Abstract
OBJECTIVE
To investigate whether lower risk HLA class II genotypes would influence the efficacy of DiaPep277 therapy in protecting β-cell function evaluated by C-peptide secretion in recent-onset type 1 diabetic subjects.
RESEARCH DESIGN AND METHODS
Data were collected from type 1 diabetic subjects enrolled in multicenter phase II studies with a randomized, double-blind, and placebo-controlled design in whom fasting and stimulated C-peptide levels were measured. HLA genotypes were classified in high, moderate, and low risk categories.
RESULTS
A total of 146 subjects (aged 4.3 to 58.5 years) were enrolled, including 76 children (<18 years old) and 70 adults. At baseline, there was a significant increase in fasting, maximal, and area under the curve (AUC) C-peptide from high to moderate and low risk HLA genotypes in adults (P for trend <0.04) but not in children. Children showed a decrease of the three parameters over time regardless of therapy and HLA genotype. DiaPep277-treated adults with low risk genotype had significantly higher maximal and AUC C-peptide versus placebo at 12 months (0.04 ± 0.07 vs. −0.28 ± 0.09 nmol/L, P < 0.01, and 0.53 ± 1.3 vs. −4.59 ± 1.5 nmol/L, P < 0.05, respectively). In the moderate risk genotype group, Δmaximal and AUC C-peptide values were significantly higher in DiaPep277-treated versus placebo-treated patients (P < 0.01 and P < 0.05, respectively).
CONCLUSIONS
This exploratory study demonstrates that type 1 diabetic adults with low and moderate risk HLA genotypes benefit the most from intervention with DiaPep277; the only subgroup with an increase of C-peptide at 12 months after diagnosis was the low risk DiaPep277-treated subgroup.
In type 1 diabetes, the autoimmune destruction of insulin-secreting pancreatic β-cells occurs as a result of complex interactions between genetic susceptibility (mainly associated with genes of the major histocompatibility complex) and environmental factors (1). There is now extensive evidence that autoimmune diabetes is a heterogeneous disease in terms of genetic, immune, metabolic, and clinical features (2). A good illustration is the correlation between age at onset and determinants of the severity of the β-cell destruction and disease progression, including younger age at onset associated with greater genetic susceptibility, more intense immune response to β-cell antigens, shorter duration of symptoms, more severe metabolic decompensation at diagnosis, and a more rapid rate of progression toward extensive β-cell destruction (3–7). Moreover, several studies indicate an independent positive correlation between HLA class II genotypes and C-peptide levels at diagnosis, as a measure of β-cell function, suggesting that the low risk genotype may induce a milder autoimmune destructive response against β-cells (8–10).
The high complexity of type 1 diabetes pathogenesis and the large interindividual heterogeneity might directly influence the efficacy of immune intervention strategies. Designing therapies that are effective in all clinical settings is therefore extremely challenging.
Thus far, there have been no consistent attempts in correlating the efficacy of an immune intervention in type 1 diabetes with HLA risk genotypes.
The aim of this study was to investigate whether therapy with DiaPep277 would be more efficacious in terms of C-peptide preservation in recent-onset type 1 diabetic subjects with lower risk HLA class II genotypes who are characterized by less extended destruction of pancreatic β-cells.
RESEARCH DESIGN AND METHODS
Data were collected from patients with type 1 diabetes enrolled in several multicenter DiaPep277 phase II studies with a prospective, randomized, double-blind, parallel-group, and placebo-controlled design. Diagnosis of type 1 diabetes and the inclusion/exclusion criteria have been previously published (11–13). Briefly, diagnosis was made according to the guidelines of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (14). A history of type 1 diabetes manifestation with acute hyperglycemia and ketonuria; type 1 diabetes of <3 months duration; positive results for at least two of the three islet antibodies, glutamic acid decarboxylase, protein tyrosine phosphatase, or islet cell antibodies; and residual basal fasting C-peptide of >0.1 nmol/L (for two of the studies) and >0.2 nmol/L (for the other two studies) were required for subjects to be enrolled (11–13). Patients who received a cytostatic corticosteroid or immunosuppressant agents were excluded. Written informed consent was obtained from all subjects. Patients received DiaPep277, the heat shock protein 60–derived peptide, or placebo subcutaneously in the upper arm at baseline and after 1, 6, and 12 months. β-cell function was evaluated by measuring both fasting and stimulated C-peptide; the maximal C-peptide response and the area under the curves (AUCs) were calculated.
HLA typing.
