Abstract
Bladder artifact during bone single-photon emission computed tomography (SPECT) is a common source of error. The extent and severity of bladder artifacts have been described for filtered back projection (FBP) reconstruction. Ordered subset expectation maximization (OSEM) may help to address this problem of bladder artifacts, which render up to 20% of the SPECT images unreadable. The objective of this study was to evaluate the relationship of the bladder to acetabulum ratio in guiding the choice of the number of iterations and subsets used for OSEM reconstruction, for reducing bladder artifacts found on FBP reconstruction. One hundred five patients with various indications for bone scans were selected and planar and SPECT images were acquired. The SPECT images were reconstructed with both FBP and OSEM using four different combinations of iterations and subsets. The images were given to three experienced nuclear physicians who were blinded to the diagnosis and type of reconstruction used. They then labeled images from the best to the worst after which the data were analyzed. The bladder to acetabulum ratio for each image was determined which was then correlated with the different iterations and subsets used. The study demonstrated that reconstruction using OSEM led to better lesion detectability compared to FBP in 87.62% of cases. It further demonstrated that the iterations and subsets used for reconstruction of an image correlate with the bladder to acetabulum ratio. Four iterations and 8 subsets yielded the best results in 48.5% of the images, whilst 2 iterations and 8 subsets yielded the best results in 33.8%. The number of reconstructed images which yielded the best results with 2 iterations and 8 subsets was the same as or more than those with 4 iterations and 8 subsets when the bladder/acetabulum ratio (A/B) was between 0.2 and 0.39. A ratio below 0.2 or above 0.39 supports the usage of 4 iterations and 8 subsets over 2 iterations and 8 subsets. We conclude that bladder to acetabulum ratio can be used to select the optimum number of iterations and subsets for reconstruction of bone SPECT for accurate characterization of lesions. This study also confirms that reconstruction with OSEM (vs. FBP) leads to better lesion detectability and characterization.
Keywords: Bladder artifact, bone single-photon emission computed tomography, ordered subset expectation maximization
Introduction
Radionuclide bone imaging of the pelvis is an important investigation for the detection of avascular necrosis of the femoral head, for the detection of metastatic tumors and other diseases such as osteomyelitis. Although planar imaging is performed routinely, single-photon emission computed tomography (SPECT) offers improved sensitivity and specificity due to its greater spatial resolution and contrast, and ability to differentiate overlying internal structures. For example, with the availability of SPECT, the sensitivity of avascular necrosis detection has gone up to 85%, compared to 55% for planar imaging alone, with no loss of specificity. However, bladder artifacts during bone SPECT imaging are a common source of errors. The extent and severity of bladder artifacts have been well documented for filtered back projection (FBP) reconstruction. Ordered Subset Expectation Maximization (OSEM) may help to overcome this anomaly, which renders up to 20% of the images unreadable.[1]
Accurate and reliable lesion detection on images is important to guide therapeutic management, improve risk stratification, and provide prognostic information in the pelvic evaluation of patients. Hence, it is crucial that the results are reliable and reproducible. The performances of OSEM and FBP have been compared in a number of other experimental and clinical studies, with a variety of reconstruction parameters employed with OSEM, as well as the use of post-reconstruction smoothing to replace noise with increasing number of iterations. To date, no consensus has been reached.[2–6]
Whilst previous studies have demonstrated better lesion detectability with attenuation correction (AC), OSEM and dynamic expectation maximum, none of these studies has determined the ideal number of iterations and subsets for any given patient or condition. The large number of combinations of iterations and subsets in OSEM may discourage the use of OSEM in the clinical setting. Again, the patients from whom these images are acquired have different physiological and pathological processes which would alter the rate of tracer excretion, extraction of tracer by bone and bone to soft tissue ratio. It is important that there is a practical simple and reproducible way of determining the best iteration and subset to use for each patient that would take into account the activity of the radiotracer in the bladder and the uptake by bone. The hip and the bladder activity (the cause of the artifacts) is easily identifiable on the whole body scan and a ratio of the counts from these provides a good individualized index against which iterations and subsets of OSEM used for reconstruction can be optimized.
