Abstract
Dyloject is a novel formulation of diclofenac intended for intravenous (IV) administration. This formulation employs the solubilizing agent hydroxypropyl-β-cyclodextrin to permit bolus IV administration. The efficacy and safety of 5 dose levels of IV diclofenac were compared with IV ketorolac and placebo following third molar extraction. This was a single-dose, randomized, double-blind, placebo- and comparator-controlled, parallel-group study. A total of 353 subjects with moderate to severe pain received placebo; ketorolac 30 mg; or IV diclofenac 3.75, 9.4, 18.75, 37.5, or 75 mg (N = 51 for all groups, except N = 47 for ketorolac). The primary endpoint was total pain relief over 6 hours (TOTPAR6) as measured by the visual analog scale (VAS). Secondary endpoints included multiple measures of pain intensity and relief; patient global evaluation; and times to pain relief and rescue medication. Dropouts and adverse effects (AEs) were also monitored. IV diclofenac was superior to placebo as measured by TOTPAR6 (P < .0001 for all doses except 3.75 mg, for which P = .0341). IV diclofenac 3.75 mg was statistically superior to placebo for TOTPAR2 and TOTPAR4. IV diclofenac at both 37.5 and 75 mg was superior to placebo (P < .05) at the earliest (5 minute) assessments of pain intensity and pain relief, but ketorolac was not. The proportion of patients reporting 30% or greater pain relief at 5 minutes was significantly greater after IV diclofenac 37.5 and 75 mg than after ketorolac 30 mg or placebo. Secondary endpoints confirmed the primary findings. Treatment-related AEs were generally mild to moderate and were typical for nonsteroidal anti-inflammatory drugs (NSAIDs). The more rapid onset of action of IV diclofenac compared with the reference injectable NSAID ketorolac suggests additional clinical benefit. If confirmed in larger series, these findings may improve the safety and efficacy of postoperative NSAID analgesia.
Keywords: Acute pain, Postoperative pain, Molar extraction, Diclofenac, Nonsteroidal anti-inflammatory drug, Cyclodextrin
Injectable nonsteroidal anti-inflammatory drugs (NSAIDs) are given worldwide for pain control after surgical procedures in which multimodal analgesia is used to hasten and enhance recovery for patients requiring analgesia with minimal opioid side effects, and for patients who are not able to tolerate oral NSAIDs. A recent meta-analysis has confirmed the clinical benefits of opioid sparing with the coadministration of NSAIDs.1 Molar extraction reliably leads to moderate to severe pain, creating challenges for pain control but also offering opportunities to evaluate analgesics in this validated, clinically relevant model.2 The contributions of nociception plus inflammation to post–molar extraction pain were observed decades ago to make it an extremely useful model for evaluation of NSAIDs.3
Dyloject (referred to herein as IV diclofenac) is a novel formulation of diclofenac sodium for injection recently approved for marketing in the United Kingdom as a 75 mg/2 mL bolus IV injection.4 The widely prescribed NSAID diclofenac is an amino-phenylacetic acid that inhibits prostaglandin biosynthesis to produce analgesic, antipyretic, and anti-inflammatory activity secondary to its nonselective inhibition of the cyclooxygenase (COX) enzymes, COX-1 and COX-2. We previously reported results of a double-blind, placebo-controlled study that compared 75 mg doses of Dyloject and Voltarol (an earlier formulation of injectable diclofenac that requires buffering, dilution, and infusion over 30 minutes) for post–molar extraction pain and demonstrated a quicker onset of action and a lower incidence of thrombophlebitis with Dyloject: 5.7% versus 12%.5 Our observation of the more rapid onset of action of Dyloject compared with Voltarol suggested that the newer formulation of diclofenac might have an enhanced potency, thereby offering a potential advantage by providing clinically meaningful analgesia at lower doses than those previously believed necessary.
First, the present study sought to explore clinical responses to a range of I.V. diclofenac doses as low as one twentieth (3.75 mg) of the standard recommended UK doses of Dyloject and Voltarol (both 75 mg). Second, we sought to extend prior findings of the rapid onset of I.V. diclofenac by comparing its speed of onset versus that of ketorolac, an NSAID with more preferential COX-1 selectivity than diclofenac, and the only NSAID approved in the United States as a sole agent for the treatment of moderate to severe acute pain.
METHODS
Participants
Male and female subjects between 18 and 75 years of age who were undergoing surgical extraction of 1 or more third molars (1 of which was a fully or partially impacted mandibular third molar requiring bone removal) were eligible for enrollment. Subjects had to be in good health as determined by the investigator on the basis of medical history and physical examination and had to have moderate or severe pain within 6 hours after completion of surgery, as measured by a categorical pain intensity scale (moderate or severe descriptor) and pain intensity of ≥50 mm on a 100 mm visual analog scale (VAS) at baseline. Female subjects of childbearing potential were required to have a negative pregnancy test and had to be practicing abstinence or a medically acceptable form of contraception plus using a spermicidal agent.
All subjects received a standard local anesthetic (lidocaine 20 mg/mL with epinephrine 12.5 µg/mL). In the lower jaw, inferior alveolar plus lingual nerve blocks were supplemented by local buccal infiltration on the side of operation. Where an ipsilateral third molar was to be removed from the upper jaw, local anesthesia at the tuberosity of the palatine foramen was supplemented by buccal and palatal infiltration.