HLA typing was performed at the central laboratory in Rome, Italy. Blood samples were collected at disease diagnosis and stored at −20°C until used for genomic extraction of DNA. Genomic DNA was extracted using QIAamp DNA Blood Kit (QIAGEN Genomics Inc., Bothell, WA). Typing for HLA-DRB1 and DQB1 loci was performed by PCR followed by a reverse line blot assay using an array of immobilized sequence-specific oligonucleotide probes. Probes were provided by Dr. H. A. Erlich and T. Bugawan (not a commercial kit; Roche Molecular System, Alameda, CA). To analyze the effect of HLA genotypes on quantitative variables, HLA genotypes were classified in three risk categories based on the absolute risk values (ARs) previously estimated in the Italian population (15): high risk (AR = 1:23) for DRB1*03-DQB1*0201/DRB1*04-DQB1*0302 genotype (DRB1*04 different from 0403, 06, 11); moderate risk (AR = 1:150) for DRB1*04-DQB1*0302/DRB1*04-DQB1*0302, DRB1*03-DQ-B1*0201/DRB1*03-DQB1*0201, DRB1*04-DQB1*0302/X (X different from DRB1*02, 03, DRB1*04-DQB1*0302 [DRB1*04 not 0403, 06, 11] or DQB1*0602), and DRB1*03/X (X different from DRB1*02, 03, DRB1*04-DQB1*0302 [DRB1*04 not 0403, 06, 11] or DQB1*0602) genotypes; and low risk (AR = 1:1100) for the remaining genotypes.
Statistical analysis.
Data were analyzed separately for children (<18 years) and adults (≥18 years). At baseline, a comparison between high, moderate, and low risk genotype for fasting, maximal, and AUC C-peptide was performed. Subjects with high risk genotypes were then grouped with subjects with moderate risk genotypes (because of the small number of subjects with high risk genotypes), each of the two groups (high/moderate and low risk) were further subdivided according to therapy received (placebo or DiaPep277), and the C-peptide values were followed up to 12 months after trial initiation. Then, moderate HLA risk genotype treated with DiaPep277 was compared with moderate HLA risk genotype treated with placebo. Baseline parameters and changes over time in C-peptide were analyzed by parametric (Student t test and ANOVA) or nonparametric (Mann-Whitney U test) tests after performing goodness-of-fit tests. When comparing high/moderate and low DiaPep277- treated versus placebo, a Bonferroni correction factor of 2 was applied. GraphPad Prism version 4.00 for Windows (GraphPad Software) was used for analysis, with P < 0.05 considered to be statistically significant.
RESULTS
A total of 146 subjects (aged 4.3 to 58.5 years) were enrolled in different phase II clinical trials of immune intervention with DiaPep277. Of these, 76 were children (42 males and 34 females) aged 11.5 ± 3.6 years and 70 were adults (53 males and 17 females) aged 28.8 ± 8.8 years. From trial participants, 13 were excluded from analysis: DNAs for HLA genotype determination were not available.
Table 1 shows the anthropometric and biochemical parameters of children and adult patients at baseline.
TABLE 1.
Clinical characteristics at baseline of adults and children with type 1 diabetes
| Adults (n = 70) | Children (n = 76) | |
|---|---|---|
| Sex (male/female) | 53/17 | 42/34 |
| Age (years) | 28.8 ± 8.8 | 11.5 ± 3.6 |
| BMI (kg/m2) | 23 ± 3.12 | 18.07 ± 2.92 |
| Fasting glucose (mmol/L) | 7.32 ± 1.64 | 8.08 ± 3.50 |
| C-peptide (mmol/L) | 0.41 ± 0.23 | 0.41 ± 0.16 |
| HbA1c (%) | 6.86 ± 1.25 | 7.75 ± 1.28 |
Data are expressed as means ± SD.
At baseline, an increase in fasting, maximal, and AUC C-peptide values from high to moderate and low risk genotype subgroups was observed in adults (P ≤ 0.04 for trend for all parameters) but not in children (Table 2).
TABLE 2.
