Abstract
Background:
Reduced upper airway muscle activity during sleep is fundamental to obstructive sleep apnea (OSA) pathogenesis. Hypoglossal nerve stimulation (HGNS) counteracts this problem, with potential to reduce OSA severity.
Study Objectives:
To examine safety and efficacy of a novel HGNS system (HGNS, Apnex Medical, Inc.) in treating OSA.
Participants:
Twenty-one patients, 67% male, age (mean ± SD) 53.6 ± 9.2 years, with moderate to severe OSA and unable to tolerate continuous positive airway pressure (CPAP).
Design:
Each participant underwent surgical implantation of the HGNS system in a prospective single-arm interventional trial. OSA severity was defined by apnea-hypopnea index (AHI) during in-laboratory polysomnography (PSG) at baseline and 3 and 6 months post-implant. Therapy compliance was assessed by nightly hours of use. Symptoms were assessed using the Epworth Sleepiness Scale (ESS), Functional Outcomes of Sleep Questionnaire (FOSQ), Calgary Sleep Apnea Quality of Life Index (SAQLI), and the Beck Depression Inventory (BDI).
Results:
HGNS was used on 89% ± 15% of nights (n = 21). On these nights, it was used for 5.8 ± 1.6 h per night. Nineteen of 21 participants had baseline and 6-month PSGs. There was a significant improvement (all P < 0.05) from baseline to 6 months in: AHI (43.1 ± 17.5 to 19.5 ± 16.7), ESS (12.1 ± 4.7 to 8.1 ± 4.4), FOSQ (14.4 ± 2.0 to 16.7 ± 2.2), SAQLI (3.2 ± 1.0 to 4.9 ± 1.3), and BDI (15.8 ± 9.0 to 9.7 ± 7.6). Two serious device-related adverse events occurred: an infection requiring device removal and a stimulation lead cuff dislodgement requiring replacement.
Conclusions:
HGNS demonstrated favorable safety, efficacy, and compliance. Participants experienced a significant decrease in OSA severity and OSA-associated symptoms.
Clinical Trial Information:
Name: Australian Clinical Study of the Apnex Medical HGNS System to Treat Obstructive Sleep Apnea. Registration Number: NCT01186926. URL: http://clinicaltrials.gov/ct2/show/NCT01186926.
Citation:
Eastwood PR; Barnes M; Walsh JH; Maddison KJ; Hee G; Schwartz AR; Smith PL; Malhotra A; McEvoy RD; Wheatley JR; O'Donoghue FJ; Rochford PD; Churchward T; Campbell MC; Palme CE; Robinson S; Goding GS; Eckert DJ; Jordan AS; Catcheside PG; Tyler L; Antic NA; Worsnop CJ; Kezirian EJ; Hillman DR. Treating obstructive sleep apnea with hypoglossal nerve stimulation. SLEEP 2011;34(11):1479-1486.
Keywords: Sleep apnea, hypoglossal nerve stimulation, implantable neurostimulator, genioglossus muscle, lung
INTRODUCTION
Obstructive sleep apnea (OSA) is a serious, potentially life-threatening condition affecting millions of people worldwide. OSA is characterized by repeated episodes of airway collapse (apnea) or narrowing (hypopnea) during sleep, often leading to hypoxemia and hypercapnia.1 Episodes are usually terminated by a brief arousal, after which sleep resumes and the cycle repeats itself, often hundreds of times per night. Prevalence of OSA is high and increasing with greater obesity and aging of populations internationally.2
Continuous positive airway pressure (CPAP) is the preferred treatment option for most patients with OSA,3 but adherence to the therapy is often poor.4 Despite efforts to improve adherence, only 40% to 60% of patients continue to use CPAP long-term or as prescribed, and many others do not seek medical attention knowing there are few acceptable alternatives. Those with untreated OSA are exposed to a significantly increased risk of sudden death,5 hypertension,6 stroke,7 coronary artery disease,8 congestive heart failure,8 type 2 diabetes,9 depression,10 motor vehicle accidents,11 occupational accidents, lost productivity,12 and decreased quality of life.13 Thus, alternative treatments are needed.
Electrical stimulation of the genioglossus muscle, the largest upper airway dilator muscle, causes tongue protrusion and stiffening of the anterior pharyngeal wall, and is therefore a potential therapeutic target for OSA. Previous studies using submental, intraoral, or intramuscular stimulating electrodes have reported improvements in upper airway diameter,14 pharyngeal collapsibility,15,16 and maximal inspiratory flow,17 as well as decreased apneas and hypopneas during sleep in patients with OSA.18,19–20 However, a major limitation with such stimulation techniques is the propensity for stimulation to induce arousal,20,21–22 presumably due to sensory stimulation, which limited their potential application as a long-term therapy for OSA.
Contraction of the genioglossus muscle can also be achieved by electrically stimulating its motor nerve, the hypoglossal nerve. The branches of the hypoglossal nerve that innervate the genioglossus predominantly contain efferent (motor) fibers, such that stimulation of those branches activates the genioglossus muscle with minimal afferent (sensory) feedback. The feasibility of chronic hypoglossal nerve stimulation (HGNS) and its potential as a therapeutic approach for the treatment of OSA was initially described by Eisele et al.23 and Schwartz et al.24 who showed that HGNS could decrease the frequency of obstructive apneic and hypopneic events and improve the severity of oxyhemoglobin desaturation without arousing patients from sleep.24 While the device used in these studies was reported to have performed well in terms of safety, a number of technical problems with the system were reported, including failure of the stimulation lead, stimulating electrode, and respiration sensor,25,26 precluding systematic evaluation of efficacy. These and other technical limitations resulted in a hiatus of 10 years during which there was no further published experience with HGNS, despite optimism from these early reports. Recent technical advances have resulted in the development of a HGNS device which addresses these limitations.
The present study reports the first clinical trial of a new generation implantable HGNS therapy system (HGNS, Apnex Medical Inc., St. Paul, MN, USA) for patients with OSA. In this device, electrical signals are generated by an implanted neurostimulator and delivered to the ipsilateral HGN via an implanted cuff electrode. Respiration is monitored via implanted thoracic leads that sense changes in bioimpedance with chest wall motion, delivering stimulation immediately prior to and during the inspiratory phase of respiration, when the upper airway is most vulnerable to sleep related narrowing and collapse.
