Abstract
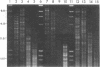
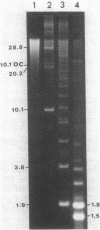
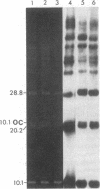
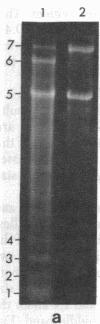
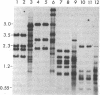
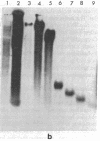
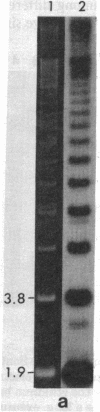
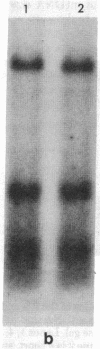
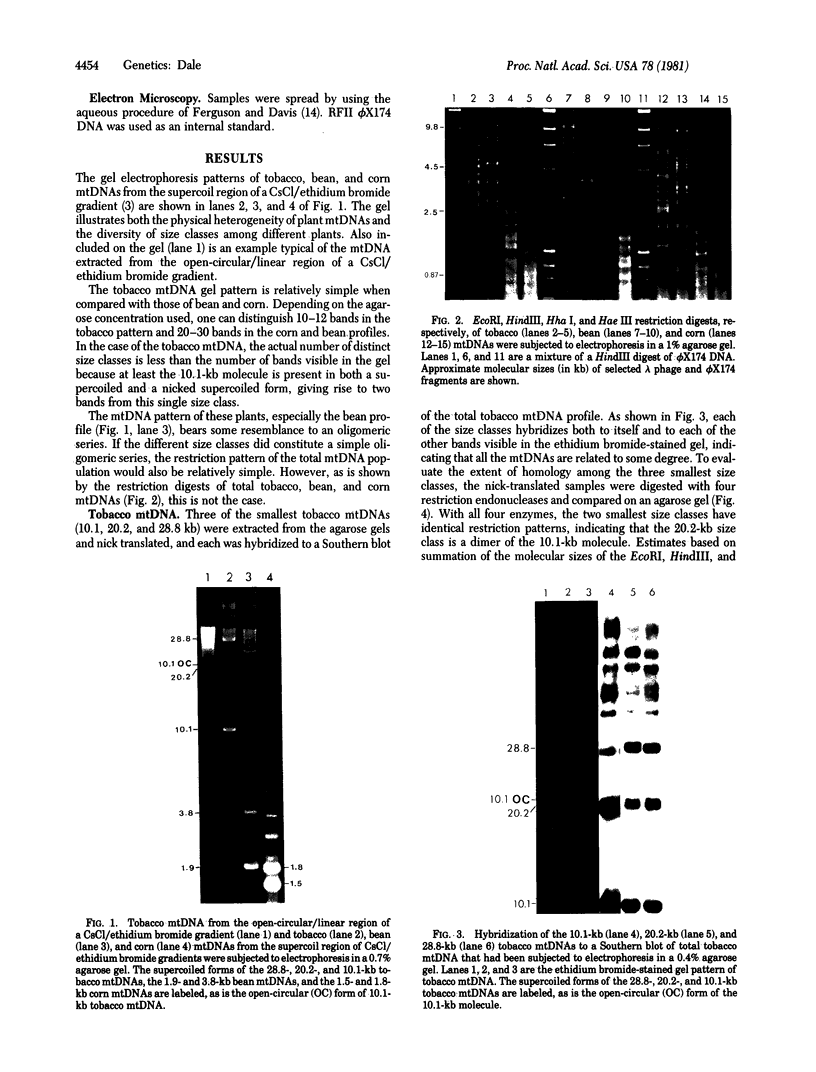
Supercoiled mtDNAs were isolated from tissue culture cells of tobacco, bean, and corn, and the smallest size classes were used to study the relationships among the different size classes of each species through restriction digests and hybridizations. Three of the smallest tobacco mtDNAs [10.1, 20.2, and 28.8 kilobases (kb)], the two smallest bean mtDNAs (1.9 and 3.8 kb), and the two smallest corn mtDNAs (1.5 and 1.8 kb) were extracted from the gels and nick translated. The 10.1-kb tobacco mtDNA hybridizes to all the other tobacco mtDNA size classes and a large percentage of the tobacco mtRNAs. Restriction digests indicate that the 20.2-kb size class is a dimer of the 10.1-kb size class. The 1.9-kb bean mtDNA hybridizes to all but three of the bean mtDNA size classes and hybridizes to two mtRNAs. Restriction digests indicate that the 3.8-kb size class is a dimer of the 1.9-kb size class. The 1.5- and 1.8-kb corn mtDNAs, which do not have any Hha I restriction fragments in common, both hybridize to many of the same size classes of the corn mtDNA profile and, in addition, each hybridizes to a few size classes not recognized by the other. The 1.5- and 1.8-kb size classes both hybridize to two RNAs, one of which they appear to have in common. However, with both the 1.9-kb bean mtDNA and the two corn mtDNAs, the molecular sizes of the two RNAs exceed those of the respective DNAs. The possible role and origin of the many size classes are discussed.
Keywords: supercoiled mtDNA, Southern hybridization, restriction enzyme analysis
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Bertrand H., Collins R. A., Stohl L. L., Goewert R. R., Lambowitz A. M. Deletion mutants of Neurospora crassa mitochondrial DNA and their relationship to the "stop-start" growth phenotype. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6032–6036. doi: 10.1073/pnas.77.10.6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P., Grivell L. A. The mitochondrial genome of yeast. Cell. 1978 Nov;15(3):705–723. doi: 10.1016/0092-8674(78)90257-x. [DOI] [PubMed] [Google Scholar]
- Bowden-Bonnett L. Isolation and Cell-free Translation of Total Messenger RNA from Germinating Castor Bean Endosperm. Plant Physiol. 1979 Apr;63(4):769–773. doi: 10.1104/pp.63.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde B. G., Oliver R. J., Leaver C. J. Variation in mitochondrial translation products associated with male-sterile cytoplasms in maize. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3841–3845. doi: 10.1073/pnas.75.8.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamborg O. L., Miller R. A., Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968 Apr;50(1):151–158. doi: 10.1016/0014-4827(68)90403-5. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. The rabbit beta-globin gene contains a large large insert in the coding sequence. Cell. 1977 Dec;12(4):1097–1108. doi: 10.1016/0092-8674(77)90172-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Synenki R. M., Levings C. S., Shah D. M. Physicochemical characterization of mitochondrial DNA from soybean. Plant Physiol. 1978 Mar;61(3):460–464. doi: 10.1104/pp.61.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuring R. W., Sanders J. P., Borst P. A freeze-squeeze method for recovering long DNA from agarose gels. Anal Biochem. 1975 May 26;66(1):213–220. doi: 10.1016/0003-2697(75)90739-3. [DOI] [PubMed] [Google Scholar]