Fasting, maximal, and AUC C-peptide values at baseline for adults and children
| Fasting C-peptide |
Maximal C-peptide |
AUC C-peptide |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High HLA | Moderate HLA | Low HLA | P value* | High HLA | Moderate HLA | Low HLA | P value* | High HLA | Moderate HLA | Low HLA | P value* | |
| Adults (n = 70) | 0.34 ± 0.12 (n = 9) | 0.37 ± 0.22 (n = 43) | 0.57 ± 0.32 (n = 18) | 0.01 | 0.60 ± 0.36 (n = 9) | 0.66 ± 0.31 (n = 43) | 0.91 ± 0.53 (n = 18) | 0.04 | 9.06 ± 4.66 (n = 9) | 10.45 ± 5.2 (n = 43) | 15.18 ± 8.76 (n = 18) | 0.01 |
| Children (n = 76) | 0.41 ± 0.16 (n = 25) | 0.40 ± 0.16 (n = 36) | 0.46 ± 0.19 (n = 15) | 0.49 | 0.71 ± 0.31 (n = 25) | 0.74 ± 0.27 (n = 36) | 0.84 ± 0.33 (n = 15) | 0.39 | 12.17 ± 4.49 (n = 25) | 12.15 ± 4.57 (n = 36) | 14.52 ± 4.73 (n = 15) | 0.20 |
Data are expressed as means ± SD. Fasting, maximal, and AUC C-peptide values (nmol/L).
*P for trend.
High/moderate and low risk HLA genotype patients were subdivided according to treatment. Placebo-treated (24 adults and 32 children) or DiaPep277-treated (46 adults and 44 children) subjects were followed up for C-peptide values up to 12 months after trial initiation (Fig. 1). Children with type 1 diabetes had a decrease of all three evaluated parameters over the observational period, and the differences between fasting, maximal, and AUC C-peptide values at 12 months versus baseline (ΔC-peptide = C-peptide at visit 12 – C-peptide at visit 1) did not differ significantly between the four subgroups (Figs. 1 and 2). In a similar manner, adults with high/moderate risk HLA genotype had a decrease of fasting, maximal, and AUC C-peptide values over time, regardless of therapy (placebo or DiaPep277). Of interest, the only subgroup that had an increase in C-peptide levels was adult subjects with low risk HLA genotype (Supplementary Table 1) who were treated with DiaPep277 