Abstract
Study Objectives:
We studied sleep duration and sleep quality in relation to cardiovascular disease (CVD) incidence.
Design/Setting:
Dutch population-based cohort study.
Participants:
20,432 men and women aged 20-65 y with no history of CVD.
Interventions:
N/A
Measurements:
Sleep duration and sleep quality were assessed by a self-administered questionnaire. Morbidity data, vital status, and causes of death were obtained through linkage with several national registries. Hazard ratios (HRs) and 95% confidence intervals (95% CIs) were calculated using Cox proportional hazards models.
Results:
During 10-15 years of follow-up, 1,486 CVD and 1,148 coronary heart disease (CHD) events occurred. Short sleepers (≤ 6 h) had a 15% higher risk of total CVD (HR: 1.15; 95%CI: 1.00-1.32) and a 23% higher risk of CHD (HR: 1.23 [1.04-1.45]) compared to normal sleepers (7 h) after adjustment for all confounders. Additional adjustment for intermediate biological risk factors attenuated these relative risks to 1.11 (0.97-1.27) for total CVD and to 1.19 (1.00-1.40) for CHD. Short sleepers with poor sleep quality had a 63% higher risk of CVD (HR: 1.63 [1.21-2.19]) and a 79% higher risk of CHD incidence (HR: 1.79 [1.24-2.58]) compared to normal sleepers with good sleep quality, after adjustments for all confounders. We observed no associations between long sleep duration (≥ 9 h) and CVD or CHD incidence.
Conclusions:
Short sleepers, especially those with poor sleep quality, have an increased risk of total CVD and CHD incidence. Future investigations should not only focus on sleep duration, but should also take sleep quality into account.
Citation:
Hoevenaar-Blom MP; Spijkerman AMW; Kromhout D; van den Berg JF; Verschuren WMM. Sleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: the MORGEN Study. SLEEP 2011;34(11):1487-1492.
Keywords: Sleep duration, sleep quality, cardiovascular disease incidence, coronary heart disease incidence
INTRODUCTION
Cohort studies reported conflicting results for the association of short sleep duration with the occurrence of cardiovascular diseases (CVD). In a recent meta-analysis incorporating 15 studies by Cappuccio et al.,1 short compared to normal sleep duration was not related to total CVD (RR: 1.03 [95%CI 0.93-1.15]), though it was related to coronary heart disease (CHD) (RR:1.48 [1.22-1.80]) and stroke (RR 1.15 [1.00-1.31]). For long compared to normal sleep duration, Cappuccio et al. reported an RR of 1.41 (1.19-1.68) for total CVD, 1.38 (1.15-1.66) for CHD, and 1.65 (1.45-1.87) for stroke.1
The relation between short sleep duration and CVD incidence could be due to an effect of short sleep on intermediate biological CVD risk factors such as BMI, blood lipids, high blood pressure, and prevalence of diabetes.2–4 The association between long sleep duration and CVD incidence may be explained by long sleep duration being an early symptom of disease and preceding clinical diagnoses.2 Also, sleep quality may modify the association between sleep duration and CVD. Sleep quality is an important factor in the physiologic recovery of the body during sleep, and good sleep quality may prevent CVD.5 Therefore, short and long sleep duration may be adequate for those with good sleep quality but not for those with poor sleep quality. Chandola et al. were the first to demonstrate that the association between short sleep duration and CHD was strongest among those with sleep disturbance.6
The purpose of the present study was to investigate the association between short and long sleep duration and total CVD and CHD incidence, independent of lifestyle related factors, subjective health and educational level. We investigated mediation by biological CVD risk factors by adjusting for these factors. In a subsample of our population, we explored the combined associations of sleep duration and sleep quality with CVD and CHD incidence.
