Abstract
OBJECTIVE
The impact of type 1 diabetes mellitus (T1DM) on the developing central nervous system is not well understood. Cross-sectional, retrospective studies suggest that exposure to glycemic extremes during development is harmful to brain structure in youth with T1DM. However, these studies cannot identify brain regions that change differentially over time depending on the degree of exposure to glycemic extremes.
RESEARCH DESIGN AND METHODS
We performed a longitudinal, prospective structural neuroimaging study of youth with T1DM (n = 75; mean age = 12.5 years) and their nondiabetic siblings (n = 25; mean age = 12.5 years). Each participant was scanned twice, separated by 2 years. Blood glucose control measurements (HbA1c, glucose meter results, and reports of severe hypoglycemia) were acquired during the 2-year follow-up. Sophisticated image registration algorithms were performed, followed by whole brain and voxel-wise statistical analyses of the change in gray and white matter volume, controlling for age, sex, and age of diabetes onset.
RESULTS
The T1DM and nondiabetic control (NDC) sibling groups did not differ in whole brain or voxel-wise change over the 2-year follow-up. However, within the T1DM group, participants with more hyperglycemia had a greater decrease in whole brain gray matter compared with those with less hyperglycemia (P < 0.05). Participants who experienced severe hypoglycemia had greater decreases in occipital/parietal white matter volume compared with those with no severe hypoglycemia (P < 0.05) and compared with the NDC sibling group (P < 0.05).
CONCLUSIONS
These results demonstrate that within diabetes, exposure to hyperglycemia and severe hypoglycemia may result in subtle deviation from normal developmental trajectories of the brain.
Type 1 diabetes mellitus (T1DM) has cumulative deleterious effects on many systems in the body. These complications can begin to develop during childhood and adolescence (1–3). The effects of T1DM on central nervous system structure are controversial and not well understood. However, recent retrospective, cross-sectional magnetic resonance imaging (MRI) brain studies have reported differences in gray or white matter integrity associated with hypoglycemia or hyperglycemia in youth and adults with T1DM (4–6). These findings suggest that exposure to glycemic extremes while the brain is developing may alter the specific pathways or regions in the brain. That determination may inform treatment strategies for children with T1DM, by the determination of the risk-to-benefit ratio of strict versus permissive glycemic control.
It has been hypothesized that prolonged exposure to hyperglycemia causes mitochondrial dysfunction and increased oxidative stress, leading to cell death in the dependent brain regions (7–12). It is not clear how these proposed mechanisms may differ in the developing brain. In addition, there is some evidence from animal models that hyperglycemia impedes myelination in the developing brain (13). In animal (14,15) and human (16–19) case studies, severe hypoglycemia has been reported to induce cell death in the brain, possibly through excitotoxic or apoptotic processes (20–22). In developing animals, a single moderate episode of hypoglycemia has been shown to induce cell death in the cortex but spare the subcortex. Limited evidence suggests that the degree of these effects in part depends on the neurodevelopmental stage at which the hypoglycemic episode is experienced (23,24). These findings, primarily from animal models, highlight the possibility that extreme glycemic states could interact with brain development to alter the normal trajectory of the brain in childhood and adolescence.
In general, youth with T1DM do not differ in brain volume compared with nondiabetic siblings in cross-sectional, retrospective MRI studies (5). Within T1DM, however, qualitatively different associations have been found between prior exposure to severe hypoglycemia or hyperglycemia and regional gray and white matter volumes. These changes were significant despite the relatively short duration of T1DM (on average 5.7 years) and limited exposure to blood glucose extremes. While retrospective analyses may identify regions that are vulnerable to long-term exposure to glycemic extremes, they cannot identify brain regions that are changing within a restricted window of time for a given age-group. In addition, they cannot rule out preexisting brain differences across groups. Longitudinal, prospective assessment is necessary to establish whether glycemic control causes short-term changes in brain volume from an established baseline during development. Indeed, logitudinal methods are preferred for studies of normal brain development because they provide greater sensitivity to detect smaller regional changes and complex trajectories (25).
To address these issues, we acquired structural brain images of youth with T1DM and their nondiabetic control (NDC) siblings twice, separated by 2 years of prospective monitoring of glucose control. Images were analyzed to determine whether exposure to hyperglycemia or hypoglycemia during those 2 years altered the developmental trajectory of brain volumes within the T1DM group.
RESEARCH DESIGN AND METHODS
Youth aged 7–17 years with T1DM were recruited from the Diabetes Clinic at the Department of Pediatrics at Washington University in St. Louis. NDC siblings of enrolled T1DM patients were also invited to participate. Potential participants were excluded for mental retardation, chronic disease other than T1DM (e.g., hypothyroidism), significant neurologic history not caused by diabetes, reported diagnosed or currently treated psychiatric disorder, and current use of psychoactive medications, birth premature more than 4 weeks and with complications, pregnancy, and contraindications to MRI (e.g., metal implants). T1DM participants were required to have been diagnosed and on insulin for at least 2 years at study entry. Handedness was assessed with a modified Edinburgh Handedness Inventory (26). Procedures were approved by the Washington University School of Medicine’s Human Research Protection Office, and all participants and their parents or guardians provided signed, informed consent. Participants attended appointments at study entry (Time 1) and after 2 years (Time 2). Imaging data from Time 1 has been analyzed and reported previously (5).
Clinical variables.
During the 2-year follow-up period, participants and their parents were asked to report any severe hypoglycemic episodes as they occurred. Study staff met with the families at every clinic visit (~3 times per year) to ask explicitly if any significant diabetes-related events had been experienced. A detailed description of each hypoglycemic event was elicited, including circumstances surrounding the event, a comprehensive list of symptoms, and how the event was treated. For analyses, “severe hypoglycemia” was defined as events requiring assistance for treatment by someone other than the patient or those involving neurologic dysfunction including seizure, loss of consciousness, or inability to arouse from sleep (27). At Time 2, patients and parents were interviewed about severe glycemic events since Time 1 in order to verify the absence of hypoglycemic episodes or catch any episodes not previously reported during the study. Medical records were also reviewed for severe hypoglycemic episodes or absence of episodes. Hyperglycemic history over the 2-year follow-up was estimated by averaging all HbA1c test results collected from participants’ medical records from the Diabetes Clinic. In addition, data from self-monitoring blood glucose devices were acquired throughout the 2-year period. Glucose meter data were downloaded at clinic visits or separate visits or were sent via e-mail in order to ensure complete ascertainment. To quantify mild hypoglycemia, the percentage of glucose readings <60 mg/dL was calculated for each subject. The percentage of readings >300 mg/dL, average, and standard deviation of blood glucose readings were also calculated. To ensure that glucose meter data reasonably represented the 2-year period, only subjects with glucose reading data on >85% of days of follow-up were included in glucose reading analyses.