compared with placebo (Figs. 1 and 2). There were statistically significant higher maximal C-peptide and AUC C-peptide values in subjects with low risk genotype treated with DiaPep277 versus placebo at 12 months compared with baseline: Δmaximal C-peptide 0.04 ± 0.07 nmol/L (DiaPep277) vs. −0.29 ± 0.1 nmol/L (placebo), P = 0.0093 (P = 0.01 after Bonferroni correction) and ΔAUC C-peptide 0.54 ± 1.3 nmol/L (DiaPep277) vs. −4.6 ± 1.5 nmol/L (placebo), P = 0.0158 (P = 0.02 after Bonferroni correction), respectively. The increase in fasting C-peptide values did not reach statistical significance: Δfasting C-peptide 0.03 ± 0.08 nmol/L (DiaPep277) vs. −0.14 ± 0.05 nmol/L (placebo), P = 0.09.
FIG. 1.
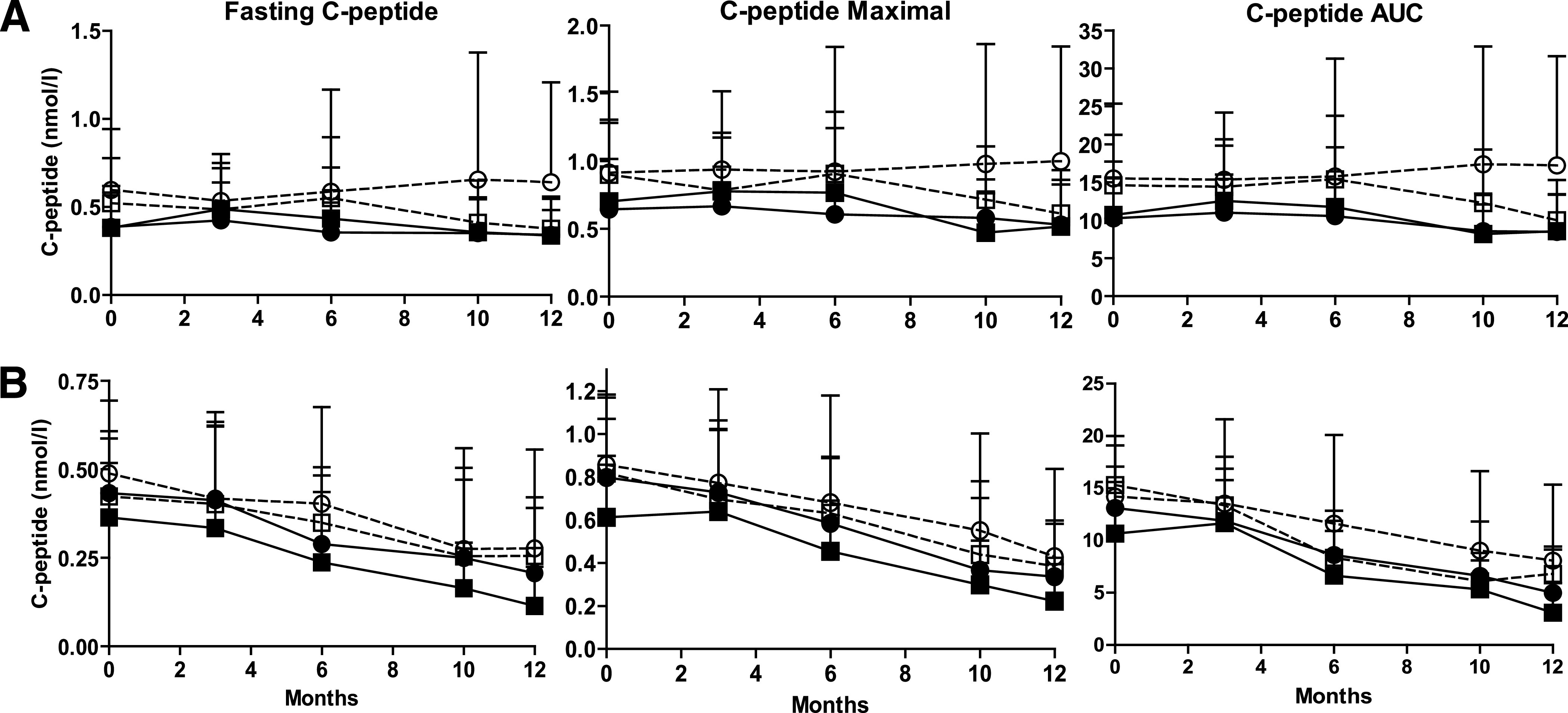
C-peptide values (fasting, maximal, and AUC) during 12 months of follow-up in subjects treated with DiaPep277 or placebo, according to the HLA risk genotype. A: Adults with type 1 diabetes. B: Children with type 1 diabetes. ■, high/moderate risk genotype, placebo treated (n = 19 adults and n = 25 children); □, low risk genotype, placebo treated (n = 5 adults and n = 7 children); ●, high/moderate risk genotype, DiaPep277 treated (n = 33 adults and n = 36 children); ○, low risk genotype, DiaPep277 treated (n = 13 adults and n = 8 children).
FIG. 2.
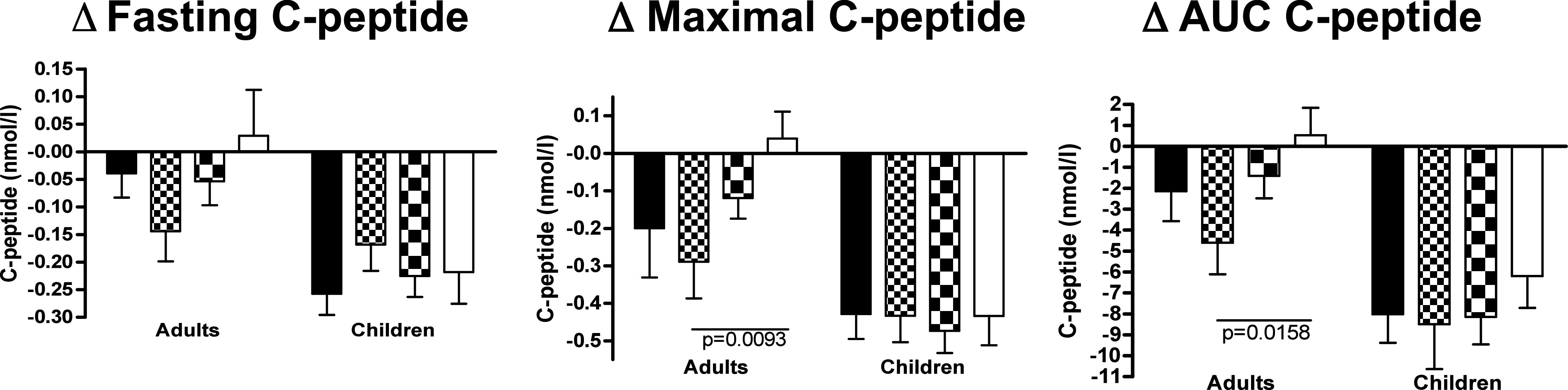
Difference in C-peptide values (fasting, maximal, and AUC) between visit 12 and baseline (visit 12 minus visit 1) for adults and children with type 1 diabetes treated with DiaPep277 or placebo. Black column, high/moderate risk genotype, placebo treated (n = 19 adults and n = 25 children); small checkered column, low risk genotype, placebo treated (n = 5 adults and n = 7 children); large checkered column, high/moderate risk genotype, DiaPep277 treated (n = 33 adults and n = 36 children); white column, low risk genotype, DiaPep277 treated (n = 13 adults and n = 8 children).
