Abstract
OBJECTIVE
Vitamin D deficiency is associated with an unfavorable metabolic profile in observational studies. The intention was to compare insulin sensitivity (the primary end point) and secretion and lipids in subjects with low and high serum 25(OH)D (25-hydroxyvitamin D) levels and to assess the effect of vitamin D supplementation on the same outcomes among the participants with low serum 25(OH)D levels.
RESEARCH DESIGN AND METHODS
Participants were recruited from a population-based study (the Tromsø Study) based on their serum 25(OH)D measurements. A 3-h hyperglycemic clamp was performed, and the participants with low serum 25(OH)D levels were thereafter randomized to receive capsules of 20,000 IU vitamin D3 or identical-looking placebo twice weekly for 6 months. A final hyperglycemic clamp was then performed.
RESULTS
The 52 participants with high serum 25(OH)D levels (85.6 ± 13.5 nmol/L [mean ± SD]) had significantly higher insulin sensitivity index (ISI) and lower HbA1c and triglycerides (TGs) than the 108 participants with low serum 25(OH)D (40.3 ± 12.8 nmol/L), but the differences in ISI and TGs were not significant after adjustments. After supplementation, serum 25(OH)D was 142.7 ± 25.7 and 42.9 ± 17.3 nmol/L in 49 of 51 completing participants randomized to vitamin D and 45 of 53 randomized to placebo, respectively. At the end of the study, there were no statistically significant differences in the outcome variables between the two groups.
CONCLUSIONS
Vitamin D supplementation to apparently healthy subjects with insufficient serum 25(OH)D levels does not improve insulin sensitivity or secretion or serum lipid profile.
Type 2 diabetes is a chronic condition associated with increased risk of micro- and macrovascular morbidity (1). The underlying pathophysiological mechanisms include insulin resistance combined with a relative deficit of insulin secretion from the pancreas, usually accompanied by systemic inflammation (2). The number of people suffering from the disease is increasing globally (2). Effective preventive means are therefore needed, and modifiable risk factors should be identified and explored.
Vitamin D insufficiency, which is reported to be highly prevalent (3), might be such a factor. The vitamin D receptor (4) and the enzyme 1-α hydroxylase (5), which is necessary for the production of the active form of the hormone 1,25(OH)2D (1,25-dihydroxyvitamin D), are present in pancreatic β-cells. Accordingly, vitamin D has been reported to increase glucose-mediated insulin secretion in animal studies (6). In vitro, 1,25(OH)2D increases the expression of the insulin receptor and enhances insulin-mediated glucose transport (7). Although less explored, the anti-inflammatory effects of vitamin D might also affect diabetes development (8).
Consistent with this, observational data from a number of epidemiological studies show an inverse association between serum 25(OH)D (25-hydroxyvitamin D) and glucose levels (9–12), insulin resistance (11–18), and prevalence of type 2 diabetes (18–20). However, to demonstrate a causal relation between vitamin D and glucose metabolism, evidence from randomized and adequately powered placebo-controlled intervention trials is needed. As recently reviewed, the studies published thus far are heterogeneous regarding dose and formulation of vitamin D treatment, duration, and inclusion criteria; most use indirect measures of insulin secretion and sensitivity; and the results are inconsistent (21).
In the sixth Tromsø Study in 2008, serum 25(OH)D was measured in nearly 12,000 subjects. On the basis of these measurements, we invited subjects with low or high serum 25(OH)D levels to a follow-up study where insulin sensitivity and secretion were evaluated with the hyperglycemic clamp technique. Thereafter, the subjects with low serum 25(OH)D levels were invited to a 6-month intervention study to compare the effect of vitamin D3 20,000 IU twice per week with placebo on the same measures. As we previously have found cross-sectional and longitudinal associations between serum 25(OH)D levels and serum lipids (22), measurements of serum lipids were also included.
RESEARCH DESIGN AND METHODS
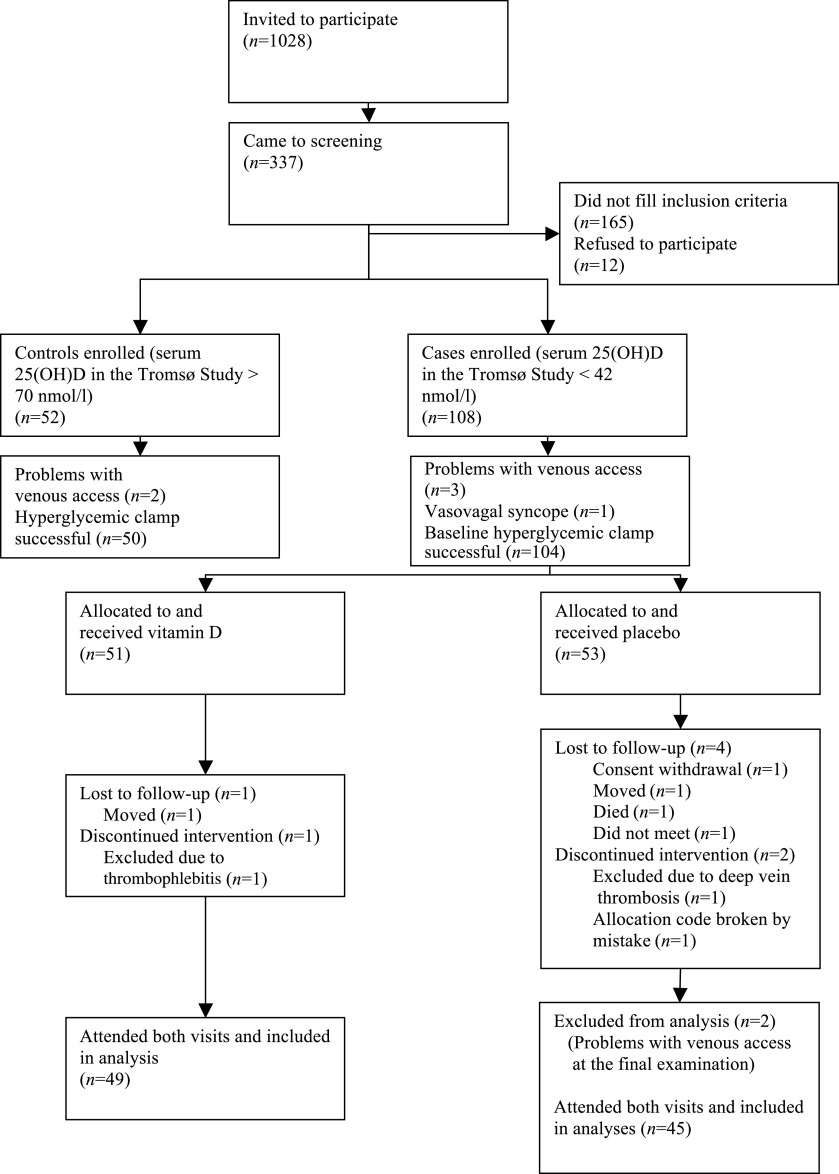
Subjects aged 30–75 years previously participating in the sixth Tromsø Study were invited to participate. The Tromsø Study is an ongoing longitudinal population-based study first performed in 1974 (23). The sixth survey was performed in 2008 and the following groups were invited: those who participated in the second phase of the fourth survey (1994–1995), a random 10% sample of subjects aged 30–39 years, all subjects aged 40–42 and 60–87 years, and a 40% random sample of subjects aged 43–59 years. In total, 19,762 subjects were invited and 12,984 subjects (65.7%) attended (23). Serum 25(OH)D measurements were performed in all the participants, and people with serum 25(OH)D between the 5th and 10th percentiles (low serum 25[OH]D; case subjects) or between the 80th and 95th percentiles (high serum 25[OH]D; control subjects) were invited to the current study by mail. Those who reported to be current smokers were not invited owing to a newly discovered interference between smoking and the assay used for serum 25(OH)D analyses in the sixth Tromsø Study (24). The invitation letter did not disclose the subject’s vitamin D status. A person not involved in the examinations administered the invitations to achieve a fairly equal distribution among case and control subjects regarding sex, age, and BMI. However, there was no head-to-head matching. Exclusion criteria were diabetes, acute myocardial infarction or stroke during the past 12 months, cancer during the past 5 years, steroid use, serum creatinine ≥130 μmol/L (males) or ≥110 μmol/L (females), possible primary hyperparathyroidism (plasma parathyroid hormone [PTH] >5.0 pmol/L combined with serum calcium >2.50 mmol/L), sarcoidosis, systolic blood pressure >175 mmHg or diastolic blood pressure >105 mmHg, and specifically for women, pregnancy, lactation, or fertile age and no contraception use. Participants were recruited between November 2008 and April 2010. Because there was a considerable time span between the first serum 25(OH)D measurement in the Tromsø Study and inclusion in the current study, a low or high serum 25(OH)D in the Tromsø Study had to be confirmed in a new serum sample before inclusion. The flowchart shows the recruitment of participants (Fig. 1).