Blocklet et al. noted that 2 iterations and 8 subsets gave acceptable iterations for most images. Fancombe et al. also noted that the use of 2 or 4 iterations gave images better than FBP; however, the number of subsets used was not mentioned. Case also used 12 by 3 subsets and iterations. Using this information with various trials on different images, 4 iteration/subsets were selected to be optimized in the population studied. These were 8 × 2, 8 × 4, 12 3 and 12 × 6.
Materials and Methods
This was a prospective study which included 105 adult patients (59 females and 46 males), who were referred for bone scintigraphy to the Department of Nuclear Medicine of the University of Pretoria between October 2008 and March 2009. All adult patients referred for bone SPECT with equivocal pelvic lesions on planar images were included in the study. Ethics approval was obtained from the Faculty of Health Sciences Research Ethics Committee, University of Pretoria, and informed written consents were obtained from all study participants.
Patients were referred for various indications and we selected those where the primary region of interest was the pelvis or instances where pelvic lesions on planar images could not be confidently characterized in the absence of SPECT imaging. One hundred and five patients consented to the study; however, 25 were lost because of incomplete or lost SPECT images. Of the remaining 80 SPECT images, there was no clearly defined preference of one iterative and subset over the other in 12 patients. The standard departmental imaging protocol was followed for all patients (adapted from current SNM and EANM guidelines) starting with the acquisition of whole body planar/spot images and proceeding to SPECT image acquisition where needed.[7,8] The SPECT images were reconstructed with both FBP and OSEM using four different combinations of iterations and subsets [Figure 1].
Figure 1.
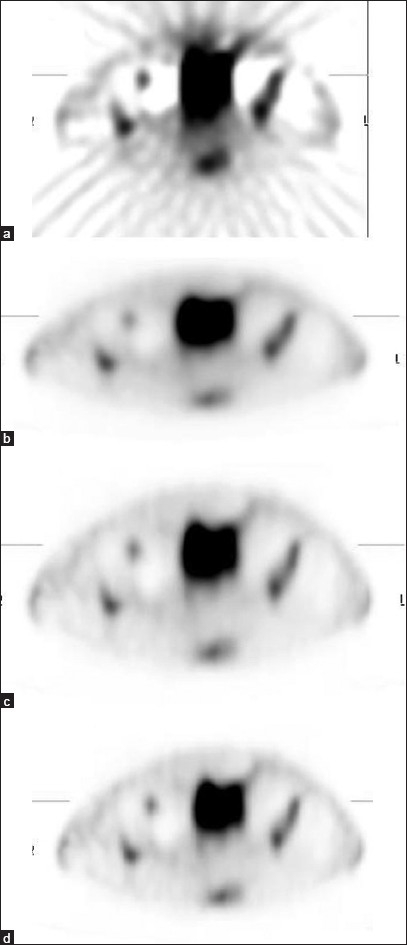
Representative images of pelvic SPECT obtained using FBP reconstruction method (a) and OSEM methods (b, c and d). Images (b), (c) and (d) were obtained using 2 × 8, 4 × 8 and 3 × 12 iterations × subsets, respectively. The results clearly demonstrate better quality images with OSEM reconstruction
FBP reconstruction was done with a Butterworth filter at 0.5 of Nyquist frequency and OSEM iterative reconstruction with various combinations of iterations and subsets. With OSEM reconstruction a non-negativity constraint was applied, which meant that negative line of response (LOR) values (because of random correction) and negative pixel values were set to 0. Limitation in terms of the number of subsets (9 different subsets) and iterations (limited to 30) programmed in the OSEM reconstruction was a restriction encountered during reconstructions. For OSEM with a subset size of 1, the number of iterations required to achieve good image quality is typically 30–50, but there is no clear guidance or recommendation for an appropriate combination.[9] Hence, a new suggestion has been made for introducing a relationship with acetabulum/bladder (A/B) ratio as a means of choosing an appropriate subset size which permits a more complete evaluation of the effect of the number of iterations on image noise and artifact. This could be a reliable and repeatable method if validated. For obtaining A/B ratio, a line profile across the acetabulum and the bladder was drawn and compared to OSEM performance.
The images were given to three experienced nuclear medicine physicians who were blinded to the type of reconstruction used. They then labeled images from the best to the worst after which the data were analyzed. The A/B ratio for each image was determined which was then correlated with the different iterations and subsets used.