Then, to further investigate the effect of DiaPep277 treatment in subjects with moderate HLA risk genotypes, the moderate risk group treated with DiaPep277 was compared with the moderate risk group treated with placebo. The only significant results were obtained in the adult patient groups where Δmaximal (12 months minus baseline) and ΔAUC (but not fasting) C-peptide values were significantly higher in the DiaPep277 treated versus placebo (−0.01 ± 0.26 vs. −0.31 ± 0.32 nmol/L, P < 0.01, and −1.62 ± 5 vs. −4.87 ± 4.61 nmol/L, P < 0.05, respectively).
DISCUSSION
Type 1 diabetes is considered a complex genetic disease with inherited genetic factors contributing to both susceptibility and resistance to the disease. There are at least four genetic loci conferring susceptibility to type 1 diabetes thought to be causally involved in the disease pathogenesis, the major genetic determinants being located in the major histocompatibility complex, in particular, alleles of the class II genes (16,17). A previous study performed in children with type 1 diabetes showed an accelerated decrease of C-peptide levels in subjects with high risk HLA genotype compared with those with lower risk HLA genotypes, which was detectable only soon after diagnosis (8). We also demonstrated that the high risk HLA genotype DRB1*03-DQB1*0201/DRB1*04-DQB1*0302 is independently associated, at the time of diagnosis, with lower C-peptide levels and a younger age at onset compared with lower risk HLA genotypes, indicating that the two variables exert a similar quantitative effect on residual β-cell function (8). More recently, we observed that low risk HLA genotypes are associated with reduced β-cell function 12 months after diagnosis, suggesting a less extensive process of β-cell destruction in subjects carrying such genotypes (10). This might be a critical aspect to take into consideration when choosing immunomodulatory therapies to apply to subjects with recent-onset type 1 diabetes.
DiaPep277 is the modified form of the dominant epitope of heat shock protein 60, a ubiquitous protein also located in mature insulin-secretory granules of pancreatic β-cells (18,19). DiaPep277 has been evaluated in clinical trials in recent-onset type 1 diabetes in virtue of its effect as an antigen immune modulator as well as by downregulating adaptive immune response and inhibiting inflammatory chemotaxis in vitro (20,21). In phase II clinical trials, DiaPep277 has shown suggestive evidence of preservation of β-cell function; the intervention group preserved mean C-peptide levels and required less exogenous insulin to obtain similar HbA1c as the placebo group at the end of the follow-up period (11,22). A similar trend toward a better maintenance of β-cell function after treatment with DiaPep277 was noted in other trials carried out in adult patients (12,23). Nevertheless, studies that evaluated the efficacy of DiaPep277 in children with type 1 diabetes report that in this age category, there was no beneficial effect in improving metabolic control or preserving β-cell function because a continuous, more rapid and pronounced deterioration was noted (12,13). On the other hand, an initial subanalysis performed in pediatric patients stratified according to HLA class II genotypes indicated more stable C-peptide responses in children with a moderate and low risk genotype treated with 1.0 mg DiaPep277 compared with placebo-treated patients possessing the same genotype (12).
This potentially interesting observation led us to evaluate the efficacy of DiaPep277 according to HLA class II genotype risk categories by looking at changes in C-peptide values. As expected, there was a significant difference in C-peptide secretion between subjects with high, moderate, and low risk HLA genotype at baseline, but this difference was apparent only for adults. This might imply that in the case of children, there are other (stronger) aspects/factors that influence the residual C-peptide secretion. Moreover, during the follow-up period after diagnosis, children presented a constant decline in C-peptide values regardless of therapy, and the difference from baseline at the end of follow-up was significantly greater as compared with adults. An important finding was that adult subjects with type 1 diabetes that have a low risk HLA genotype treated with DiaPep277 increased their C-peptide secretion (maximal and total AUC) at 12 months compared with placebo. In addition, adults with moderate risk genotype seem to benefit from therapy with DiaPep277 as well, since the moderate risk subgroup that received DiaPep277 showed a smaller decline of C-peptide secretion at 12 months compared with placebo-treated patients.