Exclusion criteria included a history of uncontrolled chronic disease that would contraindicate study participation, previous cardiovascular events (eg, myocardial infarction, stroke), or potential cardiovascular risk, including an abnormal electrocardiogram (ECG), judged by the investigator as clinically significant at screening or at baseline (ie, immediately predosing). Subjects receiving any investigational medication within 3 months before administration of study medication, or with clinically significant laboratory test results or known hypersensitivity to NSAIDs or diclofenac, were excluded.
No analgesic medications, including aspirin, opioids, other nonsteroidal anti-inflammatory drugs, or other common centrally or peripherally acting analgesic drugs, major and minor tranquilizers, muscle relaxants, or antihistamines within 24 hours before study medication administration were allowed. Subjects who had taken antidepressants, including serotonin/norepinephrine reuptake inhibitors or tricyclic antidepressants within 3 weeks of the study and subjects who had taken monoamine oxidase inhibitors, tryptophan, carbamazepine, or valproate within 2 weeks before taking study medication were also excluded.
This study was approved by the investigational review committee, Quorum Review. All subjects signed informed consent before participating in the study.
Procedure
This was a single-dose, randomized, double-blind, placebo- and comparator-controlled, parallel-group study conducted at 3 sites. At the screening visit, patients at each site were assigned a subject number in the order in which they were screened. Upon meeting all inclusion and exclusion criteria, patients were randomly assigned to receive 1 of 5 IV diclofenac doses (3.75 mg, 9.4 mg, 18.75 mg, 37.5 mg, or 75 mg), ketorolac tromethamine 30 mg, or placebo according to a computer-generated randomization schedule. Study medication was administered as an intravenous (IV) bolus injection over 15 seconds into a preplaced cannula in the arm. A third party doser who had no contact with patients except when dosing administered study treatment prepared the syringe with appropriate study treatment using a blind label within 1 hour of dosing.
Pain was assessed by each patient at baseline (time 0); at 5, 15, 30, and 45 minutes; and at 1, 1.5, 2, 3, 4, 5, 6, 7, 8, 10, 12, and 24 hours after administration of study medication. Only pain intensity was measured at baseline. At each postbaseline time period, both pain intensity (categorical and VAS) and pain relief (VAS and categorical) were measured. All pain assessments taken up to 8 hours post dosing were performed by research staff at the site. All patients were discharged after an 8 hour in-clinic observation period. Assessments taken at 10, 12, and 24 hour time points were entered directly into the patient diary by the patient. Times to onset of perceptible and meaningful pain relief were evaluated using 2 stopwatches. Patients were instructed to stop the first stopwatch at the time of perceptible pain relief and the second stopwatch at the time of meaningful pain relief. A patient global evaluation (PGE: excellent, very good, good, fair, or poor) was also recorded at the time of rescue or at the 8 hour poststudy medication assessment, whichever came first. Patients were encouraged to wait at least 60 minutes from the time of study medication administration before using rescue medication. However, patients could receive rescue medication at any time according to the usual clinical practice at each study site. The most common rescue medications taken were oral ibuprofen 400–600 mg and a combination oral analgesic containing hydrocodone 5 mg and acetaminophen 500 mg.
Safety was assessed by laboratory analyses, vital signs, 12 lead ECGs, urine pregnancy testing, adverse events (AEs), and physical examinations. ECGs and laboratory analyses were performed at screening, at baseline, 8 hours post dose, and 5 to 9 days post treatment. At these times, blood samples were analyzed for albumin, alkaline phosphatase, alanine transaminase, amylase, aspartate transaminase, bilirubin, blood urea nitrogen, calcium, chloride, creatinine kinase, glucose, lactate dehydrogenase, lipase, magnesium, phosphate, total protein, and sodium. Urine samples at these times were analyzed for specific gravity, pH, protein, glucose, ketones, bilirubin, and blood and were also examined microscopically for bacteria, casts, crystals, white blood cells, yeast, and so forth. Vital signs were performed at screening, at baseline (immediately prior to dosing), 5 minutes and 8 hours post dose, and 5 to 9 days post treatment. Physical examinations were performed at screening or at baseline and 5 to 9 days post treatment. All potentially childbearing females had pregnancy tests performed at screening, at baseline, and 5 to 9 days post treatment. AEs were monitored throughout the course of the study. Assessment of the intravenous cannula site was conducted by clinical staff at 8 hours post treatment for thrombophlebitis using a 6 point scale.6 This scale ranged from grade 0 (“no reaction”) to grade 5 (“thrombosis with overt infection”).