Image acquisition.
Structural images were acquired at entry (Time 1) and after 2 years (Time 2) for each subject on a Siemens Sonata 1.5 Tesla imaging system with a standard Siemens 30-cm circularly polarized RF head coil. At each time point, 3–5 images consisting of 128 contiguous, 1.25-mm sagittal slices were acquired using magnetization prepared rapid gradient echo (MPRAGE) (TR = 1,900 ms, TE = 3.93 ms, flip angle = 15°, matrix = 256 × 256 pixels, voxel size = 1 × 1 × 1.25 mm, each image = 7 min, 7 sec). For each subject at each time point, 3 high-quality T1 images were averaged after being coregistered by an automated, validated technique (28).
Image processing.
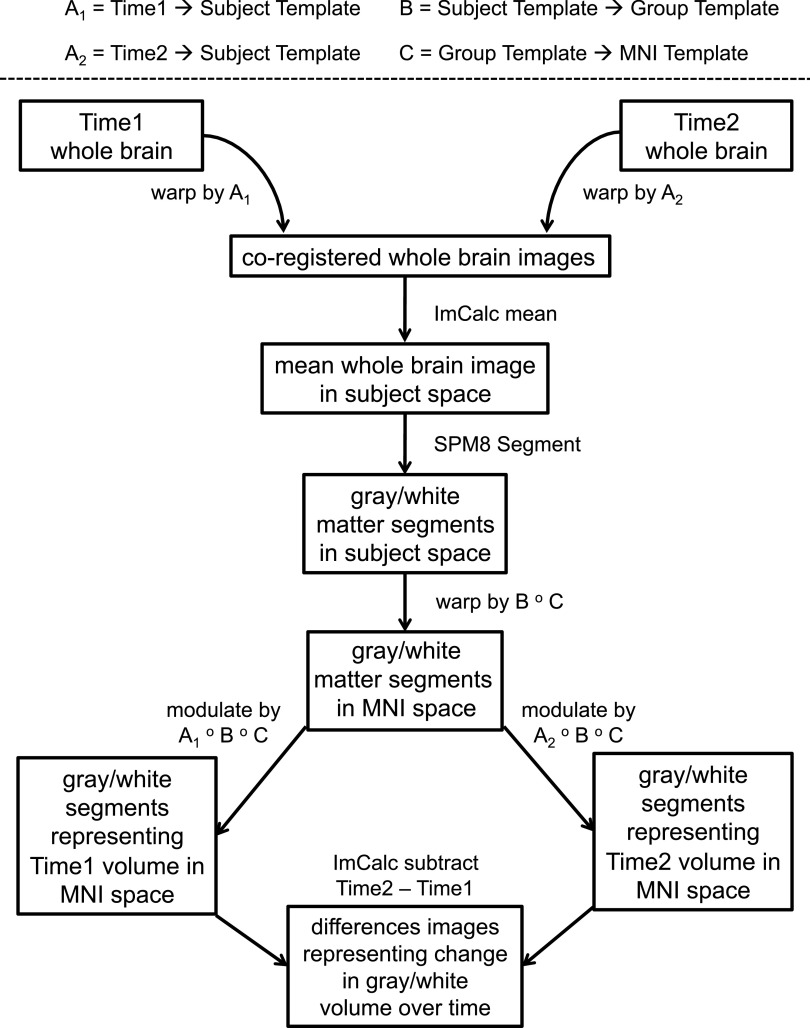
Averaged images were prepared for analysis with Statistical Parametric Mapping software (SPM8; Wellcome Department of Cognitive Neurology), as depicted in Fig. 1. This processing stream was similar to published methods (29) modified to utilize recently developed high-parameter nonlinear registration techniques (30).
FIG. 1.
Process by which images were prepared for analysis: 1) Unified segment and bias-correction. Images were segmented into gray matter, white matter, and cerebrospinal fluid, and field inhomogeneity-corrected images were produced with SPM8’s “unified segment” module (49). From the next step forward, image preparation steps were performed on gray and white matter segmented images separately. 2) DARTEL import. Gray and white matter segmented images were imported into Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL), a component of SPM8 that determines an average-shaped template from all provided images and calculates high dimensional spatial flow fields between each image and the template (30). During import, images were rigidly aligned and resampled to 1.5 mm cubic voxels. 3) Within-subject DARTEL. For each subject, DARTEL was used to calculate flow fields between Time 1 and Time 2 segmented images and a subject-specific gray or white matter template, which can be considered an image halfway between Time 1 and Time 2. We refer to the within-subject flow fields as “A1” for the warp between Time 1 and subject template and “A2” as the warp between Time 2 and subject template. 4) Between-subject DARTEL. DARTEL was used to calculate flow fields from each subject template to a simultaneously calculated group template, an image representing all 100 subjects. We refer to the warp parameters between subject template and group template as “B.” 5) A 12-parameter affine transformation from the group template to Montreal Neurologic Institute (MNI) template was calculated for ease of interpretation of coordinate results. We refer to the affine transform from group template to MNI space as “C.” 6) Within-subject flow fields (A1 and A2) were applied, respectively, to inhomogeneity-corrected whole brain Time 1 and Time 2 images (produced in Step 1). We averaged the nonzero voxels of the resulting coregistered pair of images. 7) Each subject’s mean image was segmented into gray matter and white matter tissue with SPM8’s unified segment module. 8) A composition of warps from subject template space to MNI space was calculated with SPM’s deformations utility: [subject template to group template] o [group template to MNI], or [B o C]. Composing warps so that they may be applied simultaneously prevents errors that would be introduced by resampling the images multiple times. 9) Composed warps [B o C] were applied to the gray and white matter images produced in Step 7, resulting in segmented images in MNI space. Since the spatial normalization information from subject to MNI space came from the subject-specific template, each time point contributed to the normalization, avoiding potential bias caused by applying normalization parameters of a single time point to both time points. 10) A composition of warps from each time point to MNI space was calculated with the deformations utility: [A1 o B o C] and [A2 o B o C]. 11) The MNI-registered segments were then “modulated” by (multiplied by the Jacobian determinant of) the warps from step 10 to preserve quantitative volume. The influence of independent normalization of each time point was minimized by applying Time 1 and Time 2 Jacobian determinants to the same segments. This resulted in MNI-registered Time 1 and Time 2 gray and white matter segmented images whose intensities correspond to units of volume. 12) MNI-registered Time 1 and Time 2 images were smoothed with a Gaussian kernel 8-mm full-width at half-maximum. 13) Time 1 segment images were subtracted from Time 2 segment images to create images representing change in gray or white matter volume over time. These different images were entered into statistical models to relate brain volume changes over time to variables of interest such as hypoglycemia and hyperglycemia exposure. ImCalc, image calculator.