Because of the small number of subjects in the high risk HLA genotype, we could not consider this group alone (DiaPep277 treated and placebo); we had to combine groups with high and moderate risk genotype. At the same time, as a result of the number of subjects who were treated with DiaPep277, we could not separate further DiaPep277-treated subgroups according to the dose of drug received (0.2 mg, 1 mg, and 2.5 mg). It is possible that patients treated with a higher dose of DiaPep277 would have shown higher C-peptide secretion (probably more so in the low risk genotype subjects), but this assumption has to be validated by further studies.
The obvious question remains why such differences occur between adults and children in terms of efficacy of an intervention in preserving secretory capacity of β-cells. One might assume that there are age-dependent differences in the severity and natural disease progression that can influence response to treatment. From this perspective, the timing of intervention could be a critical issue, and children—having a more aggressive β-cell destruction—might need to start the immunomodulatory treatment sooner or be treated with higher doses for the intervention to be effective.
During the last decades, there has been an upsurge in the incidence of type 1 diabetes, with a higher increase in younger age groups (24,25). Analyses of the temporal changes in the distribution of HLA genotypes in individuals with type 1 diabetes indicate that in fact, the high risk genotypes are becoming less frequent in affected individuals and, concomitantly, the relative probability of developing the disease has increased considerably in subjects with low or moderate risk HLA genotypes (26–29). These temporal trends suggest an increasing environmental pressure resulting in higher penetrance of the disease in individuals with protective or lower risk HLA genotypes. However, such evidence has been reported, thus far, only in children and adolescents (26–29). If this trend is to be confirmed also for adult patients with lower HLA risk genotypes, an increasing number of subjects with type 1 diabetes might potentially achieve significant benefit with an immunomodulatory intervention.
We are aware that our study, as a result of the limited number of patients investigated, should be considered preliminary and exploratory. Data of exploratory studies can be analyzed without multiplicity adjustments possibly resulting in rejecting, falsely, a novel and original hypothesis. To confirm our results, which have the purpose to stimulate further research in this interesting field, the corresponding hypotheses have to be tested in future confirmatory studies considering a larger number of subjects with type 1 diabetes. Until then, these data should be taken as a potential line of research deserving further investigation to be implemented in clinical trials.
In addition, similar studies should be performed in subjects with type 1 diabetes exposed to other immunomodulatory compounds so that the correlation between the HLA genetic risk and the efficacy of any due immune intervention can be further exploited.
ACKNOWLEDGMENTS
This work was supported by Centro Internazionale Studi Diabete and Fondazione DEM, both in Italy.
No potential conflicts of interest relevant to this article were reported.
R.B. wrote the manuscript. S.C. contributed to data analysis and wrote the manuscript. A.P. and M.C. contributed to data analysis. M.S. and S.Z. contributed to HLA analysis. C.G. and C.V. wrote the manuscript. P.P. planned the research protocol and wrote the manuscript.
The authors thank Professor John F. Osborn, Department of Public Health Sciences and Infectious Diseases, University Sapienza, Rome, Italy, for statistical evaluation.
APPENDIX