The primary measure of efficacy was total pain relief (TOTPAR) for the intent-to-treat (ITT) population over 0–6 hours. This score (TOPAR6) was based on pain relief scores assessed on a 100 mm VAS over a 0–6 hour time interval. Secondary measures of efficacy included time-specific pain relief (PR) (VAS and categorical); peak pain relief (PPR) (VAS and categorical); sum of pain intensity differences (SPID) over 0–2, 0–4, 0–6, 0–8, 0–10, 0–12, and 0–24 hours (VAS and categorical); time-specific pain intensity difference (PID) (VAS and categorical); peak pain intensity difference (PPID) (VAS and categorical); summed pain relief intensity differences (SPRID) over 0–2, 0–4, 0–6, 0–8, 0–10, 0–12, and 0–24 hours (VAS and categorical); time to administration of rescue medication; proportion of patients requiring rescue medication; time to meaningful pain relief; time to perceptible pain relief; and PGE. All efficacy endpoints were derived from direct, patient-reported entries in the pain assessment case report forms completed up to 8 hours postoperatively while the patient was in the clinic or (for 10 hour, 12 hour, and 24 hour self-assessments) in patient diaries completed by patients at home after leaving the clinic.
Statistical Analyses
Sample size was estimated for TOTPAR over 0–6 hours assessed using a 0–100 mm VAS, to demonstrate the superiority of IV diclofenac versus placebo. A sample size of 48 evaluable patients per group was calculated to provide 90% power to detect a difference of 90 mm hours between each IV diclofenac dosing group and placebo with a 2-sided test performed at the 5% level of significance. This calculation assumed a standard deviation of 134.2 mm hr that was obtained from a prior study of a single 75 mg dose of IV diclofenac for postoperative third molar extraction pain.5 Inclusion of a seventh cohort given IV ketorolac as a positive comparator led to an estimate of approximately 350 patients to be randomized to obtain 336 evaluable per protocol patients.
Baseline variables (age, sex, and baseline pain intensity) were summarized by treatment group. For continuous variables, comparisons between treatments were made using analysis of variance (ANOVA) models with treatment and center as independent factors. For categorical outcomes, comparisons between pairs of treatment groups were performed using Cochran-Mantel-Haenszel test with center as the stratification variable. Analyses were performed on the ITT population.
TOTPAR, PR, PPR, SPID, PID, PPID, SPRID, and GPE were analyzed using analysis of covariance (ANCOVA) with treatment, center, and baseline categorical pain intensity as factors. The possibility of interactions was investigated. Terms for treatment by center interaction and treatment by baseline categorical pain intensity were added to the model. If either of the interaction terms was significant at the .10 level, the reason for the interaction was explored. Comparisons of pain intensity and pain relief across observation times for diclofenac, placebo, and ketorolac groups were performed with Dunnett's test.
The presence of a linear dose response was tested with orthogonal contrasts for TOTPAR, SPID, and SPRID. Determination of the minimum effective dose with respect to each of these outcome measures was conducted with the Tukey, Ciminera, and Heyse step-down testing procedure using linear contrasts. This procedure involved a sequence of linear trend tests. The first test, of overall linear dose response, included placebo (defined as a dose of 0) and all IV diclofenac dose levels. The next step-down test was a linear trend test, which excluded the highest dose of IV diclofenac. Each subsequent step-down test excluded the highest remaining dose of IV diclofenac ending with the final linear trend test, which included only 3.75 mg IV diclofenac and placebo. The first of these sequential tests in which a significant linear trend was no longer noted was used to define the minimum dose with an analgesic effect discernibly different from placebo. In addition, linear, quadratic, and cubic dose response trends based on SPID from 0–6 hours (using the VAS) were tested.
Times to onset of perceptible relief and meaningful relief were analyzed with survival analysis techniques. Median time to event for each treatment group was estimated with the Kaplan-Meier product limit estimator. A 95% confidence interval for each estimated median time to event was calculated. The presence of a linear dose response for the ordered IV diclofenac dose levels was tested with orthogonal contrasts in a Cox proportional hazards regression model. Dependent variables were age at entry, gender, time from end of surgery to first study medication, and baseline pain intensity (VAS). As with area under the curve (AUC) parameters, linear trend tests using the step-down testing procedure of Tukey, Ciminera, and Heyse were performed. Time from administration of study medication to first rescue medication was analyzed in the same fashion. In addition, a figure presenting the cumulative proportion of patients rescued over time in each treatment group was constructed. The proportions of patients rescued at specific times were compared across treatment groups by means of the Cochran-Mantel-Haenszel test using center as a stratification variable.
The clinical safety of the study medications was assessed in all patients who received them. Clinical and statistical interpretations were based on tabulations of clinical laboratory results, physical examination, vital signs, ECG findings, thrombophlebitis evaluation, and AE data.
RESULTS
Five hundred seventy-five (575) patients were screened for possible study participation across 3 study sites. A total of 222/575 screened patients (38.6%) were not enrolled in the study and were classified as “screening failures.” The three primary reasons were withdrawn consent, positive drug test, or a medical history exclusion. A total of 353 patients were enrolled into the study and were randomly assigned to 1 of the 7 treatment groups. All 353 randomized patients received 1 dose of the assigned study treatment and were included in the safety population. Postbaseline efficacy data were also recorded for these 353 patients and were included in the ITT population.
Demographic variables of age, sex, ethnic origin, height, and weight were similar across treatment groups. Mean patient age was 23.7 years with 61.5% females and 38.5% males. No statistically significant demographic differences were present between treatment groups.