FIG. 1.
Flowchart showing recruitment of participants, randomization, and completion.
Protocol.
The study consisted of a nested case-control study, comparing participants with low and high serum 25(OH)D levels, and a randomized controlled trial (RCT), comparing 6 months of supplementation with high dose vitamin D3 versus placebo in the participants with low serum 25(OH)D levels.
Those who had accepted the study invitation attended a screening examination where medical history, blood pressure, and blood samples for PTH, calcium, creatinine, HbA1c, and 25(OH)D were obtained. Subjects with serum HbA1c >6.1% underwent an oral glucose tolerance test and were not included if this test showed impaired fasting glucose or reduced glucose tolerance (fasting plasma glucose >6.0 mmol/L and/or 2-h value >7.7 mmol/L). Subsequent eligible participants came fasting to a baseline visit at the Research Unit, University Hospital of North Norway, where fasting blood samples for serum lipids were drawn and a standard hyperglycemic clamp performed to study insulin secretion and sensitivity (15,25).
After the baseline clamp was finished, a sealed envelope (prepared for each participant by people not performing the examinations) was opened, revealing whether the participant was a case subject (low serum 25[OH]D) or a control subject (high serum 25[OH]D). Thus, neither the participants nor the staff knew the vitamin D status when the clamp was performed. If the participant was a control subject, involvement in the study was now completed. The case subjects were immediately randomized to either one capsule of vitamin D3 20,000 IU (Dekristol; Mibe, Brehna, Germany) or identical-looking placebo capsules to be taken twice per week. The randomization was performed by the central randomization unit at the University Hospital of North Norway, using block randomization with various block sizes. The randomization numbers with treatment allocations were given directly to the hospital pharmacy who prepared the medication boxes, which were delivered to the study nurse at baseline for each participant. Thus, the study was a randomized, double-blind, controlled trial where neither the participants, the staff performing the examinations, nor the researchers knew the randomization status of the participants during the study. A study nurse contacted the participants by phone after 1 and 3 months to ensure that the study medication was taken correctly and to register adverse events. After 6 months, participants came to the final examination where a new hyperglycemic clamp was performed. Unused study medication was returned and counted. The participants received a gift card valued at $90 for the baseline as well as the 6-month visits.
Measurements.
Physical activity was self-reported in the Tromsø Study by indicating usual level of leisure activity in the past year using one of four response categories: level 1, reading, watching television, or engaging in sedentary activities; level 2, at least 4 h a week walking, bicycling, or engaging in other types of physical activity; level 3, at least 4 h a week exercising to keep fit and participating in recreational athletics; and level 4, regular, vigorous training or participating in competitive sports several times a week. This physical activity assessment method has been observed to reflect heart rate, fitness, and metabolic profile, as well as objectively measured activity by accelerometer (26). Fat fish intake was self-reported in the Tromsø Study using the following alternatives: 1) 0–1 times per month; 2) 2–3 times per month; 3) 1–3 times per week; 4) 4–6 times per week; and 5) 1–2 times per day. Because of the small number of participants in the two highest frequency groups (three case subjects and four control subjects), groups 3–5 were assessed together in the analyses. At screening, the participants reported intake of cod liver oil or other vitamin D supplements, any sun bed use during the past year, and sunny holiday during the past 3 months. These were all coded as dichotomous variables (yes/no). Tromsø Study participants also reported number of glasses of milk (any kind) per day, sandwiches with cheese (any kind) per day, and servings of yogurt (125 mL) per week. Thus, we calculated the number of dairy product servings per week as a crude measure of calcium intake.
The hyperglycemic clamp was performed as previously described (15,25,27). The participants came fasting in the morning. Body weight and height were measured with light clothing and no shoes. The participants voided before and after the clamp for measurement of urinary glucose loss. After a stabilization period of 30 min, a bolus dose of dextrose 200 mg/mL was infused (150 mg/kg) into an antecubital vein. Blood was drawn from a cannulated dorsal vein on the opposite hand, where the blood was kept arterialized by keeping the hand in a heating device. Every 5th min, venous plasma glucose was analyzed on a YSI Life Sciences glucose analyzer (2300 STAT PLUS; Yellow Springs, OH), and the infusion was accordingly adjusted to achieve a stable glucose level of 10 mmol/L. Serum samples for insulin were drawn at 0, 2.5, 5, 7.5, 10, 15, 30, 60, 90, 120, 140, 160, and 180 min. First phase insulin release, reflecting the early insulin peak secreted from the pancreatic β-cell in response to glucose stimulation, was calculated as the area under the curve (AUC) during the first 10 min of the clamp by using the trapezium rule. Second phase insulin release, reflecting β-cell function under sustained elevated glucose levels, was calculated as AUC during the last hour (120–180 min). As a measure of glucose tolerance, glucose metabolized (M) the last hour of the clamp was calculated as M = INF − UC − SC, where INF is the glucose infusion rate, UC is the correction for urinary loss of glucose, and SC is the space correction (μmol ⋅ min−1 ⋅ kg−1) (28). Insulin sensitivity index (ISI) was calculated by dividing the mean INF (μmol ⋅ kg−1 ⋅ min−1) during the last hour by the average serum insulin level during the same period (pmol/L). To compare with other trials (29), insulin resistance from homeostasis model assessment (HOMA-IR) was calculated as fasting serum insulin (μU/mL) × fasting plasma glucose (mmol/L)/22.5 (30).
Serum 25(OH)D was measured in the Tromsø Study and at screening using an electrochemiluminescence immunoassay (Modular E170; Roche Diagnostics, Mannheim, Germany) (24). This method was later withdrawn, and sera from the baseline and final visits, stored at −70°C, were analyzed for serum 25(OH)D at the Hormone Laboratory, Haukeland University Hospital, using an in-house–developed liquid chromatography double mass spectrometry method (24). The intra-assay coefficient of variation (CV) was 1.7–2.6% for low, medium, and high levels of 25(OH)D. The other analyses were performed successively at the Department of Medical Biochemistry, University Hospital of North Norway. Serum calcium was analyzed by colorimetry using an automated clinical chemistry analyzer, serum creatinine was analyzed by an enzymatic colorimetric method (CREA plus, Roche Diagnostics), and serum phosphate was analyzed by photometric end point measurement. Modular P (Roche Diagnostics) was applied for all these analyses. Reference ranges were as follows: serum calcium 2.15–2.55 mmol/L, serum creatinine 50–90 μmol/L, and serum phosphate 0.76–1.41 mmol/L. Serum ionized calcium was analyzed by ion selectivity (ABL 800 Flex; Radiometer America Inc., Westlake, OH), reference range 1.10–1.34 mmol/L. Serum insulin was measured by electrochemiluminescence immunoassay (Modular E170, Roche Diagnostics), reference ranges of 18–173 pmol/L, whereas high-sensitivity C-reactive protein (hs-CRP) was measured by immunoturbidimetry (Modular P, Roche Diagnostics). Plasma PTH, serum glycated hemoglobin (HbA1c), total cholesterol, triglycerides (TGs), LDL cholesterol, and HDL cholesterol were analyzed as previously described (22,24,27). Before each clamp, a quality control of the YSI glucose measurement was performed using two industrial made and two human standards. The producer recommended using a CV of ≤5%; however, 89% of the controls were within a limit of CV ≤2%.
Statistical analyses.