Members of the DiaPep Trialists Group: A. Avron, DeveloGen Israel Ltd., Kiryat Weizmann, Rehovot, Israel; L. Barkai, Bajcsy-Zsilinszky Hospital, Budapest, Hungary; T. Battelino, University Children’s Hospital, Ljubljana, Slovenia; I.R. Cohen, Department of Immunology, Weizmann Institute of Science, Rehovot, Israel; R. Eldor, Department of Internal Medicine, Hadassah-Hebrew University Medical School, Jerusalem, Israel; D. Elias, DeveloGen Israel Ltd., Kiryat Weizmann, Rehovot, Israel; N. Hosszúfalusi, Bajcsy-Zsilinszky Hospital, Budapest, Hungary; G. Jermendy, Third Medical Department, Bajcsy-Zsilinsky Teaching Hospital, Budapest, Hungary; Z. Josefsberg, Institute for Endocrinology and Diabetes, National Center of Childhood Diabetes, Schneider Children's Medical Center of Israel, Petah Tikva, Israel, and Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel; A. Kovács, First Department of Medicine, Institute for Internal Medicine, Medical and Health Science Center, University of Debrecen, Hungary; L. Lazar, Institute for Endocrinology and Diabetes, National Center of Childhood Diabetes, Schneider Children's Medical Center of Israel, Petah Tikva, Israel, and Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel; C. Lengyel, Medical University, Szeged, Hungary; L. Madácsy, First Department of Pediatrics, Semmelweis University, Budapest, Hungary; G. Meierhoff, German Diabetes Clinic, German Diabetes Center, Heinrich-Heine University, Düsseldorf, Germany; M. Metzger, Department of Internal Medicine, Hadassah-Hebrew University Medical School, Jerusalem, Israel; R. Ofan, Institute for Endocrinology and Diabetes, National Center of Childhood Diabetes, Schneider Children's Medical Center of Israel, Petah Tikva, Israel; T. Oroszlán, County Hospital, Zalaegerszeg, Hungary; P. Pánczél, Third Medical Department, Bajcsy-Zsilinsky Teaching Hospital, Budapest, Hungary; M. Phillip, Institute for Endocrinology and Diabetes, National Center of Childhood Diabetes, Schneider Children's Medical Center of Israel, Petah Tikva, Israel, and Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel; I. Raz, Department of Internal Medicine, Hadassah-Hebrew University Medical School, Jerusalem, Israel; N.C. Schloot, German Diabetes Clinic, German Diabetes Center, Heinrich-Heine University, Düsseldorf, Germany; L. Symer, Department of Internal Medicine, Hadassah-Hebrew University Medical School, Jerusalem, Israel; G. Sütő, Medical University, Szeged, Hungary; J. Takács, Jahn Ferenc Hospital, Budapest, Hungary; M. Tamir, DeveloGen Israel Ltd., Kiryat Weizmann, Rehovot, Israel; G. Vándorfi, County Hospital, Zalaegerszeg, Hungary; and N. Weintrob, Institute for Endocrinology and Diabetes, National Center of Childhood Diabetes, Schneider Children's Medical Center of Israel, Petah Tikva, Israel, and Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db10-0560/-/DC1.
A complete list of the members of the DiaPep Trialists Group can be found in the appendix.
REFERENCES
- 1.Gianani R, Eisenbarth GS. The stages of type 1A diabetes: 2005. Immunol Rev 2005;204:232–249 [DOI] [PubMed] [Google Scholar]
- 2.Leslie RD, Delli Castelli M. Age-dependent influences on the origins of autoimmune diabetes: evidence and implications. Diabetes 2004;53:3033–3040 [DOI] [PubMed] [Google Scholar]
- 3.Bruno G, Cerutti F, Merletti F, et al. ; Piedmont Study Group for Diabetes Epidemiology. Residual beta-cell function and male/female ratio are higher in incident young adults than in children: the registry of type 1 diabetes of the province of Turin, Italy, 1984-2000. Diabetes Care 2005;28:312–317 [DOI] [PubMed] [Google Scholar]
- 4.Sabbah E, Savola K, Ebeling T, et al. Genetic, autoimmune, and clinical characteristics of childhood- and adult-onset type 1 diabetes. Diabetes Care 2000;23:1326–1332 [DOI] [PubMed] [Google Scholar]
- 5.Valdes AM, Thomson G, Erlich HA, Noble JA. Association between type 1 diabetes age of onset and HLA among sibling pairs. Diabetes 1999;48:1658–1661 [DOI] [PubMed] [Google Scholar]
- 6.Neu A, Ehehalt S, Willasch A, Kehrer M, Hub R, Ranke MB. Varying clinical presentations at onset of type 1 diabetes mellitus in children—epidemiological evidence for different subtypes of the disease? Pediatr Diabetes 2001;2:147–153 [DOI] [PubMed] [Google Scholar]
- 7.Seissler J, de Sonnaville JJ, Morgenthaler NG, et al. Immunological heterogeneity in type I diabetes: presence of distinct autoantibody patterns in patients with acute onset and slowly progressive disease. Diabetologia 1998;41:891–897 [DOI] [PubMed] [Google Scholar]
- 8.Petrone A, Galgani A, Spoletini M, et al. Residual insulin secretion at diagnosis of type 1 diabetes is independently associated with both, age of onset and HLA genotype. Diabetes Metab Res Rev 2005;21:271–275 [DOI] [PubMed] [Google Scholar]
- 9.Knip M, Ilonen J, Mustonen A, Akerblom HK. Evidence of an accelerated B-cell destruction in HLA-Dw3/Dw4 heterozygous children with type 1 (insulin-dependent) diabetes. Diabetologia 1986;29:347–351 [DOI] [PubMed] [Google Scholar]