METHODS
Study Population
The MORGEN Study (Monitoring Project on Risk Factors and Chronic Diseases in the Netherlands) was carried out in the Netherlands between 1993 and 1997 in 23,033 participants (10,422 men and 12,611 women). Random sex- and age-stratified samples were drawn from municipal population registers in 3 towns in the Netherlands (Doetinchem, Maastricht, and Amsterdam). The average response rate in the MORGEN Study was 45%.7 We excluded participants with prevalent CVD based on self-report and hospital admission data, women who were pregnant at baseline, those with no information on sleep duration, sleep quality, or any of the covariates, as well as those with no follow-up of vital status or of cardiovascular events. In total 20,432 participants 9,217 men and 11,215 women remained for our analyses. The Medical Ethics Committee of the Netherlands Organization for Applied Scientific Research (TNO) approved the study protocol and all participants signed an informed consent form.
Sleep Duration and Sleep Quality
Information on sleep duration and on sleep quality was obtained by a self-administered questionnaire. The average sleep duration was assessed by asking “How many hours of sleep do you usually get per 24-hour period?” with answer categories “5 hours or less,” “6 hours,” “7 hours,” “8 hours,” and “9 hours or more.” Short sleep duration was defined as sleeping ≤ 6 h, normal sleep duration as 7 (reference group) or 8 h, and long sleep duration as ≥ 9 h.
Information on sleep quality was obtained only in the first 2 years of baseline measurements (1993 and 1994; n = 8,341) as part of the Amsterdam Biographical Questionnaire on neuroticism.8 It was assessed by asking “Do you usually rise rested?” with answer categories “yes,” “don't know,” or “no.” “Good sleep quality” was defined as “yes” for rising rested and poor sleep quality was defined as “no” for rising rested. We excluded those answering “don't know” (n = 1,658, 20%) from the analyses regarding sleep quality. Thus 6,683 participants remained for our analyses on sleep quality.
Ascertainment of Non-Fatal and Fatal CVD and CHD Events
After enrolment in the MORGEN Study, the participants were followed for the occurrence of non-fatal and fatal CVD and CHD by linkage with registries. Morbidity data were provided by the National Medical Registry (NMR) using the Dutch Hospital Discharge Diagnosis Database (HDR).Vital status was obtained through linkage with the municipal population registries. Subsequently, primary and secondary causes of death were obtained through linkage with “Statistics Netherlands.” Information on morbidity and mortality follow-up was available up to January 1, 2008.7 Non-fatal and fatal CVD were defined according to ICD-99 codes 410– 414, 415.1, 427.5, 428, 430–438, 440–444, 798.1, 798.2, and 798.9; or ICD-1010 codes G45, I21-I26, I46, I50, I60-I67, I69 I70-I74, R96 for the fatal cases after 1996. CHD was defined as ICD9 codes 410-414 or ICD10 codes I20-I25.
Covariates
Information on educational level, current smoking, physical activity, alcohol and coffee consumption, subjective health and CVD risk factor medication were assessed by a self-administered questionnaire. Educational level was assessed as the highest level of education reached. Smoking was assessed as “yes,” “no,” or “former.” Information on physical activity was assessed with the validated11 and extended12 EPIC questionnaire during the period 1994-1997. We included cycling and sports as covariates, since in our population only those 2 activities were inversely associated with CVD incidence.12 Subjective health was assessed by a self-administered questionnaire by asking “How do you rate your health in general?” with 5 answer categories ranging from “excellent” to “bad.” Self-reported CVD risk factor medication was defined as using cholesterol lowering medication or antihypertensive agents. Prevalent cases of diabetes were identified through linkage with the Dutch HDR (1990–97) and by self-report. Cases detected by either of these methods were verified by consulting medical records of general practitioners.7 Standardized measurements of weight, height, and systolic and diastolic blood pressure were obtained, and blood was drawn by trained personnel during a visit to the municipal health service. Non-fasting plasma glucose and total and HDL cholesterol were measured with commonly used methods. A more detailed description of these measurements has previously been published by Beulens et al.7
Statistical Analyses
Statistical analyses were carried out using SAS statistical software version 9.2 (SAS Institute, INC., Cary, NC). Participants' characteristics are presented as mean ± SD for continuous variables, or percentages for categorical variables, in strata of sleep duration categories. Participants were followed up to the first non-fatal cardiovascular event, death, emigration, or were censored at January 1, 2008, whichever came first. Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% confidence intervals (95% CIs) of total CVD and CHD incidence in sleep categories. Participants with a normal sleep duration of 7 h were considered as the reference group. The Cox proportional hazard assumption was fulfilled according to the graphical approach and according to Schoenfeld residuals.
