Abstract
OBJECTIVE
Recently we have shown that calpain-1 activation contributes to cardiomyocyte apoptosis induced by hyperglycemia. This study was undertaken to investigate whether targeted disruption of calpain would reduce myocardial hypertrophy and fibrosis in mouse models of type 1 diabetes.
RESEARCH DESIGN AND METHODS
Diabetes in mice was induced by injection of streptozotocin (STZ), and OVE26 mice were also used as a type 1 diabetic model. The function of calpain was genetically manipulated by cardiomyocyte-specific knockout Capn4 in mice and the use of calpastatin transgenic mice. Myocardial hypertrophy and fibrosis were investigated 2 and 5 months after STZ injection or in OVE26 diabetic mice at the age of 5 months. Cultured isolated adult mouse cardiac fibroblast cells were also investigated under high glucose conditions.
RESULTS
Calpain activity, cardiomyocyte cross-sectional areas, and myocardial collagen deposition were significantly increased in both STZ-induced and OVE26 diabetic hearts, and these were accompanied by elevated expression of hypertrophic and fibrotic collagen genes. Deficiency of Capn4 or overexpression of calpastatin reduced myocardial hypertrophy and fibrosis in both diabetic models, leading to the improvement of myocardial function. These effects were associated with a normalization of the nuclear factor of activated T-cell nuclear factor-κB and matrix metalloproteinase (MMP) activities in diabetic hearts. In cultured cardiac fibroblasts, high glucose–induced proliferation and MMP activities were prevented by calpain inhibition.
CONCLUSIONS
Myocardial hypertrophy and fibrosis in diabetic mice are attenuated by reduction of calpain function. Thus targeted inhibition of calpain represents a potential novel therapeutic strategy for reversing diabetic cardiomyopathy.
Cardiovascular complications are the leading cause of diabetes-related morbidity and mortality (1). Diabetes not only exacerbates cardiac injury after myocardial infarction or ischemia/reperfusion (2–4) but also directly damages the heart, leading to a unique diabetic cardiomyopathy independent of coronary artery disease, hypertension, and hyperlipidemia (1,5). Diabetes is characterized by hyperglycemia. The correlation between hyperglycemia and cardiomyopathic changes such as cardiac hypertrophy, interstitial fibrosis, and apoptosis of cardiomyocytes is long established (6). However, the underlying cellular mechanisms responsible for these abnormalities remain partially understood.
Calpains are Ca2+-dependent intracellular proteases. Two of the best characterized calpain species are μ-calpain (calpain-1) and m-calpain (calpain-2). These isoforms are heterodimers consisting of ∼80 kDa catalytic subunits (encoded by the Capn1 and Capn2 genes, respectively) and a ∼30 kDa regulatory subunit (encoded by Capn4) (7–9). The regulatory subunit is essential for μ- and m-calpain stability and catalytic activity. Knockout of Capn4 in mice abolished calpain-1 and calpain-2 activity, resulting in embryonic lethality (10). Calpain-1 and calpain-2 activities are tightly controlled by the endogenous inhibitor calpastatin. Calpastatin appears to be specific for these two calpain isoforms but does not inhibit any other protease (7). Overexpression of calpastatin has been successfully used to block calpain activation both in vitro and in vivo (11–14). Although its precise physiological function is uncertain, calpain plays a demonstrated role in cardiovascular disease (14,15).
Studies have shown promising improvements in cardiac function after inhibition of calpain activity whether in infarction or ischemia/reperfusion models (14–17). We have recently demonstrated that calpain activity increases in high glucose–treated cardiomyocytes and in short-term streptozotocin (STZ)-induced diabetes (14). Pharmacological inhibition of calpain reduced apoptosis in both treatments. Furthermore, research has uncovered vital roles for calpain in regulating the activity of nuclear factor of activated T cells (NFAT) and nuclear factor-κB (NF-κB), two transcription factors frequently implicated in the promotion of hypertrophy and fibrosis (18–21). Therefore, we hypothesized that inhibition of calpain may have a protective effect on the myocardium in diabetes. To our knowledge, no study has previously examined the role of calpain in cardiac hypertrophy and fibrosis in diabetic conditions. Here we show that cardiomyocyte-specific calpain deletion or calpastatin overexpression reduces cardiomyopathy in mouse models of type 1 diabetes.
RESEARCH DESIGN AND METHODS
Animals.
This investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85–23). All experimental procedures were approved by the Animal Use Subcommittee at the University of Western Ontario, Canada. Breeding pairs of C57BL/6 mice and FVB(Cg)-Tg(Ins2-CALM)26OveTg(Cryaa-Tag)1Ove/PneJ transgenic mice (OVE26), a mouse model of type 1 diabetes, were purchased from the Jackson Laboratory. Mice bearing the targeted Capn4PZ allele containing loxP sites flanking essential coding exons and mice with cardiomyocyte-specific expression of Cre recombinase (Tg-Cre) under the control of α-myosin heavy chain (α-MHC) were generated as described previously (22,23). Transgenic mice overexpressing calpastatin (Tg-CAST) driven by cytomegalovirus promoter were provided by Dr. Laurent Baud (the Institut National de la Santé et de la Recherche Médicale, Paris, France) through the European Mouse Mutant Archive (11).
Experimental protocol.
Diabetes was induced in adult male mice (2 months old) by consecutive peritoneal injection of streptozotocin (STZ; 50 mg/kg per day) for 5 days. Seventy-two hours after the last injection of STZ, whole blood was obtained from the mouse tail-vein and random glucose levels were measured using the OneTouch Ultra 2 blood glucose monitoring system (LifeScan, Milpitas, CA). Mice were considered diabetic and used for the study only if they had hyperglycemia (≥15 mmol/L) 72 h after STZ injection. Citrate buffer–treated mice were used as nondiabetic controls (blood glucose <12 mmol/L). After the induction of diabetes (2 or 5 months), mice (n = 8–12 in each group) were killed for the following experiments.
Isolation of cardiac fibroblasts.
Cardiac fibroblasts were isolated as described previously (24) with some modifications. In brief, mouse ventricular tissues were excised, rinsed with cold PBS, and then placed in isolation buffer. Individual ventricles were minced and digested for 90 min in isolation buffer containing collagenase type 2 and dispase-II. Fibroblasts were collected by centrifugation, resuspended in Dulbecco’s modified Eagle’s medium containing 10% FBS, and seeded onto tissue culture dishes. All fibroblasts were used from the first two to four generations.
Histological analyses.
The collagen contents and cardiomyocyte cross-sectional area were assessed as described in our recent report (25).
Calpain activity.
Calpain activities were determined by using a fluorescence substrate N-succinyl-LLVY-AMC (Cedarlane Laboratories) (12–14) and by casein zymography as described previously (26).
NFAT and NF-κB.
NFAT and NF-κB activities were determined by using ELISA-based transcription factor Filter Plate Assay kits according to the manufacturer’s instructions (Signosis).
Matrix metalloproteinases activity.
Global matrix metalloproteinases (MMP), MMP-2, and MMP-9 activities were assessed by using commercial kits (AnaSpec), respectively.
Assessment of cell proliferation.
Cell growth was evaluated using the 3-(4, 5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay and a DNA bromodeoxyuridine (BrdU) cell proliferation assay (Millipore) according to the manufacturer’s instructions.
Real-time RT-PCR.
Real-time RT-PCR was performed to analyze mRNA expression for Capn4, β-myosin heavy chain (β-MHC), atrial natriuretic peptide (ANP), collagen I and III, and GAPDH as described in our recent report (25).
Assessment of cardiac function.
Maximal and minimal first derivatives of force (+dF/dtmax and −dF/dtmin) as the rate of contraction and relaxation were assessed as described previously (12,25).
In vivo left ventricular pressure–volume measurements.
Mice were anesthetized with ketamine/xylazine cocktail and ventilated. The chest was opened, and a Scisense mouse PV catheter (FTE-1212B-4518, 1.2F) was directly inserted into the left ventricle (LV) via the apex to measure LV pressures and volumes as we described recently (27,28). Data were recorded by the ADVantage PV System (Model FY997B, London, ON, Canada). All hemodynamic parameters were analyzed by a LabScribe2 Data Recording Software (CB Sciences, Dover, NH) according to the manufacturer’s instructions.
Statistical analysis.
All data were presented as mean ± SD. ANOVA followed by Newman-Keuls test was performed for multigroup comparisons. A value of P < 0.05 was considered statistically significant.
RESULTS
Calpain activities are increased in diabetic hearts.
