Abstract
ART is suspected to generate increased imprinting errors in the lineage. Following an intra cytoplasmic sperm injection (ICSI) procedure, a certain number of embryos fail to develop normally and imprinting disorders may be associated to the developmental failure. To evaluate this hypothesis, we analysed the methylation profile of H19DMR, a paternally imprinting control region, in high-graded blastocysts, in embryos showing developmental anomalies, in the matching sperm and in oocytes of the concerned couples when they were available. Significant hypomethylation of the paternal allele was observed in half of the embryos, independently of the stage at which they were arrested (morula, compacted morula, pre blastocyst or BC-graded blastocysts). Conversely, some embryos showed significant methylation on the maternal allele, whereas few others showed both hypomethylation of the paternal allele and abnormal methylation of the maternal allele. The matching sperm at the origin of the embryos exhibited normal methylated H19 patterns. Thus, hypomethylation of the paternal allele in the embryos does not seem inherited from the sperm but likely reflects instability of the imprint during the demethylating process, which occurred in the early embryo. Analysis of a few oocytes suggests that the defect in erasure of the paternal imprint in the maternal germ line may be responsible for the residual methylation of the maternal allele in some embryos. None of these imprinting alterations could be related to a particular stage of developmental arrest; compared with high-grade blastocysts, embryos with developmental failure are more likely to have abnormal imprinting at H19 (P<0.05).
Keywords: ART, genomic imprinting, development, H19
Introduction
Normal mammalian development requires that both paternal and maternal genome should be expressed properly. Therefore, epigenetic marks acquired in the germ line drive the monoallelic expression, according to the parent of origin, of so called ‘imprinted genes' in the embryo.1 Many imprinted genes are involved in the regulation of fetal and/or placental growth.2 Imprinted genes are regulated through DNA sequences known as imprinting control regions (ICRs) that are differentially methylated; DNA methylation at CpG sequences results typically in gene repression. Methylation of ICRs is erased early in life and reset in the germ line, according to sex.2 Another wave of genome-wide demethylation followed later by de novo methylation occurs during pre-implantation development from which imprinted genes are protected.3
Several reports have supported the idea that artificial reproductive techniques (ARTs) would favour the acquisition of imprinting errors.4, 5, 6, 7, 8 Epigenetic abnormalities in ART could be related to parental infertility, corresponding to imprinting errors in the gametes, transmitted at fertilisation, or to in vitro manipulation of gametes and embryos.9 Therefore, looking for imprinting defects in embryos that failed to develop normally and in the gametes would provide information on whether unsuitable imprint in the gametes could be transmitted and associated with developmental arrest. Thus, we have analysed the methylation profile of H19DMR (one of the three imprinting control region that acquire methylation in the paternal germ line) in control blastocysts, initially suitable for transfer, in pre-implantation embryos arrested at different stages of their development: morula, pre-blastocyst and blastocyst showing poor morphology (graded BC), in the parental sperm and in the oocytes of the couples when they were available.
H19DMR regulates the expression of two oppositely imprinted genes:10 H19 encodes an untranslated RNA with tumour suppressor activity11 and it is expressed from the maternal allele; insulin growth factor 2 (IGF2) encodes a growth factor essential for development12 and it is expressed from the paternal allele. H19DMR harbours several CTCF- (CCCTC-binding factor) binding sites.13 CTCF binds to the maternal unmethylated DMR and prevents IGF2 from access to the common enhancers. Conversely, methylation on the paternal DMR prevents the binding of CTCF, permitting IGF2 expression. Hypermethylation of H19 maternal allele is linked to Beckwith–Wiedemann syndrome (BWS) in some patients,4 whereas hypomethylation of the paternal allele is associated with Silver–Russell syndrome,14 both syndromes showing opposed growth disorders.
Materials and methods
Source of human embryos, oocytes and sperm
A total of 33 embryos from 11 different couples, derived from fertilised ICSI oocytes, were donated for research by patients of Laboratoire de Biologie de la Reproduction at Femme Mère Enfant Hospital (Bron, France), after informed consent. Women included in this study were stimulated before ICSI procedure with standard long-term stimulation protocol using FSH and HCG. The control group was constituted of five high-graded ICSI blastocysts; the abnormal embryos were distributed as follows: five pre-blastocysts, eight abnormal BC blastocysts (the inner cell mass contained several cells, loosely grouped and the trophectoderm contained very few large cells forming a loose epithelium), 13 compacted morula and 2 morula. Protocols were approved by the French legal institution for research on human embryos, ‘Agence de la Biomédecine'. ICSI indications were heterogeneous as shown in Table 1, and all embryos were originated from super-ovulated oocytes. Zona pellucida and attached cumulus cells were removed by digestion with proteinase K (9 units/ml). Denuded embryos were carefully examined under an inverted microscope with Hoffman Modulation Contrast optics (Leica DM IRB, Leica, Rueil-Malmaison, France) and only cumulus-free embryos were selected for analysis and stored individually at −80°C.