Whole brain volume analyses.
Whole brain gray and white matter volumes were calculated by summing all voxel intensities in the modulated (volume) images produced in Step 10 (Fig. 1). Repeated-measures general linear models were used to analyze the change in whole brain gray or white matter volumes over time with respect to diagnostic group (T1DM vs. NDC, covarying age and sex) or with respect to severe hypoglycemia category (no hypoglycemia vs. any hypoglycemia vs. NDC). Hierarchical linear regressions were used to analyze the continuous independent variables such as hyperglycemia exposure (mean HbA1c levels during follow-up) or mild hypoglycemia (% of readings <60 mg/dL) within the T1DM group, covarying age, sex, and age of onset. To compare effects found in these analyses within the T1DM group with those found in the NDC group, we categorized T1DM subjects into exposure groups and performed univariate or repeated-measures analyses with group as the independent variable. Hyperglycemia categories were determined by rough clinical guidelines for HbA1c levels in T1DM, as follows: low, <7.5%; medium, average 7.5–8.9%; and high, average ≥9%. If any main effects or interactions were significant from these analyses (P < 0.05), they were then explored with post hoc comparisons.
Voxel-wise analyses.
Statistical analyses of the difference images were restricted by gray or white matter masks defined as including voxels at which any subject in the model had a segmented intensity greater than 0.1 (10% probability of belonging to the tissue class) in either the Time 1 or Time 2 image. This procedure minimized inclusion of low-intensity voxels possibly belonging to other tissue classes (31), while including voxels with probabilities that may have increased or decreased over time across the threshold of 0.1. Images were analyzed using SPM8, with standard parametric tests (e.g., regressions) at each voxel, resulting in statistical parametric maps (SPMs) where every voxel’s intensity corresponds to a t value. Statistical images were thresholded to show only voxels with t values corresponding to P < 0.001 prior to multiple comparison correction (height threshold). Clusters of voxels that survived the height threshold were then examined for significance. The probability of a cluster of a given size was corrected for multiple comparisons according to the number of independent observations (resolution elements or resels), calculated by the SPM smoothness estimation, which was based on the intercorrelatedness of the voxels (using the stat_threshold script from Worsley’s fmristat package) (32). We additionally took into account the smoothness (intercorrelatedness) of the voxels within the cluster (local smoothness). With the method we used (33), if the cluster appeared in a highly intercorrelated area, a stricter threshold must be crossed to attain significance.
For severe hypoglycemia, voxel-wise independent sample t tests were performed for comparisons between groups, defining contrasts in each direction (e.g., any hypoglycemia > no hypoglycemia and any hypoglycemia < no hypoglycemia). For hyperglycemia and mild hypoglycemia according to glucose meter results, voxel-wise multiple regressions were performed using average HbA1c (hyperglycemia) or percent of readings <60 mg/dL (mild hypoglycemia) as continuous variables. Contrasts were performed in each direction (negative and positive correlations).
Age and sex were included as covariates for all models. In models using only diabetic subjects, age of diabetes onset was also covaried. Cluster-level multiple-comparison and smoothness-corrected P values <0.05 were considered significant.
RESULTS
Of the total number of subjects initially enrolled in this study at Time 1 (125 T1DM and 62 NDC), we were able to evaluate 89% of the T1DM subjects (n = 111) and 80% of the NDC subjects (n = 50) after 2 years of follow-up. The subjects that moved out of the area, chose to drop out of the study, or did not return calls (n = 26; 14 T1DM and 12 NDC from 15 families) were not different in mean age [mean age = 12.6 years; t(124) = −0.15, P = 0.88] but were lower in mean parent educational level (14.4 years) compared with those included in the neuroimaging analyses (Table 1) [t(119)=1.99, P = 0.049]. Of those that returned for follow-up, an additional 36 T1DM and 25 NDC subjects were excluded from our neuroimaging analyses for various reasons. The two most common reasons were poor image quality or missing images at either time point (orthodontia, n = 27; too young for scanning at Time 1, n = 15; movement in scanner, n = 5; claustrophobia, n = 4) and new-onset exclusion criteria during follow-up (n = 9). In addition, one T1DM subject whose Time 2 appointment occurred much later than the rest of the participants (2 years and 3 months) was excluded. The excluded participants tended to be younger (10.1 years) than those included in the neuroimaging analyses, as expected (Table 1) (t = 4.5, P < 0.001), but parent education (15.2 years) was similar to those included (Table 1) (t = 0.33, P = 0.74). After application of all exclusion criteria and MRI quality control, as described above, a total of 75 youth with T1DM (60% of the original group) and 25 NDC siblings (40% of the original group) were included in these analyses.
TABLE 1.
Demographic and clinical variables of NDC and T1DM groups
| NDC | T1DM | |
|---|---|---|
| n | 25 | 75 |
| Age at Time 1 (years) | 12.5 (2.6) | 12.5 (2.8) |
| Male/female (% male) | 12/13 (48) | 48/27 (64) |
| Right handed/other (% right handed) | 24/1 (96) | 65/10 (87) |
| Parent education (years) | 15.5 (1.9) | 15.0 (2.1) |
| Age of diabetes onset (years) | — | 7.0 (3.3) |
| Severe hypoglycemic episodes during 2-year follow-up (n) | — | 0.6 (2.0) |
| Participants with any severe hypoglycemic episodes during 2-year follow-up | — | 19 (25) |
| Mean HbA1c during 2-year follow-up | — | 8.6 (1.4) |
| Participants with ketoacidosis during 2-year follow-up | — | 10 (13) |
Data are means (SD) and n (%) unless otherwise indicated.
We received >85% of days of glucose meter data from the majority of T1DM participants in these analyses (n = 63 of 75). The 12 subjects excluded from mild hypoglycemia analyses because of insufficient glucose meter data had higher 2-year mean HbA1c levels than subjects included (mean 9.4 vs. 8.4%, P = 0.02). One additional subject with 22.8% of readings <60 mg/dL was excluded as an outlier in order to avoid skewing results. For included subjects (n = 62), the mean percentage of follow-up days of glucose meter data received was 97% (SD 3.9), and the mean number of glucose readings received was 2,827 (SD 1,308).
