Abstract
OBJECTIVE
Diabetic nephropathy and retinopathy are two important microvascular diabetes complications with a high concordance rate in diabetic patients. A recent genome-wide association study in type 1 diabetic patients of European descent identified four loci to be associated with diabetic nephropathy. The aim of this study was to test the effects of single nucleotide polymorphisms (SNPs) from these four loci on diabetic nephropathy and retinopathy in Chinese type 2 diabetic patients.
RESEARCH DESIGN AND METHODS
In stage 1, we recruited 1,276 type 2 diabetic patients, including 378 patients with diabetic nephropathy but no retinopathy, 374 patients with diabetic retinopathy but no nephropathy, 244 patients with both diabetic retinopathy and nephropathy, and 280 control subjects with diabetes for >10 years and no diabetic retinopathy or nephropathy. Fifty-five SNPs from four loci (CPVL/CHN2, FRMD3, CARS, and IRS2) were genotyped. The SNPs that showed associations to diabetic retinopathy or nephropathy were genotyped in stage 2 samples for replication.
RESULTS
SNPs from CPVL/CHN2 and FRMD3 were associated with diabetic retinopathy with rs39059 and rs10868025 as the top SNPs (odds ratio [OR] 1.292, 95% CI 1.097–1.523, P = 0.0022, for rs39059; 1.201, 1.014–1.422, P = 0.0343, for rs10868025) in stage 1 samples. In stage 2 analysis, only rs39059 showed similar effect to diabetic retinopathy (OR 1.269, 0.989–1.628, P = 0.0689), and meta-analysis showed a significant association between rs39059 and diabetic retinopathy, with an OR of 1.285 (1.120–1.474, P = 0.0003). CPVL/CHN2 rs39059 was also associated with levels of diabetic retinopathy (P = 0.0007 for trend). However, no association was detected between these SNPs and diabetic nephropathy.
CONCLUSIONS
In this study, we found CPVL/CHN2 rs39059 was associated with diabetic retinopathy in the Chinese type 2 diabetic patients.
Diabetic nephropathy and retinopathy, two important microvascular complications of diabetes, are the main causes of morbidity and mortality among diabetic patients (1). Diabetic nephropathy is the most common cause of chronic kidney failure and end-stage renal disease, whereas diabetic retinopathy is the leading cause of blindness in the adults (2,3). With the significant rise in the prevalence of diabetes, the increase of patients suffering from diabetic microvascular complications will be inevitable worldwide. Although diabetic nephropathy and retinopathy are clearly associated with the duration of diabetes and glycemic control (4), some patients develop severe complications despite well-controlled blood glucose. Conversely, not all diabetic patients with poor glycemic control develop advanced renal or retinal complications. The underlying mechanism of how these diabetic microvascular complications occur remains largely unknown, but family studies in Pima Indian and European descent populations suggest genetic factors participate in the development of these complications (5–7). However, the advance of susceptible gene identification in diabetic microvascular complications was much more limited than it was in type 1 and type 2 diabetes. Recently, a genome-wide association study using Genetics of Kidneys in Diabetes (GoKinD) samples identified four loci associated with diabetic nephropathy in the type 1 diabetic patients of European descent (8). Maeda et al. (9) further replicated the effect of one of them (rs1411766 near IRS2) in the Japanese type 2 diabetic patients. However, although diabetic retinopathy and nephropathy are two diseases with a high concordance rate in diabetic patients and might share common pathogenesis, no study reported if these single nucleotide polymorphisms (SNPs) had effects on diabetic retinopathy after stratification of the status of diabetic nephropathy. In this study, we aimed to test the effects of SNPs from these four loci on the diabetic nephropathy and retinopathy in Chinese type 2 diabetic patients.