Table 1. ICSI indication per couple.
| No of couple | ♂ Infertility factor | ♀ Infertility factor |
|---|---|---|
| 4 | Oligo-astheno-teratozoospermy | |
| 5 | Asthenozoospermy | PCOs |
| 26 | Oligo-astheno-teratozoospermy | |
| 30 | Asthenozoospermy | PCOs |
| 31 | Asthenozoospermy | PCOs+hydrosalpinx |
| 33 | Astheno-teratozoospermy | Fallopian-tube obstruction |
| 45 | Mar test +, normozoospermy | |
| 46 | Mar test +, astheno-zoospermy | |
| 50 | Normozoospermy | Fallopian-tube obstruction |
| 52 | Mar test +, astheno-teratozoospermy | PCOs |
| 56 | Normozoospermy | Fallopian-tube obstruction |
Abbreviations: PCOs, polycystic ovary syndrome; Mar test +, presence of sperm antibody.
After oocyte collection, the cumulus–oocyte complexes were partially denuded of cumulus cells by repeated pipetting in a hyaluronidase solution (150 units, type VII; Sigma, Saint Quentin Fallavier, France) and the oocytes were evaluated for maturity; the immature partially denuded oocytes, either at the germinal vesicle (GV) or at metaphase I (MI) stage, at the time of retrieval, were used for experiments. A total of 15 oocytes from two patients were included in this study: 1GV, 12 MI and 2 metaphase II (MII) that were retrieved immature and spontaneously matured in culture medium. Zona pellucida and any remaining somatic cells were removed by digestion with proteinase K (9 units/ml). After careful examination under an inverted microscope with Hoffman Modulation Contrast optics (Leica DM IRB), only cumulus-free oocytes were selected for analysis.
The ejaculated sperm samples were collected from the male partner the day when the ICSI procedure was programmed, and the methylation analysis was carried out on each individual sperm. Control sperm corresponds to sperm of normally fertile men. Routine semen analysis was carried out (volume, sperm concentration, motility and morphology), and motile sperm cells were purified on density gradient to eliminate somatic cell contamination. The sperm was washed repeatedly and placed in phosphate-buffered saline, and DNA was extracted by a standard method.
DNA methylation analysis
The methylation profile of H19 DMR was determined by bisulphite mutagenesis and sequencing as previously described.15 After treatment with bisulphite and purification, the DNA was immediately used for nested PCR. Five independent nested PCRs were performed per embryo and per sperm sample. We analysed 18 CpG sites in a 234 bp fragment of H19DMR (6097–6330 bp, AF087017) harbouring a single nucleotide polymorphism (SNP) A/C (A/T following bisulphite treatment) at nucleotide 6236. This 234 bp fragment contains the sixth CTCF-binding site. Primers specific for bisulfite-converted DNA were as follow: external forward: 5′-AATAATGAGGTGTTTTAGTTTTATGGATG-3′ external reverse: 5′-ACTTAAATCCCAAACCATAACACTAAAAC-3′ internal forward: 5′-TTGTATAGTATATGGGTATTTTTGGAGGTT-3′ internal reverse: 5′-ACTCCTATAAATATCCTATTCCCAAATAACCCC-3′. The PCR products were subcloned into pGEM-T plasmid (Promega, Charbonnière Les Bains, France). Three to six clones were sequenced for each PCR product (Biofidal, Lyon, France).
Statistics
Statistical analysis were carried out using non-parametric t-test or ANOVA, and a difference was considered significant when P≤0.05. To compute the significance of altered methylation in arrested embryos, we used Fisher's exact test and a P value ≤0.05 was considered significant.