The data were checked for normal distribution using visual inspection of histograms, and skewed variables were log transformed before statistical analyses when appropriate. For between-group comparisons of case and control subjects in the nested case-control study and for baseline values and Δ-values (6 months minus baseline) of the two treatment groups in the intervention study, Student t test or χ2 tests were used. Paired t tests were used to analyze changes from baseline to 6 months within each treatment group. To control for possible confounders, general linear models were used to compare case and control subjects at baseline. Because use of statins affects serum lipid and CRP levels, the analyses were also performed with the statin users excluded.
To compare the effect of vitamin D and placebo on the outcome variables, we also used ANCOVA models adjusting for the baseline value (31), presenting the relative effect of vitamin D to the effect of placebo (set to 1 as reference). The results from the intervention study were analyzed both as intention-to-treat analyses (with last observation carried forward) and per-protocol analyses. Tests for interactions between treatment group and sex, above or below median of HOMA-IR, BMI, or dairy servings per week, were performed for the primary outcome variable ISI.
Data are presented as mean ± SD for normally distributed variables and as median (5th to 95th percentiles) for nonnormally distributed variables, unless otherwise indicated. All statistical analyses were performed using the statistical software package SPSS 16.0 (SPSS Inc., Chicago, IL), and P < 0.05 was considered a significant finding.
Power calculations.
Power calculation prior to the study was based upon a previous work, where ISIs in healthy control subjects were 0.19 ± 0.10 mg ⋅ kg−1 ⋅ min−1/μU ⋅ mL−1 (0.15 ± 0.08 μmol ⋅ kg−1 ⋅ min−1/pmol ⋅ L−1) (15). The difference in ISI between the upper and lower half of serum 25(OH)D was 0.12 mg ⋅ kg−1 ⋅ min−1/μU ⋅ mL−1 (0.10 μmol ⋅ kg−1 ⋅ min−1/pmol ⋅ L−1). Defining a difference of 0.10 mg ⋅ kg−1 ⋅ min−1/μU ⋅ mL−1 (0.08 μmol ⋅ kg−1 ⋅ min−1/pmol ⋅ L−1) as being of clinical relevance, 40 people in each group would be needed to have a power of 90% to detect such a difference with a significance level of 0.05. To account for dropouts and nonsuccessful clamps, the inclusion of 100 case subjects and 50 control subjects was planned.
Ethics.
The study was approved by the Regional Committee for Medical Research Ethics and the Norwegian Medicines Agency. All participants signed an informed consent prior to inclusion.
RESULTS
The nested case-control study.
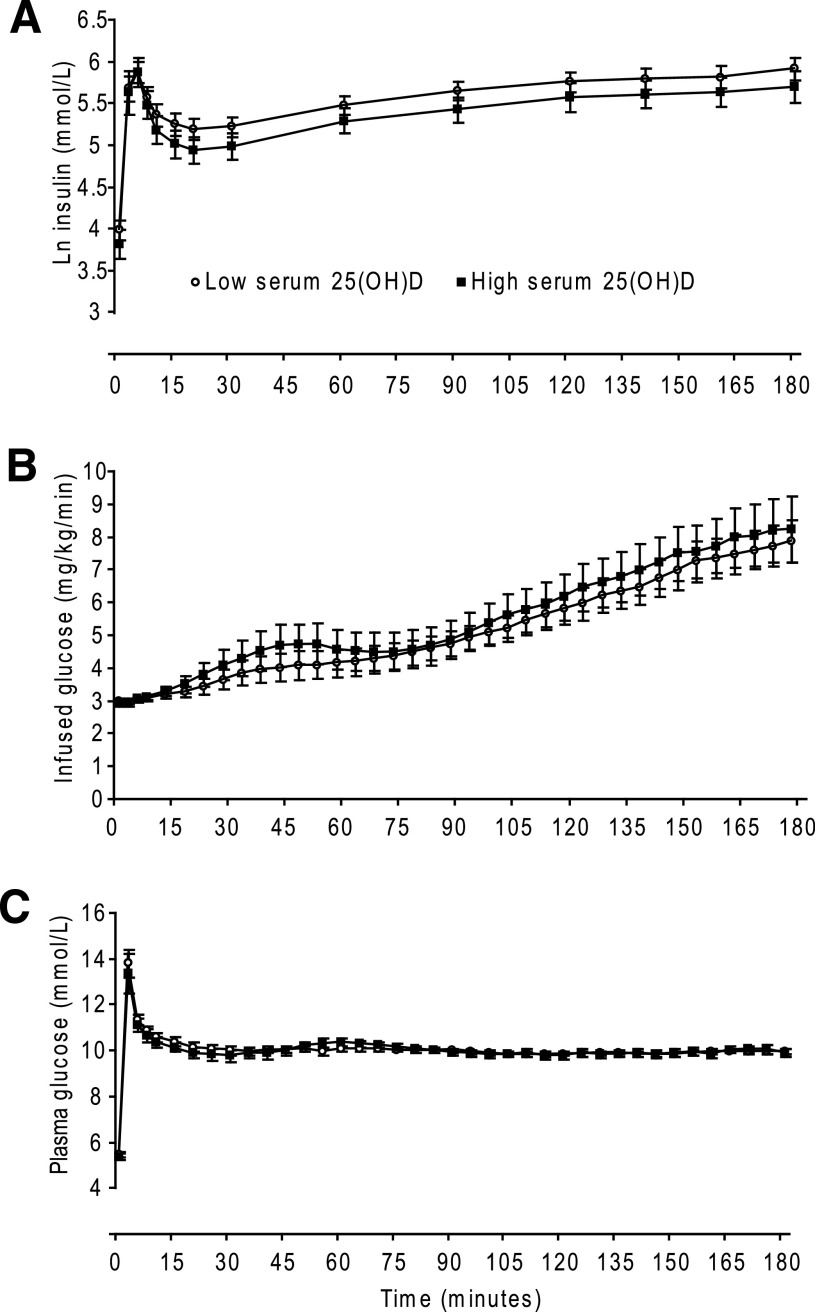
A total of 108 case subjects and 52 control subjects attended the baseline examination and a successful hyperglycemic clamp was performed in 104 and 50 participants, respectively (Fig. 1). Mean plasma glucose level the last hour of the clamp was 9.9 ± 0.3 mmol/L, with no difference between case and control subjects (P = 0.85). Figure 2 shows plasma glucose levels, infused glucose (mg/kg/min), and serum insulin levels in the case and control subjects.
FIG. 2.
Results from a 3-h hyperglycemic clamp in 104 subjects with low serum 25(OH)D (○) and 50 subjects with high serum 25(OH)D (■). A: Mean (95% CI) ln serum insulin. B: Mean (95% CI) infused glucose. C: Mean (95% CI) plasma glucose.
The serum 25(OH)D levels in the sixth Tromsø Study were 33.9 ± 5.5 for case subjects and 77.6 ± 4.9 nmol/L for control subjects, with corresponding baseline values 40.3 ± 12.8 and 85.6 ± 13.5 nmol/L (Table 1). Median time (5th to 95th percentiles) between attendance date in the sixth Tromsø Study and baseline was 13 months (8–26). As expected according to the inclusion procedure, there were no significant differences between case and control subjects at baseline regarding age, BMI, or sex. Leisure-time physical activity level was significantly higher in control subjects, as were use of vitamin D supplementation, sun bed use, and sunny holidays during the past 3 months. Serum PTH, TGs, and HbA1c were significantly higher in case subjects, while ISI was lower (Table 1 and Fig. 3). These results were similar also when statin users were excluded (data not shown).
TABLE 1.