RESEARCH DESIGN AND METHODS
We used a two-stage approach for this study. In stage 1, we recruited 1,276 type 2 diabetic patients from the Shanghai Diabetes Institute Inpatient Database of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital (10,11) and examined them for diabetic retinopathy and nephropathy. These patients included 378 individuals with diabetic nephropathy but no retinopathy, 374 with diabetic retinopathy but no nephropathy, 244 with both diabetic retinopathy and nephropathy, and 280 control subjects. The control subjects were defined as having normoalbuminuria, no retinopathy, and having diabetes for >10 years. In stage 2, we recruited 590 type 2 diabetic patients from the Shanghai Diabetic Complications Study (12) and Shanghai Diabetes Institute Inpatient Database, including 209 patients with diabetic retinopathy and 381 patients with diabetes for >5 years and without retinopathy. The basic characteristics of the study population were shown in Table 1.
TABLE 1.
Clinical characteristic of the participants
| Stage 1 |
Stage 2 |
|||||
|---|---|---|---|---|---|---|
| Control subjects | Diabetic nephropathy only | Diabetic retinopathy only | Diabetic nephropathy and retinopathy | Control subjects | Diabetic retinopathy | |
| Male/female | 102/178 | 213/165 | 157/217 | 135/109 | 170/211 | 99/110 |
| Age (years) | 67.04 ± 9.49 | 61.21 ± 13.74 | 61.24 ± 10.74 | 64.36 ± 10.56 | 64.60 ± 10.35 | 62.06 ± 11.81 |
| BMI (kg/m2) | 23.85 ± 3.16 | 25.28 ± 3.77 | 23.97 ± 3.45 | 24.43 ± 3.95 | 24.72 ± 3.49 | 25.03 ± 3.39 |
| Age at diagnosis of diabetes (years) | 52.88 ± 9.77 | 54.79 ± 12.67 | 51.76 ± 10.17 | 51.70 ± 11.38 | 53.97 ± 10.50 | 51.57 ± 11.93 |
| Duration of diabetes (years) | 12.00 (10.00–16.00) | 6.00 (0.80–10.00) | 10.00 (5.00–14.00) | 12.00 (8.00–18.00) | 9.00 (6.90–13.00) | 10.00 (5.00–15.00) |
| Hemoglobin A1c (%) | 8.47 ± 1.99 | 9.26 ± 2.34 | 8.98 ± 2.11 | 9.49 ± 2.21 | 7.88 ± 1.72 | 8.96 ± 2.53 |
| Systolic blood pressure (mmHg) | 133.90 ± 17.04 | 137.62 ± 18.58 | 135.27 ± 17.92 | 143.79 ± 19.73 | 133.42 ± 16.69 | 134.25 ± 20.98 |
| Diastolic blood pressure (mmHg) | 78.45 ± 8.68 | 82.23 ± 10.07 | 80.20 ± 9.37 | 82.98 ± 9.62 | 81.12 ± 9.22 | 80.66 ± 11.56 |
| AERs (mg/24 h) | 8.81 (6.00–13.44) | 79.90 (44.03–203.60) | 10.85 (7.17–16.28) | 163.62 (54.31–607.54) | 10.16 (5.87–29.77) | 11.67 (6.18–43.53) |
| eGFR* | 116.82 (102.05–137.85) | 112.52 (87.24–138.57) | 124.08 (105.21–149.32) | 104.72 (76.85–134.74) | 124.52 (106.74–145.06) | 122.41 (97.73–146.77) |
Data are n, means ± SD, or medians (interquartile range). eGFR, estimated glomerular filtration rate.
*eGFR was calculated by using the formula developed by the Modification of Diet in Renal Disease study group with adjustment for the Chinese ethnicity.
This study was approved by the institutional review board of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital and in accordance with the principle of the Helsinki Declaration II. Written informed consent was obtained from each participant.
Nephropathy measurement.
The 24-h albumin excretion rates (AERs) were measured in 3 consecutive days, and the mean value was recorded for each patient. Patients with AER <30 mg/24 h, 30 mg/24 h ≤ AER <300 mg/24 h, or AER ≥300 mg/24 h were classified as having normoalbuminuria, microalbuminuria, or proteinuria, respectively. Estimated glomerular filtration rate was calculated by using a formula developed by the Modification of Diet in Renal Disease study group with adjustment for Chinese ethnicity: 186 × (serum creatinine in μmol/L × 0.011)–1.154 × (age in years)–0.203 × (0.742 if female) × (1.233 if Chinese) (13).