The degree of molar impaction and resulting trauma from surgery were similar across treatment groups. A majority of patients had third molar extractions that involved only partial bony impactions (225/353; 63.7%), and the median number of third molars extracted was 2. Numbers and percentages of patients with partial bony impactions were similar across treatments (P = .9830). Surgical trauma was rated as severe in 67.1% of patients and moderate in 32.9% with a median surgical time of 7 minutes.
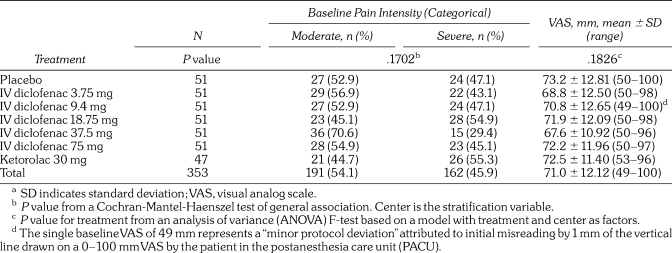
Pain intensity at the baseline evaluation (after local anesthetic effects dissipated) was similar among treatment groups, as measured by both VAS and the categorical scale (Table 1). Overall, categorical baseline pain intensity was mixed among moderate (191/353; 54.1%) and severe (162/353; 45.9%). Mean VAS baseline pain intensity was 71 ± 12.12 mm, ranging from 49 to 100 mm across treatment groups. No statistically significant differences were observed among treatment groups.
Table 1.
Baseline Pain Intensity in the Intent-to-Treat Populationa
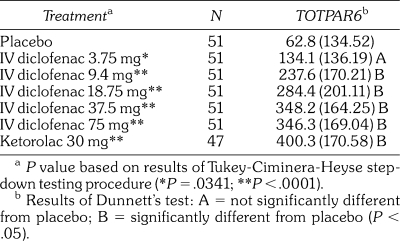
Table 2.
Mean (SD) Total Pain Relief (TOTPAR6) for the First 6 Hours Post Dose by Visual Analog Scale in the Intent-to-Treat Population
The primary study efficacy endpoint was achieved. The primary efficacy endpoint was TOTPAR6, as determined by summation of assessments of pain relief scores as measured on the 100 mm VAS over the 0–6 hour period (Table 2). IV diclofenac was statistically superior to placebo (P < .001) in providing pain relief over the 0–6 hour period following IV treatment. Tukey's step-down linear trend test demonstrated a significant linear dose response. IV diclofenac 3.75 mg was the minimum dose whose analgesic effect differed significantly from placebo (P = .0341). The per protocol analysis supported these findings; however, the lowest dose that separated from placebo in that analysis was identified as 9.4 mg. IV diclofenac 37.5 and 75 mg doses were numerically similar to ketorolac 30 mg with respect to TOTPAR6. Neither of the 2 interaction terms (treatment by center and treatment by baseline categorical pain intensity) achieved significance at the level of P < .1.
IV diclofenac 18.75 mg, 37.5 mg, and 75 mg provided pain relief as measured on the VAS scale that was statistically superior to placebo (P < .001) over TOTPAR2, TOTPAR4, TOTPAR8, TOTPAR10, TOTPAR12, and TOTPAR24. For TOTPAR8 through TOTPAR24 measures, no statistically significant differences were observed between IV diclofenac 3.75 mg and placebo treatment groups. TOTPAR24 showed no statistically significant difference between IV diclofenac 9.4 mg and placebo.
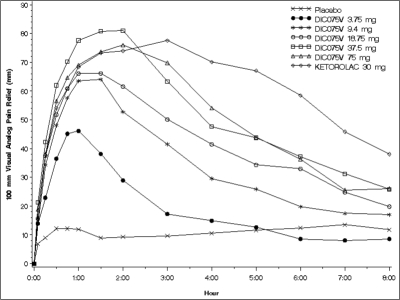
Mean pain relief scores for ketorolac and all doses of IV diclofenac increased rapidly within the first hour and, with the exception of IV diclofenac 3.75 mg, remained greater than placebo for 8 hours following administration of study drug. Statistically significant separation from placebo (P < .05) occurred at 5 minutes following drug administration for patients in the IV diclofenac 37.5 and 75 mg treatment groups and at 15 minutes following administration of ketorolac 30 mg. IV diclofenac 37.5 mg had the greatest mean pain relief scores from 30 minutes through 2 hours post dose, and ketorolac 30 mg had the greatest scores from 3 hours through 10 hours post dose; however, pain relief scores for all active treatment groups appeared to be declining monotonically beyond 3 hours. Figure 1 presents mean pain relief profile over 8 hours for each of the treatment groups. In Figures 1 through 3, “DIC075V” denotes the experimental drug name of IV diclofenac. Mean TOTPAR scores (categorical assessments) for all IV diclofenac groups and ketorolac were statistically superior to placebo (P < .0001) at 2 and 4 hours. IV diclofenac 18.75 mg, 37.5 mg, and 75 mg and ketorolac 30 mg were statistically superior to placebo (P < .0001) for all TOTPAR scores up to 0–24 hours as measured on the categorical scale. For legibility, 95% confidence intervals although calculated are not depicted in Figure 1. If these were depicted, their overlap at 6 hours and later for the 3 highest doses of IV diclofenac and ketorolac indicates that the apparently prolonged analgesic effect of ketorolac relative to IV diclofenac was not statistically significant.