The objective of this clinical study was to evaluate the safety of this therapy and the associated implant procedure. We also sought to determine its effectiveness by measuring its influence on the occurrence of apnea and hypopnea events during sleep, sleep quality, and symptoms and by assessing patient adherence.
METHODS
Study Participants
Patient inclusion criteria included previous diagnosis of moderate-to-severe OSA; failure of CPAP treatment despite persistent, supervised attempts to implement it; age between 21 and 70 years; and body mass index (BMI) ≤ 40kg/m2. On the baseline sleep study (polysomnogram, PSG), patients were required to have an apnea-hypopnea index (AHI) between 20 and 100/h, with ≥ 15/h occurring in NREM sleep, and have a predominance of hypopneas (≥ 80%) as a proportion of the sum of apnea and hypopnea events.
Primary exclusion criteria included prior surgery on palate, tongue, mandible or maxilla; enlarged tonsils; nasal obstruction uncontrolled by medication or surgery; severe retrognathia; > 5% central or mixed apneic events combined as a proportion of total apnea and hypopnea events; untreated or incompletely treated sleep disorders other than OSA; a history (within one year) of falling asleep while driving, motor vehicle accident, or near-miss secondary to excessive sleepiness; heart failure; recent history (last 3 months) of a major cardiovascular event; major disorder of the pulmonary, cardiac, renal or nervous systems; chronic narcotic use; presence of another active implantable device; systemic infection; inadequately treated major depression; and pregnancy or breastfeeding.
Study Design
A single-arm, open-label study was undertaken at 4 Australian clinical trial sites. Consenting patients who met all eligibility criteria underwent surgical implantation of a HGNS system. Therapy was initiated at approximately 30 days post-implant, following which daytime and overnight studies were used to determine the stimulation settings considered effective in reducing OSA severity. Sleep studies were repeated at 1, 3, and 6 months after implant, and primary safety and efficacy endpoints compared to baseline.
Study Device and Procedures
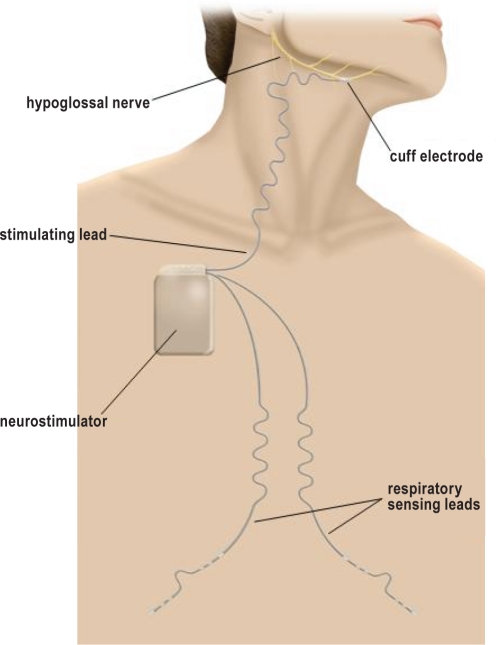
The HGNS system consists of an implantable neurostimulator that delivers current to the HGN via a stimulation lead. The current delivery is synchronized with inspiration detected by respiration sensing leads (Figure 1). The sensing leads measure changes in thoracic bioimpedance that occur with breathing. This signal is used to predict when each inspiratory effort will occur. This predictive therapy delivery algorithm allows for HGNS to begin prior to the onset of each inspiratory effort and be maintained throughout the inspiratory phase of the respiratory cycle. Therapy settings are programmed into the neurostimulator through a computer interface and programmer head. The patient can also control limited aspects (start, stop, and pause) of therapy with a handheld controller. Stimulation is normally set to start automatically according to a predefined time-of-day schedule, but it can also be operated in a manual mode depending on patient preference.
Figure 1.
The implanted components of the hypoglossal nerve stimulating system include an implantable neurostimulator that delivers safe levels of electrical stimulation to the hypoglossal nerve via a stimulation lead having a distal cuff electrode. Stimulation is delivered synchronous with inspiration as detected by respiration sensing leads using bio-impedance.
The neurostimulator, respiration sensing, and stimulation leads were surgically implanted under general anesthesia. Briefly, an incision was made below and parallel to the inferior border of the right mandible. A branch of the HGN was exposed deep to the submandibular gland and superior to the digastric tendon. The cuff of the stimulating lead was placed on the main trunk of the HGN distal to branches (typically part of the lateral branch) that innervate the retrusor muscles. There is high variability in the neuroanatomy of the HGN and final cuff placement was determined by intraoperative response of the upper airway to brief stimulation (Figure 2). The stimulation lead body was then tunnelled subplatysmally via the neck to the neurostimulator, which was implanted in an ipsilateral infraclavicular subcutaneous pocket. From the pocket, 2 respiratory sensing leads were tunnelled subcutaneously toward the midline and then bilaterally along each costal margin.
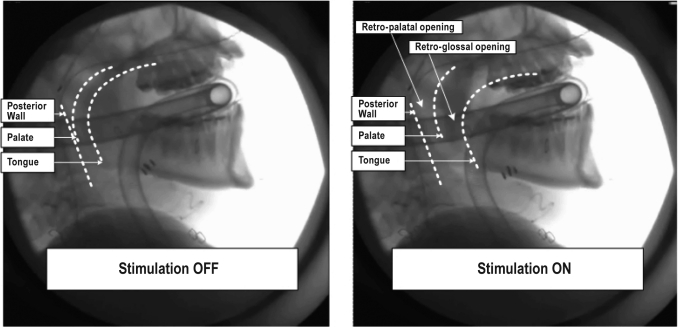
Figure 2.
Intraoperative stimulation of the hypoglossal nerve visualized using lateral fluoroscopy. The fluoroscopic imaging was undertaken to determine and verify correct electrode cuff placement (tongue protrusion/pharyngeal opening) with brief stimulation. The subject was under general anesthesia, positioned to optimize submandibular surgery (supine, head extended slightly). The endotracheal tube and connecting tubing are visible in the images.
Following surgery, a healing period of approximately 30 days was allowed, during which the device was not activated. After this procedure each participant underwent an overnight sleep study (Titration Night) during which stimulation settings (e.g., pulse width, frequency, current) were adjusted gradually, based on tolerance, to levels that consistently abolished inspiratory flow limitation. Hypnotics (e.g., temazepam 10 mg) were used as necessary to aid sleep during titration nights.