Retinal assessment.
Fundus photography was performed following a standardized protocol at the Department of Ophthalmology, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. Both eyes of each participant were photographed with a 45-degree 6.3-megapixel digital nonmydriatic camera (Canon CR6-45NM, Lake Success, NY). Level of retinopathy was defined according to the International Classification of Diabetic Retinopathy (14): mild nonproliferative diabetic retinopathy, moderate nonproliferative diabetic retinopathy, severe nonproliferative diabetic retinopathy, or proliferative diabetic retinopathy. The worse eye was recorded for each patient.
SNP selection and genotyping.
In stage 1, we genotyped 55 tagging SNPs that capture 95% of the common variants, including one reported SNP from each locus (CPVL/CHN2 rs39059, FRMD3 rs10868025, CARS rs451041, and IRS2 rs1411766). The SNPs showed associations to diabetic nephropathy or retinopathy and were genotyped in stage 2 samples. The genotyping was performed by using primer extension of multiplex products with detection by matrix-assisted laser desorption ionization – time of flight mass spectroscopy using a MassARRAY Compact Analyzer (Sequenom, San Diego, CA).
Statistical analysis.
The Hardy-Weinberg equilibrium test was performed before the association analysis. The allelic frequencies between the diabetic patients with or without complications were compared by χ2 tests using PLINK (v1.07) (15), and odd ratios (ORs) with 95% CIs were presented. Combined ORs from different studies were calculated by using a Comprehensive Meta Analysis (v2.2.057) with a fixed- or random-effect model after testing for heterogeneity. The test for homogeneity was assessed by the Cochran Q test. The genotype-disease association analyses were performed under the additive model by logistic regression with adjustment of confounding factors. The effects of SNPs on the levels of retinopathy severity were analyzed by trend analysis. Skewly distributed quantitative traits (estimated glomerular filtration rate and AER) were logarithmically transformed to approximate univariate normality before analysis. Quantitative traits were analyzed by linear regression with adjustment of confounding factors under an additive genetic model. The statistical analyses were performed using SAS for Windows (version 8.0; SAS Institute, Cary, NC) unless specified otherwise. A two-tailed P value of <0.05 was considered statistically significant.
On the basis of the previously reported effect size of these loci (∼1.40) (8), our stage 1 samples (∼600 case subjects vs. 600 control subjects) had >90% power to replicate the reported effects of SNPs with minor allele frequencies >0.2 and 75% power to replicate the effect SNP with minor allele frequency of 0.1 at a level of significance of 0.05.
RESULTS
We firstly analyzed the effects of these SNPs on diabetic retinopathy and nephropathy in stage 1 samples (Supplementary Tables 1 and 2). As shown in Table 2, four CPVL/CHN2 SNPs (rs39059, rs17756941, rs245955, and rs245962) and FRDM3 rs10868025 were nominally associated with diabetic retinopathy (P < 0.05), with rs39059 and rs10868025 showing the strongest association within each locus (OR 0.774, 95% CI 0.657–0.912, P = 0.002, for rs39059 G allele; 0.833, 0.703–0.987, P = 0.034, for rs10868025 G allele). However, none of the genotyped SNPs showed a significant association to diabetic nephropathy.
TABLE 2.