Results
A total of 15 to 30 clones were sequenced per embryo issued from five independent PCR. Because of the limited starting material and as bisulfite treatment being deleterious for DNA, identical sequences from separated PCRs are certain to represent distinct chromosomes, but identical sequences obtained from the same PCR product are counted only once, as previously discussed;15 20 to 30 clones could be scored for control embryos and 12 to 15 clones for embryos that failed to develop (see Supplementary Figures 1 bis and 2 bis).
Control blastocysts and control sperm
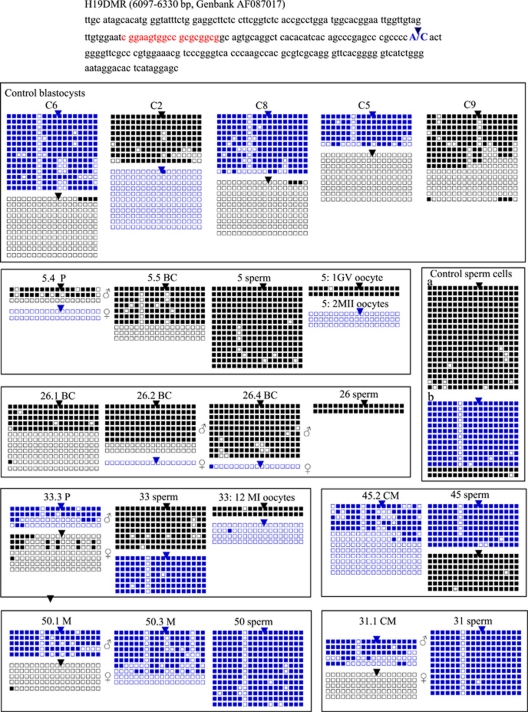
Control blastocysts were cryopreserved for 2–9 years before they were donated for research. The SNP localised within the amplified sequence allow parental allele discrimination in four out of the five analysed blastocysts as shown in Figure 1. Thus, even though the corresponding sperms were not available, all methylated sequences carrying the same SNP are most likely from paternal origin, whereas all unmethylated sequences carrying the other SNP are most likely from maternal origin. Therefore, control blastocysts appeared differentially methylated, as expected, with an average of 85.42%±2.76 methylated CpGs (615 CpGs being methylated out of 720 analysed) for the presumed paternal allele and 1.08%±0.95 methylation (8 methylated CpGs out of 738 analysed) for the presumed maternal allele. A pool of five sperms from fertile men exhibited 93.65% methylation and showed both 6236-C and 6236-A alleles, whereas the sperm from a single fertile man was homozygous 6236-C and exhibited 96.49% methylation. Thus, the highly methylated state of the paternal allele of H19 was conserved in blastocysts, even though with 8.7% significant leakage. According to the SNP depicted, the strands were distributed into two distinct profiles, 6236-C or 6236-A.
Figure 1.
Bisulphite sequencing analysis of H19DMR in control blastocysts and control sperm (a: sperm from a fertile man; b: pool of sperm from five fertile men), and in arrested embryos and their matching sperm and oocytes (when available, couples 5 and 33). Each line represents a single allele. Black squares indicate a methylated CpG and an open square denotes an unmethylated CpG. Blue lines correspond to alleles exhibiting a nucleotide 6236-A, whereas black lines correspond to alleles carrying a nucleotide 6236-C. In H19DMR, sequence from 6097 to 6330 bp, the SNP A/C, bp 6236, is indicated in blue, whereas the sixth CTCF-binding site is indicated in red. P, preblastocyst; CM, compact morula; M, morula; BC, atypic BC blastocyst;  SNP A, bp 6236; ▾ SNP C, bp 6236.
SNP A, bp 6236; ▾ SNP C, bp 6236.