Immediately after this visit the participant returned home and began using the device (Delivery Nights). Nightly stimulation was based on programmed automatic start and stop times (default) or set to allow the participant to start and stop it manually. Stimulation settings were adjusted over time as dictated by patient comfort.
In-laboratory sleep studies were performed at 1, 3, and 6 months post-implant to assess efficacy (Efficacy Nights). Hypnotics were not used during these studies, and a minimum of 6 h of sleep data were required for analysis. Information regarding at-home stimulation utilization was stored on the device and downloaded at each patient visit to the sleep laboratory.
Data from each overnight sleep study were scored and verified by a core laboratory (Clinilabs, Inc. New York, NY) using the same 2 scorers throughout. The 1999 American Academy of Sleep Medicine apnea and hypopnea definitions were used,27 except that a 4% oxygen desaturation was required for an hypopnea (i.e. modified Chicago criteria).
At baseline and at 3 and 6 months post-implant, participants completed 5 questionnaires: the Epworth Sleepiness Scale (ESS), a self-administered questionnaire measuring daytime sleepiness;28 the Functional Outcomes of Sleep Questionnaire (FOSQ), a self-administered questionnaire assessing impact of excessive sleepiness on activities of daily living;29 the Calgary Sleep Apnea Quality of Life Index (SAQLI), an interview-based questionnaire measuring within-patient change in sleep apnea related quality of life in response to therapeutic intervention;30 the Pittsburgh Sleep Quality Index (PSQI), a self-administered questionnaire measuring sleep quality and sleep disturbance retrospectively over a one month period;31 and the Beck Depression Inventory (BDI), a self-administered questionnaire rating level of depression.32
Efficacy and Safety Endpoints
The primary efficacy endpoints were the mean change in AHI and FOSQ total score at 3 and 6 months post implant compared to baseline. Secondary efficacy endpoints included change from baseline measurements of several PSG-based measures relating to sleep disordered breathing and sleep architecture, as well as scores for ESS, SAQLI, PSQI, and BDI. All adverse events were reported regardless of whether they were deemed serious and regardless of whether they were considered related to the procedure, device, or therapy. Adverse events were deemed serious if they resulted in patient death; life-threatening illness or injury; permanent impairment of body structure or function; in-patient hospitalization (> 24 h) or prolongation of existing hospitalization; medical or surgical intervention to prevent permanent impairment to body structure or body function; or a congenital anomaly/birth defect. The primary safety endpoint was the rate of freedom from serious adverse events at implant and 3 and 6 months post-implant.
Study Oversight
The study protocol was reviewed and approved by the Australian Therapeutic Goods Administration (TGA) and the ethics committee at each participating site. Adverse events were adjudicated by an independent Clinical Events Committee. An independent Data Safety Monitoring Committee provided ethical and scientific review of the study. Participants provided written informed consent prior to their involvement in any study procedure.
Statistical Analysis
This was the first clinical study of this device in humans, and there were no clinical data upon which to base formal sample size calculations a priori. However, a sample size of 21 subjects, as was used in the present study, provides 80% power to detect an effect size of 0.6 with a two-sided 0.05 α level based on a paired t-test.
Repeated measures regression models were used to assess statistical differences in outcomes between visits. In cases where the normality assumption was violated (P < 0.05 from a Shapiro-Wilk test for normality of the studentized residuals), transformations were explored to produce models with improved fits. Final transformations employed included a logarithmic transform (for apnea hypopnea index, hypopnea index, arousal index, respiratory arousal index, oxygen desaturation index [ODI] 4%, sleep latency, and time in stage N1 sleep), a negative one-half power transformation (apnea index), a square transformation (sleep efficiency), and a square root transformation (BDI). A compound symmetric covariance structure was found to fit the data adequately, except for arousal index and time in REM sleep, where an unstructured covariance structure was found to be a better fit. A linear model was used to compare the 6-month AHI values between those with BMI > 35 and < 35 kg/m2, adjusting for each subject's baseline AHI. Separately for each outcome, P-values were adjusted by the Sidak-Holm method to control for multiple comparisons. Statistical analyses were conducted in SAS version 9.2 (SAS Institute, Cary, NC). Data are presented as mean ± SD or median and percentiles, where appropriate. P < 0.05 was considered statistically significant
Adverse event profiles intraoperatively, perioperatively, and at 1, 3, and 6 months post-implant were used to estimate the rate of incidence of freedom from system (device or therapy) or procedure-related serious adverse events calculated using the Kaplan-Meier method of time-to-event analysis.
RESULTS
Subjects
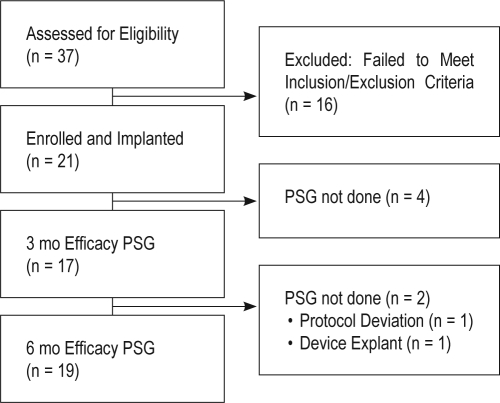
Thirty-seven subjects were assessed for eligibility; 16 of these failed to meet inclusion criteria (Figure 3). A total of 21 participants (14 male, 7 female, all Caucasian) were implanted. Demographic and baseline data are presented in Tables 1–3. At study entry, participants were overweight or obese (Table 1) and had moderate to severe OSA and reduced REM sleep (Table 2), and reported daytime sleepiness, reduced sleep quality, and mild depression (Table 3). The first 6 participants were enrolled prior to the Core Laboratory beginning to score and evaluate participation using inclusion/exclusion criteria established in the protocol. In this case, the site-scored sleep study results were used to evaluate eligibility using the inclusion/exclusion criteria established in the protocol.
Figure 3.
Flow diagram showing the progress of all participants through the trial. PSG, laboratory-based polysomnography.
Table 1.