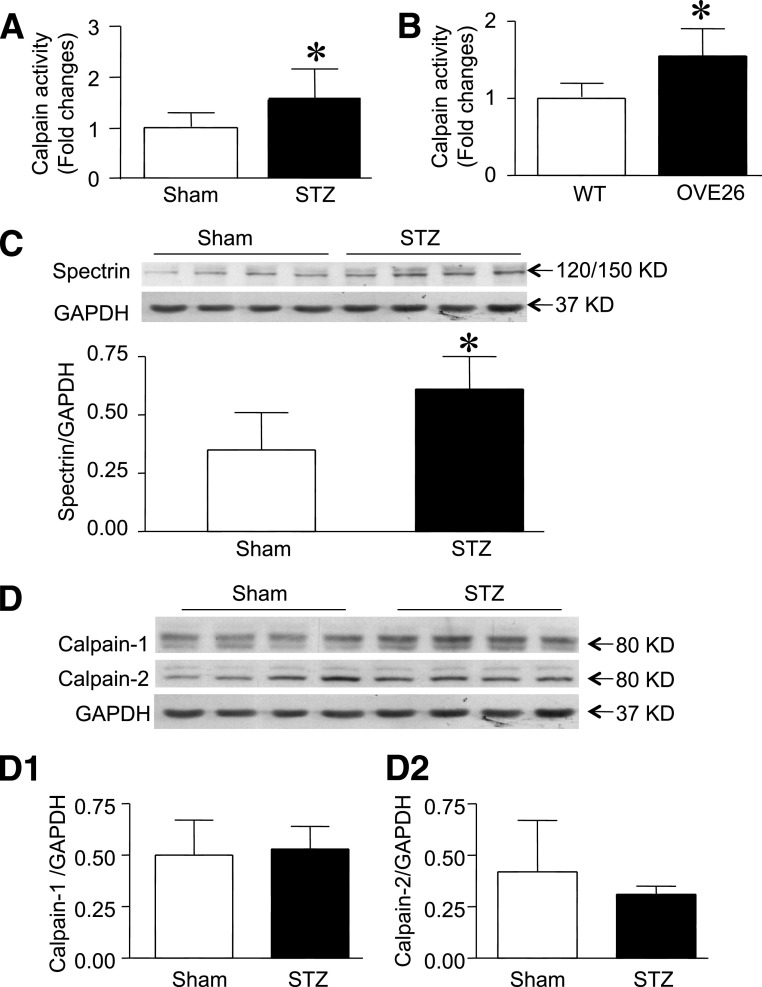
To establish calpain as a protease of interest in understanding the mechanisms behind cardiomyopathy in the diabetic heart, we measured calpain activity in heart tissues from STZ-injected and OVE26 diabetic mice. Consistent with our recent report in short-term STZ-induced diabetes (14), both STZ and OVE26 hearts showed a significant increase in calpain activity over nondiabetic hearts (Fig. 1A and B). The activation of calpain was further confirmed by upregulation of cleaved α-spectrin in diabetic hearts (Fig. 1C). The upregulation of calpain activity was not as a result of alterations in the ratio of calpain to calpastatin expression because the protein and mRNA levels of calpain-1 and calpain-2 as well as calpastatin were not significantly altered in diabetic hearts (Fig. 1D and Supplementary Fig. 1). These results confirm the association between increased calpain activity and diabetes.
FIG. 1.
Calpain activity and protein expression in STZ-injected and OVE26 mice. Enzymatic calpain activity was measured in sham and STZ-treated hearts (A) or wild-type (WT) and OVE26 hearts (B). C: The cleavage of α-spectrin (120/150 KD) was determined in sham and STZ-injected hearts by Western blot analysis. Top: A representative Western blot for cleaved fragments of α-spectrin from four of eight different hearts in each group; bottom: quantification of cleaved fragments of α-spectrin relative to GAPDH. D: Representative Western blot for calpain-1, calpain-2, and GAPDH proteins from four of eight hearts in each group (sham and STZ-induced hearts). D1 and D2 are the quantification of calpain-1 and calpain-2 protein relative to GAPDH. Data are mean ± SD; n = 8. KD, kDa. *P < 0.05 vs. sham or WT.
Generation of cardiac-specific Capn4 knockout mice.
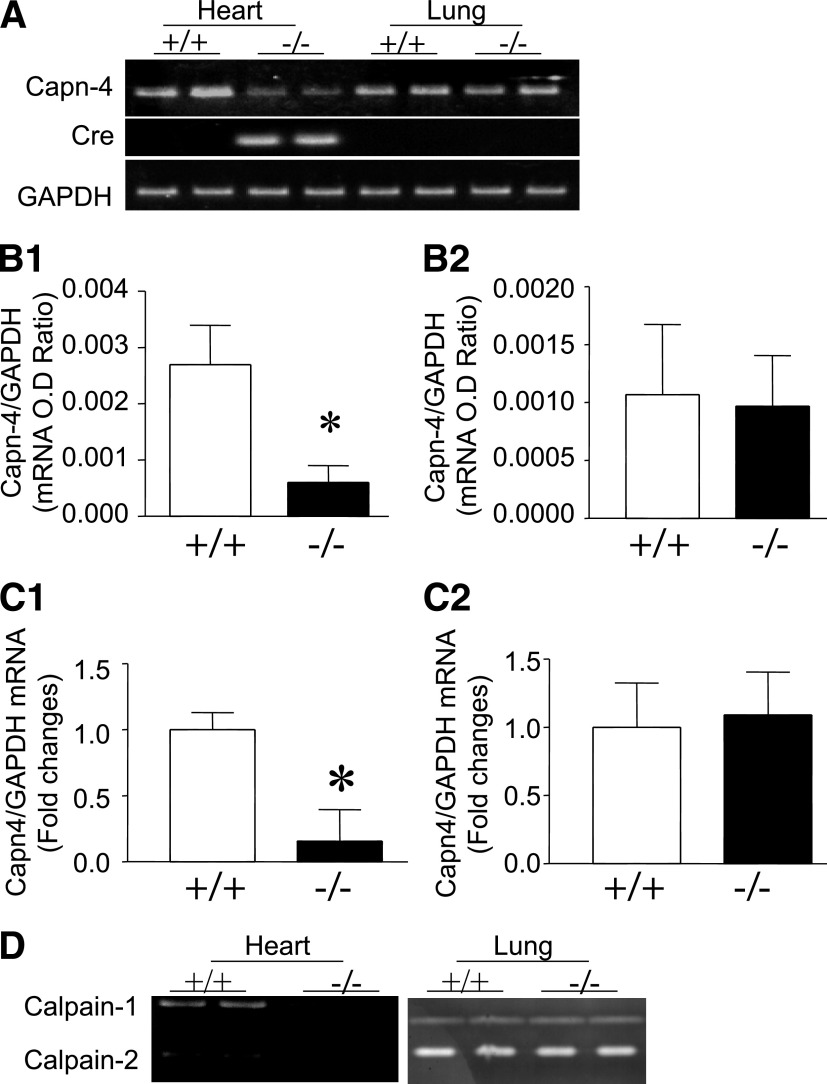
To investigate the role of calpain, we generated mice with cardiomyocyte-specific disruption of calpain by crossing floxed Capn4PZ mice with transgenic mice overexpressing Cre under the control of the α-MHC promoter (Tg-Cre). All of the mice used in this study, including controls, were littermates of the same generation. PCR analysis of tail DNA confirmed untargeted (Capn4+/+), either homozygous (Capn4PZ/PZ) or heterozygous (Capn4+/PZ) for the floxed Capn4 gene, and the Cre transgene (Supplementary Fig. 2A). Mice homozygous or heterozygous for the floxed Capn4 gene and positive for the α-MHC-Cre transgene were identified as potential homozygous (Capn4−/−) or heterozygous knockouts (Capn4+/−), respectively. To confirm decreased Capn4 expression in the heart, Capn4 mRNA was analyzed in Capn4+/+ and Capn4−/− mice using primers specifically amplifying the deleted region of Capn4 mRNA. Myocardial Capn4 mRNA was reduced by 78% in Capn4−/− mice, as compared with Capn4+/+ mice (Fig. 2A and B). In contrast, Capn4 mRNA levels in lung tissues were similar between Capn4−/− and Capn4+/+ mice (Fig. 2A and B), supporting cardiac-specific Capn4 knockout. To further confirm that the deletion of Capn4 was restricted in cardiomyocytes, the mRNA levels of Capn4 were significantly decreased in isolated cardiomyocytes of Capn4−/− mice, whereas their levels were comparable between Capn4−/− and Capn4+/+ fibroblasts (Fig. 2C). Because the Capn4-encoded regulatory subunit is required for the stability and proteolytic activities of calpain-1 and calpain-2 catalytic subunits (10), Capn4 deletion results in the disruption of calpain-1 and calpain-2 activities. Indeed, casein zymography analysis of myocardial tissues showed a robust reduction of both calpain-1 and calpain-2 activities in Capn4−/− compared with Capn4+/+ mice (Fig. 2D), confirming the functional knockout of Capn4. Similar to Capn4 mRNA expression, both calpain-1 and calpain-2 activities were not decreased in Capn4−/− lung tissues (Fig. 2D).
FIG. 2.