Figure 1.
Mean pain relief (on a 0–100 mm visual analog scale) versus time after administration of single, graded doses intravenous (IV) diclofenac (“DIC-075V”), placebo, or the positive comparator ketorolac 30 mg. Confidence intervals surrounding each point are omitted for legibility. If these were depicted, their overlap at 6 hours and later for the 3 highest doses of IV diclofenac and ketorolac indicates that the apparently prolonged analgesic effect of ketorolac relative to IV diclofenac was not statistically significant.
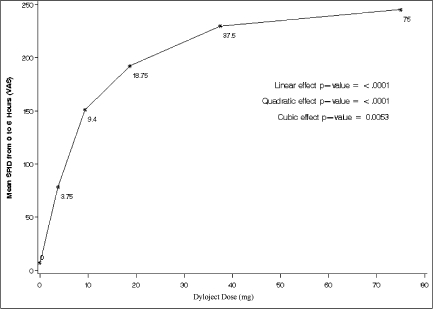
Figure 2.
Mean sum of pain intensity differences (on a 0–100 mm visual analog scale) from 0–6 hours following administration of single, graded doses of IV diclofenac. A significant dose response with a plateau above 37.5 mg was evident according to linear, quadratic, and cubic models.
Figure 3.
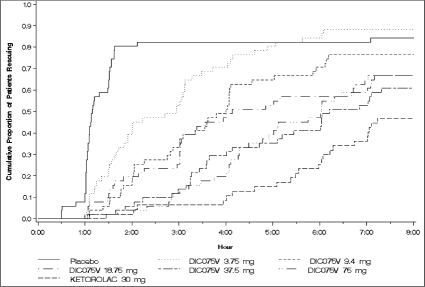
Cumulative proportions of patients requiring rescue medication during the 8 hours after single, graded doses of IV diclofenac (“DIC075V”), ketorolac 30 mg, or placebo.
Pain intensity was measured by the 0–100 mm VAS and 5 point categorical scales. The pain intensity difference (PID) was derived by subtracting the pain intensity at each observation point from the baseline pain intensity. Mean PID VAS scores increased rapidly through the first hour after administration for all doses of DIC075V and ketorolac, and remained higher than placebo throughout 24 hours. From 5 minutes through 1.5 hours, DIC075V 37.5 mg maintained the greatest mean PID VAS score at each assessment. From 2 through 24 hours, ketorolac 30 mg had the highest mean PID VAS scores. Similar results were observed for mean pain intensity scores as measured on the categorical scale. PID scores were summed through 2, 4, 6, 8, 10, 12, and 24 hours post dose. Mean SPID scores, based on VAS assessments, indicated that IV diclofenac 18.75, 37.5, and 75 mg and ketorolac were statistically superior to placebo (P < .0001) and were similar to one another at 0–2, 0–4, 0–6, 0–8, 0–10, 0–12, and 0–24 hours post dose, except SPID 0–24 hours for IV diclofenac 18.75 mg (P = .0003). IV diclofenac 3.75 mg was not statistically superior to placebo at 0–10, 0–12, and 0–24 hours. IV diclofenac 9.4 mg was not statistically superior to placebo at 0–24 hours. When assessed on the categorical scale, similar results for mean SPID scores were observed for IV diclofenac 18.75, 37.5, and 75 mg and for ketorolac.
To further explore the linear dose-response trend for IV diclofenac, an additional analysis based on mean SPID from 0–6 hours was conducted to test for linear, quadratic, and cubic effects. Placebo was included as a dose of 0 mg. As seen in Figure 2, highly significant (P < .0001) linear and quadratic dose effects were evident, and a significant (P < .0053) cubic effect as well. These results indicate that a clear, statistically significant relationship was noted between increasing doses of IV diclofenac from 3.75 through 37.5 mg, as were greater reductions in pain intensity, with little incremental effectiveness between the 37.5 and 75 mg doses of IV diclofenac.
PRID scores were summed through 2, 4, 6, 8, 10, 12, and 24 hours post dose. Mean SPRID scores, based on VAS assessments, indicated that IV diclofenac 18.75, 37.5, and 75 mg and ketorolac were statistically superior to placebo (P < .0001) at 0–2, 0–4, 0–6, 0–8, 0–10, 0–12, and 0–24 hours post dose. When assessed on the categorical scale, similar results for mean SPRID scores were observed for IV diclofenac 18.75, 37.5, and 75 mg and ketorolac.
Patient requests for rescue medication (recorded up to 24 hours post dosing) were indicative of the duration of analgesic effect. Median times to rescue medication in the IV diclofenac 75 and 37.5 mg groups were 6 hours 2 minutes and 6 hours 12 minutes, respectively, compared with 1 hour 9 minutes for the placebo treatment group. IV diclofenac 18.75 and 9.4 mg both had approximately 4 hours until rescue, and IV diclofenac 3.75 mg had approximately 3 hours. All IV diclofenac and ketorolac treatment groups were statistically superior to placebo (P < .05). Cumulative proportions of patients in each treatment group who required rescue medication are shown in Figure 3.