Baseline subject characteristics
| N | Mean (± SD) | Range | |
|---|---|---|---|
| Age at implant (years) | 21 | 53.6 (9.2) | 34.3 - 68.5 |
| Body mass index (kg/m2) | 21 | 32.7 (3.6) | 26.7 - 38.7 |
| Waist circumference (cm) | 20* | 107.5 (11.9) | 80.0 - 132.0 |
| Neck circumference (cm) | 19* | 41.4 (4.9) | 33.0 - 51.2 |
| Systolic blood pressure (mm Hg) | 21 | 131.6 (13.2) | 105.0 - 156.0 |
| Diastolic blood pressure (mm Hg) | 21 | 79.4 (9.0) | 60.0 - 93.0 |
N, sample size.
Waist circumference was not collected at baseline for one subject and neck circumference for two subjects.
Table 3.
Change in symptoms
| Scale | Baseline (n = 21) | 3 Months (n = 19) | P-value | 6 Months (n = 19) | P-value |
|---|---|---|---|---|---|
| ESS | 12.1 (4.7) | 7.9 (4.0) | < 0.001 | 8.1 (4.4) | < 0.001 |
| FOSQ | 14.4 (2.0) | 17.0 (2.0) | < 0.001 | 16.7 (2.2) | < 0.001 |
| SAQLI | 3.2 (1.0) | 4.8 (1.3) | < 0.001 | 4.9 (1.3) | < 0.001 |
| PSQI | 10.1 (2.6) | 7.5 (3.8) | 0.025 | 8.7 (3.9)† | 0.19 |
| BDI | 15.8 (9.0) | 8.8 (7.5) | < 0.001 | 9.7 (7.6) | < 0.001 |
ESS, Epworth Sleepiness Scale; FOSQ, Functional Outcomes of Sleep Questionnaire; SAQLI, Sleep Apnea Quality of Life Index; PSQI, Pittsburgh Sleep Quality Index; BDI, Beck Depression Index; N, sample size.
n = 17,
n = 18.
All values are mean (± SD). P-values are all versus Baseline. Data are also presented as median and ranges in Supplementary Table S2.
Table 2.
Change in sleep parameters
| Baseline (n = 21) | 3 Months (n = 17) | P-value | 6 Months (n = 19) | P-value | |
|---|---|---|---|---|---|
| Sleep Disordered Breathing | |||||
| Apnea hypopnea index (events/h) | 43.1 (17.5) | 19.0 (10.7) | < 0.001 | 19.5 (16.7) | < 0.001 |
| Apnea index (events/h) | 4.8 (7.3) | 2.6 (3.4) | 0.26 | 1.3 (2.2) | 0.002 |
| Hypopnea index (events/h) | 38.3 (14.8) | 16.4 (8.8) | < 0.001 | 18.3 (16.0) | < 0.001 |
| Arousal index (events/h) | 43.8 (19.5) | 23.4 (9.6) | 0.015 | 23.5 (15.4) | < 0.001 |
| Respiratory arousal index (events/h) | 31.3 (20.2) | 10.5 (5.8) | < 0.001 | 11.0 (13.8) | < 0.001 |
| Oxygen desaturation index 4% (events/h) | 16.8 (14.4) | 8.0 (7.8) | < 0.001 | 9.1 (16.7) | < 0.001 |
| Sleep Architecture | |||||
| Sleep latency (min) | 17.8 (15.5) | 12.1 (14.1) | 0.14 | 10.1 (9.7) | 0.098 |
| Total sleep time (min) | 340.5 (64.0) | 363.0 (65.7) | 0.43 | 349.5 (57.7) | 0.88 |
| Sleep efficiency (%) | 76.6 (11.3) | 82.5 (12.5) | 0.04 | 81.7 (11.6) | 0.03 |
| Time in N1 (% total sleep time) | 27.4 (10.4) | 17.6 (7.5) | 0.003 | 20.8 (11.5) | 0.003 |
| Time in N2 (% total sleep time) | 47.9 (7.2) | 49.2 (8.5) | 0.96 | 50.6 (9.6) | 0.62 |
| Time in N3 (% total sleep time) | 11.2 (7.7) | 14.8 (9.5) | 0.34 | 11.6 (9.3) | 0.99 |
| Time in REM (% total sleep time) | 13.5 (5.5) | 18.4 (4.4) | 0.006 | 17.0 (5.6) | 0.02 |
All values are mean (± SD).
N, sample size.
P-values are all versus Baseline. Data are also presented as median and ranges in Supplementary Table S1.
Study Outcomes and Therapy Utilization
Polysomnography
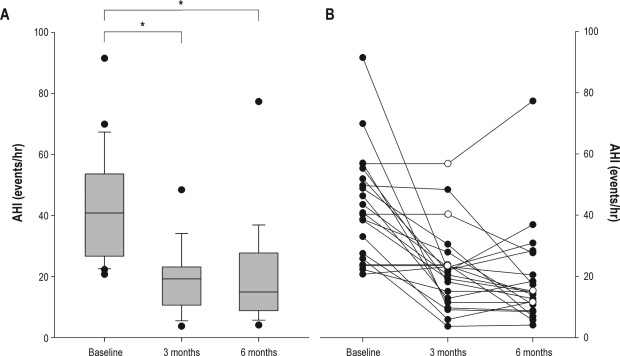
Relative to baseline values, AHI decreased by 56% at 3 months (P < 0.001) and 55% at 6 months (P < 0.001) (Figure 4, Table 2). The number of respiratory-related arousals, total arousals and oxygen desaturations also were reduced at 3 and 6 months (P < 0.01 for all). At 6 months, the AHI in those with a BMI < 35 kg/m2 (n = 13) was less than those with a BMI > 35 kg/m2 (n = 6) (14.0 ± 7.7 vs 31.5 ± 24.6 events/h, respectively, P = 0.03), despite both groups having a similar AHI at baseline (43.0 ± 19.5 vs 44.5 ± 13.6 events/h, respectively).
Figure 4.
Apnea-hypopnea index (AHI) scores at Baseline and at 3 and 6 months following implant. (A) Boxplots of group data showing the median values indicated by the thick horizontal lines, and the 25th and 75th percentiles indicated by the upper and lower margins of the box, respectively at Baseline (n = 21), 3 months (n = 17), and 6 months (n = 19) post-implant. Error bars represent 5th and 95th percentiles. Extreme values are indicated by closed circles, *P < 0.001. (B) Line graph showing individual data (n = 21). A missing data point in any given subject was derived using the last value carried forward method and is shown as an open circle.
