labelling of the 66 000 dalton chain of the acetylcholine receptor by the covalent non-competitive blocker 5-azido-[3H]trimethisoquin. FEBS Lett. 1980 Jul 11;116(1):30–36. doi: 10.1016/0014-5793(80)80522-9. [DOI] [PubMed] [Google Scholar]
- Saitoh T., Wennogle L. P., Changeux J. P. Factors regulating the susceptibility of the acetylcholine receptor protein to heat inactivation. FEBS Lett. 1979 Dec 15;108(2):489–494. doi: 10.1016/0014-5793(79)80595-5. [DOI] [PubMed] [Google Scholar]
- Sanes J. R., Hall Z. W. Antibodies that bind specifically to synaptic sites on muscle fiber basal lamina. J Cell Biol. 1979 Nov;83(2 Pt 1):357–370. doi: 10.1083/jcb.83.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze S. M. The acetylcholine channel open time in chick muscle is not decreased following innervation. J Physiol. 1980 Jun;303:111–124. doi: 10.1113/jphysiol.1980.sp013274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel A., Heidmann T., Hofler J., Changeux J. P. Distinct protein components from Torpedo marmorata membranes carry the acetylcholine receptor site and the binding site for local anesthetics and histrionicotoxin. Proc Natl Acad Sci U S A. 1978 Jan;75(1):510–514. doi: 10.1073/pnas.75.1.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel A., Weber M., Changeux J. P. Large-scale purification of the acetylcholine-receptor protein in its membrane-bound and detergent-extracted forms from Torpedo marmorata electric organ. Eur J Biochem. 1977 Oct 17;80(1):215–224. doi: 10.1111/j.1432-1033.1977.tb11874.x. [DOI] [PubMed] [Google Scholar]
- Teichberg V. I., Changeux J. P. Evidence for protein phosphorylation and dephosphorylation in membrane fragments isolated from the electric organ of Electrophorus electricus. FEBS Lett. 1977 Feb 15;74(1):71–76. doi: 10.1016/0014-5793(77)80755-2. [DOI] [PubMed] [Google Scholar]
- Teichberg V. I., Changeux J. P. Presence of two forms of acetylcholine receptor with different isoelectric points in the electric organ of Electrophorus electricus and their catalytic interconversion in vitro. FEBS Lett. 1976 Sep 1;67(3):264–268. doi: 10.1016/0014-5793(76)80543-1. [DOI] [PubMed] [Google Scholar]
- Teichberg V. I., Sobel A., Changeux J. P. In vitro phosphorylation of the acetylcholine receptor. Nature. 1977 Jun 9;267(5611):540–542. doi: 10.1038/267540a0. [DOI] [PubMed] [Google Scholar]
- Vandlen R. L., Wu W. C., Eisenach J. C., Raftery M. A. Studies of the composition of purified Torpedo californica acetylcholine receptor and of its subunits. Biochemistry. 1979 May 15;18(10):1845–1854. doi: 10.1021/bi00577a001. [DOI] [PubMed] [Google Scholar]
- Weinberg C. B., Hall Z. W. Antibodies from patients with myasthenia gravis recognize determinants unique to extrajunctional acetylcholine receptors. Proc Natl Acad Sci U S A. 1979 Jan;76(1):504–508. doi: 10.1073/pnas.76.1.504. [DOI] [PMC free article] [PubMed] [Google Scholar]