Effects of the SNPs on diabetic retinopathy and nephropathy in stage 1 samples
| Chromosome |
SNP |
Position |
Minor/major allele |
Diabetic retinopathy | Diabetic nephropathy | ||
|---|---|---|---|---|---|---|---|
| OR (95% CI) |
P | OR (95% CI) | P | ||||
| 7 | rs3812398 | 29237994 | C,T | 0.970 (0.816–1.153) | 0.728 | 1.087 (0.915–1.292) | 0.341 |
| 7 | rs3812389 | 29244759 | A,G | 0.839 (0.679–1.036) | 0.103 | 0.960 (0.778–1.185) | 0.704 |
| 7 | rs2269903 | 29247409 | C,A | 1.202 (0.976–1.481) | 0.084 | 1.026 (0.832–1.264) | 0.813 |
| 7 | rs3812388 | 29249198 | G,C | 1.190 (0.967–1.464) | 0.100 | 1.055 (0.858–1.298) | 0.611 |
| 7 | rs17756941 | 29250007 | G,A | 0.747 (0.598–0.933) | 0.010 | 0.941 (0.755–1.174) | 0.593 |
| 7 | rs11981737 | 29250335 | A,G | 1.064 (0.736–1.536) | 0.743 | 0.834 (0.578–1.204) | 0.334 |
| 7 | rs39059 | 29255470 | G,A | 0.774 (0.657–0.912) | 0.002 | 0.959 (0.814–1.131) | 0.621 |
| 7 | rs39065 | 29262601 | A,G | 0.973 (0.711–1.331) | 0.862 | 1.180 (0.862–1.614) | 0.300 |
| 7 | rs17157658 | 29274254 | C,G | 0.858 (0.719–1.024) | 0.090 | 1.051 (0.881–1.254) | 0.577 |
| 7 | rs245955 | 29276307 | C,T | 0.784 (0.668–0.921) | 0.003 | 0.989 (0.843–1.161) | 0.896 |
| 7 | rs245962 | 29290153 | A,G | 0.800 (0.681–0.939) | 0.006 | 0.989 (0.842–1.161) | 0.893 |
| 7 | rs39099 | 29293095 | A,G | 0.910 (0.762–1.085) | 0.293 | 1.042 (0.874–1.243) | 0.646 |
| 7 | rs39101 | 29294462 | A,G | 1.049 (0.846–1.301) | 0.664 | 0.884 (0.712–1.096) | 0.261 |
| 9 | rs11140139 | 86145409 | A,G | 1.018 (0.848–1.221) | 0.854 | 1.010 (0.841–1.211) | 0.918 |
| 9 | rs4877788 | 86146950 | C,T | 1.029 (0.866–1.221) | 0.750 | 1.044 (0.880–1.240) | 0.620 |
| 9 | rs7849075 | 86149610 | C,T | 0.972 (0.787–1.199) | 0.787 | 0.971 (0.787–1.198) | 0.783 |
| 9 | rs1888746 | 86155392 | T,C | 0.815 (0.538–1.234) | 0.334 | 0.928 (0.602–1.380) | 0.661 |
| 9 | rs11140156 | 86163694 | G,C | 0.922 (0.717–1.186) | 0.528 | 0.919 (0.714–1.183) | 0.511 |
| 9 | rs10868025 | 86164176 | G,A | 0.833 (0.703–0.987) | 0.034 | 0.905 (0.764–1.072) | 0.249 |
| 9 | rs11535575 | 86165034 | T,C | 0.671 (0.439–1.025) | 0.065 | 0.762 (0.501–1.160) | 0.205 |
| 9 | rs6559732 | 86168692 | C,T | 1.152 (0.948–1.399) | 0.154 | 1.080 (0.889–1.311) | 0.440 |
| 9 | rs7470287 | 86172665 | C,G | 0.840 (0.687–1.027) | 0.090 | 0.963 (0.788–1.177) | 0.712 |
| 9 | rs3934902 | 86177401 | A,G | 1.103 (0.938–1.298) | 0.237 | 1.018 (0.865–1.197) | 0.835 |
| 9 | rs4451390 | 86179563 | T,C | 1.000 (0.848–1.180) | 0.998 | 1.042 (0.883–1.229) | 0.628 |
| 9 | rs11793821 | 86184504 | G,A | 1.116 (0.861–1.447) | 0.407 | 0.915 (0.706–1.187) | 0.503 |
| 11 | rs3764895 | 2945945 | T,C | 0.994 (0.802–1.231) | 0.954 | 0.897 (0.724–1.112) | 0.321 |
| 11 | rs2583442 | 2956166 | A,G | 1.021 (0.860–1.211) | 0.812 | 0.993 (0.837–1.179) | 0.940 |