Characterization of mice with cardiac-specific Capn4 knockout. A: Representative RT-PCR for Cre and Capn4 mRNA from two of six mice in each group. Cre mRNA was detected in Capn4−/− hearts (lanes 3 and 4). Levels of Capn4 mRNA were significantly reduced in Capn4−/− hearts. In contrast, the Capn4 mRNA was not decreased in Capn4−/− lungs. B: Capn4 mRNA was quantified by real-time RT-PCR in Capn4+/+ and Capn4−/− hearts (B1) and lung tissues (B2). C1 and C2 are the quantification of Capn4 mRNA in isolated cardiomyocytes (C1) and cardiac fibroblasts (C2) from Capn4+/+ and Capn4−/− mice. D: Representative casein zymography for calpain-1 and calpain-2 activities from two of six hearts and lungs, respectively, showing downregulation of calpain-1 and calpain-2 activities in Capn4−/− hearts but not in lungs. Data are mean ± SD; n = 5–9. *P < 0.05 vs. +/+. OD, optical density.
Capn4−/− mice grew normally into adulthood with no obvious health problems. In nondiabetic mice, there were no differences in heart weight, body weight, and in Capn4+/+ or Capn4−/− mice (Supplementary Table 1). Glucose tolerance test showed no difference between Capn4+/+ and Capn4−/− (Supplementary Fig. 2B). Histological analysis showed no pathological changes in Capn4−/− hearts (Supplementary Fig. 2C). These observations suggest that cardiomyocyte-specific deletion of Capn4 is not deleterious to the heart and rules out potential adverse effects of Cre expression in the hearts of Capn4−/− mice.
Disruption of calpain reduces myocardial hypertrophy in mouse models of type 1 diabetes.
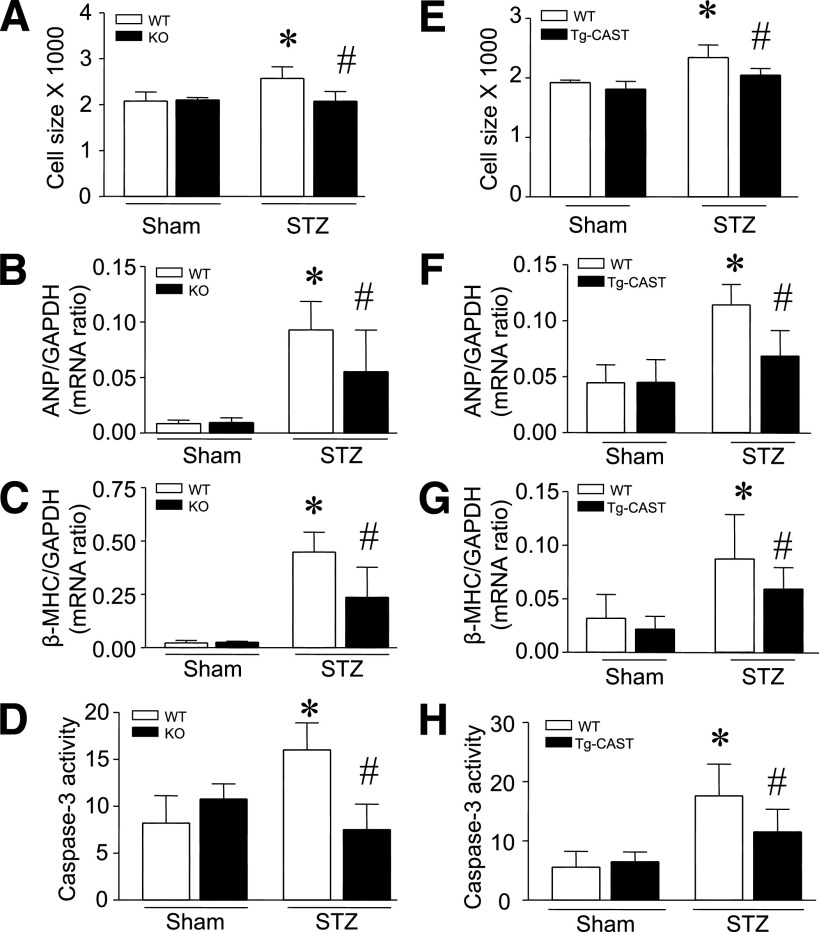
Capn4−/− and Capn4+/+ mice were rendered diabetic with STZ. Two months after STZ injection, mice exhibited hyperglycemia, hyperphagia, polydipsia, and decreased body weight versus vehicle-treated animals. There were no evident differences in body weight, heart weight, and blood glucose concentration between Capn4−/− and Capn4+/+ diabetic mice (Supplementary Table 1). Consistent with our recent report (25), histological analysis of cardiomyocyte cross-sectional areas in wild-type mice revealed increased cardiomyocyte size in diabetic versus nondiabetic hearts, indicative of cardiomyocyte hypertrophy. However, cardiomyocyte sizes were not increased in diabetic Capn4−/− hearts (Fig. 3A). Furthermore, gene expression measurements of diabetic hearts showed upregulation of both ANP and β-MHC mRNA (Fig. 3B and C), providing further evidence of a hypertrophic phenotype. Although both genes were also upregulated in Capn4−/− diabetic hearts, those increases were significantly reduced relative to wild-type mice (Fig. 3B and C). Interestingly, there were no differences in cardiomyocyte size and hypertrophic gene expression when comparing Capn4−/− and Capn4+/+ nondiabetic hearts (Fig. 3B and C). These results demonstrated that Capn4 knockout prevents myocardial hypertrophy in a mouse model of STZ-induced type 1 diabetes. Deletion of Capn4 also decreased myocardial caspase-3 activity (Fig. 3D) and cytoplasmic histone-associated DNA fragments (Supplementary Fig. 3), suggesting a reduction of apoptosis, which is consistent with our recent report (14). Similarly, 5 months after STZ injection, cardiomyocyte size and hypertrophic gene expression were reduced in Capn4−/− compared with Capn4+/+ hearts (Supplementary Fig. 4A and B), suggesting that the protective effects of Capn4 deletion may be sustained in diabetic cardiomyopathy.
FIG. 3.
Assessment of cardiac hypertrophy in diabetic Capn4−/− (knockout [KO]) and Tg-CAST mice. Cardiac hypertrophy was determined by measuring cardiomyocyte cross-sectional areas and hypertrophic gene expression (ANP and β-MHC). A–D: Deficiency of Capn4 reduced cardiomyocyte cross-sectional areas (A), the mRNA levels of ANP (B) and β-MHC (C), and caspase-3 activity (D) in diabetic Capn4−/− hearts. E–H: Cardiomyocyte cross-sectional areas (E), the mRNA levels of ANP (F) and β-MHC (G), and caspase-3 activity (H) were decreased in diabetic Tg-CAST hearts. Data are mean ± SD; n = 8. *P < 0.05 vs. sham in wild-type (WT); #P < 0.05 vs. STZ in WT.
Calpain-1 and calpain-2 activities are tightly and specifically regulated by calpastatin (8). Overexpression of calpastatin has been successfully used to block calpain activation both in vitro and in vivo (11–14). To further understand the role of calpain in diabetic cardiac hypertrophy, we rendered transgenic calpastatin mice (Tg-CAST) and their wild-type littermates diabetic by STZ treatment and assessed cardiac hypertrophic changes 2 months after STZ injection. In nondiabetic mice, there were no differences in cardiomyocyte sizes and hypertrophic gene expression in hearts of Tg-CAST and wild-type mice (Fig. 3E–G). In diabetic mice, overexpression of calpastatin did not correlate with altered blood glucose levels and body and heart weight (Supplementary Table 1); however, diabetes-induced increases in cardiomyocyte cross-sectional areas, ANP and β-MHC expression, and caspase-3 activity were significantly attenuated in Tg-CAST compared with wild-type mice (Fig. 3E–H). These results further argue that inhibition of calpain reduces myocardial hypertrophy in STZ-induced diabetic mice.