Total sleep time was unchanged from baseline, while sleep latency decreased at 6 months (P = 0.03). Sleep efficiency increased at 3 months (P = 0.04) and 6 months (P = 0.03). At 3 months, the percent of total sleep time in stage N1 sleep was reduced (P < 0.001), and time in REM sleep was increased (P < 0.001). These changes were sustained at 6 months. The time in stage N2 and N3 sleep was unchanged from baseline values.
Symptoms and quality of life
The FOSQ scores increased by 2.6 points at 3 months (P < 0.001) and 2.3 points at 6 months (P < 0.001). The ESS score changed from 12.0 at baseline to 7.9 and 8.0 at 3- and 6 months, respectively (P < 0.001 for both). The SAQLI increased at 3 and 6 months (P < 0.001 for both). The PSQI (P < 0.05) decreased by 2.6 points at 3 months (P < 0.02) and 1.7 points at 6 months (P < 0.04). The BDI was 15.8 at baseline and decreased to 8.8 at 3 months (P < 0.001) and 8.9 at 6 months (P < 0.001).
Therapy utilization
Utilization data were available for 21 participants who were followed for 142 ± 42 (mean ± SD) nights (range 52-226). Therapy was used a mean of 89% ± 15% of nights (range 47% to 100%) for 5.8 ± 1.6 h/night (range 2.3-8.4 h).
Safety
The implant procedure time was approximately 3 h (189 ± 58 min) from incision to closure. Eight of 21 participants required intraoperative cuff lead repositioning at least once during the course of the procedure to optimize upper airway opening (Supplementary Table S1).
There were no deaths in the study and no unanticipated adverse device effects. Two participants had the device explanted. One elected to have the device explanted after deciding to have upper airway surgery as an alternative treatment for OSA. The second had the device explanted secondary to a procedure-related hematoma and infection. In addition, a third had a cuff dislodgement that required a subsequent procedure to replace it. These participants and the circumstances surrounding the explants and lead replacement are described in detail in Supplementary Table S2.
At least one adverse event related to the implantation procedure or therapy occurred in 71% and 67% of the participants, respectively. The most common procedure-related events were numbness/pain at the incision sites, and the most common therapy-related events were abrasions on the ventral surface of the tongue, caused by movement of the tongue over the lower incisors, which were of short duration and most often treated with plastic guards placed over the mandibular dentition, with resolution in all cases. These adverse events are listed in Supplementary Tables S3 and S4. The rate of freedom from system (device or therapy) or procedure-related serious adverse events at 3 months was 90.2% (19/21 participants) and at 6 months was 85.2% (18/21 participants).
DISCUSSION
Treatment of OSA with this implantable HGNS system is a safe and effective way to treat individuals with moderate to severe OSA who have failed or do not tolerate positive airway pressure therapy. When assessed at 3 and 6 months post-implant, there were decreases in the severity of OSA and daytime sleepiness, as well as improvements in sleep architecture and daytime function. Therapy usage was high.
The most commonly used definition of surgical success includes a postoperative reduction of the AHI to < 20 events/h and > 50% postoperative reduction of AHI.33 Twelve of 18 participants (67%) met these criteria after 6 months of HGNS. For the whole group, the mean AHI decreased by 55% to 19.5 events/h at 6 months, which was not attributable to worsening arousals, as the number of respiratory-related arousals, total arousals, and number and severity of oxygen desaturations all significantly decreased. These results are consistent with earlier studies which showed that HGNS during sleep can reduce upper airway collapsibility and AHI.23,24
These changes were associated with a clinically meaningful decrease in daytime sleepiness and improvement in daytime function. Specifically, the 2.3 point increase in FOSQ scores exceeds the 2-point change considered to be a clinically meaningful improvement in activities of daily life.29 The ESS score changed from 12.0 at baseline (indicating abnormal sleepiness) to 8.0, which is within the normal range on the ESS.28 The increase in SAQLI and decrease in PSQI both indicate an improvement in sleep quality, although PSQI scores > 5 still are considered to be in the range of poor sleep quality.30,31 Improved sleep quality was also reflected in the decreased total time spent in stage N1 sleep and increased time spent in REM sleep.
The feasibility of chronic HGNS and its potential as a therapeutic approach for the treatment of OSA have been previously described by Schwartz et al.24 While the device used in their study was reported to have performed well in terms of safety, a number of technical problems with the system were reported.25,26 Numerous design differences were incorporated into the present HGNS device to overcome these problems. Principal among these were the development of a cuff electrode designed to encompass securely and safely the nerve branch and the development of an impedance-based respiratory sensing system that allowed HGNS synchronously with inspiration.
Similar to CPAP, optimal therapeutic stimulation levels are determined during a sleep titration night. However, HGNS and its associated tongue protrusion require a period of time for acclimatization to this movement. Because of this delay, the stimulator is initially set at a low level, with full titration by 3 months post-implant in most subjects. Compliance with the therapy was high, with participants using it on average for 5.8 hours each night over the 6-month period. This level of compliance compares very favorably with CPAP compliance, which is often incomplete.4 Indeed the relatively high compliance favorably influences comparative effectiveness of the therapy, which is a combined function of efficacy and compliance.
Because the direct effect of HGNS is tongue protrusion, its therapeutic effectiveness is dependent on predominant retrolingual obstruction or, in the case of predominant velopharyngeal obstruction or multisite obstruction, good coupling between tongue displacement and the velopharyngeal airway via the fauces and tissues of the soft palate. Patients who have very crowded airways with obstruction at multiple levels may not receive as much benefit from this approach unless they have good mechanical coupling between movement of the oro-and velopharyngeal airways. Indeed, the finding that individuals with a BMI < 35 kg/m2 had a lower AHI at 6 months post-implant than those with a BMI > 35 kg/m2 suggests that global obesity-related pharyngeal crowding may be a predictor of poor therapeutic response.
As a feasibility trial, this study had a limited number of participants, no control group, and a follow-up period of only 6 months of active treatment. The long-term effectiveness of this therapy is unknown at this time, and further evaluation in a well-controlled trial is needed. A pivotal clinical trial using this system will soon be conducted, its design having been informed by the results of this study.
In conclusion, this study has shown that HGNS has a favorable safety and efficacy profile with a high compliance that magnifies the overall effectiveness of the therapy. Subjects experienced both a significant decrease in OSA severity as measured by AHI and a reduction in OSA-associated symptoms.