| 11 | rs4758576 | 2973880 | G,A | 0.956 (0.726–1.259) | 0.751 | 0.912 (0.692–1.201) | 0.512 |
| 11 | rs11024758 | 2981782 | T,C | 1.180 (0.640–2.174) | 0.596 | 1.590 (0.853–2.962) | 0.144 |
| 11 | rs4758504 | 3000179 | A,G | 0.963 (0.724–1.279) | 0.794 | 0.901 (0.678–1.198) | 0.473 |
| 11 | rs4758621 | 3009640 | A,G | 1.008 (0.851–1.194) | 0.925 | 0.990 (0.836–1.173) | 0.911 |
| 11 | rs12363575 | 3030104 | G,A | 1.007 (0.783–1.294) | 0.958 | 1.104 (0.859–1.420) | 0.438 |
| 11 | rs12421922 | 3035070 | T,C | 1.024 (0.868–1.209) | 0.778 | 0.996 (0.844–1.175) | 0.961 |
| 11 | rs2071101 | 3050137 | A,G | 1.042 (0.885–1.225) | 0.624 | 0.939 (0.798–1.105) | 0.451 |
| 11 | rs572373 | 3055361 | C,T | 1.061 (0.878–1.281) | 0.541 | 1.032 (0.854–1.247) | 0.745 |
| 11 | rs451041 | 3060725 | A,G | 1.046 (0.881–1.243) | 0.605 | 1.010 (0.851–1.200) | 0.906 |
| 11 | rs7111857 | 3068106 | A,G | 1.069 (0.876–1.306) | 0.511 | 1.044 (0.855–1.274) | 0.676 |
| 11 | rs6578318 | 3072442 | T,C | 1.009 (0.792–1.284) | 0.945 | 0.981 (0.770–1.248) | 0.873 |
| 11 | rs2290000 | 3073838 | T,A | 1.019 (0.801–1.296) | 0.878 | 0.969 (0.762–1.233) | 0.799 |
| 11 | rs406598 | 3076285 | C,T | 1.016 (0.859–1.203) | 0.850 | 1.002 (0.847–1.185) | 0.980 |
| 11 | rs10833173 | 3094505 | A,T | 1.033 (0.837–1.275) | 0.760 | 0.950 (0.770–1.173) | 0.635 |
| 11 | rs2084239 | 3106659 | G,A | 0.983 (0.831–1.163) | 0.845 | 0.958 (0.810–1.133) | 0.617 |
| 13 | rs914270 | 110243017 | C,G | 0.995 (0.847–1.169) | 0.954 | 0.955 (0.812–1.122) | 0.573 |
| 13 | rs2391776 | 110243425 | C,T | 0.937 (0.735–1.194) | 0.599 | 1.006 (0.790–1.281) | 0.963 |
| 13 | rs1041466 | 110244322 | C,T | 1.264 (0.945–1.691) | 0.115 | 0.782 (0.584–1.048) | 0.100 |
| 13 | rs11069790 | 110244401 | A,G | 0.937 (0.784–1.121) | 0.477 | 1.102 (0.921–1.317) | 0.288 |
| 13 | rs4462453 | 110251328 | G,A | 1.246 (0.902–1.719) | 0.182 | 0.869 (0.629–1.199) | 0.392 |
| 13 | rs1411766 | 110252160 | T,C | 1.094 (0.828–1.445) | 0.530 | 0.991 (0.750–1.310) | 0.951 |
| 13 | rs12184748 | 110253930 | C,T | 1.001 (0.845–1.186) | 0.990 | 1.028 (0.868–1.217) | 0.754 |
| 13 | rs2150481 | 110256550 | G,C | 1.079 (0.917–1.269) | 0.362 | 1.030 (0.876–1.212) | 0.717 |
| 13 | rs2391778 | 110258553 | T,A | 1.048 (0.888–1.236) | 0.582 | 1.037 (0.879–1.223) | 0.666 |
| 13 | rs1547241 | 110273605 | G,C | 1.108 (0.824–1.490) | 0.497 | 0.858 (0.637–1.155) | 0.311 |
| 13 | rs10161791 | 110281789 | G,A | 0.938 (0.786–1.121) | 0.483 | 1.106 (0.926–1.321) | 0.264 |
| 13 | rs9587939 | 110284951 | A,C | 0.965 (0.810–1.150) | 0.693 | 1.055 (0.885–1.257) | 0.549 |
| 13 | rs4773068 | 110288967 | A,G | 0.924 (0.771–1.107) | 0.390 | 1.160 (0.968–1.390) | 0.108 |
P values <0.05 are shown in boldface. ORs with 95% CIs were calculated for the minor allele.