Baseline characteristics in subjects with low serum 25(OH)D and subjects with high serum 25(OH)D
| Low serum 25(OH)D (case subjects, n = 108) | High serum 25(OH)D (control subjects, n = 52) | P value | |
|---|---|---|---|
| Age (years) | 52.1 ± 9.3 | 53.9 ± 10.5 | 0.28 |
| Female, n (%) | 53 (49.1) | 25 (48.1) | 0.91 |
| BMI (kg/m2) | 26.5 ± 3.1‡ | 26.3 ± 3.0† | 0.65 |
| Statin user, n (%) | 5 (4.6) | 6 (11.5) | 0.11 |
| Fat fish intake, n (%) | 0.054 | ||
| ≤1 time per month | 28 (25.9) | 6 (11.5) | |
| 2–3 times per month | 35 (32.4) | 17 (32.7) | |
| ≥1 time per week | 41 (38.0) | 25 (55.8) | |
| Missing | 4 (3.7) | 0 | |
| Physical activity level, leisure time, n (%) | 0.04 | ||
| Sedentary | 25 (23.1) | 6 (11.5) | |
| Moderate | 59 (54.6) | 24 (46.2) | |
| Hard | 19 (17.6) | 17 (32.7) | |
| Vigorous | 3 (2.8) | 4 (7.7) | |
| Missing | 2 (1.9) | 1 (1.9) | |
| Dairy products, servings per week | 16 (9–46) | 21 (4–58) | 0.08 |
| Vitamin D supplementation, n (%) | 26 (24.1) | 30 (57.7) | <0.01 |
| Sun bed use past year, n (%) | 6 (5.6) | 8 (15.4) | 0.04 |
| Sunny holiday past 3 months, n (%) | 8 (7.4) | 17 (32.7) | <0.01 |
| Serum 25(OH)D (nmol/L) | 40.3 ± 12.8‡ | 85.6 ± 13.5† | <0.01 |
| Plasma PTH (pmol/L) | 5.95 ± 1.56* | 5.08 ± 1.65* | <0.01 |
| Serum calcium (mmol/L) | 2.25 (2.15–2.40) | 2.25 (2.15–2.42) | 0.84 |
| Serum ionized calcium (mmol/L) | 1.22 ± 0.03‖ | 1.22 ± 0.03† | 0.92 |
| Fasting plasma glucose (mmol/L) | 5.42 ± 0.61* | 5.35 ± 0.60† | 0.54 |
| Serum HbA1c (%) | 5.55 ± 0.35 | 5.37 ± 0.36 | <0.01 |
| Serum cholesterol (mmol/L) | 5.5 (4.0–7.6)* | 5.3 (4.1–7.4)* | 0.51 |
| Serum HDL cholesterol (mmol/L) | 1.4 (1.0–2.3)* | 1.5 (1.0–2.4)* | 0.19 |
| Serum LDL cholesterol (mmol/L) | 3.54 ± 0.99* | 3.45 ± 0.90* | 0.58 |
| Serum TG (mmol/L) | 0.9 (0.5–2.9)*¶ | 0.9 (0.5–2.2)*# | 0.03 |
| Serum hs-CRP (mg/L) | 1.10 (0.29–8.41)* | 1.17 (0.27–4.94)* | 0.40 |
| Fasting serum insulin (pmol/L) | 53 (18–171)‡ | 45 (14–145)† | 0.13 |
| AUC insulin 0–10 min (pmol/L ⋅ min) | 2,630 (1,013–7,827)‡ | 2,826 (727–6,026)† | 0.68 |
| AUC insulin 120–180 min (pmol/L ⋅ min) | 19,650 (6,782–59,888)‖ | 16,390 (5,171–40,235)† | 0.08 |
| HOMA-IR | 1.83 (0.56–5.93)§ | 1.51 (0.44–5.27)‡ | 0.14 |
| M 120–180 min (μmol ⋅ min−1 ⋅ kg−1) | 34.7 (17.9–66.2)§ | 37.9 (18.6–68.1)† | 0.31 |
| ISI 120–180 min (μmol ⋅ min−1 ⋅ kg−1/pmol−1 ⋅ L−1) | 0.12 (0.03–0.34)‖ | 0.15 (0.03–0.43)† | 0.04 |
Unless otherwise stated, data are means ± SD if normally distributed and median (5th to 95th percentiles) if skewed. Skewed variables were log transformed before further analyses. Comparisons between case and control subjects were performed with t tests for independent groups for continuous data and χ2 test for categorical data.
*Data missing in one participant.
†Data missing in two participants.
‡Data missing in three participants.
§Data missing in four participants.
‖Data missing in five participants.
¶Geometric mean 1.07 mmol/L.
#Geometric mean 0.89 mmol/L.
FIG. 3.
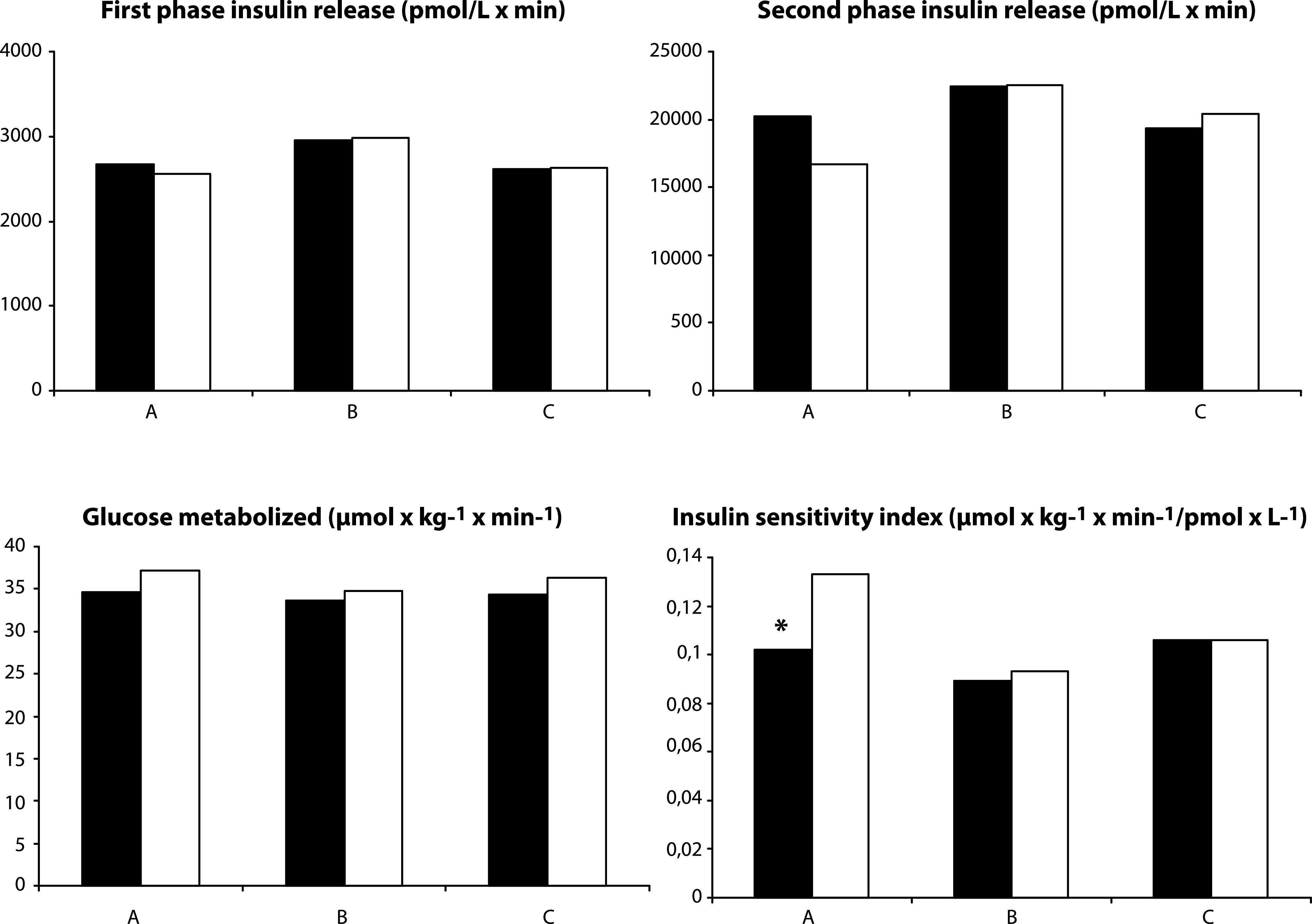
First phase insulin release (AUC 0–10 min), second phase insulin release (AUC 120–180 min), M, and ISI from a 3-h hyperglycemic clamp comparing participants with low and high serum 25(OH)D at baseline, and at baseline and after completion in the RCT. Data are geometric means. A: Subjects with low serum 25(OH)D (n = 104) (black bars) and high serum 25(OH)D (n = 50) (white bars) levels at baseline. B: Subjects with low serum 25(OH)D (n = 49) randomized to high dose vitamin D3 at baseline (black bars) and after 6 months (white bars). C: Subjects with low serum 25(OH)D (n = 45) randomized to placebo at baseline (black bars) and after 6 months (white bars). *P < 0.05.