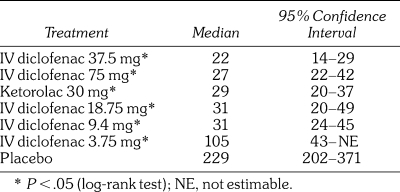
The estimated median time to perceptible pain relief based on a Kaplan-Meier analysis was significantly longer in the placebo group than in all of the active treatment groups (P < .05). All active treatment groups were statistically superior to placebo (P < .05) with regard to onset of meaningful pain relief. IV diclofenac 37.5 mg (22 minutes) and 75 mg (27 minutes) treatment groups had numerically faster median times to meaningful pain relief (TMPR) than the ketorolac 30 mg group (29 minutes). Table 3 presents median times to onset of meaningful pain relief. According to Cochran-Mantel-Haenszel testing, the proportions of patients receiving IV diclofenac 37.5 and 75 mg who reported a 30% reduction in pain intensity at 5 minutes were greater than those following placebo (P = .0150 and P = .0455, respectively). The proportion of patients who reported a 30% reduction in pain intensity at 5 minutes following ketorolac 30 mg did not differ from placebo (P = .649).
Table 3.
Median Time to Onset (Minutes) of Meaningful Pain Relief in the Intent-to-Treat Population
The PGE, which was completed by all patients prior to administration of rescue medication or at the end of the 8 hour postdose period (whichever came first), served as a means for patients to express their assessment of the study drugs' overall effectiveness, onset of action, and duration of effect. Consistent with the other study results, PGE showed statistically significant superiority of IV diclofenac groups and ketorolac over placebo. Overall, 42 of 51 (82.4%) patients who received IV diclofenac 37.5 mg indicated “good” or better on their global evaluation compared with 42 of 47 (89.3%) patients who received ketorolac. Patients who received IV diclofenac 75 mg indicated “good” or better in 78.4% of cases (40/51) on their global evaluation.
Both IV diclofenac and ketorolac were well tolerated. No deaths or withdrawals due to AEs were reported. A total of 191 of 353 (54.1%) patients reported at least 1 treatment-emergent AE, but most treatment-related AEs in all groups were mild to moderate in severity, were transient, and subsided without intervention. Although this prevalence of AEs at first glance appears high, it is not unusually so for studies involving prospective assessment of AE in the acute postoperative setting. One serious AE was reported in the placebo treatment group (appendicitis with onset 4 days after dosing) and was deemed unrelated to study treatment. The most frequently reported AEs with a ≥5% incidence in the overall treatment group were post pain (12.2%), procedural site reaction (9.3%), nausea (8.2%), headache (6.8%), and dry socket (6.2%). This study provided no evidence of a dose response or treatment effect for AEs, including laboratory abnormalities. No palpable swelling or thrombosis was noted at the IV infusion site in any of the treatment groups (ie, no patient in any treatment group reported a grade >1). Overall, patients treated with placebo or IV diclofenac 37.5 mg reported the highest incidence of grade 1 (tenderness along the vein)—5/51 (9.8%); those who received IV diclofenac 18.75 mg reported the lowest incidence—1/51 (2%). The ketorolac group reported an incidence of grade 1 in 3 of 47 patients (6.4%). Consistent with the lesser degree of inhibition of platelet function by diclofenac compared with ketorolac,7 incidences of postprocedural hemorrhage in the IV diclofenac groups ranged from 2.0 to 3.9% compared with 8.5% in the ketorolac group. This numeric difference was not statistically significant.
A total of 37 clinical laboratory parameters per patient were examined during this study, and 15 of 353 (4.2%) patients had clinically significant differences in these attributed to study drug. All abnormal laboratory events were mild, with the exception of 1 moderate event in the ketorolac group. One report described a notable or greater (≥3 × upper limit of normal [ULN]) elevation of alanine aminotransferase (ALT) or aspartate aminotransferase (AST), which occurred in the lowest-dose IV diclofenac group (3.75 mg). Only 4 renal events were reported, all mild and none requiring intervention. Two abnormal vital sign events were reported in the ketorolac group and none in the IV diclofenac groups. Two isolated ECG events reported in the IV diclofenac groups (a single tracing with a T-wave abnormality in a patient given 75 mg, and 1 other tracing with a T-wave inversion in a patient given 9.4 mg) were judged by investigators as “not related” and “unlikely related,” respectively, to study medication. Both resolved spontaneously without intervention before the next ECG tracing.
DISCUSSION
This randomized, double-blind, placebo- and comparator-controlled, dose-response study demonstrated that both IV diclofenac and ketorolac were well tolerated and statistically superior to placebo (P < .0001 for TOTPAR6) in relieving moderate to severe postsurgical pain for 6 hours following IV administration. The analgesic response of the 2 highest IV diclofenac doses was maintained for an equally long duration of action as evidenced by the median time to rescue medication—approximately 6 hours for IV diclofenac 37.5 and 75 mg, the latter consistent with findings of a previous efficacy study5 in which only the 75 mg dose was evaluated.