Blood glucose checks were conducted before or after the MRI scan, as needed. We analyzed blood glucose levels obtained within 30 min of the scan time when these data were available. Of the 75 T1DM subjects, we obtained valid glucose levels from 49 subjects at Time 1 and 54 subjects at Time 2. The average blood glucose level at the Time 1 scan was 181 mg/dL (SD 84; acquired on average 14 min from scan time). The average blood glucose at the Time 2 scan was 191 mg/dL (SD 96; acquired on average 10 min from scan time). Time 2 glucose levels did not correlate with average HbA1c over the 2-year follow-up period (r = 0.006, P = 0.97) and were not different between those who experienced hypoglycemia and those who did not (t = 1.4, P = 0.17).
T1DM versus NDC subjects
Demographic and clinical variables.
The mean follow-up duration was 2 years and 6 days (736 days; SD 11 days, range 718–772 days). T1DM and NDC groups did not differ significantly in age at Time 1 (t = −0.12, P = 0.91), duration of interval follow-up (t = −1.08, P = 0.28), parent education (t = 1.09, P = 0.28), or racial/ethnic minority status (χ2 = 0.31, P = 0.58) (Table 1). Groups did not differ in the proportion of male to female subjects (χ2 = 2.00, P = 0.16) (Table 1) or in the proportion of left-handed or ambidextrous subjects (χ2 = 1.76, P = 0.19).
Whole brain volume analyses.
For whole brain gray matter, repeated-measures general linear modeling revealed a main effect of time [F(1,95) = 12.1, P = 0.001] but no main effect of group [T1DM vs. NDC; F(1,96) = 2.01, P = 0.16)] or interaction between time and group [F(1,96) = 0.09, P = 0.77]. Likewise, for whole brain white matter, there was a main effect of time [F(1,96) = 56.8, P < 0.001], but no main effect of group [F(1,96) = 1.3, P = 0.26] or interaction between time and group [F(1,96) = 0.29, P = 0.59) (Table 3).
TABLE 3.
Whole brain gray and white volume at each time point for NDC and T1DM groups and T1DM subgroups (in milliliters)
| NDC | T1DM | No hypo | Any hypo | HbA1c (%) |
|||
|---|---|---|---|---|---|---|---|
| <7.5 | 7.5–8.9 | ≥9 | |||||
| n | 25 | 75 | 49 | 19 | 15 | 44 | 16 |
| Whole brain gray volume | |||||||
| Time 1 | 820.09 (82.09) | 853.94 (74.24) | 859.51 (78.75) | 847.94 (57.77) | 873.42 (86.45) | 854.96 (63.92) | 832.89 (87.43) |
| Time 2 | 809.87 (81.75) | 844.84 (75.97) | 850.72 (81.99) | 838.81 (54.94) | 863.63 (86.31) | 849.16 (66.02) | 815.36 (87.69) |
| Whole brain white volume | |||||||
| Time 1 | 448.16 (51.11) | 466.69 (49.48) | 472.81 (52.94) | 457.32 (40.34) | 481.49 (53.02) | 462.38 (44.11) | 464.66 (60.00) |
| Time 2 | 452.18 (49.18) | 471.71 (50.29) | 451.16 (43.16) | 461.27 (40.21) | 484.39 (50.55) | 469.48 (46.40) | 465.98 (60.90) |
Data are means (SD) unless otherwise indicated. Hypo, hypoglycemia.
Voxel-wise analysis.
After correction for multiple comparisons, there were no regional differences between the groups.
Hyperglycemia
Clinical and demographic variables.
The mean number of HbA1c tests per T1DM participant during the 2 years of follow-up was 5.4 (SD 1.0; range 3–8 tests); the mean 2-year HbA1c level for all T1DM participants was 8.6% (SD 1.4). Given that only 10 participants (13%) experienced a severe hyperglycemic event (hospitalization for ketoacidosis or high blood glucose with vomiting), these data were not analyzed.
Although mean HbA1c was used for voxel-wise and whole brain correlational analyses, we also divided T1DM subjects into HbA1c subgroups so that they could be directly compared with the NDC group. HbA1c subgroups were defined as low (mean <7.5%), medium (mean 7.5–9%), and high (mean ≥9%) and were similar in age and other characteristics (Table 2). Since mean glucose levels from meter readings, percentage of readings >300 mg/dL, and SD of glucose readings correlated highly with 2-year mean HbA1c (r > 0.84, P < 0.001), these variables were not investigated further.
TABLE 2.
Demographic and clinical variables for T1DM subgroups
| No hypo | Any hypo | HbA1c (%) |
|||
|---|---|---|---|---|---|
| <7.5 | 7.5–8.9 | ≥9 | |||
| n | 49 | 19 | 15 | 44 | 16 |
| Age at Time 1 (years) | 12.8 (2.7) | 11.8 (3.1) | 13.0 (3.6) | 12.2 (2.7) | 12.9 (2.6) |
| Male/female (% male) | 31/18 (63) | 13/6 (68) | 11/4 (73) | 29/15 (66) | 8/8 (50) |
| Right handed/other (% right handed) | 43/6 (88) | 16/3 (84) | 13/2 (87) | 38/6 (86) | 14/2 (88) |
| Parent education (years) | 14.6 (2.2) | 15.6 (1.9) | 15.4 (2.3) | 15.0 (2.1) | 14.5 (1.9) |
| Age of diabetes onset (years) | 7.0 (3.6) | 6.8 (2.9) | 6.8 (3.0) | 6.7 (3.5) | 7.7 (3.4) |
| Severe hypoglycemic episodes during 2-year follow-up | 0 | 2.3 (0.6) | 0.2 (0.4) | 0.8 (2.7) | 0.5 (0.7) |
| Participants with any severe hypoglycemic episodes during 2-year follow-up | 0 (0) | 19 (100) | 3 (20) | 11 (25) | 5 (31) |
| Mean HbA1c during 2-year follow-up | 8.5 (1.4) | 8.5 (1.3) | 7.1 (0.4) | 8.2 (0.4) | 10.8 (1.2) |
| Participants with ketoacidosis during 2-year follow-up | 5 (10) | 3 (16) | 1 (6) | 6 (14) | 3 (19) |
Data are means (SD) and n (%) unless otherwise indicated. Hypo, hypoglycemia.
Whole brain analyses.