Embryos with developmental failure and matching sperms
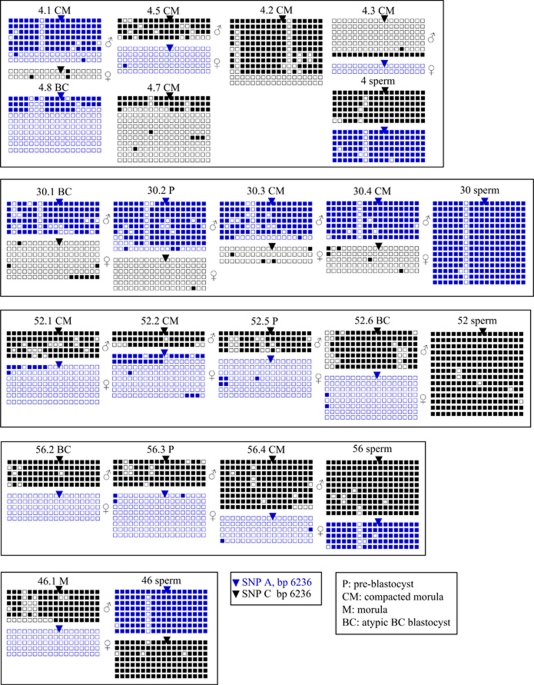
Sperms that matched embryos showing developmental failure belonged to various patients, presenting normal or altered sperm parameters, either severe sperm morphology abnormalities (teratozoospermy), mobility defect (asthenozoospermy) or associated sperm morphology, mobility and concentration defects (oligo-astheno-teratozoospermy), as shown in Table 1. All exhibited normal high methylation at H19 as shown in Figures 1 and 2, with an average of 95.16% methylated CpGs±2.61, which is comparable to that observed in sperms from fertile men. Six were homozygous (6236-C or 6236-A) and five were heterozygous (6236-C and 6236-A) for the SNP described.
In 21 embryos out of 28, the observed SNP permitted parental allele discrimination, as shown in Figures 1 and 2. Embryos included in this study were blocked at various stages, from morula to compacted morula and pre-blastocyst stage, while five embryos reached the blastocyst stage but with poor morphology (graded BC). Embryos from the same couple could be arrested at different stages of development, and there was no correlation between the stage of blockage and any infertility factor (Tables 1 and 2). We observed significant relaxation of H19 paternal imprint in 8 embryos out of 21 (4.1; 4.3; 5.4; 26.2; 30.2; 30.3; 31.1; and 33.3) where parental allele discrimination was possible, the altered strands being partially to totally unmethylated (Figures 1 and 2). Moreover, eight of these embryos (4.1; 30.1; 30.3; 30.4; 33.3; 52.1; 52.2; and 52.5) exhibited significant, even minor, methylation of the maternal allele, with two embryos, 33.3 and 52.2, showing up to 15.87 and 20.14% methylation, respectively. Altered metylation patterns on the paternal and the maternal alleles were not correlated, nor were they associated with a particular stage of developmental arrest (Table 2).
Figure 2.
Bisulphite sequencing analysis of H19DMR in arrested embryos and their matching sperm. Each line represents a single allele. Black squares indicate a methylated CpG and an open square denotes an unmethylated CpG. Blue lines correspond to alleles exhibiting a nucleotide 6236-A, whereas black lines correspond to alleles carrying a nucleotide 6236-C.
Table 2. Methylation of H19DMR in embryos, in which parental allele discrimination could be carried out and in sperms.
| No of embryo | Embryo type | ♂ Allele % methylation | ♀ Allele % methylation | Sperm % methylation |
|---|---|---|---|---|
| 4.1 | CM | 68.75 (99/144)* | 8.34 (3/36)* | 97.68 (211/216) |
| 4.3 | CM | 15.07 (19/145)* | 0 (0/36) | 97.68 (211/216) |
| 4.5 | CM | 79.17 (57/72) | 1.11 (1/90) | 97.68 (211/216) |
| 5.4 | P | 51.85 (28/54)* | 0 (0/36) | 96.66 (261/270) |
| 26.2 | BC | 77.78 (126/162)* | 0 (0/18) | 100 (36/36) |
| 26.4 | BC | 94.11 (170/180) | 5.56 (1/17) | 100 (36/36) |
| 30.1 | BC | 78.70 (85/108) | 2.56 (9/117)* | 94.07 (254/270) |
| 30.2 | P | 75.93 (123/162)* | 0.93 (1/108) | 94.07 (254/270) |
| 30.3 | CM | 75.39 (95/126)* | 7.40 (4/50)* | 94.07 (254/270) |
| 30.4 | CM | 90.48 (114/126) | 5.55 (5/90)* | 94.07 (254/270) |
| 31.1 | CM | 69.44 (75/108)* | 0 (0/90) | 93.98 (203/216) |
| 33.3 | P | 54.17 (39/72)* | 15.87 (20/126)* | 93.70 (253/270) |