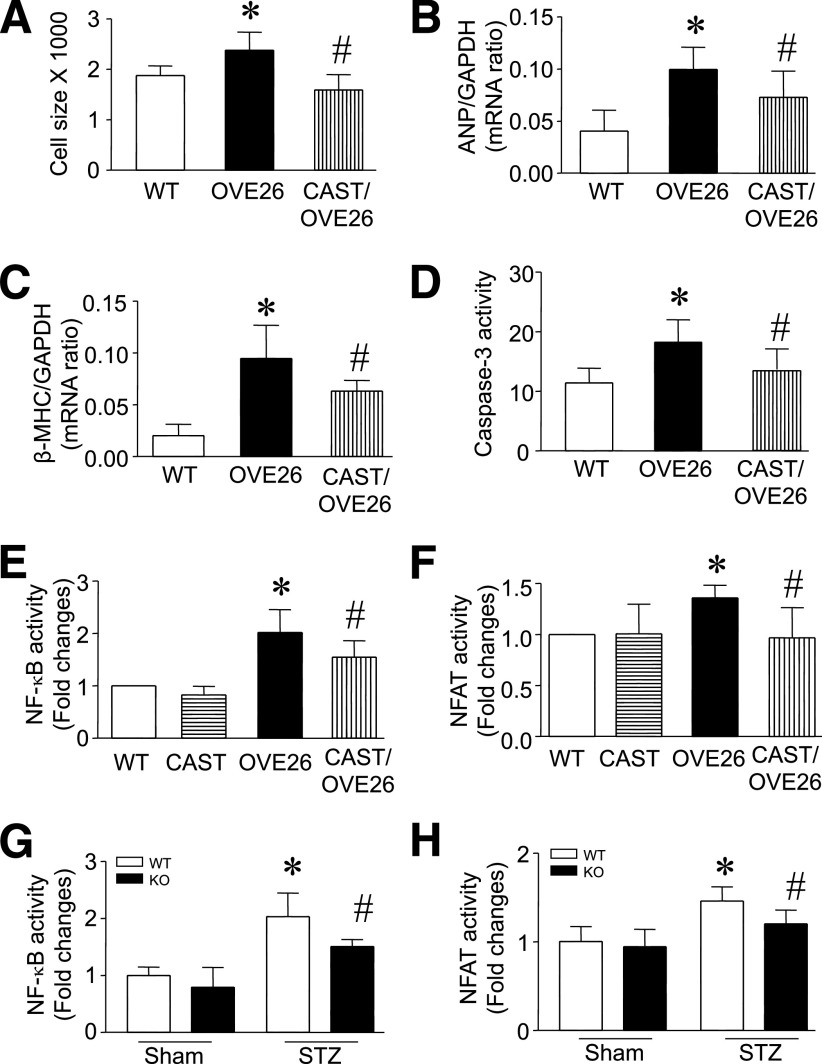
To rule out the possibility that STZ treatment had systemic actions independent of β-cell destruction, we repeated these experiments in the OVE26 type 1 diabetic mouse model and crossed them with Tg-CAST mice to inhibit calpain activity. Expression of transgenic calpastatin in the OVE26 heart was confirmed by RT-PCR (Supplementary Fig. 5A). At the age of 5 months, cardiomyocyte cross-sectional areas, the levels of ANP and β-MHC mRNA, and caspase-3 activity were significantly increased in OVE26 mice compared with their wild-type littermates (Fig. 4A–D), indicative of cardiac hypertrophy. There were no differences in blood glucose levels and body and heart weight between OVE26 and Tg-CAST/OVE26 mice (Supplementary Table 1); however, the increases in cardiomyocyte cross-sectional areas, ANP and β-MHC expression, and caspase-3 in OVE26 hearts were normalized in Tg-CAST/OVE26 mice (Fig. 4A–D). These results in the OVE26 model further support the antihypertrophic effect of calpain inhibition in type 1 diabetes.
FIG. 4.
Assessment of cardiac hypertrophy in OVE26 mice. A–D: Cardiomyocyte cross-sectional areas, the mRNA levels of ANP (B) and β-MHC (C), and caspase-3 activity (D) increased in OVE26 relative to wild-type (WT) hearts, but were reduced in Tg-CAST/OVE26 mice. E–H: NFAT and NF-κB activities were increased in OVE26 and STZ-injected WT hearts but were reduced in Tg-CAST/OVE26 and Capn4 knockout (KO) hearts. Data are mean ± SD; n = 6–8. *P < 0.05 vs. WT; #P < 0.05 vs. OVE26 or STZ in WT.
To explore potential downstream signaling in calpain-mediated myocardial hypertrophy, we focused on two transcription factors, NFAT and NF-κB, which play central roles in the regulation of cardiac hypertrophy (18,29–32). It is generally accepted that calpain-dependent activation of the serine/threonine protein phosphatase calcineurin controls NFAT activity in cardiomyocytes (18). Studies also suggest that calpain mediates degradation of the cytosolic NF-κB inhibitor, inhibitor κ Bα (IκBα), a step that is a prerequisite in the activation of NF-κB (33). In this regard, we measured the activities of both NF-κB and NFAT in OVE26 and Capn4−/− diabetic hearts. When compared with the nondiabetic heart of wild-type or Tg-CAST mice, the activities of both NF-κB and NFAT were increased in OVE26 and STZ-injected Capn4+/+ hearts. However, overexpression of calpastatin in Tg-CAST/OVE26 mice and Capn4 deletion in Capn4−/− STZ mice correlated with significantly lowered NF-κB and NFAT activities relative to OVE26 and STZ-injected Capn4+/+ hearts (Fig. 4E–H). These results suggest that the calpain/calpastatin system may be involved in the activation of NF-κB and NFAT pathways in the complex regulation of cardiac hypertrophy in diabetic hearts.
Calpain is required for myocardial fibrosis in mouse models of type 1 diabetes.
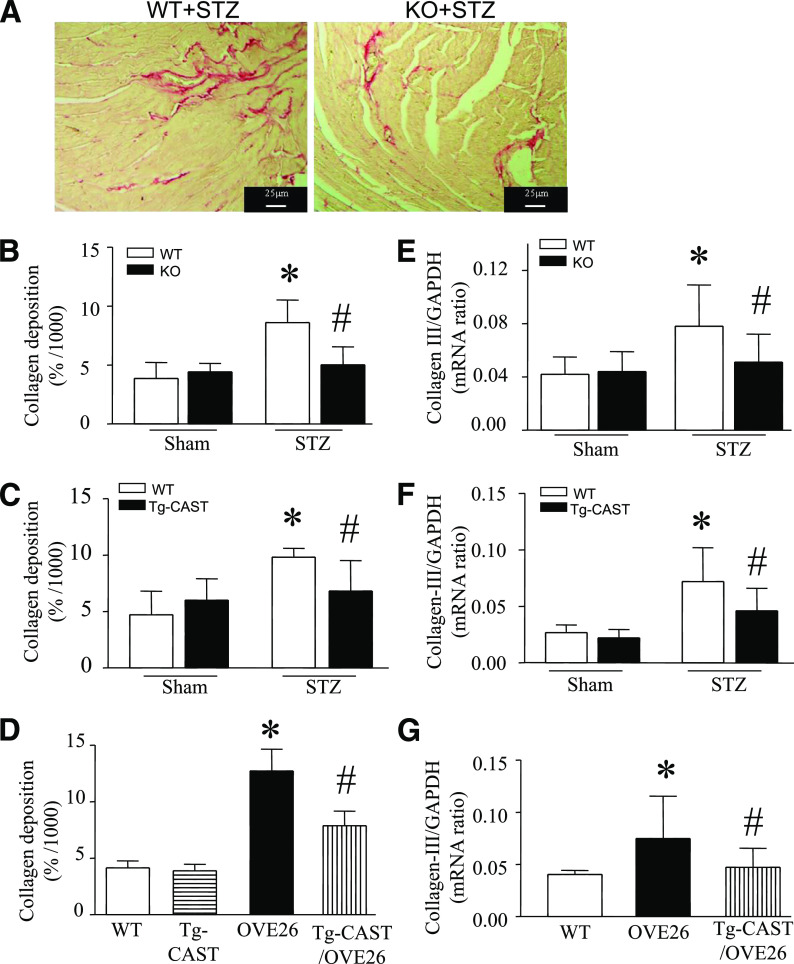
Myocardial fibrosis is commonly observed in diabetic cardiomyopathy and is characterized by excessive production and accumulation of extracellular collagen (1,34). To assess fibrosis, myocardial tissues were stained with picrosirius red to highlight collagen. Ratios of collagen area to total area were measured. Deposition of collagen was increased in STZ-induced diabetic hearts; however, Capn4 deletion in Capn4−/− mice and calpastatin overexpression in Tg-CAST mice correlated with a significant reduction in those levels after STZ injection (Fig. 5A–C). In line with altered collagen deposition, deletion of Capn4 and overexpression of calpastatin lowered the mRNA levels of collagens I and III in diabetic Capn4−/− and Tg-CAST hearts, respectively (Fig. 5E and F and Supplementary Fig. 6A). Similarly, this inhibitory effect of calpain deletion on myocardial fibrosis was also observed 5 months after STZ injection (Supplementary Fig. 4C–E). Thus, inhibition or targeted disruption of calpain prevented myocardial fibrosis in the STZ-induced mouse model of type 1 diabetes.
FIG. 5.