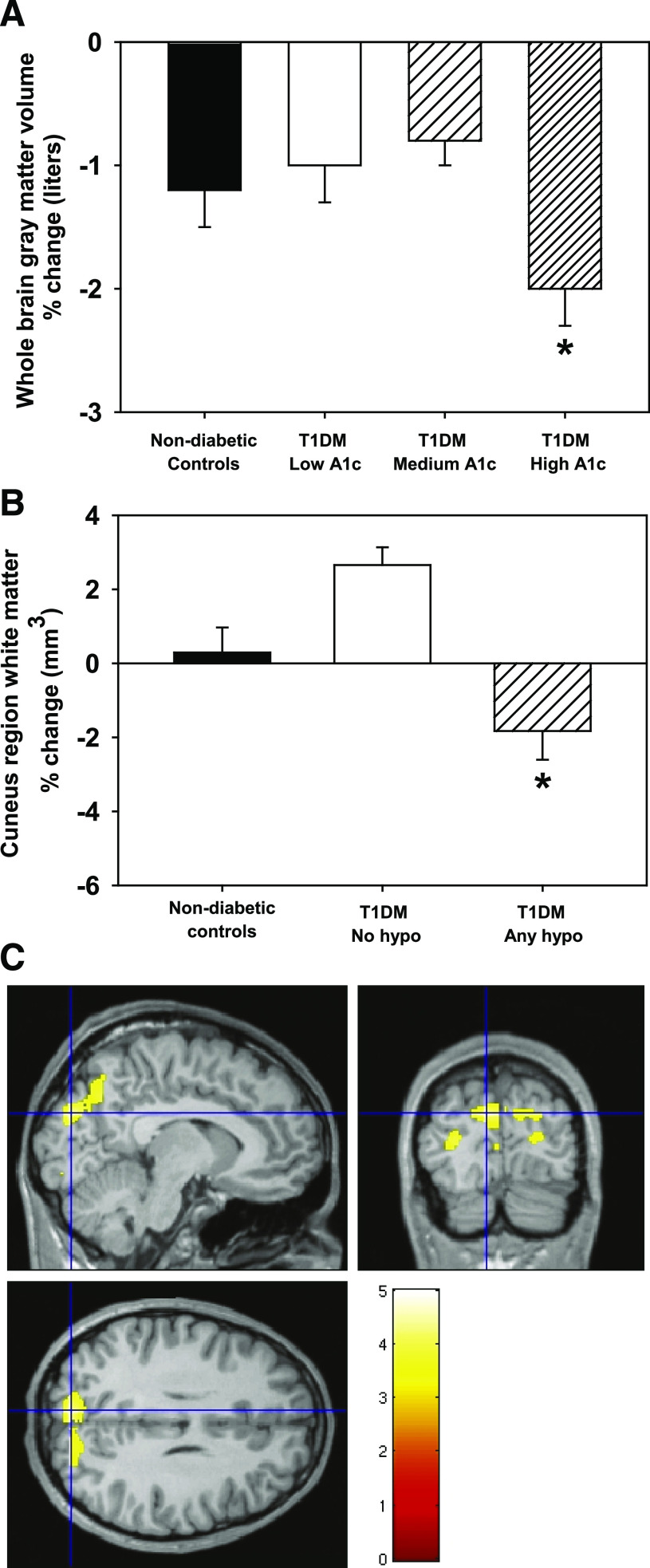
Hierarchical linear regression revealed that higher 2-year mean HbA1c was associated with greater decreases in whole brain gray matter after controlling for age, sex, and age of onset [F change(1,62) = 6.1, P = 0.017]. To compare with NDC, we performed general linear modeling analyses using HbA1c subgroups and the NDC group. This analysis revealed a main effect of time [F(1,94) = 10.2, P = 0.002], and an interaction between time and group [F(3,94) = 3.3, P = 0.025] (Table 3). Pairwise comparisons showed that the high-HbA1c group had a significantly greater percent decrease in whole brain gray matter over time than the low-HbA1c (P = 0.04) and medium-HbA1c subgroups (P = 0.002), but not compared with the NDC group (P = 0.06) (Fig. 2A). Change in whole brain white matter was not associated with mean HbA1c [F change(1,62) = 0.45, P = 0.51].
FIG. 2.
A: Mean ± SEM percent change in whole brain gray matter across hyperglycemia subgroups and NDCs. *Different from other HbA1c groups (P < 0.05) and marginally different from NDC (P = 0.06). B: Occipital/parietal white matter across severe hypoglycemia subgroups and NDCs. *Different from other groups (P < 0.05). C: Statistical image showing occipital/parietal region where T1DM with any hypoglycemia differ from T1DM with no hypoglycemia. Hypo, hypoglycemia. (A high-quality digital representation of this figure is available in the online issue.)
Voxel-wise analyses.
Voxel-wise analyses revealed no relationships between HbA1c and gray or white matter regions.
Hypoglycemia
Clinical and demographic variables.
Severe hypoglycemia: Nineteen T1DM subjects (25%) experienced one or more episodes meeting our criteria for severe hypoglycemia during the 2-year follow-up. This included 12 participants with a single episode, 4 with two episodes, and 3 with three or more episodes. Of these 19 participants, 7 experienced at least one episode with loss of consciousness and 4 experienced episodes with seizures. Analyses of the effects of severe hypoglycemia excluded 7 participants whose medical charts or interviews mentioned hypoglycemia but lacked sufficient details to categorize according to our strict definition of severe hypoglycemia. The 19 participants with severe hypoglycemic events during follow-up were compared with 49 participants with no severe hypoglycemia during follow-up. Age, sex, 2-year mean HbA1c, handedness, duration of diabetes, and age of onset of diabetes did not differ between these groups. T1DM participants with severe hypoglycemia during follow-up were more likely to have a prior history of severe hypoglycemia (χ2 = 5.2, P = 0.02).
Mild hypoglycemia:The percentage of glucose meter readings <60 mg/dL ranged from 0.1 to 10.6% (mean 4.9%, SD 2.7) and did not differ between participants with (n = 15) and without (n = 43) severe hypoglycemic episodes during follow-up.
Whole brain volume analyses.
For whole brain gray matter, repeated-measures analyses revealed a main effect of time [F(1,88) = 11.1, P = 0.001] but no main effect of severe hypoglycemia group (no hypoglycemia, any hypoglycemia, NDC; F(1,88) = 1.7, P = 0.19] or interaction between time and group [F(1,88) = 0.50, P = 0.61]. For whole brain white matter, there was also a main effect of time [F(1,88) = 55.9, P < 0.001) but no main effect of group [F(1,88) = 1.6, P = 0.21] or interaction between time and group [F(1,88) = 2.53, P = 0.09]. Rate of mild hypoglycemia did not correlate with whole brain gray or white matter change (Table 4).
TABLE 4.
Summary of whole brain and voxel-wise analyses and results by group and variable
| Significant effects of diagnosis or exposure | |
|---|---|
| T1DM vs. NDC | |
| Whole brain | |
| Gray | None |
| White | None |
| Voxel-wise | |
| Gray | None |
| White | None |
| Hyperglycemia | |
| Whole brain | |
| Gray | Higher HbA1c → greater decrease over time |
| White | None |
| Voxel-wise | |
| Gray | None |
| White | None |
| Severe hypoglycemia | |
| Whole brain | |
| Gray | None |
| White | None |
| Voxel-wise | |
| Gray | None |
| White | Any severe hypoglycemia → greater decrease in occipital/parietal region over time |
| Mild hypoglycemia | |
| Whole brain | |
| Gray | None |
| White | None |
| Voxel-wise | |
| Gray | None |
| White | None |
Voxel-wise analyses.