These differences between the groups regarding ISI, HbA1c, and TGs were still significant after adjustment for age, sex, and BMI. Adjustment for physical activity level attenuated the differences so that they became nonsignificant for ISI and TGs (Table 2). The difference in HbA1c remained significant also after additional adjustment for fat fish intake, dairy servings per week, vitamin D supplementation, sunny holidays, and sun bed use (Table 2).
TABLE 2.
Comparisons of participants with low and high baseline serum 25(OH)D
| Low serum 25(OH)D (case subjects, n = 105) | High serum 25(OH)D (control subjects, n = 50) | P value | |
|---|---|---|---|
| Ln ISI 120–180 min (μmol ⋅ min−1 ⋅ kg−1/pmol−1 ⋅ L−1) | |||
| Model 1 | −2.27 (−2.39 to −2.16)† | −2.03 (−2.20 to −1.87) | 0.02 |
| Model 2 | −2.25 (−2.36 to −2.14)† | −2.08 (−2.25 to −1.92) | 0.10 |
| Model 3 | −2.25 (−2.37 to −2.13)‡ | −2.11 (−2.29 to −1.92)* | 0.24 |
| HbA1c (%) | |||
| Model 1 | 5.56 (5.50 to 5.62) | 5.36 (5.27 to 5.44) | <0.01 |
| Model 2 | 5.56 (5.50 to 5.62) | 5.35 (5.27 to 5.44) | <0.01 |
| Model 3 | 5.57 (5.50 to 5.63)† | 5.33 (5.23 to 5.42)* | <0.01 |
| Ln TG (mmol/L) | |||
| Model 1 | 0.050 (−0.040 to 0.140) | −0.097 (−0.228 to 0.034) | 0.07 |
| Model 2 | 0.046 (−0.043 to 0.135) | −0.088 (−0.219 to 0.043) | 0.10 |
| Model 3 | 0.054 (−0.042 to 0.150)† | −0.093 (−0.241 to 0.054)* | 0.12 |
| Ln TG (mmol/L)§ | |||
| Model 1 | 0.041 (−0.050 to 0.132) | −0.140 (−0.278 to −0.003) | 0.03 |
| Model 2 | 0.036 (−0.055 to 0.127) | −0.129 (−0.267 to 0.008) | 0.051 |
| Model 3 | 0.038 (−0.058 to 0.135)† | −0.123 (−0.278 to 0.031)* | 0.10 |
Data are estimated means (95% CI), using general linear models. Model 1: means adjusted for age, sex, and BMI; model 2: model 1 plus leisure time physical activity; and model 3: model 2 plus fat fish intake, dairy servings per week, sun bed use, sunny holidays past 3 months, and vitamin D supplementation.
*Data missing in one participant.
†Data missing in two participants.
‡Data missing in four participants.
§Statin users excluded (five case subjects and six control subjects).
The intervention study.
From the 108 case subjects with low serum 25(OH)D, 51 were randomized to vitamin D treatment and 53 to placebo, and 49 and 45 case subjects, respectively, completed the study. Figure 1 shows the reasons for dropouts and exclusions. Compliance was 98 and 97% in the treatment and placebo group, respectively, and all but 1 subject in each group had a compliance >80%.
Serum 25(OH)D increased to a mean of 142.7 nmol/L in the treatment group and remained low in the placebo group (42.9 nmol/L) with no overlap between the groups (Table 3). Conversely, plasma PTH decreased in the treatment group while remaining unchanged in the placebo group. There were no statistically significant differences between the two groups after 6 months regarding measures of insulin secretion, insulin sensitivity, or lipids (Table 3 and Figs. 3 and 4). The results were essentially the same if statin users were excluded, if the analyses were performed as intention-to-treat analyses or per-protocol analyses, or if adjustments for baseline BMI, sex, and age were performed. The treatment group had a statistically significant increase in fasting glucose and HbA1c during the study and also a slight decrease in BMI; however, these changes were not significantly different from the placebo group (Table 3). There were no statistical interactions between treatment group and sex, below/above median HOMA-IR, BMI, or dairy servings per week on the effect on ISI (P = 0.94, 0.19, 0.38, and 0.09, respectively). Accordingly, analyses stratified by sex, above/below baseline median of HOMA-IR (1.83), BMI (26.8 kg/m2), or dairy servings per week (18 servings) revealed similar results (data not shown).
TABLE 3.
Baseline, final, and Δ-values and relative differences between treatment and placebo groups in the 94 subjects who completed the intervention study
| Vitamin D group (n = 49) |
Placebo group (n = 45) |
P for difference between groups at baseline | P for difference between Δ-values | Relative differences (95% CI) between Δ-values adjusted for baseline values (placebo group as reference) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 months | Δ-value | Baseline | 6 months | Δ-value | P | ||||
| Age (years) | 51.5 ± 8.8 | 52.7 ± 9.7 | 0.53 | |||||||
| Female, n (%) | 22 (45) | 23 (51) | 0.55 | |||||||
| BMI (kg/m2) | 27.2 ± 3.1 | 26.8 ± 3.2*† | −0.31 ± 0.85† | 26.3 ± 2.9 | 26.2 ± 2.8 | −0.09 ± 0.96 | 0.15 | 0.24 | 0.99 (0.98–1.01) | 0.28 |