To assess sleep duration and sleep quality in relation to total CVD and CHD incidence, four models were used. The first model was adjusted for age and sex. In the second model we additionally included current smoking (yes, no), alcohol consumption (male: none, > 0-2, > 2 glasses per day/female: none, > 0-1, > 1 glass per day) and coffee consumption (< 6, ≥ 6 cups a day) in the model. In the third model we additionally included educational level (low [lower vocational training or primary school], medium [secondary school and intermediate vocational training], or high [higher vocational training or university]) and subjective health (bad-reasonable, good-excellent). In the fourth model we assessed mediation by biological risk factors, by additionally adjusting for BMI (continuously), total-/HDL cholesterol ratio, systolic blood pressure (continuously), CVD risk factor medication (yes, no), and prevalent type 2 diabetes (yes, no).
To test for interaction in the association between sleep duration and total CVD with age (younger or older than 50 years), sex, current smoking, hypertension, BMI categories (< 25, 25-30, ≥ 30 kg/m2) and sleep quality, we added interaction terms to models adjusted for age and sex. Only the interaction term between sleep quality and sleep duration was statistically significant at α < 0.05.
To minimize the possibility that sleeping habits have changed in response to subclinical disease, the analyses were repeated after exclusion of cases in the first 2 years of CVD follow-up. Additionally, sensitivity analyses were performed in which participants with underweight (BMI < 18.5; n = 327), prevalent diabetes (n = 189), and those with CVD risk factor medication (n = 1015) were excluded.
RESULTS
During 10-15 years of follow-up (mean 11.9 years) 1,486 total CVD events and 1,148 CHD events occurred. In total 3,387 (17%) participants were short sleepers, and 1,480 (7%) long sleepers. At baseline, short sleepers were generally older, more likely to be male, and had a less favorable risk profile than normal sleepers (Table 1). Long sleepers were of the same age, less likely to be male, had less favorable levels of lifestyle factors, and similar levels of biological risk factors compared to normal sleepers (Table 1).
Table 1.
Baseline characteristics of men and women; the MORGEN Study, 1993-1997
| Sleep Duration |
||||
|---|---|---|---|---|
| ≤ 6 h (n = 3387) | 7 h (n = 8216) | 8 h (n = 7349) | ≥ 9 h (n = 1480) | |
| Demographic Factors | ||||
| Age (years)a | 44.3 (10.3) | 41.7 (10.6) | 41.5 (11.8) | 41.4 (12.9) |
| Sex (male, %) | 54 | 50 | 38 | 32 |
| Education (low, %) | 54 | 44 | 49 | 57 |
| Lifestyle Factors (%) | ||||
| Current cigarette smoking | 40 | 35 | 33 | 35 |
| Cyclingb | 70 | 80 | 79 | 72 |
| Sportsb | 36 | 46 | 43 | 36 |
| Alcohol | ||||
| None | 15 | 10 | 15 | 23 |
| Moderatec | 52 | 58 | 60 | 54 |
| Coffee consumption (≥ 6 cups a day) | 44 | 39 | 31 | 28 |
| General Health (%) | ||||
| Good subjective health | 82 | 92 | 91 | 79 |
| Sleep qualityd | ||||
| Good | 39 | 53 | 58 | 44 |
| Don't know | 21 | 21 | 19 | 16 |
| Poor | 40 | 26 | 24 | 40 |
| Biological Risk Factors | ||||
| BMI (kg/m2)a | 25.7 (4.1) | 24.9 (3.8) | 24.8 (3.9) | 24.9 (4.3) |
| Overweight (25-30 kg/m2,%) | 39 | 35 | 33 | 34 |
| Obesity (≥ 30 kg/m2,%) | 14 | 9 | 9 | 11 |
| Total cholesterol (mmol/L)a | 5.4 (1.1) | 5.2 (1) | 5.3 (1.1) | 5.3 (1.1) |
| HDL cholesterol (mmol/L)a | 1.3 (0.4) | 1.4 (0.4) | 1.4 (0.4) | 1.4 (0.4) |
| Glucose (mmol/L)a | 5.4 (1.5) | 5.2 (1.2) | 5.2 (1.3) | 5.3 (1.5) |
| Systolic blood pressure (mm Hg)a | 123 (17) | 121 (16) | 120 (16) | 120 (16) |