Severe hypoglycemia: There was no significant effect of the categorical presence/absence of severe hypoglycemia on regional gray matter. However, there was a significant effect of severe hypoglycemia on regional white matter in the occipital/parietal cortex (P = 0.02; peak coordinates −9, −86, 27; extent 1,018 voxels or 3.4 ml; near Brodmann’s areas 7, 18, and 19) (Fig. 2C). To explore how our severe hypoglycemia subgroups differed from NDC subjects in this region, the volume in this cluster was examined with repeated-measures general linear modeling analyses with group as the between-subjects variable and time as the repeated measure, covarying age and sex. White matter in this region decreased over time at a greater rate in T1DM subjects with severe hypoglycemia than in T1DM subjects with no severe hypoglycemia or NDC [univariate ANOVA, main effect of group, F(2,88) = 9.6, P < 0.001; post hoc comparisons, any hypoglycemia group different from other groups, P < 0.05] (Fig. 2B).
Mild hypoglycemia: Rate of mild hypoglycemia among all T1DM subjects with valid glucose meter data (n = 62) did not correlate with any regional gray or white matter change.
Blood glucose at the time of scans.
Blood glucose at the Time 1 scan did not correlate with whole brain gray matter (r = 0.11, P = 0.38), white matter (r = 0.02, P = 0.85), or cuneus volume at Time 1 (r = −0.10, P = 0.43) after controlling for age and sex (n = 49). Blood glucose at the Time 2 scan did not correlate with whole brain gray matter (r = −0.11, P = 0.38), white matter (r = −0.12, P = 0.33), or cuneus volume at Time 2 (r = −0.13, P = 0.32) after controlling for age and sex (n = 54).
DISCUSSION
This study represents an important step toward understanding how glycemic extremes associated with diabetes affect the brain during development. Our unique longitudinal neuroimaging design revealed that, in general, youth with diabetes do not differ significantly from control subjects in brain development over a 2-year time period. However, within the diabetes group, we found qualitatively different effects of hyperglycemia and severe hypoglycemia on brain development (Table 4). These effects could be detected after 2 years of observation, and for some of the results these effects were also significantly different from those of NDC subjects, suggesting a subtle deviation from normal developmental patterns.
In previous cross-sectional neuroimaging studies (4–6), severe hypoglycemia and hyperglycemia have been shown to be associated with qualitatively different brain volume patterns. This finding would be expected if glycemic extremes caused these differences, as each are thought to have different neurobiological consequences. An alternative interpretation, which we cannot rule out based on cross-sectional studies, might be that these differences precede altered glycemic exposure and are merely markers of individual cognitive or physiologic differences that influence whether someone is more or less likely to have poor glycemic control. The strength of the current longitudinal study design is that it examines change from a baseline in response to prospectively ascertained glycemic control. The results shown here bolster the hypothesis that extreme glycemic exposure plays a causal role in producing structural brain changes during development. This interpretation is supported by the work of Northam and colleagues (4,34,35), who find that newly diagnosed children with T1DM have cognitive function similar to that of healthy control subjects but that selective cognitive impairments begin to develop with time and exposure to hyperglycemia or severe hypoglycemia.
Given that longitudinal neuroimaging studies of T1DM subjects have not been previously published, our results here can only be compared with the existing cross-sectional studies, which differ in the time span assessed (e.g., lifetime exposure vs. our 2-year follow-up) and could be confounded by individual differences at baseline. For example, our retrospective study (5) revealed effects of lifetime exposure to hyperglycemia (occipital/parietal gray and matter volume reductions) and severe hypoglycemia (lateral temporal-parietal-occipital gray matter volume reduction) on the brain, but the regions and effects were different from what we report here in our prospective design. While consistency between analyses might have been easier to interpret, the discrepancy is not unexpected given the many differences between the two study designs. For instance, the independent variables (exposure from diagnosis to Time 1 vs. exposure over a 2-year period) and dependent variables (brain volume at Time 1 vs. change in brain volume over a 2-year period) are qualitatively different. The age-range over which glycemic exposure variables were derived were also different (retrospective: ages 1–16 years; prospective ages 7–18 years), which could be important given the possibility of differential effects on the brain of exposure to glycemic extremes depending on the developmental stage at which they were experienced (23,24,36). Thus, these analyses are complementary and necessary to obtain the most comprehensive understanding of interactions between brain development and glycemic exposure.
We found that hyperglycemia exposure was associated with an accentuated decrease in whole brain gray matter over a 2-year time period in youth with T1DM. This finding is consistent with studies in T1DM adults that found indications of decreased total brain volume compared with control subjects (37–39). These findings also suggest that hyperglycemia may have a broad impact on gray matter across the brain, particularly during development. Understanding the mechanisms behind these findings will require further investigation.
We found that exposure to severe hypoglycemia over a 2-year period was associated with greater decreases in regional white matter volume in the precuneus/cuneus region compared with those individuals who did not experience severe hypoglycemia. Interestingly, occipital white matter damage and more general white matter abnormalities have been noted following neonatal hypoglycemia (40–42). Impaired white matter microstructural integrity in occipital cortex has also been reported in adults with diabetes (43), although these changes were not linked to hypoglycemia. Interestingly, slice culture studies have reported that hypoglycemia can impair myelinated fiber formation and trigger apoptotic cell death in oligodendrocyte precursor cells (44), providing a possible mechanism for these brain imaging findings. Notably, our previous work (5) found reduced white matter in the precuneus/cuneus region associated with hyperglycemia, not hypoglycemia, exposure. Overall, these results highlight the potential vulnerability of occipital and parietal white matter in T1DM, but also suggest that the mechanism of the vulnerability (hypoglycemia vs. hyperglycemia vs. T1DM) may be more difficult to discern. This region of the brain is associated with higher-order visual function as well as other more complex cognitive processes (e.g., monitoring internal thoughts, memory retrieval) (45,46). Memory and visuospatial function has been found to be affected by diabetes and by exposure to extreme glycemic states (47,48); however, data supporting a direct link between reduced white matter volume in this region and alterations in cognitive function in diabetes have not been reported.
This report has several important strengths. We present analyses from a novel, longitudinal study using sophisticated image registration and conservative statistics. The high-parameter registration process enables detection of subtle changes within subjects and valid comparisons between subjects, accounting for minor variations in anatomy. Modulation of mutually aligned time points reduced the possibility that observed differences were caused by disparities in registration of each time point. This feature is especially important in a population of children and adolescents, whose brains may be changing substantially between measurements. Our methods also allowed us to more confidently ascribe change in brain structures to exposure of severe glycemic states than has been possible previously.