Assessment of fibrosis in diabetic hearts. Myocardial collagen deposition was determined by picrosirius red staining and collagen III mRNA was quantified by real-time RT-PCR. A: Representative picrosirius red staining for collagen deposition from diabetic Capn4−/− and Capn4+/+ hearts (red color). Collagen deposition was reduced in hearts of Capn4−/− (knockout [KO]; B), Tg-CAST (C), and Tg-CAST/OVE26 (D) relative to diabetic wild-type (WT), Capn4+/+ (KO), and OVE26 diabetic mice, respectively. Collagen III mRNA was decreased in diabetic Capn4−/− (E), Tg-CAST (F), and Tg-CAST/OVE26 mice (G). Data are mean ± SD; n = 8 to 9. *P < 0.05 vs. sham or nondiabetic WT; #P < 0.05 vs. STZ in WT or OVE26. (A high-quality color representation of this figure is available in the online issue.)
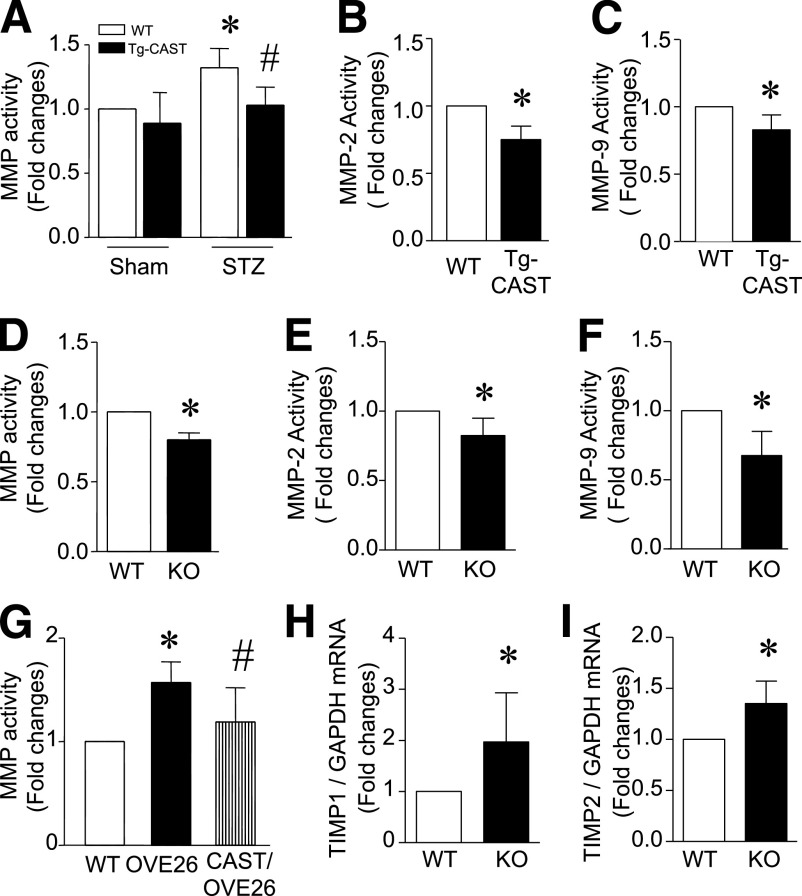
MMPs play key roles in maintenance and degradation of the extracellular matrix (ECM), contributing to the process of myocardial fibrosis (35), so we next measured MMP activity in the myocardium. STZ-induced diabetes correlated with 34% elevation in total MMP activity, but this increase was significantly reduced in diabetic Tg-CAST and Capn4−/− mice (Fig. 6A and D). Because MMP-2 and MMP-9 are the most important MMPs in the regulation of myocardial remodeling, we measured their activities in the diabetic heart. Similarly, both MMP-2 and MMP-9 activities were reduced in Tg-CAST and Capn4−/− mice (Fig. 6B, C, E, and F). The role of MMPs is negatively regulated by tissue inhibitors of metalloproteinases (TIMPs). We therefore also measured TIMP-1 and TIMP-2 expression in the diabetic heart. The mRNA levels of TIMP-1 and TIMP-2 were significantly increased in diabetic Capn4−/− compared with Capn+/+ mice (Fig. 6H and I). Taken together, our data suggest that disruption of calpain may inhibit MMP activities through downregulation of TIMP-1 and TIMP-2 in the diabetic heart.
FIG. 6.
MMP activities and TIMP expression in diabetic hearts. MMP activities were determined in Capn4−/−, Tg-CAST, and OVE26 hearts. A: MMP activities were increased in diabetic hearts. Overexpression of calpastatin reduced total MMP activities and MMP-2 and MMP-9 activities in diabetic Tg-CAST hearts (A–C). D–F: Deficiency of Capn4 reduced total MMP activities and MMP-2 and MMP-9 activities in Capn4−/− (knockout [KO]) relative to nondiabetic wild-type (WT) hearts. G: Total MMP activities were elevated in OVE26 relative to WT hearts and were decreased in Tg-CAST/OVE26 hearts. H and I: TIMP-1 and TIMP-2 mRNA levels were decreased in Capn4−/− (KO) relative to nondiabetic WT hearts. Data are mean ± SD; n = 6–8. *P < 0.05 vs. WT; #P < 0.05 vs. STZ-treated WT or OVE26.
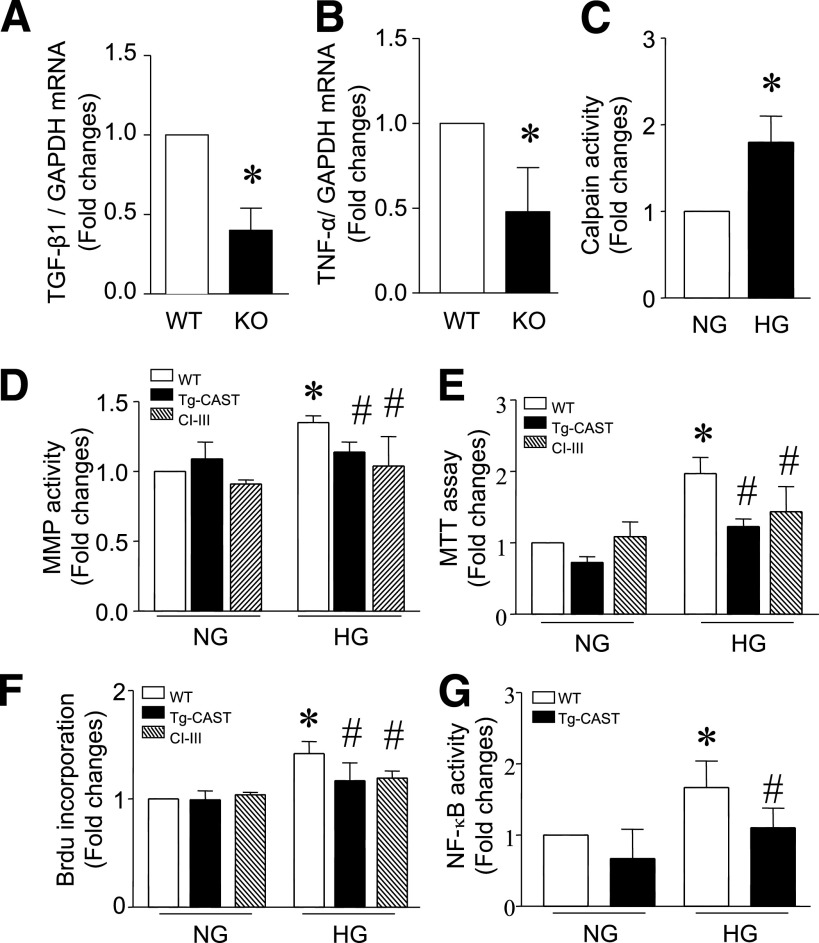
Inflammation plays an important role in myocardial fibrosis. Our recent study demonstrated that expression of proinflammatory cytokines correlates with myocardial fibrosis in the diabetic heart (25). In line with the inhibition of myocardial fibrosis, deletion of Capn4 significantly decreased TNF-α and TGF-β1 expression in diabetic Capn4−/− compared with Capn4+/+ mice (Fig. 7A and B). In addition, the number of mast cells was increased in the diabetic heart, which was reduced in Capn4−/− mice (Supplementary Fig. 7A and B).
FIG. 7.
Proinflammatory cytokine expression in diabetic Capn4 knockout hearts. Five months after STZ injection, the mRNA levels of TGF-β1 (A) and TNF-α (B) in Capn4−/− (knockout; KO) and Capn4+/+ (wild-type; WT) hearts were determined by real-time RT-PCR. Data are mean ± SD from six different hearts in each group. *P < 0.05 vs. WT. Effects of calpain inhibition on MMP activity and cell proliferation. Cardiac fibroblast cells were isolated from WT and Tg-CAST mice and were incubated with normal (NG; 5.5 mmol/L) and high glucose (HG; 30 mmol/L) in the presence and absence of calpain inhibitor-III (10 μmol/L) for 24 h. Calpain activity in fibroblast cells (C) and MMP activity (D) in culture media were measured. Cell proliferation was determined by the 3-(4, 5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay (E) and BrdU incorporation assay (F). NF-κB activity was measured in fibroblasts (G; see details in research design and methods). Data are mean ± SD from at least three different cultures. *P < 0.05 vs. NG; #P < 0.05 vs. WT cells cultured in HG.