To further validate the effects of rs39059 and rs10868025 on diabetic retinopathy, we genotyped both SNPs in stage 2 samples. We found only CPVL/CHN2 rs39059 showed similar effects to diabetic retinopathy, as identified in the first stage (OR 1.269, 95% CI 0.989–1.628, P = 0.061, for rs39059; 1.014, 0.789–1.302, P = 0.9147, for rs10868025). We then performed a meta-analysis with the fixed-effect model and found rs39059 was associated with diabetic retinopathy, with an OR of 1.285 (1.120–1.474, P = 0.0003) (Table 3). This association remained significant after adjusting confounding factors, including hemoglobin A1c levels, duration of diabetes, systolic and diastolic blood pressure, and BMI (OR 1.242, 1.074–1.437, P = 0.0034).
TABLE 3.
Association of rs39059 in CPVL/CHN2 with diabetic retinopathy in Chinese type 2 diabetic patients
|
n (case/control) |
Risk allele frequencies | OR (95% CI)* |
P |
||
|---|---|---|---|---|---|
| Case subjects | Control subjects | ||||
| Diabetic retinopathy vs. control subjects | 365/273 | 0.619 | 0.564 | 1.256 (1.003–1.575) | 0.047 |
| Diabetic nephropathy and retinopathy vs. diabetic nephropathy | 231/363 | 0.636 | 0.567 | 1.334 (1.049–1.695) | 0.018 |
| Stage 2 validation | 209/381 | 0.660 | 0.605 | 1.269 (0.989–1.628) | 0.061 |
| Meta-analysis | 805/1,017 | 0.633 | 0.581 | 1.285 (1.120–1.474) | 0.0003† |
*ORs with 95% CIs were shown for risk allele.
†P values of meta-analysis were calculated using the fixed-effect model; homogeneity test P = 0.932.
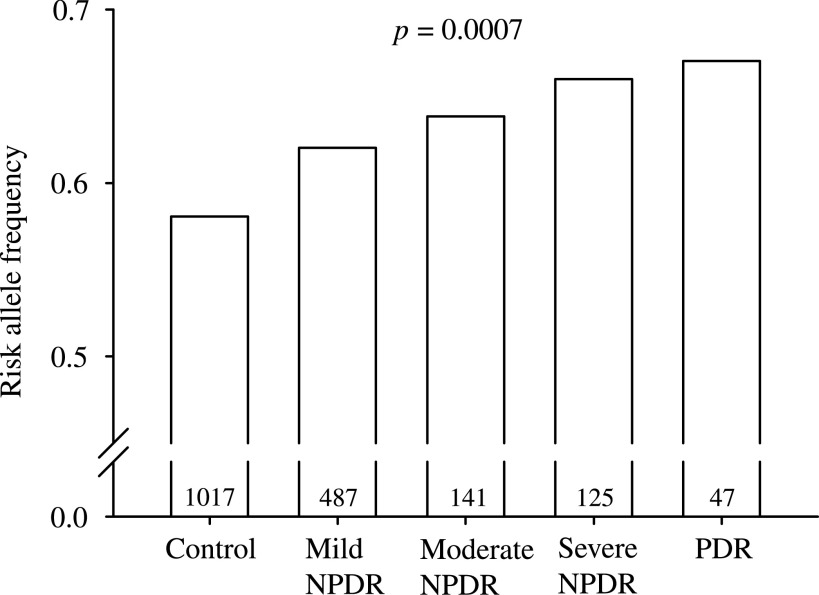
We then analyzed the effects of CPVL/CHN2 rs39059 on the disease severity of diabetic retinopathy in all the samples. As shown in Fig. 1, CPVL/CHN2 rs39059 showed an association to the levels of diabetic retinopathy, with the risk allele more frequent in the more severe retinopathy patients (P = 0.0007 for trend analysis).