Images were assessed on a 4-point scale [Table 1] for the presence of artifacts and the clinical impact of artifacts on diagnosis of pelvic abnormalities. A blinded analysis technique was used in an attempt to eliminate bias, whereby the FBP result was hidden from the analysts until reviewers agreed - based on properties of the data set from OSEM. Correlation analysis was performed between A/B ratio and various OSEM reconstruction parameters and P-values less than 0.05 were considered significant.
Table 1.
Four-point scale for image assessment

Results
One hundred and five patients (59 females and 46 males) were studied. The average age was 55 years, with a standard deviation of 15 years. It was observed that out of the 105 images reconstructed using FBP reconstruction method, only 13 images (12.38%) were rated as grade 4 high-quality images. Hence, the remaining 92 images were reconstructed using OSEM method of reconstruction, which resulted in high-quality (grade 4) images. Reconstruction of imaging using OSEM led to better lesion detectability compared to FBP in all 92 cases. It further demonstrated that the iterations and subsets used for reconstruction of an image correlate with the A/B ratio. Four iterations and 8 subsets yielded the best results in 48.5% of the images, whilst 2 iterations and 8 subsets yielded the best results in 33.8% [Table 2]. The number of reconstructed images which yielded the best results with 2 iterations and 8 subsets was the same as or more than those with 4 iterations and 8 subsets when the A/B ratio was between 0.2 and 0.39. A ratio below 0.2 or above 0.39 supported the use of 8 iterations and 4 subsets over 8 iterations and 2 subsets. Although less common, should the ratio be above 0.69, then 12 iterations and 3 subsets will provide image qualities of grade 3 and 4 [Figure 2].
Table 2.
Relationship between iterations and subsets versus best quality images

Figure 2.
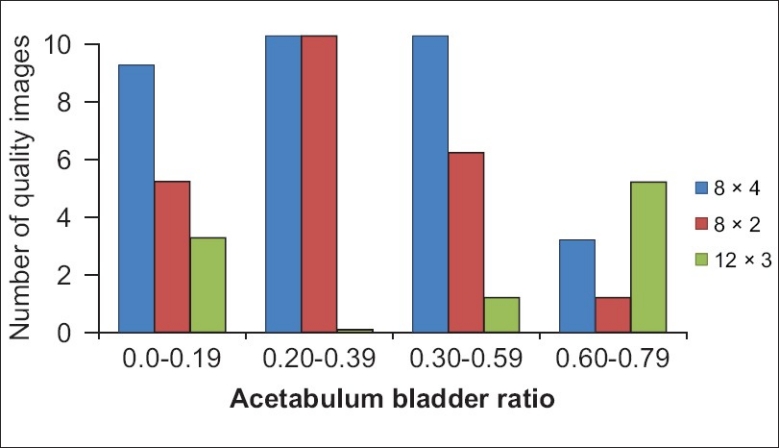
Relationship between iterations and subsets used for reconstruction versus Acetabulum/Bladder (A/B) ratio
Out of all reconstructed images, OSEM reconstruction method led to a significant reduction in bladder artifacts when compared to FBP. Images reconstructed using FBP method completely differed from images reconstructed using OSEM method. The OSEM method of reconstruction significantly reduced (P = 0.0001) the bladder artifacts in the pelvis in SPECT imaging compared to FBP. It improved the uniformity and symmetry of bone tracer uptake, and thus optimized lesion detectability. The reduction of pelvic bladder artifacts in the OSEM reconstructed images was independent of diagnosis, age or gender of the patients.
Discussion
SPECT imaging of the pelvis has been well established as an important diagnostic test in clinical practice for various benign and malignant pathologies. These include avascular necrosis (AVN) of the femoral heads, metastatic bone disease and osteomyelitis, among others. However, two important confounding issues frequently limit the accuracy of pelvic bone SPECT, leading to both false-positive and false-negative results. Firstly, the attenuation of emitted activity due to non-homogenous attenuation distribution may result in inconsistent projection measurements of the radiotracer distribution. As a result of these inconsistent measurements, it is possible for streaking artifacts to appear in reconstructed images, which may reduce lesion contrast within the pelvic region.[1] This effect may be reduced by acquiring transmission measurements using an external radioactive source and incorporating attenuation compensation into the image reconstruction process.