| Hypertension (%)e | 23 | 18 | 17 | 18 |
| Self-reported CVD risk factor medication (%)f | 7 | 5 | 5 | 6 |
| Diabetes mellitus type 2 (%) | 1.4 | 0.6 | 0.8 | 1.4 |
| Cardiovascular Events | ||||
| Non-fatal CVD (n/%)g | 309/9.2 | 531/6.5 | 456/6.3 | 97/6.7 |
| Non-fatal CHD (n/%)h | 209/6.2 | 345/4.3 | 260/3.6 | 51/3.5 |
| Fatal CVD (n/%)g | 42/1.2 | 58/0.7 | 64/0.9 | 13/0.9 |
| Fatal CHD (n/%)h | 25/0.7 | 25/0.3 | 33/0.5 | 5/0.3 |
Numbers are given as mean (SD);
Numbers are based on baseline data from 1994-1997;
Moderate alcohol consumption: ≤ 2 glasses per day for men and ≤ 1 glass for women;
Numbers are based on baseline data from 1993 and 1994;
Systolic blood pressure ≥ mm Hg and/or diastolic blood pressure ≥ 90 mm Hg and/or usage of antihypertensive medication;
Cholesterol lowering and/or antihypertensive medication;
CVD were defined according to ICD-9 codes 410-414, 415.1,427.5, 428, 430-438, 440-444, 798.1, 798.2 and 798.9 and corresponding ICD-10 codes for the fatal cases after 1996;
CHD was defined according to ICD-9 codes 410-414 and corresponding ICD-10 codes for the fatal cases after 1996.
After adjustment for relevant confounders (age, sex, lifestyle factors, subjective health, and educational level; model 3), short sleepers had a 15% higher risk of total CVD incidence (Table 2; HR: 1.15; 95%CI: 1.00-1.32) and a 23% higher risk of CHD incidence (HR: 1.23 [1.04-1.45]) compared to normal sleepers (Table 2). In the subgroup of participants with information on physical activity (n = 16,183), additional adjusting for cycling (yes, no) and sports (yes, no)12 did not further attenuate the association (results not shown). Adjustment for intermediate biological risk factors (BMI, total-/HDL cholesterol ratio, systolic blood pressure, CVD risk factor medication, and prevalent type 2 diabetes; model 4) diminished the relative risks to 1.11% for total CVD incidence (HR: 1.11 [0.97-1.27]) and to 1.19% for CHD incidence (HR: 1.19 [1.00-1.40]). Long sleep duration was not associated with total CVD incidence (HR: 0.94 [0.76-1.16]; model 3), although long sleepers tended to have a lower risk of CHD incidence (HR: 0.77 [0.58-1.02]; model 3).
Table 2.
Hazard ratios (95% CI) of incident total CVD and CHD by sleep duration category; the MORGEN Study, 1993-1997
| Sleep Duration |
||||
|---|---|---|---|---|
| ≤ 6 h | 7 h | 8 h | ≥ 9 h | |
| Persons at risk, no. | 3387 | 8216 | 7349 | 1480 |
| Incident total CVD, no. | 331 | 563 | 488 | 104 |
| Person years | 39 738 | 97 980 | 87 967 | 17 468 |
| Model 1a | 1.25 (1.09-1.43) | 1.00 (ref) | 0.99 (0.88-1.12) | 1.04 (0.84-1.28) |
| Model 2b | 1.20 (1.05-1.37 | 1.00 (ref) | 0.98 (0.87-1.11) | 1.00 (0.81-1.23) |
| Model 3c | 1.15 (1.00-1.32) | 1.00 (ref) | 0.97 (0.86-1.10) | 0.94 (0.76-1.16) |
| Model 4d | 1.11 (0.97-1.27) | 1.00 (ref) | 0.95 (0.84-1.08) | 0.96 (0.77-1.18) |
| Incident CHD, no. | 227 | 361 | 280 | 55 |
| Person years | 40185 | 98969 | 88929 | 17715 |
| Model 1a | 1.33 (1.13-1.57) | 1.00 (ref) | 0.89 (0.76-1.04) | 0.85 (0.64-1.14) |
| Model 2b | 1.29 (1.09-1.53) | 1.00 (ref) | 0.88 (0.75-1.03) | 0.82 (0.61-1.09) |
| Model 3c | 1.23 (1.04-1.45) | 1.00 (ref) | 0.87 (0.74-1.02) | 0.77 (0.58-1.02) |
| Model 4d | 1.19 (1.00-1.40) | 1.00 (ref) | 0.85 (0.73-1.00) | 0.78 (0.58-1.04) |
Model 1: analyses adjusted for age and sex;
Model 2: model 1 + smoking, alcohol, and coffee;
Model 3: model 2 + subjective health and educational level;
Model 4: model 3 + BMI, total-/HDL cholesterol ratio, systolic blood pressure, CVD risk factor medication, and prevalence of type 2 diabetes.