OVE26 mice also displayed myocardial fibrosis as determined by increases in collagen deposition (Fig. 5D), mRNA levels of collagen I and collagen III (Fig. 5G and Supplementary Fig. 6B), and elevation of MMP activity (Fig. 6G). All these indications of fibrosis were normalized by calpastatin overexpression in Tg-CAST/OVE26 mice. These results further argue for the protective effects of calpain inhibition in diabetic hearts in mouse models of type 1 diabetes.
To gain further insights into the role of calpain in fibrosis, we isolated and cultured cardiac fibroblast cells, which are the putative mediators of fibrosis. High glucose (30 mmol/L) was used to mimic the diabetic condition in cultured fibroblasts. High glucose raised calpain (Fig. 7C) and MMP activities (Fig. 7D) as well as cell proliferation relative to normal glucose (5.5 mmol/L; Fig. 7E and F). These effects were abrogated by calpain inhibitor-III. Consistently, fibroblasts from Tg-CAST hearts showed much lower MMP activity and less cell proliferation than those from wild-type hearts in response to high glucose (Fig. 7D–F). These effects of calpain inhibition correlated with the changes of NF-κB activation (Fig. 7G). Further in vivo evidence to support the inhibitory effect of calpain inhibition on fibroblast proliferation in the diabetic heart were that 1) α-smooth muscle actin (α-SMA) expression was reduced in diabetic Capn4−/− compared with Capn4+/+ mice and 2) the number of noncardiomyocytes was relatively less in diabetic Capn4−/− than that in Capn4+/+ hearts (Supplementary Fig. 7D and E). Thus, inhibition of MMP activity and reduction of fibroblast cell proliferation by calpain inhibition may represent important mechanisms for reducing fibrosis in diabetic hearts.
Myocardial function in mouse model of STZ-induced type 1 diabetes.
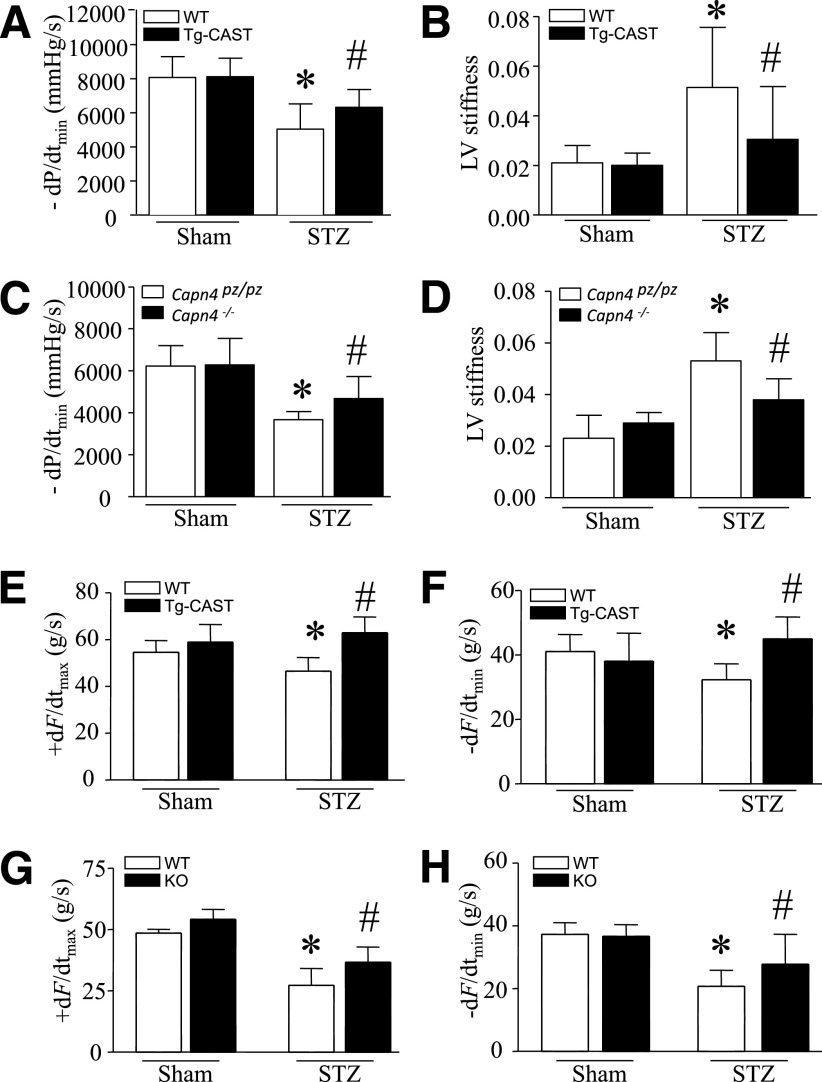
Having demonstrated the ability of calpastatin and cardiac Capn4 deletion to reverse cardiomyocyte hypertrophy and fibrosis in the diabetic myocardium, we performed in vivo LV pressure–volume measurements and a functional analysis of heart contraction and relaxation using the Langendorff system as an extension of those results. The hemodynamic parameters were shown in Supplementary Tables 2 and 3. As expected, pressure–volume analysis showed significant decreases in maximal positive and minimal negative first derivatives of LV pressure (+dP/dtmax and −dP/dtmin) and increase in LV stiffness in diabetic hearts. However, these changes of −dP/dtmin and stiffness were relatively attenuated in diabetic Capn4−/− and Tg-CAST compared with their wild-type littermates (Fig. 8A–D). Consistently, the decreases of heart contraction and relaxation were also attenuated in isolated calpastatin transgenic and Capn4 knockout hearts after STZ injection (Fig. 8E–H). Taken together, targeted disruption of calpain preserves myocardial function in diabetic cardiomyopathy of STZ-induced type 1 diabetes.
FIG. 8.
Cardiac function. Tg-CAST, Capn4−/− (knockout [KO]), and wild-type (WT) mice were injected with STZ. Two (Tg-CAST) or five months (Capn4−/−) after STZ injection, in vivo hemodynamic measurement was performed. Changes in −dP/dtmin (A and C) and LV stiffness (B and D) are shown. Data are mean ± SD; n = 6–10 per group. *P < 0.05 vs. WT sham; #P < 0.05 vs. WT STZ. Two months after STZ injection, mouse hearts were isolated and perfused in a Langendorff system. Contractile function of the heart was determined. Changes in the rate of contraction (+dF/dtmax; E and G) and relaxation (−dF/dtmin; F and H) are presented. Data are mean ± SD; n = 8 to 9 per group. *P < 0.05 vs. WT sham; #P < 0.05 vs. WT STZ.
DISCUSSION
Although calpain activity is elevated in the diabetic heart (14), its pathophysiological significance is incompletely understood. In the current study, we generated novel mouse models including cardiac-specific Capn4 knockout mice and OVE26 mice with calpastatin overexpression and investigated the role of calpain in diabetic cardiomyopathy. We observed that cardiac-specific deletion of Capn4 and calpastatin overexpression reduced cardiac hypertrophy and fibrosis in both STZ-induced and OVE26 type 1 diabetic mice, leading to the improvement of myocardial function. Given the fact that both calpastatin overexpression and Capn4 deletion inhibit only calpain-1 and calpain-2 activity out of the 14 known mammalian isoforms (7), the current study reveals a pivotal role for these two calpain isoforms in diabetic cardiomyopathy.
An important cellular hallmark of diabetic cardiomyopathy is cardiac hypertrophy (1,5,36). In its initial stages, cardiomyocyte enlargement may be an adaptive response intended to improve ventricular performance (37). However, sustained hypertrophic growth of the myocardium may be associated with pathological LV remodeling (37). The role of calpain in cardiac hypertrophy is poorly understood. A recent study reported that transgenic overexpression of calpastatin reduced myocardial hypertrophy in a mouse model of angiotensin-II–induced hypertension (38), suggesting that calpain may be a contributing factor to cardiac hypertrophy. By impeding calpain function we observed a clear reversal of cardiac hypertrophy in both STZ-induced and OVE26 type 1 diabetic mice. These data suggest that calpains may play a role in cardiac hypertrophy of diabetic cardiomyopathy. We further showed that calpain activation correlated with increased NFAT and NF-κB activity, each of which mediates prohypertrophic signaling pathways (18,19,39,40). Indeed, calpain has been identified as a key activator of the NF-κB pathway leading to myocardial hypertrophy in angiotensin-II–induced hypertension (38). Thus, the dramatic reduction of NF-κB activity levels in diabetic hearts after Capn4 deletion and calpastatin overexpression underscores the importance of this pathway in diabetic cardiomyopathy (41). It has been shown that calpain degrades IκB, the inhibitor of NF-κB, which allows NF-κB to translocate freely into the nucleus and transcribe prohypertrophic genes such as β-MHC. We therefore postulate that inhibition of calpain will increase IκB and limit NF-κB nuclear translocation, thereby limiting cardiac hypertrophy in diabetic hearts. Calpain also activates calcineurin, which is another important mediator of cardiac hypertrophy (19,20). Activated calcineurin dephosphorylates NFAT, which binds to GATA-4 upon nuclear translocation and further augments the transcriptional response leading to myocardial hypertrophy (19). In this regard, we showed that inhibition of calpain reduced NFAT activity in diabetic hearts, suggesting that the calcineurin/NFAT pathway is an additional mechanism by which calpain mediates cardiac hypertrophy in diabetic cardiomyopathy. These results contrast with a recent study that demonstrated that the NFAT pathway was not involved in inhibition of myocardial hypertrophy by calpastatin overexpression in angiotensin-II–induced hypertension (38). The difference between these two studies suggests that calpain may mediate cardiac hypertrophy via multiple mechanisms depending on the underlying pathological conditions.