Secondly, during pelvic SPECT acquisition, inconsistent projection data are acquired as a result of accumulation of activity into the bladder during the data acquisition process. When reconstructed with conventional image reconstruction procedures such as FBP, image artifacts will appear as streaks through the bladder region.[10] The extent of these streaks is dependent on both the amount of activity accumulating in the bladder as well as the rate of accumulation of radioactivity in the bladder. When the amount or rate of accumulation is not significant, these streaks will not appear as significant. In many cases, however, the amount and/or rate of uptake is significant and produces streak artifacts. The above-mentioned artifacts may mask other regions within the pelvis, thus possibly affecting lesion detection. They may appear as anomalous blobs of apparent activity, which may be mistaken for tumors (false positives), or as dark shadows, which may hide true lesions (false negatives)[6,10] and mimic the photon-deficient regions of avascular necrosis.[11] The bladder-filling artifacts that occur in pelvic imaging are particularly severe, rendering as many as 20% of SPECT scans of this region unusable.[1] The following have been suggested as possible solutions.
AC has been shown to improve bone SPECT image quality in other regions of the body, such as the cervical spine,[3] and to improve lesion detection in thoracic SPECT.[6] Positron emission tomography (PET) images of the pelvis have also been shown to benefit from AC.[12] Unfortunately, AC alone may not be sufficient for pelvic SPECT because changing activity in the bladder throughout the acquisition contributes to the artifact. Catheterization is a possible means of mitigating this effect, but it has an associated risk of infection and consequently is unattractive for general application.
FBP versus OSEM
FBP has been the standard technique for tomographic image reconstruction in clinical nuclear medicine. However, FBP can result in the generation of artifacts, which mainly consist of streaking and negative counts near the borders of hot objects.[11,13] There are myriad iterative reconstruction algorithms that can be used as alternative reconstruction techniques to FBP. However, many of these, such as maximum likelihood expectation maximization (MLEM), are computationally intensive and have never been used in clinical practice.[14] Various methods have been developed to accelerate the speed of these algorithms. The most widely used acceleration technique is the ordered subset procedure of Hudson and Larkin,[9] which resulted in the development of the OSEM technique. The OSEM algorithm recently has become available on many commercial nuclear medicine computer systems and is now being used in routine clinical practice.[2,15]
Bladder artifacts in pelvic SPECT are known to be caused by the non-uniform attenuating media and changing bladder activity,[11] both of which also lead to incomplete cancellation of side lobes in FBP, and so iterative reconstruction would be expected to reduce the magnitude of the artifact. With the availability of faster hardware and more efficient iterative reconstruction techniques, algorithms such as OSEM are now moving from the research environment into routine clinical use. It is important to understand the quality control requirements that such algorithms place on imaging systems.
Whilst iterative methods of reconstruction have gained wide clinical acceptability in relatively newer nuclear medicine techniques such as PET, their use for the relatively older procedures has not gained wide clinical acceptability. The numerous amounts of iterative and subsets one must use to get an optimum image interrupts the usual work flow in busy nuclear medicine department. An index that would reduce the number of trials of reconstruction would provide an acceptable method and probably encourage the use of OSEM in clinical bone SPECT. This study revealed that for A/B ratios less than 0.59, the best images would be produced by 8 iterations and 4 subsets; as the ratio increases, a higher number of iterations (12 × 3) would be required. The improvement at higher level is however lost at higher iterations and subsets because it accentuates the noise, compromising the quality of the images as noted with 12 × 6.
Many comparison studies have shown that iterative reconstruction outperforms FBP in terms of image quality, signal-to-noise ratio, and resolution and contrast,[16] and improves lesion detection.[17] It has been highlighted that the characteristics of the reconstructed images are bound to the chosen number of iterations and to the source distribution.[18] Convergence studies have shown that the optimal number of iterations depends on the statistics of the input scan. The higher the statistics, the higher is the number of iterations to be used. The results of previous studies aimed at determining the number of iterations and subsets enabling the most accurate parameter estimation were never validated.[19] The optimal number of MLEM equivalent updates (iterations × subsets) is object dependent and convergence does not occur at the same iteration for the whole image. The finding of the most appropriate parameters is even more complicated for bladder artifacts. In this study, it was found that OSEM shows a clear advantage in the quality of the reconstructed image, but there is understandably a concern over the price paid in reconstruction time which may introduce delays into the daily work flow.