DISCLOSURE STATEMENT
This study was funded by Apnex Medical and Phillips Respironics provided medical equipment. Dr. Antic has received honorarium from ResMed and AtheroGenics and is a co-investigator on the partially industry supported SAVE study. Drs. Eastwood, Hillman, Schwartz, and Kezirian serve as on the medical advisory board and are consultants for Apnex Medical. Dr. Hillman has received honorarium and medical equipment from ResMed Inc. Dr. Eckert has received research support from Sepracor Pharmaceuticals. Dr. Schwartz also serves as a consultant or on the advisory board of Cardiac Concepts, Sleep Science, Sova Pharmaceutical and has received medical equipment from ResMed. Dr. Kezirian also serves as a consultant or is on the advisory board for ArthroCare, Medtronic, Pavad Medical, and ReVent Medical. Dr. Malhotra has received consulting and/or research income from NIH, AHA, Apnex, Apnicure, Philips, Ethicon, Medtronic, Pfizer, Merck, SGS, SHC, Cephalon, Sepracor, and Galleon. Drs. Eckert, Smith, Hee, and Jordan serve as consultants for Apnex Medical. Drs. Eastwood, Jordan, Barnes, and Maddison have received research equipment from ResMed Inc. Dr. McEvoy has received research grants from Respironics Foundation, ResMed, and Fisher and Paykel. Dr. Rochford has received research funding from Compumedics Australia Limited. Dr. Barnes has also received salary support for a research assistant from Bird (Pyt) Ltd. and research support from Fisher & Paykel, Actelion Pharmaceuticals, Boehringer-Ingelheim, Glaxo Smith Kline, Novartis, Hunter Immunology, and Sanofi Aventis. Dr. Wheatley has received research support from Actelion Pharmaceuticals, Boehringer Ingelheim, and Glaxo Smith Kline. Dr. Tyler has received research support from Actelion Pharmaceuticals. Dr. Worsnop has received speaking honorarium from Medinah International, The University of Melbourne, Glaxo Smith Kline, AstraZeneca, Pfizer, Nycomed, Novartis, Actelion Pharmaceuticals, and Boehringer Ingelheim.
ACKNOWLEDGMENTS
Dr. Eastwood is funded by a National Health and Medical Research Council of Australia (NHMRC) Senior Research Fellowship No. 513704. Dr. Kezirian was supported by a career development award from the National Center for Research Resources (NCRR) of the National Institutes of Health and a Triological Society Research Career Development Award of the American Laryngological, Rhinological, and Otological Society. Dr. Eckert is supported by an Overseas Based Biomedical (CJ Martin) Fellowship from the NHMRC (510392) and the American Heart Association (10SDG3510018). The authors acknowledge the assistance of Mr. Chris Mullin from Apnex Medical Inc, with the statistical analyses.The authors note with sadness the untimely death of their collaborator Dr. Sam Robinson and dedicate this manuscript to him.
Footnotes
A commentary on this article appears in this issue on page 1455.
Table S1.
Implant procedure results
| Characteristic | N | mean ± SD [median] (range) or % (n/N) |
|---|---|---|
| Surgical time | ||
| Anesthesia time (start to stop) (min) | 21 | 246.6 ± 60.1 [243] (148.0, 387.0 ) |
| Skin-to-skin time (incision to closure) (min) | 21 | 189.4 ± 58.1 [176] (111.0, 337.0 ) |
| Hypoglossal nerve diameter (mm) | 21 | 2.8 ± 0.5 [3] (2.0, 4.0) |
| Implant details | ||
| Neurostimulator location | ||
| Right | 21 | 100.0% (21/21) |
| Neurostimulator position | ||
| Subcutaneous pocket | 21 | 100.0% (21/21) |
| Intraoperative Lead cuff repositioning required | 21 | 38.1% (8/21) |
The mean procedure time was 189.4 min (± 58.1 min) (approximately 3.25 h) from incision to closure. The implantable neurostimulator (INS) was implanted on the right side in a subcutaneous pocket in all patients. Stimulation lead (STL) cuff position was verified intraoperatively guided by fluoroscopic imaging of the upper airway. When stimulation resulted in inadequate upper airway opening based on lateral fluoroscopy, the stimulation lead cuff was immediately repositioned until adequate opening was observed. Eight of 21 patients required cuff lead repositioning intraoperatively at least once to obtain adequate upper airway opening.
Table S2.
HGNS system explants and replacements
| Patient ID | Reason | Type | Description |
|---|---|---|---|
| S14 | Elective removal | Neurostimulator and leads | Explanted without replacement |
| S20 | Adverse Event – Infection | Neurostimulator and leads | Explanted without replacement |
| S17 | Adverse Event – Cuff Dislodgement | Stimulation Lead | Replaced |
Two patients had the device explanted. Patient S14 elected to have the device explanted after deciding to have upper airway surgery as an alternative treatment for OSA. Patient S20 had the device explanted secondary to a device- and procedure-related hematoma and infection. A third patient (S17) had a cuff dislodgement that required a lead replacement.
Table S3.
Procedure-related adverse events by severity
| Severity | Adverse Event | No. subjects with event |
|---|---|---|
| Mild | Pain | 3 |
| Mild | Skin irritation (soreness, itchiness) | 3 |
| Mild | Bleeding (hemorrhage) | 1 |
| Mild | Edema (swelling) of tissues or nerves | 1 |
| Mild | Change in salivary flow | 1 |
| Mild | Numbness | 5 |
| Mild | Bruising | 1 |
| Mild | Gastrointestinal abnormality | 1 |
| Mild | Incision fluid leak, drainage or separation | 1 |
| Mild | Muscle or skin tightness | 1 |
| Mild | Abnormal scarring (keloid or hypertrophic) | 1 |
| Total with at least 1 mild adverse event | 12/21 | |
| Moderate | Infection | 5 |
| Moderate | Pain | 1 |
| Moderate | Edema (swelling) of tissues or nerves | 2 |
| Moderate | Hematoma | 1 |
| Moderate | Nausea/vomiting | 3 |
| Moderate | Damage to hypoglossal nerve | 1 |
| Moderate | Headache/dizziness | 1 |
| Moderate | Lip Weakness | 2 |
| Moderate | Muscle or skin tightness | 2 |
| Moderate | Musculoskeletal abnormality | 1 |
| Total with at least 1 moderate adverse event | 12/21 | |
| Severe | Infection | 1 |
| Severe | Cuff dislodgement | 1 |
| Severe | Spinal accessory nerve damage | 1 |
| Total with at least 1 severe adverse event | 2/21 |
Adverse events determined by the Clinical Events Committee to be related to the procedure. Totals refer to number of patients with an event (i.e., one subject may have had > 1 event of a given severity).