FIG. 1.
The effects of CPVL/CHN2 rs39059 on the disease severity of diabetic retinopathy. Numbers within the bar represent the number of participants of each group. NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy.
DISCUSSION
In the current study, we analyzed the effects of SNPs from four loci on diabetic retinopathy and nephropathy in Chinese type 2 diabetic patients. We first reported that CPVL/CHN2 rs39059 was associated with diabetic retinopathy. Although the association we observed was solid by replication in independent samples and the P value remained significant after Bonferroni correction of analysis on multiple SNPs (corrected P = 0.033, on the basis of the association analysis between 55 SNPs and two traits), we still cannot fully exclude the possibility that the association detected was false positive because of the relatively small sample size of this study. But considering we also found an association between rs39059 and disease severity in our samples, which supported the role of this locus in diabetic retinopathy from another aspect, the chance of a false-positive finding was limited. Although this locus was originally identified to be associated with diabetic nephropathy in a previous genome-wide association study (since the concordance rate of retinopathy and nephropathy was high in diabetic patients [16] and the genome-wide association study in the GoKinD samples (8) did not exclude the patients with retinopathy), CPVL/CHN2 may be a susceptible locus of diabetic retinopathy other than nephropathy. However, whether this effect is restricted to type 2 diabetes is still unknown and needs to be investigated in studies with type 1 diabetic patients.
The SNP rs39059 locates in the intron of CHN2, which encodes β-2 chimerin that have been shown to regulate cell growth, proliferation, and migration (17). Previous studies showed decreased expression of CHN2 is associated with high-grade malignant gliomas, breast cancer, and duodenal adenocarcinoma, whereas increased expression of CHN2 is reported to be associated with lymphomas (17,18). One other gene within the same haplotype block of rs39059 is CPVL. It encodes a carboxypeptidase that cleaves a single amino acid from the COOH termini of peptides (19). However, the function of this gene is still largely unknown. Although hemoglobin A1c levels and systolic blood pressure were also associated with diabetic retinopathy in our samples (P = 0.0065 for systolic blood pressure and P = 7.0 × 10−9 for hemoglobin A1c), since rs39059 showed association to none of these traits (P = 0.5031 for systolic blood pressure and P = 0.1561 for hemoglobin A1c), our data suggest this locus did not participate in the pathogenesis of diabetic retinopathy through the effects on blood pressure and glucose levels. Thus, the mechanism how this locus affected diabetic retinopathy susceptibility remains to be investigated. And the causal variant also remains to be identified by fine mapping studies.
In this study, we failed to replicate the associations between these four loci and diabetic nephropathy in Chinese type 2 diabetic patients, although we had enough statistical power. Because we used a tagging SNP approach and captured most of the common variants within these loci, it was unlikely that causal variants were captured by different SNPs in different populations. One possible explanation may be the complicated phenotypes in type 2 diabetic patients. In our study, >60% of the diabetic nephropathy patients also suffered from hypertension; although we statistically adjusted blood pressure as a confounding factor, the impact of hypertension cannot be ignored. In a previous replication study in Japanese type 2 diabetic patients, Maeda et al. (9) also failed to replicate the effects of most of these loci in four independent samples; thus, it is highly possible that the effects of these loci on diabetic nephropathy are restricted in the European descent population or type 1 diabetic patients. Further studies with type 1 diabetic patients are needed to confirm the effects of these loci.
In summary, we found CPVL/CHN2 rs39059 was associated with diabetic retinopathy in Chinese type 2 diabetic patients. Further studies are needed to replicate this finding.
ACKNOWLEDGMENTS
This work was supported by grants from the National 973 Program (2011CB504001), the Project of National Natural Science Foundation of China (30800617), the major program of Shanghai Municipality for Basic Research (08dj1400601), the National 863 Program (2006AA02A409), the Shanghai Rising-Star Program (09QA1404400), “Chen Guang” Project (09CG07), and the Young Talent Program of Shanghai Municipal Health Bureau.
No potential conflicts of interest relevant to this article were reported.