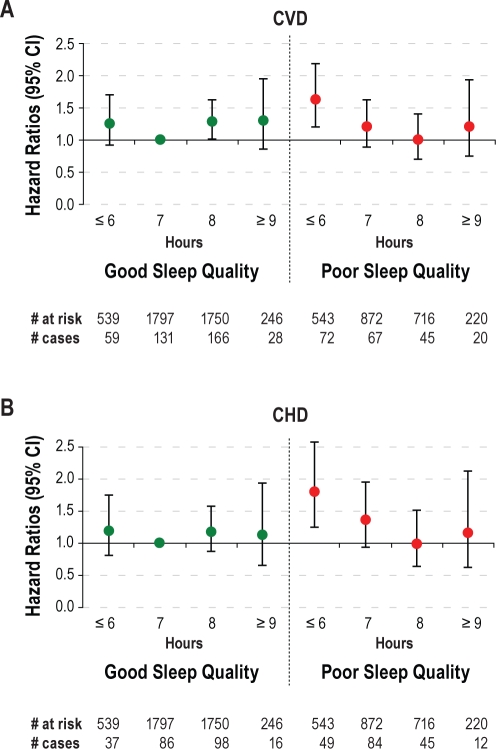
In a subgroup of 6,683 participants data was available on sleep quality. After adjustment for age and sex, participants with poor sleep quality had a 22% (HR: 1.22 [1.02-1.44]) higher risk of CVD and a 34% (HR: 1.34 [1.08-1.66]) higher risk of CHD incidence than those with good sleep quality (Table 3). After additional adjustment for sleep duration, lifestyle factors, subjective health, and educational level, this higher risk was no longer statistically significant (HR CVD: 1.05 [0.87-1.26]/HR CHD: 1.18 [0.94-1.49]). Interactions between sleep duration and sleep quality in relation to CVD and to CHD were significant (both P < 0.05). After adjustment for all relevant confounders and compared to participants with a normal sleep duration and good sleep quality, those with short sleep duration or with poor sleep quality were not at higher risk of CVD or CHD incidence, whereas those with both short sleep duration and poor sleep quality had a 63% higher risk of CVD (HR: 1.63 [1.21-2.19]) and a 79% higher risk of CHD incidence (HR: 1.79 [1.24-2.58]) (Figure 1). Among short sleepers, in our full model (adjusted for age, sex, smoking, alcohol consumption, coffee, subjective health, and educational level) we found an HR of 1.32 (0.95-1.84) for poor compared to good quality in relation to total CVD and an HR of 1.57 (1.05-2.36) for CHD.
Table 3.