Table S4.
Therapy-related adverse events by severity
| Severity | Adverse Event | No. subjects with event |
|---|---|---|
| Mild | Skin irritation (soreness, itchiness) | 3 |
| Mild | Nausea/vomiting | 1 |
| Mild | Tongue soreness | 1 |
| Mild | Pain or discomfort due to electrical stimulation | 1 |
| Mild | Change in salivary flow | 1 |
| Mild | Tongue abrasion with or without visible lesion(s) | 4 |
| Total with at least 1 mild adverse event | 9/21 | |
| Moderate | Pain | 1 |
| Moderate | Tongue muscle fatigue, weakness or soreness | 3 |
| Moderate | Tongue abrasion with or without visible lesion(s) | 8 |
| Moderate | Bruising | 1 |
| Moderate | Lip Weakness | 1 |
| Moderate | Genitourinary abnormality | 1 |
| Moderate | Abnormal scarring (keloid or hypertrophic) | 1 |
| Moderate | Musculoskeletal abnormality | 1 |
| Total with at least 1 moderate adverse event | 9/21 | |
| Severe | Infection* | 1 |
| Severe | Cuff dislodgement* | 1 |
| Total with at least 1 severe adverse event | 2/21 |
Adverse events determined by the Clinical Events Committee to be related to HGNS therapy. Totals refer to number of patients with an event (i.e., one subject may have had > 1 event of a given severity).
Serious adverse event, also considered to be related to the implant procedure.
REFERENCES
- 1.Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–8. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–6. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 3.Gay P, Weaver T, Loube D, Iber C. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006;29:381–401. doi: 10.1093/sleep/29.3.381. [DOI] [PubMed] [Google Scholar]
- 4.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:887–95. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 5.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 7.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 8.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 9.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172:1590–5. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peppard PE, Szklo-Coxe M, Hla KM, Young T. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med. 2006;166:1709–15. doi: 10.1001/archinte.166.16.1709. [DOI] [PubMed] [Google Scholar]
- 11.Mulgrew AT, Nasvadi G, Butt A, et al. Risk and severity of motor vehicle crashes in patients with obstructive sleep apnoea/hypopnoea. Thorax. 2008;63:536–41. doi: 10.1136/thx.2007.085464. [DOI] [PubMed] [Google Scholar]
- 12.Lindberg E, Carter N, Gislason T, Janson C. Role of snoring and daytime sleepiness in occupational accidents. Am J Respir Crit Care Med. 2001;164:2031–5. doi: 10.1164/ajrccm.164.11.2102028. [DOI] [PubMed] [Google Scholar]
- 13.Baldwin CM, Griffith KA, Nieto FJ, O'Connor GT, Walsleben JA, Redline S. The association of sleep-disordered breathing and sleep symptoms with quality of life in the Sleep Heart Health Study. Sleep. 2001;24:96–105. doi: 10.1093/sleep/24.1.96. [DOI] [PubMed] [Google Scholar]
- 14.Mann EA, Burnett T, Cornell S, Ludlow CL. The effect of neuromuscular stimulation of the genioglossus on the hypopharyngeal airway. Laryngoscope. 2002;112:351–6. doi: 10.1097/00005537-200202000-00027. [DOI] [PubMed] [Google Scholar]
- 15.Oliven A, O'Hearn DJ, Boudewyns A, et al. Upper airway response to electrical stimulation of the genioglossus in obstructive sleep apnea. J Appl Physiol. 2003;95:2023–9. doi: 10.1152/japplphysiol.00203.2003. [DOI] [PubMed] [Google Scholar]
- 16.Oliven A, Tov N, Geitini L, et al. Effect of genioglossus contraction on pharyngeal lumen and airflow in sleep apnoea patients. Eur Respir J. 2007;30:748–58. doi: 10.1183/09031936.00131106. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz AR, Eisele DW, Hari A, Testerman R, Erickson D, Smith PL. Electrical stimulation of the lingual musculature in obstructive sleep apnea. J Appl Physiol. 1996;81:643–52. doi: 10.1152/jappl.1996.81.2.643. [DOI] [PubMed] [Google Scholar]
- 18.Miki H, Hida W, Chonan T, Kikuchi Y, Takishima T. Effects of submental electrical stimulation during sleep on upper airway patency in patients with obstructive sleep apnea. Am Rev Respir Dis. 1989;140:1285–9. doi: 10.1164/ajrccm/140.5.1285. [DOI] [PubMed] [Google Scholar]
- 19.Hida W, Okabe S, Miki H, et al. Effects of submental stimulation for several consecutive nights in patients with obstructive sleep apnoea. Thorax. 1994;49:446–52. doi: 10.1136/thx.49.5.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Decker MJ, Haaga J, Arnold JL, Atzberger D, Strohl KP. Functional electrical stimulation and respiration during sleep. J Appl Physiol. 1993;75:1053–61. doi: 10.1152/jappl.1993.75.3.1053. [DOI] [PubMed] [Google Scholar]
- 21.Guilleminault C, Powell N, Bowman B, Stoohs R. The effect of electrical stimulation on obstructive sleep apnea syndrome. Chest. 1995;107:67–73. doi: 10.1378/chest.107.1.67. [DOI] [PubMed] [Google Scholar]
- 22.Smith PL, Eisele DW, Podszus T, et al. Electrical stimulation of upper airway musculature. Sleep. 1996;19:S284–7. [PubMed] [Google Scholar]
- 23.Eisele DW, Smith PL, Alam DS, Schwartz AR. Direct hypoglossal nerve stimulation in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 1997;123:57–61. doi: 10.1001/archotol.1997.01900010067009. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz AR, Bennett ML, Smith PL, et al. Therapeutic electrical stimulation of the hypoglossal nerve in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2001;127:1216–23. doi: 10.1001/archotol.127.10.1216. [DOI] [PubMed] [Google Scholar]
- 25.Eisele DW, Schwartz AR, Smith PL. Tongue neuromuscular and direct hypoglossal nerve stimulation for obstructive sleep apnea. Otolaryngol Clin North Am. 2003;36:501–10. doi: 10.1016/s0030-6665(02)00178-0. [DOI] [PubMed] [Google Scholar]