| 46 | CM | 78.70 (85/108) | 0 (0/72) | 95.55 (258/270) |
| 50.1 | M | 81.11 (73/90)) | 1.11 (1/90) | 90.87 (229/252) |
| 52.1 | CM | 84.44 (76/90) | 6.35 (8/126)* | 98.01 (247/252) |
| 52.2 | CM | 88.89 (48/54) | 20.14 (29/144)* | 98.01 (247/252) |
| 52.5 | P | 80.56 (58/72) | 3.96 (5/126)* | 98.01 (247/252) |
| 52.6 | BC | 81.75 (103/126) | 1.39 (2/144) | 98.01 (247/252) |
| 56.2 | BC | 93.33 (84/90) | 0 (0/90) | 94.44 (255/270) |
| 56.3 | P | 88.89 (80/90) | 2.08 (3/144) | 94.44 (255/270) |
| 56.4 | CM | 93.21 (151/162) | 2.22 (2/90) | 94.44 (255/270) |
| 2 | Control | 89.50 (145/162) | 0 (0/198) | |
| 5 | Control | 83.33 (90/108) | 0 (0/162) | |
| 6 | Control | 84.92 (214/252) | 0 (0/198) | |
| 8 | Control | 85.18 (184/216) | 1.66 (3/177) | |
| Control sperm | ||||
| a | 96.49 (275/285) | |||
| b | 93.65 (236/252) | |||
Abbreviations: M, morula; CM, compacted morula; BC, abnormal BC blastocysts; P, pre-blastocysts; control, high-graded blastocysts, suitable for transfer. a, sperm from a fertile man; b, pool of sperms from five fertile men.
*P≤0.005.
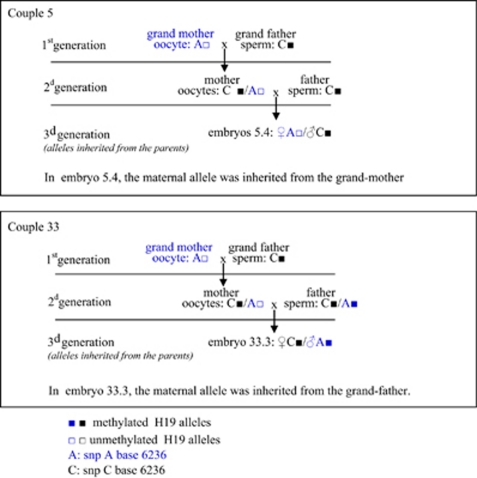
The methylation status of the oocytes could be analysed for two couples (Figure 1). In couple 5 the father was homozygous C, whereas the mother was heterozygous A or C. One GV oocyte, carrying the SNP 6236-C, exhibited hypermethylated alleles, whereas a pool of two mature MII oocytes, carrying the SNP 6236-A, showed normal hypomethylation. As hypothesised in Figure 3, the hypermethylated 6236-C alleles would be inherited from the grand father and would correspond to alleles that escaped erasure of paternal marks, early during oogenesis. Thus, embryo 5.4 inherited a paternal 6236-C allele, the corresponding sperm being homozygous 6236-C and a maternal 6236-A allele showing normal hypomethylation that is likely to be inherited from the grand mother. The paternally inherited 6236-C allele exhibited significant hypomethylation (P<0.001). In couple 33, both the father and the mother were heterozygous 6236-C and 6236-A. In all, 12 immature MI oocytes could be collected and analysed. They showed two different allelic profiles: 6236-A, normally hypomethylated and presumably inherited from the mother or 6236-C, highly methylated (35 methylated CpGs out of 36 analysed) and presumably paternally inherited, corresponding to alleles that escaped erasure of paternal marks early during oogenesis, as previously discussed. Thus, embryo 33.3 is likely to originate from a paternal spermatozoon carrying a 6236-A methylated allele, as the two methylated 6236-A strands carried an umethylated 6194-C nucleotide, as can be seen in all 6236-A sperm alleles, and an oocyte carrying a 6236-C methylated allele for H19, likely inherited from the grand father as speculated in Figure 2. The presumed paternal allele is significantly hypomethylated (39 methylated CpGs out of 72 analysed P<0.001), whereas the presumed maternal allele is significantly methylated (20 methylated CpGs out of 126 analysed, P<0.001).
Figure 3.
Proposed origin of the methylation alterations observed in embryos 5.4 and 33.3.
Compared with high-grade blastocysts, embryos with developmental failure are more likely to have abnormal imprinting at H19 (13/21 versus 0/4, P<0.05).