However, there are also limitations of our study. The fact that our control sample was relatively small may limit our power to detect more subtle deviations in the T1DM group from normal development. Further, despite our careful prospective ascertainment of severe hypoglycemic episodes, there may have been episodes of severe hypoglycemia, including nocturnal hypoglycemia, that were unknown to the families or were not reported to us. Exposure to severe hypoglycemia was very limited during our 2-year follow-up, also restricting our power. Glucose meter readings, while useful, are not comprehensive and may be biased by an individual’s treatment regimen or ascertainment related to the subject’s blood glucose monitoring practices. Finally, we cannot rule out the possibility that the patterns of change that we observe could be different with very early exposure to glycemic extremes (e.g., before age 7 years).
These findings highlight brain regions that may be vulnerable to glycemic extremes during development. Hyperglycemia accentuated the normal whole brain gray matter reductions during this time, possibly through accelerated pruning or direct cell damage. Severe hypoglycemia reversed the normal increase in white matter volume in the occipital/parietal region, possibly by interfering with normal myelination or direct damage to white matter. Mechanisms behind these vulnerabilities need to be better understood and may require work in animal models to carefully control exposure-related variables. For example, it is not known whether the changes we observed are a direct result of damage or are compensatory in some way. Finally, we need to understand whether there are any functional consequences of this altered neurodevelopment at this stage of exposure. Examination of how exposure to glycemic extremes and regional brain volume changes relate to cognitive functioning over this time period will be important in shedding light on these issues.
ACKNOWLEDGMENTS
This work was supported by the Dana Foundation and the National Institutes of Health (grants DK064832, DK064832-06, DK064832-06S1, DK064832-06S2, and UL1 RR024992).
No potential conflicts of interest relevant to this article were reported.
D.C.P. researched data, contributed to discussions, and wrote, reviewed, and edited the manuscript. J.M.K. researched data, contributed to discussions, and reviewed and edited the manuscript. P.M.W. researched data and reviewed and edited the manuscript. H.M.L. researched data, contributed to discussions, and reviewed and edited the manuscript. K.J.B. contributed to discussions and reviewed and edited the manuscript. N.H.W. contributed to discussions and reviewed and edited the manuscript. T.H. researched data, contributed to discussions, and wrote, reviewed, and edited the manuscript.
The authors thank the participants and their families for their support.
Footnotes
Clinical trial reg. no. NCT00879203, clinicaltrials.gov.
REFERENCES
- 1.Olsen BS, Sjølie A, Hougaard P, et al. A 6-year nationwide cohort study of glycaemic control in young people with type 1 diabetes. Risk markers for the development of retinopathy, nephropathy and neuropathy. J Diabetes Complications 2000;14:295–300 [DOI] [PubMed] [Google Scholar]
- 2.Chkhartishvili D, Khachapuridze N, Natriashvili G, Geladze N, Kapanadze N. Nerve conduction abnormalities in children with type I diabetes. Annals of Biomedical Research and Education 2002;2:331–334 [Google Scholar]
- 3.Riihimaa PH, Suominen K, Tolonen U, Jäntti V, Knip M, Tapanainen P. Peripheral nerve function is increasingly impaired during puberty in adolescents with type 1 diabetes. Diabetes Care 2001;24:1087–1092 [DOI] [PubMed] [Google Scholar]
- 4.Northam EA, Rankins D, Lin A, et al. Central nervous system function in youth with type 1 diabetes 12 years after disease onset. Diabetes Care 2009;32:445–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perantie DC, Wu J, Koller JM, et al. Regional brain volume differences associated with hyperglycemia and severe hypoglycemia in youth with type 1 diabetes. Diabetes Care 2007;30:2331–2337 [DOI] [PubMed] [Google Scholar]
- 6.Ho MS, Weller NJ, Ives FJ, et al. Prevalence of structural central nervous system abnormalities in early-onset type 1 diabetes mellitus. J Pediatr 2008;153:385–390 [DOI] [PubMed] [Google Scholar]
- 7.Di Marzio D, Mohn A, Mokini ZH, Giannini C, Chiarelli F. Macroangiopathy in adults and children with diabetes: from molecular mechanisms to vascular damage (part 1). Horm Metab Res 2006;38:691–705 [DOI] [PubMed] [Google Scholar]
- 8.Folli F, Guzzi V, Perego L, et al. Proteomics reveals novel oxidative and glycolytic mechanisms in type 1 diabetic patients’ skin which are normalized by kidney-pancreas transplantation. PLoS ONE 2010;5:e9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rask-Madsen C, King GL. Mechanisms of disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab 2007;3:46–56 [DOI] [PubMed] [Google Scholar]
- 10.Wessels AM, Simsek S, Remijnse PL, et al. Voxel-based morphometry demonstrates reduced grey matter density on brain MRI in patients with diabetic retinopathy. Diabetologia 2006;49:2474–2480 [DOI] [PubMed]
- 11.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001;414:813–820 [DOI] [PubMed] [Google Scholar]
- 12.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005;54:1615–1625 [DOI] [PubMed] [Google Scholar]
- 13.Vlassara H, Brownlee M, Cerami A. Excessive nonenzymatic glycosylation of peripheral and central nervous system myelin components in diabetic rats. Diabetes 1983;32:670–674 [DOI] [PubMed] [Google Scholar]
- 14.Auer RN, Siesjö BK. Biological differences between ischemia, hypoglycemia, and epilepsy. Ann Neurol 1988;24:699–707 [DOI] [PubMed] [Google Scholar]
- 15.Auer RN, Olsson Y, Siesjö BK. Hypoglycemic brain injury in the rat. Correlation of density of brain damage with the EEG isoelectric time: a quantitative study. Diabetes 1984;33:1090–1098 [DOI] [PubMed] [Google Scholar]