- 26.Goding GS, Jr, Eisele DW, Testerman R, Smith PL, Roertgen K, Schwartz AR. Relief of upper airway obstruction with hypoglossal nerve stimulation in the canine. Laryngoscope. 1998;108:162–9. doi: 10.1097/00005537-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 27.American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 28.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–81. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 29.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–43. [PubMed] [Google Scholar]
- 30.Flemons WW, Reimer MA. Measurement properties of the calgary sleep apnea quality of life index. Am J Respir Crit Care Med. 2002;165:159–64. doi: 10.1164/ajrccm.165.2.2010008. [DOI] [PubMed] [Google Scholar]
- 31.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 32.Lasa L, Ayuso-Mateos JL, Vazquez-Barquero JL, Diez-Manrique FJ, Dowrick CF. The use of the Beck Depression Inventory to screen for depression in the general population: a preliminary analysis. J Affect Disord. 2000;57:261–5. doi: 10.1016/s0165-0327(99)00088-9. [DOI] [PubMed] [Google Scholar]
- 33.Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19:156–77. doi: 10.1093/sleep/19.2.156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Implant procedure results
| Characteristic | N | mean ± SD [median] (range) or % (n/N) |
|---|---|---|
| Surgical time | ||
| Anesthesia time (start to stop) (min) | 21 | 246.6 ± 60.1 [243] (148.0, 387.0 ) |
| Skin-to-skin time (incision to closure) (min) | 21 | 189.4 ± 58.1 [176] (111.0, 337.0 ) |
| Hypoglossal nerve diameter (mm) | 21 | 2.8 ± 0.5 [3] (2.0, 4.0) |
| Implant details | ||
| Neurostimulator location | ||
| Right | 21 | 100.0% (21/21) |
| Neurostimulator position | ||
| Subcutaneous pocket | 21 | 100.0% (21/21) |
| Intraoperative Lead cuff repositioning required | 21 | 38.1% (8/21) |
The mean procedure time was 189.4 min (± 58.1 min) (approximately 3.25 h) from incision to closure. The implantable neurostimulator (INS) was implanted on the right side in a subcutaneous pocket in all patients. Stimulation lead (STL) cuff position was verified intraoperatively guided by fluoroscopic imaging of the upper airway. When stimulation resulted in inadequate upper airway opening based on lateral fluoroscopy, the stimulation lead cuff was immediately repositioned until adequate opening was observed. Eight of 21 patients required cuff lead repositioning intraoperatively at least once to obtain adequate upper airway opening.
Table S2.
HGNS system explants and replacements
| Patient ID | Reason | Type | Description |
|---|---|---|---|
| S14 | Elective removal | Neurostimulator and leads | Explanted without replacement |
| S20 | Adverse Event – Infection | Neurostimulator and leads | Explanted without replacement |
| S17 | Adverse Event – Cuff Dislodgement | Stimulation Lead | Replaced |
Two patients had the device explanted. Patient S14 elected to have the device explanted after deciding to have upper airway surgery as an alternative treatment for OSA. Patient S20 had the device explanted secondary to a device- and procedure-related hematoma and infection. A third patient (S17) had a cuff dislodgement that required a lead replacement.
Table S3.
Procedure-related adverse events by severity
| Severity | Adverse Event | No. subjects with event |
|---|---|---|
| Mild | Pain | 3 |
| Mild | Skin irritation (soreness, itchiness) | 3 |
| Mild | Bleeding (hemorrhage) | 1 |
| Mild | Edema (swelling) of tissues or nerves | 1 |
| Mild | Change in salivary flow | 1 |
| Mild | Numbness | 5 |
| Mild | Bruising | 1 |
| Mild | Gastrointestinal abnormality | 1 |
| Mild | Incision fluid leak, drainage or separation | 1 |
| Mild | Muscle or skin tightness | 1 |
| Mild | Abnormal scarring (keloid or hypertrophic) | 1 |
| Total with at least 1 mild adverse event | 12/21 | |
| Moderate | Infection | 5 |
| Moderate | Pain | 1 |
| Moderate | Edema (swelling) of tissues or nerves | 2 |
| Moderate | Hematoma | 1 |
| Moderate | Nausea/vomiting | 3 |
| Moderate | Damage to hypoglossal nerve | 1 |
| Moderate | Headache/dizziness | 1 |
| Moderate | Lip Weakness | 2 |
| Moderate | Muscle or skin tightness | 2 |
| Moderate | Musculoskeletal abnormality | 1 |
| Total with at least 1 moderate adverse event | 12/21 | |
| Severe | Infection | 1 |
| Severe | Cuff dislodgement | 1 |
| Severe | Spinal accessory nerve damage | 1 |
| Total with at least 1 severe adverse event | 2/21 |
Adverse events determined by the Clinical Events Committee to be related to the procedure. Totals refer to number of patients with an event (i.e., one subject may have had > 1 event of a given severity).
Table S4.
Therapy-related adverse events by severity
| Severity | Adverse Event | No. subjects with event |
|---|---|---|
| Mild | Skin irritation (soreness, itchiness) | 3 |
| Mild | Nausea/vomiting | 1 |
| Mild | Tongue soreness | 1 |
| Mild | Pain or discomfort due to electrical stimulation | 1 |
| Mild | Change in salivary flow | 1 |
| Mild | Tongue abrasion with or without visible lesion(s) | 4 |
| Total with at least 1 mild adverse event | 9/21 | |
| Moderate | Pain | 1 |
| Moderate | Tongue muscle fatigue, weakness or soreness | 3 |
| Moderate | Tongue abrasion with or without visible lesion(s) | 8 |
| Moderate | Bruising | 1 |
| Moderate | Lip Weakness | 1 |
| Moderate | Genitourinary abnormality | 1 |
| Moderate | Abnormal scarring (keloid or hypertrophic) | 1 |
| Moderate | Musculoskeletal abnormality | 1 |
| Total with at least 1 moderate adverse event | 9/21 | |
| Severe | Infection* | 1 |
| Severe | Cuff dislodgement* | 1 |
| Total with at least 1 severe adverse event | 2/21 |
Adverse events determined by the Clinical Events Committee to be related to HGNS therapy. Totals refer to number of patients with an event (i.e., one subject may have had > 1 event of a given severity).
Serious adverse event, also considered to be related to the implant procedure.