Discussion
Imprinting is both heritable and reversible; in the early embryo, epigenetic information is erased from the genome on a large scale to permit the return of developmental pluripotency to embryonic cells, and this limits the amount of epigenetic information that can be inherited across generation.16 Imprinted genes that carry such heritable epigenetic information must be protected against this powerful remodelling of the epigenome proceeding during pre-implantation development. Considering the sensor gene H19, the paternal allele normally escapes the active demethylation of the male genome that occurs in the pronucleus soon after fertilisation and the maternal allele is protected against de novo methylation of the zygotic DNA from morula to blastocyst stage.3
Very little is known concerning the differential methylation of imprinted genes in pre-implantation human embryos. In the present work, using an SNP within H19DMR, we can determine the differential methylation status of 4 out of 5 control blastocysts and of 21 out of 28 abnormal embryos, the paternally inherited alleles being generally methylated, whereas the maternally inherited alleles were unmethylated. Our results from control embryos confirmed that, as observed in the mouse,17 H19 is differentially methylated in human blastocysts conceived via ART. In contrast, 38% of the embryos that failed to develop exhibited significant hypomethylation of the paternal allele, independently of the type of developmental failure: blockage at the compacted morula or pre-blastocyst stage, or poor morphology at the blastocyst stage. As normal methylation of the paternal allele was observed in the other abnormal embryos, imprinting errors at H19/IGF2 are likely not to be primarily involved in the developmental failure of human ICSI embryos. Culture conditions were identical for control and developmentally failing embryos and thus could not be the cause of abnormal methylation.
Embryos showing hypomethylated paternal alleles may originate from sperm, in which resetting of the imprint has been only partially accomplished. Recently, Kobayashi et al18 showed that hypomethylation of H19DMR in aborted ART concepti originated from the parental sperm. A case of Silver–Russel syndrome due to paternal H19DMR hypomethylation in an ICSI patient has been likewise documented.19 In fact, we found no alteration of the normal methylated pattern of H19DMR in all the examined parental sperm, whatever their biological characteristics were, from normozoospermic to oligo-astheno-teratospermic, demonstrating that the alterations in DNA methylation seen in some arrested embryos did not pre-exist in the father's germ line. It is interesting to note that, in sperm carrying the SNP A, the cytosine corresponding to the fourth CpG of the sixth CTCF-binding site (nucleotide 6194) commonly appeared as a thymidine (in 84 out of 87 analysed alleles), presumably corresponding to an unmethylated CpG in the original strand. Renda et al20 demonstrated that methylation of the first and the second CpGs of the CTCF-binding sequence was sufficient to inhibit the binding of CTCF, the methylation of the first CpG being more powerful. On the other hand, in most arrested embryos, hypomethylation of the paternal allele did not particularly target the key CpGs, suggesting normal expression of IFG2. However, the number of samples analysed remained limited, and sperm-transmitted aberrant hypomethylation of the paternal allele of H19 in arrested embryos cannot be totally excluded, as H19 hypomethylation in sperm has been associated with paternal infertility in a number of articles.21, 22, 23 Thus, considering that the resetting of methylation during gametogenesis has normally proceeded, it could be hypothesised that the hypomethylation observed is due to an alteration of the methylation maintenance in the early embryo. Maintenance of DNA methylation at imprinting DMRs in pre-implantation embryos has recently been assigned to the somatic and the oocyte form of DNMT1, in cooperation. Maternal deletion of both isoforms, DNMT1s and DNMT1o, caused the loss of methylation at multiple imprinted loci in mouse blastocysts.24 Other trans-acting factors critical for the maintenance of methylation at imprinted genes have been identified, such as MBD3, which is required to maintain the methylation of the paternal H19 allele and its silencing.25
Some embryos exhibited significant methylation of the maternal allele. Results from couples 5 and 33 whose oocytes were available allow speculating. In both couples the paternal allele shows some relaxation of the imprint, probably due to alterations in DNA methylation maintenance as previously discussed. In both couples some immature GV and MI oocytes were highly methylated. We previously evidenced such altered pattern of methylation in human oocytes, particularly in oocytes that were immature on the day of retrieval, but also in mature oocytes.15 Although the maternally originating H19 strands were not methylated in embryo 5.4, they were significantly methylated in embryo 33.3. Both the father and the mother were heterozygous in couple 33. The allele likely inherited from the father carried the A SNP; it was initially methylated as the sperm appeared normally methylated, and was then partially demethylated in the course of cell divisions. The allele likely inherited from the mother carried the C SNP and showed abnormal methylation. Methylated H19 strands in the oocytes may correspond to the acquisition of abnormal methylation on the maternal allele during oogenesis or lack of erasure of the paternal imprint early in the gonocytes. Because the oocytes from the mother, which could be analysed showed either unmethylated A-SNP-H19 strands or methylated C-SNP-H19 strands, methylation apposition during oogenesis is unlikely, otherwise, it would also have randomly mark A-SNP-H19 strands. Lack of erasure of the copies originating from the grand father in the gonocytes of the mother is a more credible scenario. Thus, all methylated copies seen in the oocytes are likely originated from paternal copies, the imprint of which has not been erased, as postulated in Figure 3. Such a gain of maternal methylation and a loss of paternal methylation at H19DMR have been recently documented in mouse blastocysts and were attributed to superovulation induced imprinting disorders.17 The authors did not give information on the imprint in the oocytes themselves. On the other hand, we found almost no residual methylation on the maternal allele of control human blastocysts while Market-Velker et al17 showed an average methylation of the maternal allele of H19 of up to 15% in mouse blastocysts obtained from spontaneously ovulated female, demonstrating one more time the discrepancies existing between species at the level of epigenome regulation.