- 16.Auer RN, Hugh J, Cosgrove E, Curry B. Neuropathologic findings in three cases of profound hypoglycemia. Clin Neuropathol 1989;8:63–68 [PubMed] [Google Scholar]
- 17.Fujioka M, Okuchi K, Hiramatsu KI, Sakaki T, Sakaguchi S, Ishii Y. Specific changes in human brain after hypoglycemic injury. Stroke 1997;28:584–587 [DOI] [PubMed] [Google Scholar]
- 18.Kalimo H, Olsson Y. Effects of severe hypoglycemia on the human brain. Neuropathological case reports. Acta Neurol Scand 1980;62:345–356 [DOI] [PubMed] [Google Scholar]
- 19.Sieber FE, Traystman RJ. Special issues: glucose and the brain. Crit Care Med 1992;20:104–114 [PubMed] [Google Scholar]
- 20.McCall AL. The impact of diabetes on the CNS. Diabetes 1992;41:557–570 [DOI] [PubMed] [Google Scholar]
- 21.Wieloch T. Hypoglycemia-induced neuronal damage prevented by an N-methyl-D-aspartate antagonist. Science 1985;230:681–683 [DOI] [PubMed] [Google Scholar]
- 22.Ouyang YB, He QP, Li PA, Janelidze S, Wang GX, Siesjö BK. Is neuronal injury caused by hypoglycemic coma of the necrotic or apoptotic type? Neurochem Res 2000;25:661–667 [DOI] [PubMed] [Google Scholar]
- 23.Yamada KA, Rensing N, Izumi Y, et al. Repetitive hypoglycemia in young rats impairs hippocampal long-term potentiation. Pediatr Res 2004;55:372–379 [DOI] [PubMed] [Google Scholar]
- 24.Ennis K, Tran PV, Seaquist ER, Rao R. Postnatal age influences hypoglycemia-induced neuronal injury in the rat brain. Brain Res 2008;1224:119–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson PM, Sowell ER, Gogtay N, et al. Structural MRI and brain development. Int Rev Neurobiol 2005;67:285–323 [DOI] [PubMed] [Google Scholar]
- 26.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971;9:97–113 [DOI] [PubMed] [Google Scholar]
- 27.The DCCT Research Group . Epidemiology of severe hypoglycemia in the Diabetes Control and Complications Trial. Am J Med 1991;90:450–459 [PubMed] [Google Scholar]
- 28.Black KJ, Snyder AZ, Koller JM, Gado MH, Perlmutter JS. Template images for nonhuman primate neuroimaging: 1. Baboon. Neuroimage 2001;14:736–743 [DOI] [PubMed] [Google Scholar]
- 29.Kipps CM, Duggins AJ, Mahant N, Gomes L, Ashburner J, McCusker EA. Progression of structural neuropathology in preclinical Huntington’s disease: a tensor based morphometry study. J Neurol Neurosurg Psychiatry 2005;76:650–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage 2007;38:95–113 [DOI] [PubMed] [Google Scholar]
- 31.Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage 2000;11:805–821 [DOI] [PubMed] [Google Scholar]
- 32.Worsley KJ, Liao CH, Aston J, et al. A general statistical analysis for fMRI data. Neuroimage 2002;15:1–15 [DOI] [PubMed] [Google Scholar]
- 33.Moorhead TW, Job DE, Spencer MD, Whalley HC, Johnstone EC, Lawrie SM. Empirical comparison of maximal voxel and non-isotropic adjusted cluster extent results in a voxel-based morphometry study of comorbid learning disability with schizophrenia. Neuroimage 2005;28:544–552 [DOI] [PubMed] [Google Scholar]
- 34.Northam EA, Anderson PJ, Werther GA, Warne GL, Adler RG, Andrewes D. Neuropsychological complications of IDDM in children 2 years after disease onset. Diabetes Care 1998;21:379–384 [DOI] [PubMed] [Google Scholar]
- 35.Northam EA, Anderson PJ, Jacobs R, Hughes M, Warne GL, Werther GA. Neuropsychological profiles of children with type 1 diabetes 6 years after disease onset. Diabetes Care 2001;24:1541–1546 [DOI] [PubMed] [Google Scholar]
- 36.Hershey T, Perantie DC, Warren SL, Zimmerman EC, Sadler M, White NH. Frequency and timing of severe hypoglycemia affects spatial memory in children with type 1 diabetes. Diabetes Care 2005;28:2372–2377 [DOI] [PubMed] [Google Scholar]
- 37.Ferguson SC, Blane A, Perros P, et al. Cognitive ability and brain structure in type 1 diabetes: relation to microangiopathy and preceding severe hypoglycemia. Diabetes 2003;52:149–156 [DOI] [PubMed] [Google Scholar]
- 38.Ferguson SC, Blane A, Wardlaw J, et al. Influence of an early-onset age of type 1 diabetes on cerebral structure and cognitive function. Diabetes Care 2005;28:1431–1437 [DOI] [PubMed] [Google Scholar]
- 39.Lobnig BM, Krömeke O, Optenhostert-Porst C, Wolf OT. Hippocampal volume and cognitive performance in long-standing type 1 diabetic patients without macrovascular complications. Diabet Med 2006;23:32–39 [DOI] [PubMed] [Google Scholar]
- 40.Karimzadeh P, Tabarestani S, Ghofrani M. Hypoglycemia-occipital syndrome: a specific neurologic syndrome following neonatal hypoglycemia? J Child Neurol 2010;26:152–159 [DOI] [PubMed]
- 41.Burns CM, Rutherford MA, Boardman JP, Cowan FM. Patterns of cerebral injury and neurodevelopmental outcomes after symptomatic neonatal hypoglycemia. Pediatrics 2008;122:65–74 [DOI] [PubMed] [Google Scholar]
- 42.Barkovich AJ, Ali FA, Rowley HA, Bass N. Imaging patterns of neonatal hypoglycemia. AJNR Am J Neuroradiol 1998;19:523–528 [PMC free article] [PubMed] [Google Scholar]
- 43.Kodl CT, Franc DT, Rao JP, et al. Diffusion tensor imaging identifies deficits in white matter microstructure in subjects with type 1 diabetes that correlate with reduced neurocognitive function. Diabetes 2008;57:3083–3089 [DOI] [PMC free article] [PubMed]
- 44.Yan H, Rivkees SA. Hypoglycemia influences oligodendrocyte development and myelin formation. Neuroreport 2006;17:55–59 [DOI] [PubMed] [Google Scholar]
- 45.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 2006;129:564–583 [DOI] [PubMed] [Google Scholar]
- 46.Vincent JL, Snyder AZ, Fox MD, et al. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol 2006;96:3517–3531 [DOI] [PubMed] [Google Scholar]
- 47.Blasetti A, Chiuri RM, Tocco AM, et al. The effect of recurrent severe hypoglycemia on cognitive performance in children with type 1 diabetes: a meta-analysis. J Child Neurol. 13 May 2011 [Epub ahead of print] [DOI] [PubMed]
- 48.Naguib JM, Kulinskaya E, Lomax CL, Garralda ME. Neuro-cognitive performance in children with type 1 diabetes: a meta-analysis. J Pediatr Psychol 2008;34:271–282 [DOI] [PubMed]
- 49.Ashburner J, Friston KJ. Unified segmentation. Neuroimage 2005;26:839–851 [DOI] [PubMed] [Google Scholar]