| Statin user, n (%) | 2 (4.1) | 3 (6.7) | 0.58 | |||||||
| Serum 25(OH)D (nmol/L) | 42.2 ± 13.9 | 142.7 ± 25.2** | 100.5 ± 27.8 | 39.2 ± 12.1 | 42.9 ± 17.3 | 3.7 ± 17.5 | 0.27 | <0.01 | 3.44 (3.07–3.86) | <0.01 |
| Plasma PTH (pmol/L) | 6.11 ± 1.36 | 5.15 ± 1.28**† | −0.99 ± 1.06† | 5.86 ± 1.80 | 5.77 ± 1.95 | −0.09 ± 1.19 | 0.45 | <0.01 | 0.86 (0.80–0.94) | <0.01 |
| Serum calcium (mmol/L) | 2.26 ± 0.07 | 2.26 ± 0.07 | −0.004 ± 0.074 | 2.25 ± 0.08 | 2.24 ± 0.08 | −0.018 ± 0.075 | 0.60 | 0.37 | 1.01 (1.00–1.02) | 0.21 |
| Serum ionized calcium (mmol/L) | 1.22 ± 0.03‡ | 1.22 ± 0.04 | 0.002 ± 0.028‡ | 1.22 ± 0.04† | 1.22 ± 0.04† | −0.005 ± 0.028‡ | 0.92 | 0.30 | 1.01 (1.00–1.02) | 0.29 |
| Fasting plasma glucose (mmol/L) | 5.40 ± 0.59 | 5.61 ± 0.69* | 0.21 ± 0.57 | 5.44 ± 0.59 | 5.44 ± 0.52 | −0.00 ± 0.58 | 0.74 | 0.07 | 1.03 (0.99–1.07) | 0.10 |
| Serum HbA1c (%) | 5.54 ± 0.36 | 5.64 ± 0.38*† | 0.11 ± 0.32† | 5.59 ± 0.37 | 5.64 ± 0.28 | 0.05 ± 0.28 | 0.44 | 0.35 | 1.01 (0.99–1.03) | 0.60 |
| Serum cholesterol (mmol/L) | 5.47 ± 1.02 | 5.50 ± 0.87 | 0.03 ± 0.58 | 5.68 ± 1.14 | 5.57 ± 1.01 | −0.10 ± 0.66 | 0.37 | 0.31 | 1.02 (0.97–1.06) | 0.47 |
| Serum HDL cholesterol (mmol/L) | 1.50 ± 0.41 | 1.52 ± 0.40 | 0.02 ± 0.19 | 1.51 ± 0.39 | 1.51 ± 0.37 | −0.00 ± 0.25 | 0.83 | 0.53 | 1.02 (0.96–1.08) | 0.52 |
| Serum LDL cholesterol (mmol/L) | 3.43 ± 0.92 | 3.48 ± 0.87 | 0.05 ± 0.46 | 3.59 ± 1.06 | 3.61 ± 0.93 | 0.02 ± 0.56 | 0.44 | 0.77 | 0.99 (0.94–1.05) | 0.84 |
| Serum TG (mmol/L) | 0.9 (0.5–3.2) | 1.0 (0.5–2.8) | 0.06 ± 0.87 | 0.9 (0.5–2.7) | 1.0 (0.6–2.7) | −0.38 ± 0.79 | 0.91 | 0.59 | 1.02 (0.87–1.19) | 0.81 |
| Serum hs-CRP (mg/L) | 1.24 (0.28–15.93) | 1.12 (0.26–8.51) | −0.81 ± 4.26 | 0.83 (0.28–3.78) | 1.20 (0.24–7.73) | 0.20 ± 3.75 | 0.03 | 0.23 | 0.93 (0.67–1.28) | 0.64 |
| Fasting serum insulin (pmol/L) | 52.0 (18.0–248.0) | 65.0 (20.5–191.3)† | 1.7 ± 36.6† | 54.0 (15.7–125.7) | 60.0 (14.2–137.3) | 1.5 ± 29.4 | 0.55 | 0.97 | 1.11 (0.93–1.32) | 0.23 |
| AUC insulin 0–10 min (pmol/L ⋅ min) | 2,755 (1,078–8,723) | 2,873 (1,064–9,846)† | −177 ± 2,315† | 2,630 (884–6,150) | 2,740 (947–7,909) | 22 ± 1,395 | 0.36 | 0.62 | 1.03 (0.89–1.20) | 0.65 |
| AUC insulin 120–180 min (pmol/L ⋅ min) | 20,460 (6,680–92,115) | 22,940 (8,372–68,539)† | −1,024 ± 10,596† | 18,705 (7,235–53,640)† | 19,870 (6,782–55,202) | 1,840 ± 13,046† | 0.28 | 0.25 | 1.01 (0.86–1.19) | 0.87 |
| HOMA-IR | 1.83 (0.59–9.74) | 2.24 (0.64–8.94)† | 0.19 ± 1.41† | 1.98 (0.50–5.01)† | 2.00 (0.51–5.45) | 0.05 ± 1.20† | 0.62 | 0.62 | 1.15 (0.96–1.39) | 0.14 |
| M 120–180 min (μmol ⋅ min−1 ⋅ kg−1) | 33.9 (15.5–59.8) | 38.5 (15.7–62.8) | 1.2 ± 10.3 | 35.1 (14.1–65.3) | 38.0 (17.2–73.5) | 2.1 ± 11.9 | 0.83 | 0.70 | 0.97 (0.87–1.09) | 0.64 |
| ISI 120–180 min (μmol ⋅ min−1 ⋅ kg−1/pmol−1 ⋅ L−1) | 0.102 (0.020–0.279) | 0.118 (0.021–0.273)† | 0.001 ± 0.030† | 0.116 (0.034–0.348)† | 0.094 (0.036–0.343) | −0.005 ± 0.063† | 0.26 | 0.55 | 0.99 (0.86–1.15) | 0.93 |
Data (baseline, 6 months, and Δ-values) are means ± SD if normally distributed and median (5th to 95th percentiles) if skewed. Skewed variables were log transformed before further analyses. The Δ-values are the 6-month values minus baseline values. Paired t tests were used to study change from baseline to 6 months within each treatment group. Independent t tests were used to compare baseline and Δ-values between the treatment groups. To allow adjustment for baseline values when comparing effect of vitamin D against placebo, ANCOVA models were used and the results presented as the relative effect of vitamin D, with the effect of placebo set to 1 as reference.
*P < 0.05 vs. baseline.
**P < 0.01 vs. baseline.
†Data missing in one participant.
‡Data missing in two participants.
FIG. 4.
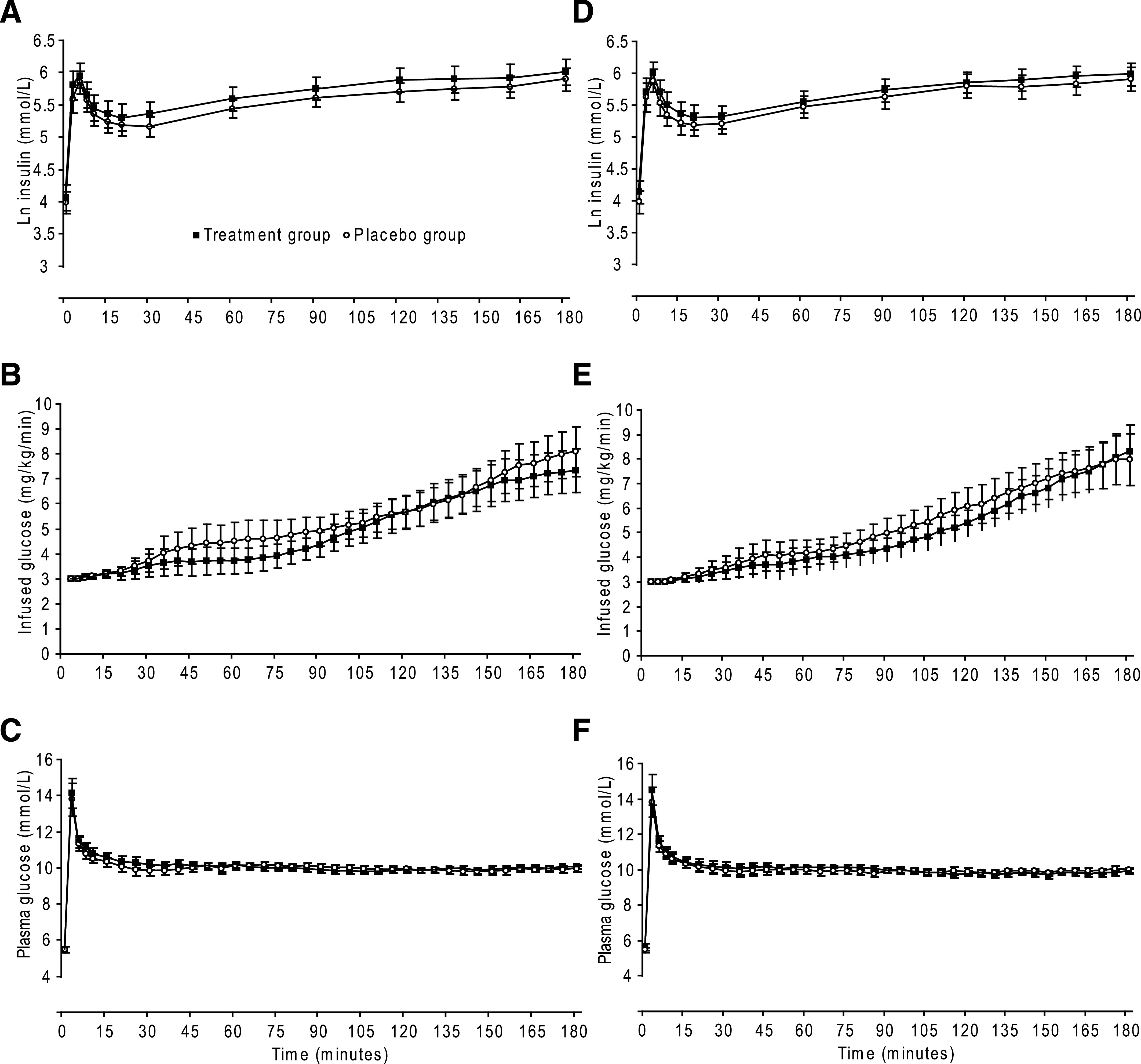
Baseline (A–C) and final (D–F) results in completers of the RCT comparing 6 months of high dose vitamin D3 (n = 49) (■) and placebo (n = 45) (○) in vitamin D–insufficient participants. A and D: Mean (95% CI) ln serum insulin. B and E: Mean (95% CI) infused glucose. C and F: Mean (95% CI) plasma glucose.
Side effects.
There were no significant differences between the groups regarding side effects, with 45 and 46 events in the vitamin D and placebo group, respectively. No cases of hypercalcemia or kidney stones were observed. One subject in the placebo group died by unknown cause.
Complications related to the clamp procedure included vasovagal syncopes (n = 7), extravasal infusion of glucose (n = 1), pain in the infused arm (n = 2), and thrombophlebitis in the infused vein (n = 2).