Hazard ratios (95% CI) of incident CVD and CHD by sleep duration and sleep quality; the MORGEN Study, 1993 and 1994a
| Sleep Duration |
Sleep Quality |
|||||
|---|---|---|---|---|---|---|
| ≤ 6 h | 7 h | 8 h | ≥ 9 h | Good | Poor | |
| Persons at risk, no. | 1082 | 2669 | 2466 | 466 | 4332 | 2351 |
| Incident CVD, no. | 131 | 198 | 211 | 48 | 384 | 204 |
| Model 1b | 1.49 (1.19-1.85) | 1.00 (ref) | 1.18 (0.97-1.43) | 1.36 (0.99-1.86) | 1.00 (ref) | 1.22 (1.02-1.44) |
| Model 2c | 1.44 (1.15-1.80) | 1.00 (ref) | 1.18 (0.97-1.44) | 1.33 (0.96-1.82) | 1.00 (ref) | 1.17 (0.98-1.39) |
| Model 3d | 1.34 (1.07-1.68) | 1.00 (ref) | 1.14 (0.94-1.39) | 1.17 (0.85-1.62) | 1.00 (ref) | 1.05 (0.87-1.26) |
| Model 4e | 1.26 (1.01-1.58) | 1.00 (ref) | 1.12 (0.92-1.36) | 1.19 (0.86-1.64) | 1.00 (ref) | 1.04 (0.87-1.26) |
| Incident CHD, no. | 86 | 132 | 125 | 28 | 237 | 134 |
| Model 1b | 1.45 (1.11-1.91) | 1.00 (ref) | 1.05 (0.82-1.34) | 1.16 (0.77-1.75) | 1.00 (ref) | 1.34 (1.08-1.66) |
| Model 2c | 1.38 (1.05-1.82) | 1.00 (ref) | 1.05 (0.82-1.35) | 1.12 (0.74-1.69) | 1.00 (ref) | 1.29 (1.03-1.60) |
| Model 3d | 1.29 (0.98-1.71) | 1.00 (ref) | 1.03 (0.80-1.32) | 1.01 (0.67-1.52) | 1.00 (ref) | 1.18 (0.94-1.49) |
| Model 4e | 1.19 (0.90-1.58) | 1.00 (ref) | 1.01 (0.79-1.29) | 1.00 (0.66-1.51) | 1.00 (ref) | 1.19 (0.95-1.50) |
These are the first 2 years of baseline measurement, this is therefore a subpopulation.
Model 1: analyses adjusted for age and sex;
Model 2: model 1 + sleep duration or sleep quality;
Model 3: model 2 + smoking, alcohol, coffee, subjective health, and educational level;
Model 4: model 3 + BMI, total-/HDL cholesterol ratio, systolic blood pressure, CVD risk factor medication, and prevalence of type 2 diabetes.
Figure 1.
Hazard ratios (95% CI) of incident CVD (A) and CHD (B) by sleep duration and sleep quality, adjusted for age, sex, smoking, alcohol, coffee, subjective health and educational level; the MORGEN Study, 1993 and 1994.
The exclusion of participants with a CVD event during the first 2 years of follow-up did not attenuate our results; neither did exclusion of participants with prevalent type 2 diabetes, with underweight, or those with CVD risk factor medication.
DISCUSSION
For short sleepers, we observed a 15% higher risk of total CVD and a 23% higher risk of CHD compared to normal sleepers after adjustment for relevant confounders. These associations could partially be explained by intermediate biological risk factors. Compared to participants with a normal sleep duration and good sleep quality, those with both short sleep duration and poor sleep quality had a 63% higher risk of total CVD incidence and a 79% higher risk of CHD incidence. Long sleepers did not have an increased risk of total CVD or CHD incidence.
The finding that short sleep duration was associated with increased total CVD2,13–19 and CHD incidence1,2,13,15,16,18,20,21 has previously been reported. Epidemiological studies showed that short sleep duration was associated with higher incidence of overweight, obesity, and hypertension, with higher levels of blood pressure, total cholesterol, hemoglobin A (1c), and triglycerides.2–4 Also, blood pressure declines to its lowest levels during nighttime sleep when the parasympathetic activity is highest.22 Consequently, the arteries of people with short sleep duration benefit the least from this positive effect. Indeed, part of the association was explained by adjusting for BMI, total-/HDL cholesterol ratio, systolic blood pressure, and prevalence of type 2 diabetes. Unfortunately, we were unable to assess the effects of hemoglobin A (1c), triglycerides, or nocturnal blood pressures on our results.