- 10.Spoletini M, Petrone A, Zampetti S, et al. ; IMDIAB Group. Low-risk HLA genotype in Type 1 diabetes is associated with less destruction of pancreatic B-cells 12 months after diagnosis. Diabet Med 2007;24:1487–1490 [DOI] [PubMed] [Google Scholar]
- 11.Raz I, Avron A, Tamir M, et al. Treatment of new-onset type 1 diabetes with peptide DiaPep277 is safe and associated with preserved beta-cell function: extension of a randomized, double-blind, phase II trial. Diabetes Metab Res Rev 2007;23:292–298 [DOI] [PubMed] [Google Scholar]
- 12.Schloot NC, Meierhoff G, Lengyel C, et al. Effect of heat shock protein peptide DiaPep277 on beta-cell function in paediatric and adult patients with recent-onset diabetes mellitus type 1: two prospective, randomized, double-blind phase II trials. Diabetes Metab Res Rev 2007;23:276–285 [DOI] [PubMed] [Google Scholar]
- 13.Lazar L, Ofan R, Weintrob N, et al. Heat-shock protein peptide DiaPep277 treatment in children with newly diagnosed type 1 diabetes: a randomised, double-blind phase II study. Diabetes Metab Res Rev 2007;23:286–291 [DOI] [PubMed] [Google Scholar]
- 14.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2000;23(Suppl. 1):S4–S19 [PubMed] [Google Scholar]
- 15.Petrone A, Battelino T, Krzisnik C, et al. Similar incidence of type 1 diabetes in two ethnically different populations (Italy and Slovenia) is sustained by similar HLA susceptible/protective haplotype frequencies. Tissue Antigens 2002;60:244–253 [DOI] [PubMed] [Google Scholar]
- 16.Cerná M. Genetics of autoimmune diabetes mellitus. Wien Med Wochenschr 2008;158:2–12 [DOI] [PubMed] [Google Scholar]
- 17.Maier LM, Wicker LS. Genetic susceptibility to type 1 diabetes. Curr Opin Immunol 2005;17:601–608 [DOI] [PubMed] [Google Scholar]
- 18.Birk OS, Elias D, Weiss AS, et al. NOD mouse diabetes: the ubiquitous mouse hsp60 is a beta-cell target antigen of autoimmune T cells. J Autoimmun 1996;9:159–166 [DOI] [PubMed] [Google Scholar]
- 19.Brudzynski K, Martinez V, Gupta RS. Immunocytochemical localization of heat-shock protein 60-related protein in beta-cell secretory granules and its altered distribution in non-obese diabetic mice. Diabetologia 1992;35:316–324 [DOI] [PubMed] [Google Scholar]
- 20.Zanin-Zhorov A, Cahalon L, Tal G, Margalit R, Lider O, Cohen IR. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest 2006;116:2022–2032 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Nussbaum G, Zanin-Zhorov A, Quintana F, Lider O, Cohen IR. Peptide p277 of HSP60 signals T cells: inhibition of inflammatory chemotaxis. Int Immunol 2006;18:1413–1419 [DOI] [PubMed] [Google Scholar]
- 22.Raz I, Elias D, Avron A, Tamir M, Metzger M, Cohen IR. β-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet 2001;358:1749–1753 [DOI] [PubMed] [Google Scholar]
- 23.Huurman VA, Decochez K, Mathieu C, Cohen IR, Roep BO. Therapy with the hsp60 peptide DiaPep277 in C-peptide positive type 1 diabetes patients. Diabetes Metab Res Rev 2007;23:269–275 [DOI] [PubMed] [Google Scholar]
- 24.Gale EA. The rise of childhood type 1 diabetes in the 20th century. Diabetes 2002;51:3353–3361 [DOI] [PubMed] [Google Scholar]
- 25.Global Burden of Youth Diabetes: Perspectives and Potential. Chapter One: Diabetes in children: epidemiology. Pediatr Diabetes 2007;8(Suppl. 8):10–18 [DOI] [PubMed] [Google Scholar]
- 26.Vehik K, Hamman RF, Lezotte D, et al. Trends in high-risk HLA susceptibility genes among Colorado youth with type 1 diabetes. Diabetes Care 2008;31:1392–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hermann R, Knip M, Veijola R, et al. ; FinnDiane Study Group. Temporal changes in the frequencies of HLA genotypes in patients with Type 1 diabetes—indication of an increased environmental pressure? Diabetologia 2003;46:420–425 [DOI] [PubMed] [Google Scholar]
- 28.Gillespie KM, Bain SC, Barnett AH, et al. The rising incidence of childhood type 1 diabetes and reduced contribution of high-risk HLA haplotypes. Lancet 2004;364:1699–1700 [DOI] [PubMed] [Google Scholar]
- 29.Fourlanos S, Varney MD, Tait BD, et al. The rising incidence of type 1 diabetes is accounted for by cases with lower-risk human leukocyte antigen genotypes. Diabetes Care 2008;31:1546–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]