Fibrosis is another important hallmark of diabetic cardiomyopathy (1,5,36). It is believed to be a maladaptive response independent of cellular hypertrophy that is characterized by excess collagen accumulation (1,5,36). We consistently observed that myocardial collagen deposition was significantly increased in both STZ-induced and OVE26 type 1 diabetic mice. It is noteworthy that both cardiac-specific deletion of Capn4 and overexpression of calpastatin reduced collagen deposition and expression levels of the main components of the ECM, collagens I and III. This finding suggests that cardiomyocyte-expressed calpain may contribute to fibrosis via indirect (paracrine) or direct mechanisms. However, it is likely that calpain in noncardiomyocytes may also play an important role in myocardial fibrosis in diabetes since Tg-CAST mice displayed a significant reduction in peri vascular fibrosis in response to angiotensin II stimulation when compared with wild-type mice (38). Additionally, a significant decrease in angiotensin II–induced synthesis and secretion of collagen occurred in vascular smooth muscle cells from Tg-CAST mice compared with wild-type mice (38).
Fibroblasts play an important role in fibrosis (42). Cardiac fibroblasts may differentiate into myofibroblasts, which induce fibroblast proliferation. Our recent study has shown an increase in myofibroblast marker α-SMA in diabetic hearts (25), suggesting a possible differentiation of myofibroblasts. In this regard, we demonstrated a significant reduction of α-SMA expression in diabetic Capn4 knockout hearts. In line with the downregulation of α-SMA expression, the number of noncardiomyocytes was reduced in diabetic Capn4−/− compared with Capn4PZ/PZ mice. Furthermore, cultured cardiac fibroblasts undergo significant hyperplasia in response to high glucose, and that was reduced by the inhibition of calpain. Fibroblast proliferation is induced by a number of factors, including possibly NF-κB. Evidence suggests the IκB–NF-κB complex is influenced by calpain activation, which may contribute to the development of diabetic cardiomyopathy through the promotion of fibrosis (43,44). Specifically, NF-κB is required for the induction of proinflammatory cytokines such as TNF-α, which is also overexpressed in the diabetic heart (25,40). Proinflammatory cytokines can further induce expression of cytokines such as TGF-β1, which is also elevated in the diabetic heart (25), and is known to be a profibrogenic inducer (45). Indeed, our data showed that deletion of Capn4 reduced TNF-α and TGF-β1 expression in the diabetic heart. The profibrogenic effect of these cytokines may also include MMP activation. Derangement of MMP activity alters the balance between synthesis and degradation of the ECM resulting in excessive collagen deposition and reduced structural integrity in the myocardium (35). Studies have shown upregulation of MMP-9 activity (46) and downregulation of MMP-2 activity in the diabetic heart (47). Inhibition of MMP activity has been suggested to be cardiac protective in diabetes (48). In the current study, we demonstrated that total MMP activity was increased in both fibroblasts in vitro in response to high glucose and in the diabetic heart in vivo, which in both cases was reversed by calpain inhibition. Specifically, calpain inhibition also reduced MMP-2 and MMP-9 activities in the diabetic heart. This finding reveals a link between calpain and MMPs in diabetic conditions. Prior work suggests that calpain induces MMP-9 activity in homocysteine-stimulated cardiac microvascular endothelial cells (49). Taken together with our findings a general role for calpain in the modulating ECM composition in the heart is likely.
It is currently unknown whether hematopoietic cells contribute to the fibrotic processes in our type 1 diabetes models. The expression of CD68, a marker of macrophage, was comparable between diabetic Capn4−/− and Capn4PZ/PZ hearts (Supplementary Fig. 6C), potentially excluding the involvement of macrophage infiltration in the inhibitory effect of Capn4 deletion in diabetic myocardial fibrosis. However, we demonstrated that the number of mast cells was increased in the diabetic heart, which was significantly reduced in Capn4−/− compared with Capn4PZ/PZ mice. Whether this reduction of mast cells plays a role in the inhibitory effect of Capn4 deletion in diabetic myocardial fibrosis is beyond the scope of this study and merits future studies.
In summary, the current study has demonstrated that targeted inhibition of calpain is protective against hypertrophy and fibrosis, two general hallmarks of diabetic cardiomyopathy, and preserves myocardial function in mouse models of type 1 diabetes. These effects of calpain inhibition may be mediated through NFAT-, NF-κB-, and MMP-dependent signaling pathways. Cardiac hypertrophy and deposition of collagen, in particular collagen I and III, were associated with cardiac dysfunction in diabetes (1,5,36) and were all restored by calpain inhibition, thereby supporting the important in vivo functional significance of the calpain/calpastatin system in the diabetic heart. Therefore, calpain may be a potential therapeutic target for diabetes heart diseases.
ACKNOWLEDGMENTS
This study was supported by an operating grant awarded to T.P. from the Canadian Institutes of Heart Research (MOP93657). T.P. is a recipient of a New Investigator Award from the Heart & Stroke Foundation of Canada.
No potential conflicts of interest relevant to this article were reported.
Y.L., J.M., and H.Z. researched data. M.S. researched data and wrote and edited the manuscript. D.H. wrote the manuscript, reviewed the manuscript, and contributed to discussion. P.A.G. contributed to Capn4PZ mice and reviewed the manuscript. J.M.A. contributed to discussion and reviewed the manuscript. E.D.A. contributed the MHC-Cre mice and discussed, reviewed, and edited the manuscript. T.P. researched data and wrote, reviewed, and edited the manuscript.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-1333/-/DC1.