In our study, long sleep duration was not associated with CVD and tended to be protective for CHD (HR: 0.77 [0.58-1.02]). The latter result was not found in earlier research. Cappuccio et al. reported in their meta-analysis of long compared to normal sleep duration, a RR of 1.41 (95%CI 1.19-1.68) for total CVD and 1.38 (1.15-1.66) for CHD.1 However, our trend towards a protective association between long sleep duration and CVD is consistent with the results of King et al., who observed that the longer the (actigraphically measured) sleep duration, the lower the 5-year incidence of coronary artery calcifications.23
We observed that short sleepers did not have a higher risk of CVD or CHD incidence compared to normal sleepers when both had good sleep quality. Only short sleepers with poor sleep quality had a 63% higher risk of CVD and a 79% higher risk of CHD. When looking within short sleepers only, poor compared to good sleep quality increased the risk of CHD. The results also suggest an increased risk of total CVD, but this association did not reach statistical significance. This is consistent with our findings that are presented in Figure 1. In line with our results, Chandola et al. found in the Whitehall II cohort that the association of short sleep (≤ 6 h) with CHD risk was greatest among those who reported some sleep disturbance.6 These findings support the hypothesis that for some people, sleep duration of 6 hours or less may be adequate for the restorative physiologic processes accompanied by sleep, but not for others.6 Therefore, sleep quality should be taken into account in addition to sleep duration when investigating sleep.
We obtained data on non-fatal CVD and CHD incidence through linkage with the Dutch Hospital Discharge Diagnosis Database (HDR). In a validation study, with an approximate 33% overlap of participants from our study, the HDR was compared with the detailed clinical registry of cardiovascular patients of the Cardiology department of Maastricht University Hospital.24 A high sensitivity (84%) and positive predictive value (97%) were observed for CHD hospital admissions.24 Causes of death were obtained through linkage with “Statistics Netherlands” in the present study. In a study in which causes of death were coded again 2 years after initial coding, agreement ranged from 77% to 89% for cardiovascular diseases.25 If non-fatal or fatal CVD or CHD cases in our study have been missed, this is unlikely to be related to sleep duration and will therefore not have biased our results.
Our results must be considered in the context of the inherent limitations of research on sleep duration. As in most other epidemiological studies, a self-reported single survey item was used to assess sleep duration and sleep quality. It was not feasible to obtain more detailed and objective measures of sleep, such as actigraphic or polysomnographic measures, from such a large population.6 Reported sleep durations may represent time spent in bed rather than physiologic sleep duration.26 Lauderdale et al. found that actigraphically measured sleep duration was shorter than self-reported questionnaire sleep duration (6.06 h vs. 6.65 h on a weekday).27 This measurement error may have biased our results since it is related to age, sex, and subjective sleep quality.28 Sleep quality was assessed in the first two years of the study. The question on sleep quality was dropped due to space limitations when in the third year additional questions on other topics were added to the questionnaire. Since sample selection has not changed over the years, it is unlikely that this group is different from the people measured in subsequent years. We therefore do not suspect any selection bias. Sleep quality was assessed as rising rested which is an indirect measure of sleep quality. As in other studies assessing sleep quality by questionnaire, this question was not validated. However, those not rising rested are likely to have poor sleep quality, even detecting people with micro-arousals or disturbances in sleep phases who might not even know they have poor sleep quality. These people only experience not rising rested in the morning. Moreover, we did not have the power to analyze the “don't know” category of the rising rested question separately. Therefore, we excluded those answering “don't” know from our analyses pertaining to sleep quality. Finally, data on physical activity, which could be an important confounder, were available for only 77% of the participants. Multivariate analyses with and without adjustment for physical activity yielded similar risk estimates for sleep duration and CVD incidence. Therefore, residual confounding by physical activity is not a major issue in the present study.
A major strength of the present study was the comprehensive data collection from a large study population that allowed us to adjust for a large number of potential confounders. Another advantage was that we could investigate intermediate risk factors and explore the interaction of sleep duration with sleep quality in relation to CVD and CHD incidence.