In this work, we show that a significant number of arrested pre-implantation embryos have altered H19 imprints on both paternal and maternal alleles, whereas blastocysts suitable for transfer were normally imprinted. Our results suggested both a failure of erasure of the paternally inherited imprint in the maternal germ line and a lack of methylation maintenance on the paternal allele. The question remains whether there is a link between imprinted errors and parental infertility. No evident link appeared between imprint anomalies and a particular stage of development in failing embryos. However, the data presented show a significant association between developmental failure of embryos and abnormal imprinting of H19.
Acknowledgments
We are very grateful to all the couples that donated embryos and gametes for research and to the staff of ‘Service de Biologie de la Reproduction' at FME Hospital, Bron, particularly to Jacqueline Lornage and Astrid Perret. This work was supported by Université Claude Bernard Lyon 1.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary Material
References
- Surani MA. Imprinting and the initiation of gene silencing in the germ line. Cell. 1998;93:309–312. doi: 10.1016/s0092-8674(00)81156-3. [DOI] [PubMed] [Google Scholar]
- Hajkova P, Erhardt S, Lane N, et al. Epigenetic reprogramming in mouse primordial germ cells. Mech Dev. 2002;117:15–23. doi: 10.1016/s0925-4773(02)00181-8. [DOI] [PubMed] [Google Scholar]
- Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- DeBaun MR, Niemitz EL, Feinberg AP. Association of in vitro fertilization with Beckwith-Wiedemann syndrome and epigenetic alterations of LIT1 and H19. Am J Hum Genet. 2003;72:156–160. doi: 10.1086/346031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gicquel C, Gaston V, Mandelbaum J, Siffroi JP, Flahault A, Le Bouc Y. In vitro fertilization may increase the risk of Beckwith-Wiedemann syndrome related to the abnormal imprinting of the KCN1OT gene. Am J Hum Genet. 2003;72:1338–1341. doi: 10.1086/374824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher ER, Brueton LA, Bowdin SC, et al. Beckwith-Wiedemann syndrome and assisted reproduction technology (ART) J Med Genet. 2003;40:62–64. doi: 10.1136/jmg.40.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orstavik KH, Eiklid K, van der Hagen CB, et al. Another case of imprinting defect in a girl with Angelman syndrome who was conceived by intracytoplasmic semen injection. Am J Hum Genet. 2003;72:218–219. doi: 10.1086/346030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowdin S, Allen C, Kirby G, et al. A survey of assisted reproductive technology births and imprinting disorders. Hum Reprod. 2007;22:3237–3240. doi: 10.1093/humrep/dem268. [DOI] [PubMed] [Google Scholar]
- Grace KS, Sinclair KD. Assisted reproductive technology, epigenetics, and long-term health: a developmental time bomb still ticking. Semin Reprod Med. 2009;27:409–416. doi: 10.1055/s-0029-1237429. [DOI] [PubMed] [Google Scholar]
- Zemel S, Bartolomei MS, Tilghman SM. Physical linkage of two mammalian imprinted genes, H19 and insulin-like growth factor 2. Nat Genet. 1992;2:61–65. doi: 10.1038/ng0992-61. [DOI] [PubMed] [Google Scholar]
- Yoshimizu T, Miroglio A, Ripoche MA, et al. The H19 locus acts in vivo as a tumor suppressor. Proc Natl Acad Sci USA. 2008;105:12417–12422. doi: 10.1073/pnas.0801540105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik W, Constancia M, Dean W, et al. Igf2 imprinting in development and disease. Int J Dev Biol. 2000;44:145–150. [PubMed] [Google Scholar]