DISCUSSION
Results from this population-based case-control study confirm the observed inverse relationship described in previous studies between serum 25(OH)D and TGs and HbA1c and the positive association with ISI (9,14–17,22). These effects, however, were attenuated after adjustment for physical activity and no longer significant for TGs and ISI. In addition, there were no beneficial effects on the same outcomes after 6 months of supplementation with vitamin D as compared with placebo.
The major strength of the study is the sophisticated methodology used to measure insulin secretion and sensitivity. Insulin sensitivity measured by hyperglycemic clamp technique correlates well with the gold standard euglycemic clamp technique (25), includes both hepatic and peripheral insulin resistance (32), and provides measures of insulin secretion.
Another strength of the study is that low and high serum 25(OH)D levels were confirmed through two different measurements, some of which were >2 years apart. The doses used were high enough to achieve a substantial increase in serum 25(OH)D levels, which was verified in serum measurements. Good compliance and high retention increased the validity of the findings. Finally, we had the opportunity to adjust for possible confounding factors, such as physical activity and fat fish intake.
The study also has several limitations. Most important, the study was powered to detect a difference between the groups after treatment of 0.08 μmol ⋅ kg−1 ⋅ min−1/pmol ⋅ L−1. However, the difference between the case and control groups at baseline was only 0.03 μmol ⋅ kg−1 ⋅ min−1/pmol ⋅ L−1, which would have been a more realistic intervention goal. We lacked power to detect such a small difference and cannot exclude that a larger study would have disclosed an effect of vitamin D intervention on ISI. On the other hand, the relative effect of vitamin D supplementation on ISI in our study was 0.99, and we find it unlikely that we have missed a pronounced effect of vitamin D on glucose metabolism. Since the intervention lasted only 6 months, we cannot assess long-term effects on glucose and lipid metabolism or safety. Furthermore, our subjects were almost exclusively Caucasians, and the results may not apply to other ethnic groups. Thus, administration of a similar dose (4,000 IU/day) of vitamin D3 for 6 months lowered fasting insulin and increased insulin sensitivity in South-Asian women (29), and an increase in oral glucose insulin sensitivity in vitamin D–supplemented (120,000 IU three times, 2 weeks apart) Indian men after only 6 weeks has also been reported (33). The participants included in these studies were more vitamin D deficient and insulin resistant at baseline than in our study. It is therefore possible that vitamin D–deficient people with abnormal glucose metabolism still could benefit from vitamin D treatment. However, stratified analyses in our participants according to baseline HOMA-IR or BMI did not reveal an effect of vitamin D supplementation.
Furthermore, our results are consistent with previous studies showing that 1-year supplementation with 20,000 or 40,000 IU/week vitamin D3 did not improve glucose metabolism or serum lipids in 330 overweight or obese Caucasian subjects (34). Nor did injection of two doses of 100,000 IU vitamin D3 improve fasting glucose or insulin sensitivity in 33 primarily Caucasian adults with serum 25(OH)D <50 nmol/L (35). No effect on fasting glucose, insulin, HOMA-IR, or development of diabetes during 7 years of supplementation with 1,000 mg calcium and 400 IU vitamin D3 in >33,000 women was reported from the Women’s Health Initiative study (36), and intervention studies with vitamin D in subjects with established type 2 diabetes also fail to show improvement in glycemic control (37,38). Other minor studies, mainly without control groups, report conflicting results, as reviewed recently (21).
Although there was a significant difference in serum TGs between case and control subjects at baseline, we found no effect of vitamin D supplementation on serum TGs or other lipids. This is in accordance with several other reports (29,33,34,38–40), although a decrease in serum TGs and an increase in LDL were found in the group supplemented with 83 μg (3,320 IU)/day vitamin D3 as compared with placebo in a 1-year study of 200 overweight subjects (41). None of these studies include participants based on presence of hyperlipidemia or hypertriglyceridemia, and RCTs in participants with such traits are still needed.
In the case-control study, adjustment for physical activity attenuated the differences between case and control subjects regarding TGs and ISI but not HbA1c. To address confounding related to vitamin D in this regard is challenging because many factors fulfill the criteria for being confounders while at the same time serving as important contributors to the vitamin D level itself. Thus, overadjustment might occur. For instance, the effect of physical activity on ISI might in part be mediated by the higher level of vitamin D in physically active subjects as a result of increased outdoor time (42).
Worth noticing is that the final serum 25(OH)D in the intervention study was higher than the serum levels in the high serum 25(OH)D group in the nested case-control study. We might therefore have exceeded the ideal serum concentration, assuming a U-shaped association between serum 25(OH)D and dysregulation of glucose metabolism (43). Such an association has recently been reported between serum 25(OH)D levels and mortality (44), frailty (45), and some cancers (46). However, there were no differences in changes in ISI, TGs, or HbA1c when the participants were stratified by quartiles of final serum 25(OH)D level, irrespective of treatment group (data not shown).
The ideal serum 25(OH)D level for multiple health outcomes is still not settled but suggested to be at least 75–100 nmol/L (47). Our results do not support that a higher level would be preferable in relation to insulin sensitivity and secretion in glucose tolerant individuals, and although not statistically significant from the placebo group, the finding that both fasting glucose and HbA1c increased in the supplemented group is of concern. The role of vitamin D in glucose metabolism among glucose intolerant people remains unsettled and warrants further research, preferably through RCTs.
ACKNOWLEDGMENTS
This work was supported by the Norwegian Council of Cardiovascular Disease.
No potential conflicts of interest relevant to this article were reported.
G.G. researched data and wrote the manuscript. Y.F., B.A., and R.J. researched data, contributed to discussion, and reviewed and edited the manuscript.
The superb assistance from the staff at the Research Unit and the Department of Medical Biochemistry at the University Hospital of North Norway, as well as Otto Baarholm at Hormone Laboratory, Haukeland University Hospital, is greatly acknowledged. The authors thank Vidar Anderssen, Clinical Research Centre, University Hospital of North Norway, for help with preparing the figures. The authors also thank the Tromsø Study for providing data necessary for the inclusion of the participants.
Footnotes
Clinical trial reg. no. NCT00809744, clinicaltrials.gov.