Importantly, this study is the first to report on a relationship between A/B ratio and the choice of the number of iterations and subsets used for OSEM reconstruction. Hence, the results of this study offer a huge potential to reduce the reconstruction time by selecting either 2 iterations and 8 subsets or 4 iterations and 8 subsets when the A/B ratio is between 0.2 and 0.39. Four iterations and 8 subsets should be used if the ratio is below 0.2 or above 0.39. If confirmed by other authors, this methodology would also help in addressing the issue of reproducibility and reliability in follow-up studies. This can thus be standardized by vendors on various work stations. To overcome the reconstruction dilemma, the installation of faster hardware or use of a large subset size (between 4 and 8) to speed up the reconstruction[20] and reduce the processing time will also be of benefit.
These requirements are well known for FBP, and some work needs to be done to determine the uniformity requirements for algorithms such as OSEM. It has been reported previously that in clinical practice, the use of iterative reconstruction techniques in place of FBP does not appear to alter the basic requirements for good gamma camera uniformity. However, the accuracy and validity of this information has not been critically examined as the results were obtained from limited data using a subset size of 1 and 40 iterations were set at 40 (in OSEM reconstruction method).[20] The current study also did not critically analyze the uniformity requirements for the reconstructed methods used having fixed the pixel size and the amount of post reconstruction filtering.
Despite the diversity in diagnosis, images reconstructed with OSEM method of reconstruction showed the best reduction of pelvic bladder artifacts, irrespective of the age or gender of the patients, when compared to images reconstructed with FBP method of reconstruction. In cases where avascular necrosis of the head of femur is suspected, very high resolution planar images of the region (acquired using a pinhole collimator) have an advantage over SPECT pelvic images reconstructed using OSEM. In some cases, a simple additional delayed (6–24 hours) planar image may result in higher target to background ratio and permit better evaluation of the pelvis if it was obscured by the bladder, thus excluding the need for pelvic SPECT imaging. Hence, the results obtained are restricted to comparing the FBP and OSEM methods of reconstruction in reducing bladder artifacts, when SPECT pelvic imaging is necessary for accurate localization and detection of lesion.
To conclude, the bladder-filling artifacts were significantly reduced in most patients, and subjective evaluation of image quality demonstrated a significant difference between OSEM and FBP. Importantly, our study is the first to demonstrate the relationship of the bladder to acetabulum ratio in guiding the choice of the number of iterations and subsets used for reconstruction, which is most likely to lead to accurate lesion localization and/or characterization.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Collier BD, Carrera GF, Johnson RP, Isitman AT, Hellman RS, Knobel J, et al. Detection of femoral head avascular necrosis in adults by SPECT. J Nucl Med. 1985;26:979–87. [PubMed] [Google Scholar]
- 2.Blocklet D, Seret A, Popa N, Schoutens A. Maximum likelihood reconstruction with ordered subsets in bone SPECT. J Nucl Med. 1999;40:1978–84. [PubMed] [Google Scholar]
- 3.Case JA, Licho K, King MA, Weaver JP. Bone SPECT of the spine: A comparison of attenuation correction techniques. J Nucl Med. 1999;40:604–13. [PubMed] [Google Scholar]
- 4.Kauppinen T, Koskinen MO, Alenius S, Vanninen E, Kuikka JT. Improvement of brain perfusion SPET using iterative reconstruction with scatter and non - uniform attenuation correction. Eur J Nucl Med. 2000;27:1380–6. doi: 10.1007/s002590000291. [DOI] [PubMed] [Google Scholar]