REFERENCES
- 1.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation 2007;115:3213–3223 [DOI] [PubMed] [Google Scholar]
- 2.Di Filippo C, Marfella R, Cuzzocrea S, et al. Hyperglycemia in streptozotocin-induced diabetic rat increases infarct size associated with low levels of myocardial HO-1 during ischemia/reperfusion. Diabetes 2005;54:803–810 [DOI] [PubMed] [Google Scholar]
- 3.Greer JJ, Ware DP, Lefer DJ. Myocardial infarction and heart failure in the db/db diabetic mouse. Am J Physiol Heart Circ Physiol 2006;290:H146–H153 [DOI] [PubMed] [Google Scholar]
- 4.Schramm TK, Gislason GH, Køber L, et al. Diabetes patients requiring glucose-lowering therapy and nondiabetics with a prior myocardial infarction carry the same cardiovascular risk: a population study of 3.3 million people. Circulation 2008;117:1945–1954 [DOI] [PubMed] [Google Scholar]
- 5.Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev 2004;25:543–567 [DOI] [PubMed] [Google Scholar]
- 6.Poornima IG, Parikh P, Shannon RP. Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ Res 2006;98:596–605 [DOI] [PubMed] [Google Scholar]
- 7.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev 2003;83:731–801 [DOI] [PubMed] [Google Scholar]
- 8.Hanna RA, Campbell RL, Davies PL. Calcium-bound structure of calpain and its mechanism of inhibition by calpastatin. Nature 2008;456:409–412 [DOI] [PubMed] [Google Scholar]
- 9.Suzuki K, Hata S, Kawabata Y, Sorimachi H. Structure, activation, and biology of calpain. Diabetes 2004;53(Suppl. 1):S12–S18 [DOI] [PubMed] [Google Scholar]
- 10.Arthur JS, Elce JS, Hegadorn C, Williams K, Greer PA. Disruption of the murine calpain small subunit gene, Capn4: calpain is essential for embryonic development but not for cell growth and division. Mol Cell Biol 2000;20:4474–4481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peltier J, Bellocq A, Perez J, et al. Calpain activation and secretion promote glomerular injury in experimental glomerulonephritis: evidence from calpastatin-transgenic mice. J Am Soc Nephrol 2006;17:3415–3423 [DOI] [PubMed] [Google Scholar]
- 12.Li X, Li Y, Shan L, Shen E, Chen R, Peng T. Over-expression of calpastatin inhibits calpain activation and attenuates myocardial dysfunction during endotoxaemia. Cardiovasc Res 2009;83:72–79 [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Arnold JM, Pampillo M, Babwah AV, Peng T. Taurine prevents cardiomyocyte death by inhibiting NADPH oxidase-mediated calpain activation. Free Radic Biol Med 2009;46:51–61 [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Li Y, Feng Q, Arnold M, Peng T. Calpain activation contributes to hyperglycaemia-induced apoptosis in cardiomyocytes. Cardiovasc Res 2009;84:100–110 [DOI] [PubMed] [Google Scholar]
- 15.Chen M, Won DJ, Krajewski S, Gottlieb RA. Calpain and mitochondria in ischemia/reperfusion injury. J Biol Chem 2002;277:29181–29186 [DOI] [PubMed] [Google Scholar]
- 16.Khalil PN, Neuhof C, Huss R, et al. Calpain inhibition reduces infarct size and improves global hemodynamics and left ventricular contractility in a porcine myocardial ischemia/reperfusion model. Eur J Pharmacol 2005;528:124–131 [DOI] [PubMed] [Google Scholar]
- 17.Mani SK, Balasubramanian S, Zavadzkas JA, et al. Calpain inhibition preserves myocardial structure and function following myocardial infarction. Am J Physiol Heart Circ Physiol 2009;297:H1744–H1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkins BJ, Dai YS, Bueno OF, et al. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res 2004;94:110–118 [DOI] [PubMed] [Google Scholar]
- 19.Bourajjaj M, Armand AS, da Costa Martins PA, et al. NFATc2 is a necessary mediator of calcineurin-dependent cardiac hypertrophy and heart failure. J Biol Chem 2008;283:22295–22303 [DOI] [PubMed] [Google Scholar]
- 20.Colella M, Grisan F, Robert V, Turner JD, Thomas AP, Pozzan T. Ca2+ oscillation frequency decoding in cardiac cell hypertrophy: role of calcineurin/NFAT as Ca2+ signal integrators. Proc Natl Acad Sci USA 2008;105:2859–2864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang D, Ma S, Tan Y, et al. Increased expression of calpain and elevated activity of calcineurin in the myocardium of patients with congestive heart failure. Int J Mol Med 2010;26:159–164 [DOI] [PubMed] [Google Scholar]
- 22.Tan Y, Dourdin N, Wu C, De Veyra T, Elce JS, Greer PA. Conditional disruption of ubiquitous calpains in the mouse. Genesis 2006;44:297–303 [DOI] [PubMed] [Google Scholar]
- 23.Abel ED, Kaulbach HC, Tian R, et al. Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J Clin Invest 1999;104:1703–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bashey RI, Donnelly M, Insinga F, Jimenez SA. Growth properties and biochemical characterization of collagens synthesized by adult rat heart fibroblasts in culture. J Mol Cell Cardiol 1992;24:691–700 [DOI] [PubMed] [Google Scholar]
- 25.Li J, Zhu H, Shen E, Wan L, Arnold JM, Peng T. Deficiency of rac1 blocks NADPH oxidase activation, inhibits endoplasmic reticulum stress, and reduces myocardial remodeling in a mouse model of type 1 diabetes. Diabetes 2010;59:2033–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dourdin N, Bhatt AK, Dutt P, et al. Reduced cell migration and disruption of the actin cytoskeleton in calpain-deficient embryonic fibroblasts. J Biol Chem 2001;276:48382–48388 [DOI] [PubMed] [Google Scholar]
- 27.Peng T, Lu X, Lei M, Moe GW, Feng Q. Inhibition of p38 MAPK decreases myocardial TNF-alpha expression and improves myocardial function and survival in endotoxemia. Cardiovasc Res 2003;59:893–900 [DOI] [PubMed] [Google Scholar]
- 28.Peng T, Zhang T, Lu X, Feng Q. JNK1/c-fos inhibits cardiomyocyte TNF-alpha expression via a negative crosstalk with ERK and p38 MAPK in endotoxaemia. Cardiovasc Res 2009;81:733–741 [DOI] [PubMed] [Google Scholar]
- 29.van Rooij E, Doevendans PA, de Theije CC, Babiker FA, Molkentin JD, de Windt LJ. Requirement of nuclear factor of activated T-cells in calcineurin-mediated cardiomyocyte hypertrophy. J Biol Chem 2002;277:48617–48626 [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Ha T, Gao X, et al. NF-kappaB activation is required for the development of cardiac hypertrophy in vivo. Am J Physiol Heart Circ Physiol 2004;287:H1712–H1720 [DOI] [PubMed] [Google Scholar]
- 31.Wilkins BJ, Dai YS, Bueno OF, et al. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res 2004;94:110–118 [DOI] [PubMed] [Google Scholar]
- 32.Zelarayan L, Renger A, Noack C, et al. NF-kappaB activation is required for adaptive cardiac hypertrophy. Cardiovasc Res 2009;84:416–424 [DOI] [PubMed] [Google Scholar]
- 33.Chen F, Lu Y, Kuhn DC, et al. Calpain contributes to silica-induced I kappa B-alpha degradation and nuclear factor-kappa B activation. Arch Biochem Biophys 1997;342:383–388 [DOI] [PubMed] [Google Scholar]
- 34.Asbun J, Villarreal FJ. The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J Am Coll Cardiol 2006;47:693–700 [DOI] [PubMed] [Google Scholar]
- 35.Wilson EM, Spinale FG. Myocardial remodelling and matrix metalloproteinases in heart failure: turmoil within the interstitium. Ann Med 2001;33:623–634 [DOI] [PubMed] [Google Scholar]
- 36.Taegtmeyer H, McNulty P, Young ME. Adaptation and maladaptation of the heart in diabetes: Part I: general concepts. Circulation 2002;105:1727–1733 [DOI] [PubMed] [Google Scholar]
- 37.McKinsey TA, Kass DA. Small-molecule therapies for cardiac hypertrophy: moving beneath the cell surface. Nat Rev Drug Discov 2007;6:617–635 [DOI] [PubMed] [Google Scholar]
- 38.Letavernier E, Perez J, Bellocq A, et al. Targeting the calpain/calpastatin system as a new strategy to prevent cardiovascular remodeling in angiotensin II-induced hypertension. Circ Res 2008;102:720–728 [DOI] [PubMed] [Google Scholar]
- 39.Gupta S, Young D, Maitra RK, et al. Prevention of cardiac hypertrophy and heart failure by silencing of NF-kappaB. J Mol Biol 2008;375:637–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van der Heiden K, Cuhlmann S, Luong A, Zakkar M, Evans PC. Role of nuclear factor kappaB in cardiovascular health and disease. Clin Sci (Lond) 2010;118:593–605 [DOI] [PubMed] [Google Scholar]
- 41.Heidrich FM, Ehrlich BE. Calcium, calpains, and cardiac hypertrophy: a new link. Circ Res 2009;104:e19–e20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takeda N, Manabe I, Uchino Y, et al. Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload. J Clin Invest 2010;120:254–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamid T, Guo SZ, Kingery JR, Xiang X, Dawn B, Prabhu SD. Cardiomyocyte NF-kappaB p65 promotes adverse remodeling, apoptosis and endoplasmic reticulum stress in heart failure. Cardiovasc Res 2011;89:129–138 [DOI] [PMC free article] [PubMed]
- 44.Onai Y, Suzuki J, Maejima Y, et al. Inhibition of NF-kappaB improves left ventricular remodeling and cardiac dysfunction after myocardial infarction. Am J Physiol Heart Circ Physiol 2007;292:H530–H538 [DOI] [PubMed] [Google Scholar]
- 45.Petrov VV, Fagard RH, Lijnen PJ. Stimulation of collagen production by transforming growth factor-beta1 during differentiation of cardiac fibroblasts to myofibroblasts. Hypertension 2002;39:258–263 [DOI] [PubMed] [Google Scholar]
- 46.Camp TM, Tyagi SC, Senior RM, Hayden MR, Tyagi SC. Gelatinase B(MMP-9) an apoptotic factor in diabetic transgenic mice. Diabetologia 2003;46:1438–1445 [DOI] [PubMed] [Google Scholar]
- 47.Westermann D, Rutschow S, Jäger S, et al. Contributions of inflammation and cardiac matrix metalloproteinase activity to cardiac failure in diabetic cardiomyopathy: the role of angiotensin type 1 receptor antagonism. Diabetes 2007;56:641–646 [DOI] [PubMed] [Google Scholar]
- 48.Yaras N, Sariahmetoglu M, Bilginoglu A, et al. Protective action of doxycycline against diabetic cardiomyopathy in rats. Br J Pharmacol 2008;155:1174–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moshal KS, Singh M, Sen U, et al. Homocysteine-mediated activation and mitochondrial translocation of calpain regulates MMP-9 in MVEC. Am J Physiol Heart Circ Physiol 2006;291:H2825–H2835 [DOI] [PubMed] [Google Scholar]