Although their duration of action was similar, IV diclofenac appeared to provide a more rapid onset of action by several measures when compared with ketorolac. Rapid onset of action, obviously desirable from the patient's point of view, facilitates early discharge from outpatient dental and other ambulatory surgery in which NSAIDs play an important role in postoperative analgesia, both as analgesics per se and by virtue of their opioid-sparing effect.1,8 In contrast, earlier formulations of diclofenac5 or current formulations of other nonopioid analgesics such as acetaminophen9 or ibuprofen10 whose IV dosing requires reconstitution, dilution, and/or slow infusion are at an intrinsic disadvantage with regard to speed of onset compared with agents such as IV diclofenac that are ready to use as a rapid, small-volume bolus.
NSAIDs and acetaminophen are the foundation of multimodal analgesia,11 now well established worldwide as the standard of care for acute postoperative pain.12,13 In practice, the goal of multimodal analgesia is to reduce opioid requirements so as to reduce opioid side effects,14 which are common and costly to treat in postsurgical patients.15 However, unless postoperative opioid requirements are reduced by a third or more, little evidence suggests that opioid side effects are lessened.1 Indeed, nonopioids such as IV acetaminophen, which on average reduce postoperative opioid consumption by 20%, do not lead to a diminution of opioid side effects.16,17 Substantial differences in intrinsic efficacy are also found between NSAIDs,13 which comprise a diverse group of compounds not only with respect to relative COX-1 and COX-2 selectivity, but also with respect to effects upon unrelated mechanisms such as ion channels or nuclear regulatory factors. Compared with other parenteral NSAIDs, diclofenac has a high intrinsic efficacy as manifested, for example, by superior degrees of opioid sparing evident in postoperative trials comparing it with indomethacin,18 ketoprofen,19,20 and ketorolac.21 Diclofenac also has the benefit of an extensive safety database based on decades of use for acute and chronic pain in diverse formulations.
The well-known differences in intrinsic efficacy of various NSAIDs, together with results of the present study, in which patients with moderate to severe pain at baseline responded well to IV diclofenac given as a single agent without opioids, contradict the conventional view that “while useful analgesic adjuncts, NSAIDs are inadequate as the sole analgesic agent in the treatment of severe postoperative pain.”13 Although it is true that IV acetaminophen and certain IV NSAIDs such as ibuprofen are intended for use as sole agents for the treatment of mild to moderate pain, or (for ibuprofen) for moderate to severe pain when given concurrently with an opioid, the present study demonstrates that IV diclofenac as a single agent is sufficient to provide analgesia for patients with moderate to severe pain.
Findings from this study suggest that a substantially lower IV diclofenac dose than that previously thought necessary (37.5 mg vs 75 mg) is sufficient to treat moderate to severe acute pain following third molar extraction. If the present findings are extended to multiple-dose, multiday trials in other models of postoperative pain, such as orthopedic and abdominal surgery, this could present an attractive alternative to ketorolac, the only NSAID currently approved as a sole analgesic for moderate to severe acute pain. Confirming the analgesic efficacy of low IV diclofenac doses in larger-scale trials in diverse models and populations would have the potential to improve the benefit-to-risk ratio of perioperative NSAID analgesia compared with ketorolac, particularly in at-risk groups such as the elderly, those with renal insufficiency, and those who receive postoperative anticoagulation.
Acknowledgments
The study was sponsored by Javelin Pharmaceuticals Inc, which was subsequently acquired by Hospira Inc. The study was conducted and data were collected by Premier Research Group (formerly the Scirex Corporation). Portions of this work were presented in preliminary form at the Fifth Congress of the European Federation of Chapters of the International Association for the Study of Pain, Istanbul, September 2006. Robert Colucci, PharmD, Daniel Gawarecki, PhD, Michael Moshman, MBA, and Curtis Wright, MD, participated in the early phases of this study.