REFERENCES
- 1.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet 2005;365:1333–1346 [DOI] [PubMed] [Google Scholar]
- 2.Fujimoto WY. The importance of insulin resistance in the pathogenesis of type 2 diabetes mellitus. Am J Med 2000;108(Suppl. 6a):9S–14S [DOI] [PubMed] [Google Scholar]
- 3.Mithal A, Wahl DA, Bonjour JP, et al. ; IOF Committee of Scientific Advisors (CSA) Nutrition Working Group. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int 2009;20:1807–1820 [DOI] [PubMed] [Google Scholar]
- 4.Johnson JA, Grande JP, Roche PC, Kumar R. Immunohistochemical localization of the 1,25(OH)2D3 receptor and calbindin D28k in human and rat pancreas. Am J Physiol 1994;267:E356–E360 [DOI] [PubMed] [Google Scholar]
- 5.Bland R, Markovic D, Hills CE, et al. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in pancreatic islets. J Steroid Biochem Mol Biol 2004;89-90:121–125 [DOI] [PubMed] [Google Scholar]
- 6.Cade C, Norman AW. Vitamin D3 improves impaired glucose tolerance and insulin secretion in the vitamin D-deficient rat in vivo. Endocrinology 1986;119:84–90 [DOI] [PubMed] [Google Scholar]
- 7.Maestro B, Campión J, Dávila N, Calle C. Stimulation by 1,25-dihydroxyvitamin D3 of insulin receptor expression and insulin responsiveness for glucose transport in U-937 human promonocytic cells. Endocr J 2000;47:383–391 [DOI] [PubMed] [Google Scholar]
- 8.Danescu LG, Levy S, Levy J. Vitamin D and diabetes mellitus. Endocrine 2009;35:11–17 [DOI] [PubMed] [Google Scholar]
- 9.Ford ES, Ajani UA, McGuire LC, Liu S. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care 2005;28:1228–1230 [DOI] [PubMed] [Google Scholar]
- 10.Need AG, O’Loughlin PD, Horowitz M, Nordin BE. Relationship between fasting serum glucose, age, body mass index and serum 25 hydroxyvitamin D in postmenopausal women. Clin Endocrinol (Oxf) 2005;62:738–741 [DOI] [PubMed] [Google Scholar]
- 11.Forouhi NG, Luan J, Cooper A, Boucher BJ, Wareham NJ. Baseline serum 25-hydroxy vitamin D is predictive of future glycemic status and insulin resistance: the Medical Research Council Ely Prospective Study 1990-2000. Diabetes 2008;57:2619–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu E, Meigs JB, Pittas AG, et al. Plasma 25-hydroxyvitamin D is associated with markers of the insulin resistant phenotype in nondiabetic adults. J Nutr 2009;139:329–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baynes KC, Boucher BJ, Feskens EJ, Kromhout D. Vitamin D, glucose tolerance and insulinaemia in elderly men. Diabetologia 1997;40:344–347 [DOI] [PubMed] [Google Scholar]
- 14.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr 2004;79:820–825 [DOI] [PubMed] [Google Scholar]
- 15.Kamycheva E, Jorde R, Figenschau Y, Haug E. Insulin sensitivity in subjects with secondary hyperparathyroidism and the effect of a low serum 25-hydroxyvitamin D level on insulin sensitivity. J Endocrinol Invest 2007;30:126–132 [DOI] [PubMed] [Google Scholar]
- 16.Kayaniyil S, Vieth R, Retnakaran R, et al. Association of vitamin D with insulin resistance and beta-cell dysfunction in subjects at risk for type 2 diabetes. Diabetes Care 2010;33:1379–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alvarez JA, Ashraf AP, Hunter GR, Gower BA. Serum 25-hydroxyvitamin D and parathyroid hormone are independent determinants of whole-body insulin sensitivity in women and may contribute to lower insulin sensitivity in African Americans. Am J Clin Nutr 2010;92:1344–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scragg R, Sowers M, Bell C; Third National Health and Nutrition Examination Survey. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care 2004;27:2813–2818 [DOI] [PubMed] [Google Scholar]
- 19.Knekt P, Laaksonen M, Mattila C, et al. Serum vitamin D and subsequent occurrence of type 2 diabetes. Epidemiology 2008;19:666–671 [DOI] [PubMed] [Google Scholar]
- 20.Grimnes G, Emaus N, Joakimsen RM, et al. Baseline serum 25-hydroxyvitamin D concentrations in the Tromsø Study 1994-95 and risk of developing type 2 diabetes mellitus during 11 years of follow-up. Diabet Med 2010;27:1107–1115 [DOI] [PubMed] [Google Scholar]
- 21.Alvarez JA, Ashraf A. Role of vitamin D in insulin secretion and insulin sensitivity for glucose homeostasis. Int J Endocrinol 2010;2010:351385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jorde R, Figenschau Y, Hutchinson M, Emaus N, Grimnes G. High serum 25-hydroxyvitamin D concentrations are associated with a favorable serum lipid profile. Eur J Clin Nutr 2010;64:1457–1464 [DOI] [PubMed] [Google Scholar]
- 23.Jacobsen BK, Eggen AE, Mathiesen EB, Wilsgaard T, Njølstad I. Cohort profile: The Tromso Study. Int J Epidemiol. 21 April 2011 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grimnes G, Almaas B, Eggen AE, et al. Effect of smoking on the serum levels of 25-hydroxyvitamin D depends on the assay employed. Eur J Endocrinol 2010;163:339–348 [DOI] [PubMed] [Google Scholar]
- 25.Mitrakou A, Vuorinen-Markkola H, Raptis G, et al. Simultaneous assessment of insulin secretion and insulin sensitivity using a hyperglycemia clamp. J Clin Endocrinol Metab 1992;75:379–382 [DOI] [PubMed] [Google Scholar]
- 26.Emaus A, Degerstrøm J, Wilsgaard T, et al. Does a variation in self-reported physical activity reflect variation in objectively measured physical activity, resting heart rate, and physical fitness? Results from the Tromso study. Scand J Public Health 2010;38(Suppl. 5):105–118 [DOI] [PubMed] [Google Scholar]
- 27.Solbu MD, Jenssen TG, Eriksen BO, Toft I. Changes in insulin sensitivity, renal function, and markers of endothelial dysfunction in hypertension—the impact of microalbuminuria: a 13-year follow-up study. Metabolism 2009;58:408–415 [DOI] [PubMed] [Google Scholar]
- 28.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 29.von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient—a randomised, placebo-controlled trial. Br J Nutr 2010;103:549–555 [DOI] [PubMed] [Google Scholar]
- 30.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–1495 [DOI] [PubMed] [Google Scholar]
- 31.Vickers AJ, Altman DG. Statistics notes: Analysing controlled trials with baseline and follow up measurements. BMJ 2001;323:1123–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffman RP. Indices of insulin action calculated from fasting glucose and insulin reflect hepatic, not peripheral, insulin sensitivity in African-American and Caucasian adolescents. Pediatr Diabetes 2008;9:57–61 [DOI] [PubMed] [Google Scholar]
- 33.Nagpal J, Pande JN, Bhartia A. A double-blind, randomized, placebo-controlled trial of the short-term effect of vitamin D3 supplementation on insulin sensitivity in apparently healthy, middle-aged, centrally obese men. Diabet Med 2009;26:19–27 [DOI] [PubMed] [Google Scholar]
- 34.Jorde R, Sneve M, Torjesen P, Figenschau Y. No improvement in cardiovascular risk factors in overweight and obese subjects after supplementation with vitamin D3 for 1 year. J Intern Med 2010;267:462–472 [DOI] [PubMed] [Google Scholar]
- 35.Tai K, Need AG, Horowitz M, Chapman IM. Glucose tolerance and vitamin D: effects of treating vitamin D deficiency. Nutrition 2008;24:950–956 [DOI] [PubMed] [Google Scholar]
- 36.de Boer IH, Tinker LF, Connelly S, et al. ; Women’s Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of incident diabetes in the Women’s Health Initiative. Diabetes Care 2008;31:701–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jorde R, Figenschau Y. Supplementation with cholecalciferol does not improve glycaemic control in diabetic subjects with normal serum 25-hydroxyvitamin D levels. Eur J Nutr 2009;48:349–354 [DOI] [PubMed] [Google Scholar]
- 38.Witham MD, Dove FJ, Dryburgh M, Sugden JA, Morris AD, Struthers AD. The effect of different doses of vitamin D(3) on markers of vascular health in patients with type 2 diabetes: a randomised controlled trial. Diabetologia 2010;53:2112–2119 [DOI] [PubMed] [Google Scholar]
- 39.Scragg R, Khaw KT, Murphy S. Effect of winter oral vitamin D3 supplementation on cardiovascular risk factors in elderly adults. Eur J Clin Nutr 1995;49:640–646 [PubMed] [Google Scholar]
- 40.Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab 2001;86:1633–1637 [DOI] [PubMed] [Google Scholar]
- 41.Zittermann A, Frisch S, Berthold HK, et al. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr 2009;89:1321–1327 [DOI] [PubMed] [Google Scholar]
- 42.Scragg R, Camargo CA, Jr. Frequency of leisure-time physical activity and serum 25-hydroxyvitamin D levels in the US population: results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol 2008;168:577–586; discussion 587–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robinson JG, Manson JE, Larson J, et al. Lack of association between 25(OH)D levels and incident type 2 diabetes in older women. Diabetes Care 2011;34:628–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michaëlsson K, Baron JA, Snellman G, et al. Plasma vitamin D and mortality in older men: a community-based prospective cohort study. Am J Clin Nutr 2010;92:841–848 [DOI] [PubMed] [Google Scholar]
- 45.Ensrud KE, Ewing SK, Fredman L, et al. ; Study of Osteoporotic Fractures Research Group. Circulating 25-hydroxyvitamin D levels and frailty status in older women. J Clin Endocrinol Metab 2010;95:5266–5273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toner CD, Davis CD, Milner JA. The vitamin D and cancer conundrum: aiming at a moving target. J Am Diet Assoc 2010;110:1492–1500 [DOI] [PubMed] [Google Scholar]
- 47.Bischoff-Ferrari HA. Optimal serum 25-hydroxyvitamin D levels for multiple health outcomes. Adv Exp Med Biol 2008;624:55–71 [DOI] [PubMed] [Google Scholar]