- Takai D, Gonzales FA, Tsai YC, Thayer MJ, Jones PA. Large scale mapping of methylcytosines in CTCF-binding sites in the human H19 promoter and aberrant hypomethylation in human bladder cancer. Hum Mol Genet. 2001;10:2619–2626. doi: 10.1093/hmg/10.23.2619. [DOI] [PubMed] [Google Scholar]
- Gicquel C, Rossignol S, Cabrol S, et al. Epimutation of the telomeric imprinting center region on chromosome 11p15 in Silver-Russell syndrome. Nat Genet. 2005;37:1003–1007. doi: 10.1038/ng1629. [DOI] [PubMed] [Google Scholar]
- Borghol N, Lornage J, Blachere T, Garret AS, Lefèvre A. Epigenetic status of the H19 locus in human oocytes following in vitro maturation. Genomics. 2006;87:417–426. doi: 10.1016/j.ygeno.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Santos F, Hendrich B, Reik W, Dean W. Dynamic reprogramming of DNA methylation in the early mouse embryo. Dev Biol. 2002;241:172–182. doi: 10.1006/dbio.2001.0501. [DOI] [PubMed] [Google Scholar]
- Market-Velker BA, Zhang L, Magri LS, Bonvissuto AC, Mann MR. Dual effects of superovulation: loss of maternal and paternal imprinted methylation in a dose-dependent manner. Hum Mol Genet. 2010;19:36–51. doi: 10.1093/hmg/ddp465. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Hiura H, John RM, et al. DNA methylation errors at imprinted loci after assisted conception originate in the parental sperm. Eur J Hum Genet. 2009;17:1582–1591. doi: 10.1038/ejhg.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra M, Amor DJ, Sutton L, Algar E, Mowat D. Russell-Silver syndrome due to paternal H19/IGF2 hypomethylation in a patient conceived using intracytoplasmic sperm injection. Reprod Biomed Online. 2010;20:843–847. doi: 10.1016/j.rbmo.2010.02.025. [DOI] [PubMed] [Google Scholar]
- Renda M, Baglivo I, Burgess-Beusse B, et al. Critical DNA binding interactions of the insulator protein CTCF: a small number of zinc fingers mediate strong binding, and a single finger-DNA interaction controls binding at imprinted loci. J Biol Chem. 2007;282:33336–33345. doi: 10.1074/jbc.M706213200. [DOI] [PubMed] [Google Scholar]
- Boissonnas CC, Abdalaoui HE, Haelewyn V, et al. Specific epigenetic alterations of IGF2-H19 locus in spermatozoa from infertile men. Eur J Hum Genet. 2010;18:73–80. doi: 10.1038/ejhg.2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques CJ, Costa P, Vaz B, et al. Abnormal methylation of imprinted genes in human sperm is associated with oligozoospermia. Mol Hum Reprod. 2008;14:67–74. doi: 10.1093/molehr/gam093. [DOI] [PubMed] [Google Scholar]
- Poplinski A, Tuttelmann F, Kanber D, Horsthemke B, Gronoll J. Idiopathic male infertility is strongly associated with aberrant methylation of MEST and IGF2/H19 ICR1. Int J Androl. 2010;33:642–649. doi: 10.1111/j.1365-2605.2009.01000.x. [DOI] [PubMed] [Google Scholar]
- Hirasawa R, Chiba H, Kaneda M, et al. Maternal and zygotic Dnmt1 are necessary and sufficient for the maintenance of DNA methylation imprints during preimplantation development. Genes Dev. 2008;22:1607–1616. doi: 10.1101/gad.1667008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese KJ, Lin S, Verona RI, Schultz RM, Bartolomei MS. Maintenance of paternal methylation and repression of the imprinted H19 gene requires MBD3. PLoS Genet. 2007;3:e137. doi: 10.1371/journal.pgen.0030137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.