- 5.Vanhove C, Defrise M, Frankers PR, Evernert H, Deconinck F, Bossyut A. Interest of the ordered subsets expectation maximization (OSEM) algorithm in pinhole single-photon emission tomography reconstruction: A phantom study. Eur J Nucl Med. 2000;27:140–6. doi: 10.1007/s002590050019. [DOI] [PubMed] [Google Scholar]
- 6.Wells GR, King MA, Simkin PH, Judy PF, Brill AB, Gifford HC, et al. Comparing filtered back projection and ordered subsets expectation maximization for small- lesions detection and localization in 67Ga SPECT. J Nucl Med. 2000;41:1391–9. [PubMed] [Google Scholar]
- 7.Donohoe KJ, Henkin RE, Royal HD, Brown ML, Collier BD, O’Mara RE, et al. Procedure guideline for bone scintigraphy.Society of Nuclear Medicine. J Nucl Med. 1996;37:1903–6. [PubMed] [Google Scholar]
- 8.Bombardieri E, Aktolun C, Baum RP, Bishof-Delaloye A, Buscombe J, Chatal JF, et al. Bone Scintigraphy: Procedure Guideline for Tumour Imaging. Eur J Nucl Med Mol Imaging. 2003;30:BP99–106. doi: 10.1007/s00259-003-1347-2. [DOI] [PubMed] [Google Scholar]
- 9.Hudson HM, Larkin RS. Accelerated image reconstruction using ordered subsets of projection data. IEEE Trans Med Imaging. 1994;13:601–9. doi: 10.1109/42.363108. [DOI] [PubMed] [Google Scholar]
- 10.King MA, Tsui BM, Pan TS, Glick SJ, Soares EJ. Attenuation compensation for cardiac single-photon emission computed tomographic imaging: Part 2.Attenuation compensation algorithms. J Nucl Cardiol. 1996;3:55–63. doi: 10.1016/s1071-3581(96)90024-0. [DOI] [PubMed] [Google Scholar]
- 11.Gillen G, McKillop J, Hilditch T, Davidson J, Elliot A. Digital filtering of the bladder in SPECT bone studies of the pelvis. J Nucl Med. 1988;29:1587–95. [PubMed] [Google Scholar]
- 12.Turkington T, Coleman R. Effects of reconstruction methods and attenuation correction on hot bladder artefacts in PET [abstract] J Nucl Med. 2000;41(suppl):194P. [Google Scholar]
- 13.Forstrom LA, Dunn WL, O’Connor MK, Decklever TD, Hardyman TJ, Howarth DM. Technical pitfalls in image acquisition, processing and display. Semin Nucl Med. 1996;26:278–94. doi: 10.1016/s0001-2998(96)80004-3. [DOI] [PubMed] [Google Scholar]
- 14.Hutton BF, Hudson HM, Beekman FJ. A clinical perspective of accelerated statistical reconstruction. Eur J Nucl Med. 1997;24:797–808. doi: 10.1007/BF00879671. [DOI] [PubMed] [Google Scholar]
- 15.Alush DS, Tsui BM. Performance of ordered-subset reconstruction algorithms under conditions of extreme attenuation and truncation in myocardial SPECT. J Nucl Med. 2000;41:737–44. [PubMed] [Google Scholar]
- 16.Bouchareb K, Thielemans T, Spinks O, Rimoldi , Camici PG. Comparison of analytic and iterative reconstruction methods for quantitative cardiac PET studies in 3D using Oxygen-15 water scans. IEEE Nucl Sci Symp Conf Rec. 2005;4:2120–3. [Google Scholar]
- 17.Lartizien C, Kinahan PE, Swensson R, Comtat C, Lin M, Villemagne V, et al. Evaluating image reconstruction methods for tumor detection in 3-dimensional whole-body PET oncology imaging. J Nucl Med. 2003;44:276–90. [PubMed] [Google Scholar]
- 18.Gutman F, Gardin I, Delahaye N, Rakotonirina H, Hitzel A, Manrique A, et al. Optimisation of the OS-EM algorithm and comparison with FBP for image reconstruction on a dual-head camera: A phantom and a clinical 18F-FDG study. Eur J Nucl Med Mol Imaging. 2003;30:1510–9. doi: 10.1007/s00259-003-1246-6. [DOI] [PubMed] [Google Scholar]
- 19.Reilhac A, Tomeï S, Buvat I, Michel C, Keheren F, Costes N. Simulation-based evaluation of OSEM iterative reconstruction methods in dynamic brain PET studies. Neuroimage. 2008;39:359–68. doi: 10.1016/j.neuroimage.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 20.Leong LK, Kruger RL, O’Connor MK. A Comparison of the Uniformity Requirements for SPECT Image Reconstruction Using FBP and OSEM Techniques. J Nucl Med Technol. 2001;29:79–83. [PubMed] [Google Scholar]