C.H. researched data, contributed to discussion, and wrote the manuscript. R.Z. and W.Y. researched data. J.W. contributed to discussion and wrote the manuscript. C.W. researched data. C.P. contributed to discussion. X.M. researched data. Y.B. and K.X. contributed to discussion. W.J. contributed to discussion and reviewed and edited the manuscript.
The authors thank all the research participants and the nursing and medical staff at Shanghai Clinical Center for Diabetes for their dedication and professionalism in conducting this study.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-0028/-/DC1.
REFERENCES
- 1.Sheetz MJ, King GL. Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA 2002;288:2579–2588 [DOI] [PubMed] [Google Scholar]
- 2.Ritz E. Nephropathy in type 2 diabetes. J Intern Med 1999;245:111–126 [DOI] [PubMed] [Google Scholar]
- 3.Thylefors B, Négrel AD, Pararajasegaram R, Dadzie KY. Global data on blindness. Bull World Health Organ 1995;73:115–121 [PMC free article] [PubMed] [Google Scholar]
- 4.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seaquist ER, Goetz FC, Rich S, Barbosa J. Familial clustering of diabetic kidney disease: evidence for genetic susceptibility to diabetic nephropathy. N Engl J Med 1989;320:1161–1165 [DOI] [PubMed] [Google Scholar]
- 6.Pettitt DJ, Saad MF, Bennett PH, Nelson RG, Knowler WC. Familial predisposition to renal disease in two generations of Pima Indians with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1990;33:438–443 [DOI] [PubMed] [Google Scholar]
- 7.Diabetes Control and Complications Trial Research Group. Clustering of long-term complications in families with diabetes in the diabetes control and complications trial. Diabetes 1997;46:1829–1839 [PubMed] [Google Scholar]
- 8.Pezzolesi MG, Poznik GD, Mychaleckyj JC, et al. Genome-wide association scan for diabetic nephropathy susceptibility genes in type 1 diabetes. Diabetes 2009;58:1403–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maeda S, Araki S, Babazono T, et al. Replication study for the association between four loci identified by a genome-wide association study on European American subjects with type 1 diabetes and susceptibility to diabetic nephropathy in Japanese subjects with type 2 diabetes. Diabetes 2010;59:2075–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu C, Wang C, Zhang R, et al. Association of genetic variants of NOS1AP with type 2 diabetes in a Chinese population. Diabetologia 2010;53:290–298 [DOI] [PubMed] [Google Scholar]
- 11.Hu C, Zhang R, Wang C, et al. Effects of GCK, GCKR, G6PC2 and MTNR1B variants on glucose metabolism and insulin secretion. PLoS ONE 2010;5:e11761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia W, Gao X, Pang C, et al. Prevalence and risk factors of albuminuria and chronic kidney disease in Chinese population with type 2 diabetes and impaired glucose regulation: Shanghai Diabetic Complications Study (SHDCS). Nephrol Dial Transplant 2009;24:3724–3731 [DOI] [PubMed] [Google Scholar]
- 13.Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 2006;17:2937–2944 [DOI] [PubMed] [Google Scholar]
- 14.Wilkinson CP, Ferris FL, 3rd, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003;110:1677–1682 [DOI] [PubMed] [Google Scholar]
- 15.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007;81:559–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girach A, Vignati L. Diabetic microvascular complications: can the presence of one predict the development of another? J Diabetes Complications 2006;20:228–237 [DOI] [PubMed] [Google Scholar]
- 17.Bruinsma SP, Baranski TJ. Beta2-chimaerin in cancer signaling: connecting cell adhesion and MAP kinase activation. Cell Cycle 2007;6:2440–2444 [DOI] [PubMed] [Google Scholar]
- 18.Nishiu M, Yanagawa R, Nakatsuka S, et al. Microarray analysis of gene-expression profiles in diffuse large B-cell lymphoma: identification of genes related to disease progression. Jpn J Cancer Res 2002;93:894–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahoney JA, Ntolosi B, DaSilva RP, Gordon S, McKnight AJ. Cloning and characterization of CPVL, a novel serine carboxypeptidase, from human macrophages. Genomics 2001;72:243–251 [DOI] [PubMed] [Google Scholar]