REFERENCES
- 1.Marret E., Kurdi O., Zufferey P., Bonnet F. Effects of nonsteroidal antiinflammatory drugs on patient controlled analgesia morphine side effects: meta-analysis of randomized controlled trials. Anesthesiology. 2005;102:1249–1260. doi: 10.1097/00000542-200506000-00027. [DOI] [PubMed] [Google Scholar]
- 2.Forbes J. A. Oral surgery. In: Max M. B., Portenoy R. K., Laska E. M., editors. The Design of Analgesic Clinical Trials: Advances in Pain Research and Therapy, vol 18. New York: Raven Press; 1991. pp. 347–374. [Google Scholar]
- 3.Cooper S. A., Beaver W. T. A model to evaluate mild analgesics in oral surgery outpatients. Clin Pharmacol Ther. 1976;20:241–250. doi: 10.1002/cpt1976202241. [DOI] [PubMed] [Google Scholar]
- 4.McCormack P. L., Scott L. J. Diclofenac sodium injection (Dyloject) in postoperative pain. Drugs. 2008;68:123–130. doi: 10.2165/00003495-200868010-00008. [DOI] [PubMed] [Google Scholar]
- 5.Leeson R., Harrison C., Ernst C. C., et al. Dyloject, a novel injectable diclofenac formulation, offers greater safety and efficacy than Voltarol for postoperative dental pain. Reg Anesth Pain Med. 2007;32:303–310. doi: 10.1016/j.rapm.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 6.Dinley R. J. Venous reactions related to indwelling plastic cannulae: a prospective clinical trial. Curr Med Res Opin. 1976;3:607–617. [Google Scholar]
- 7.Bauer K. A., Gerson W., Wright C., et al. Platelet function following administration of a novel formulation of intravenous diclofenac sodium versus active comparators: a randomized, single dose, crossover study in healthy male volunteers. J Clin Anesth. 2010;22:510–518. doi: 10.1016/j.jclinane.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Sandhu N., Karuvannur S., Harmon D. Postoperative pain management in the ambulatory setting. In: Shorten G., Carr D. B., Harmon D., Puig M. M., Browne J., editors. Postoperative Pain Management: An Evidence-Based Guide to Practice. Philadelphia: Saunders; 2006. pp. 249–258. [Google Scholar]
- 9.Sinatra R. S., Jahr J. S., Reynolds L. W., Viscusi E. R., Groudine S. B., Payen-Champenois C. Efficacy and safety of single and repeated administration of 1 gram intravenous acetaminophen injection (paracetamol) for pain management after major orthopedic surgery. Anesthesiology. 2005;102:822–831. doi: 10.1097/00000542-200504000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Southworth S., Peters J., Rock A., Pavliv L. A multicenter, randomized, double-blind, placebo-controlled trial of intravenous ibuprofen 400 and 800 mg every 6 hours in the management of postoperative pain. Clin Ther. 2009;31:1922–1935. doi: 10.1016/j.clinthera.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 11.Pergolizzi J., Wills L. M. Multimodal analgesic therapy. In: Shorten G., Carr D. B., Harmon D., Puig M. M., Browne J., editors. Postoperative Pain Management: An Evidence-Based Guide to Practice. Philadelphia: Saunders; 2006. pp. 182–196. [Google Scholar]
- 12.Ashburn M. A., Caplan R. A., Carr D. B., et al. Practice guidelines for acute pain management in the perioperative setting. Anesthesiology. 2004;100:1573–1581. doi: 10.1097/00000542-200406000-00033. [DOI] [PubMed] [Google Scholar]
- 13.Macintyre P. E., Schug S. A., Scott D. A., Visser E. J., Walker S. M. APMSE Working Group of the Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine. Acute Pain Management: Scientific Evidence. 3rd ed. Melbourne: ANZCA and FPM; 2010. pp. 71–75. Available at: http://www.anzca.edu.au/resources/books-and-publications/. [Google Scholar]
- 14.White P. F., Kehlet H., Neal J. M., Schricker T., Carr D. B., Carli F. Fast-Track Surgery Study Group. The role of the anesthesiologist in fast-track surgery: from multimodal analgesia to perioperative medical care. Anesth Analg. 2007;104:1380–1396. doi: 10.1213/01.ane.0000263034.96885.e1. [DOI] [PubMed] [Google Scholar]
- 15.Oderda G., Evans R. S., Lloyd J., et al. Cost of opioid-related adverse drug events in surgical patients. J Pain Symptom Manage. 2003;25:276–283. doi: 10.1016/s0885-3924(02)00691-7. [DOI] [PubMed] [Google Scholar]
- 16.Elia N., Lysakowski C., Tramer M. R. Does multimodal analgesia with acetaminophen, nonsteroidal anti-inflammatory drugs, or selective cyclooxygenase-2 inhibitors and patient-controlled analgesia morphine offer advantages over morphine alone? Anesthesiology. 2005;103:1296–1304. doi: 10.1097/00000542-200512000-00025. [DOI] [PubMed] [Google Scholar]
- 17.Remy C., Marret E., Bonnet F. Effects of acetaminophen on morphine side-effects and consumption after major surgery: meta-analysis of randomized controlled trials. Br J Anaesth. 2005;94:505–513. doi: 10.1093/bja/aei085. [DOI] [PubMed] [Google Scholar]
- 18.Laitinen J., Nuutinen L., Kiiskila E-L., Freudenthal Y., Ranta P., Karvonen J. Comparison of intravenous diclofenac, indomethacin and oxycodone as post-operative analgesics in patients undergoing knee surgery. Eur J Anaesth. 1992;9:29–33. [PubMed] [Google Scholar]
- 19.Niemi L., Tuominen M., Pitkanen M., Rosenberg P. H. Comparison of parenteral diclofenac and ketoprofen for postoperative pain relief after maxillofacial surgery. Acta Anaesthesiol Scand. 1995;39:96–99. doi: 10.1111/j.1399-6576.1995.tb05599.x. [DOI] [PubMed] [Google Scholar]
- 20.Silvanto M., Lappi M., Rosenberg P. Comparison of the opioid-sparing efficacy of diclofenac and ketoprofen for 3 days after knee arthroplasty. Acta Anaesthesiol Scand. 2002;46:322–328. doi: 10.1034/j.1399-6576.2002.t01-1-460316.x. [DOI] [PubMed] [Google Scholar]
- 21.Alexander R., El-Moalem H. E., Gan T. J. Comparison of the morphine-sparing effects of diclofenac sodium and ketorolac tromethamine after major orthopedic surgery. J Clin Anesth. 2002;14:187–192. doi: 10.1016/s0952-8180(01)00382-8. [DOI] [PubMed] [Google Scholar]