In conclusion, we observed that short sleep duration was associated with total CVD and CHD incidence, whereas long sleep duration was not. This association could partially be explained by intermediate biological CVD risk factors. The strongest association with CVD and CHD incidence was found in short sleepers with poor sleep quality. Future investigations should not only focus on sleep duration, but should also take sleep quality into account.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This investigation was performed at the National Institute for Public Health and the Environment of the Netherlands in Bilthoven, the Netherlands. The Monitoring Project on Risk Factors and Chronic Diseases in the Netherlands (MORGEN) Study was supported by the Ministry of Health, Welfare and Sport of the Netherlands, the National Institute of Public Health and the Environment, Bilthoven, the Netherlands and the Europe Against Cancer Program of the European Union. The authors thank the epidemiologists and field workers of the Municipal Health Services in Amsterdam, Doetinchem, and Maastricht for their important contribution to the data collection for this study. The project steering committee consisted of Dr. H.B. Bueno de Mesquita, Prof. H.A. Smit, Dr. W.M.M. Verschuren and Prof. J.C. Seidell (Project Director). Data management was provided by A. Blokstra.
Footnotes
A commentary on this article appears in this issue on page 1457.
REFERENCES
- 1.Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011 doi: 10.1093/eurheartj/ehr007. In press. [DOI] [PubMed] [Google Scholar]
- 2.Ikehara S, Iso H, Date C, et al. Association of sleep duration with mortality from cardiovascular disease and other causes for Japanese men and women: the JACC study. Sleep. 2009;32:295–301. doi: 10.1093/sleep/32.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33:414–20. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cappuccio FP, Stranges S, Kandala NB, et al. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension. 2007;50:693–700. doi: 10.1161/HYPERTENSIONAHA.107.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang ET. The impact of daytime naps on the relation between sleep duration and cardiovascular events. Arch Intern Med. 2009;169:717. doi: 10.1001/archinternmed.2009.29. [DOI] [PubMed] [Google Scholar]
- 6.Chandola T, Ferrie JE, Perski A, Akbaraly T, Marmot MG. The effect of short sleep duration on coronary heart disease risk is greatest among those with sleep disturbance: a prospective study from the Whitehall II cohort. Sleep. 2010;33:739–44. doi: 10.1093/sleep/33.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beulens JW, Monninkhof EM, Verschuren WM, et al. Cohort Profile: The EPIC-NL study. Int J Epidemiol. 2009;39:1170–8. doi: 10.1093/ije/dyp217. [DOI] [PubMed] [Google Scholar]
- 8.Ormel J. Neuroticism and well-being inventories: measuring traits or states? Psychol Med. 1983;13:165–76. doi: 10.1017/s0033291700050170. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organisation. Geneva: 1977. International classification of diseases, 9th revision (ICD-9) [Google Scholar]
- 10.World Health Organisation. Geneva: 1990. International classification of diseases, 10th revision (ICD-10) [Google Scholar]
- 11.Pols MA, Peeters PH, Ocke MC, Slimani N, Bueno-de-Mesquita HB, Collette HJ. Estimation of reproducibility and relative validity of the questions included in the EPIC Physical Activity Questionnaire. Int J Epidemiol. 1997;26(Suppl 1):S181–9. doi: 10.1093/ije/26.suppl_1.s181. [DOI] [PubMed] [Google Scholar]
- 12.Hoevenaar-Blom MP, Wendel-Vos GC, Spijkerman AM, Kromhout D, Verschuren WM. Cycling and sports, but not walking, are associated with 10-year cardiovascular disease incidence: the MORGEN Study. Eur J Cardiovasc Prev Rehabil. 2010;18:41–7. doi: 10.1097/HJR.0b013e32833bfc87. [DOI] [PubMed] [Google Scholar]
- 13.Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003;163:205–9. doi: 10.1001/archinte.163.2.205. [DOI] [PubMed] [Google Scholar]
- 14.Chen JC, Brunner RL, Ren H, et al. Sleep duration and risk of ischemic stroke in postmenopausal women. Stroke. 2008;39:3185–92. doi: 10.1161/STROKEAHA.108.521773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shankar A, Koh WP, Yuan JM, Lee HP, Yu MC. Sleep duration and coronary heart disease mortality among Chinese adults in Singapore: a population-based cohort study. Am J Epidemiol. 2008;168:1367–73. doi: 10.1093/aje/kwn281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amagai Y, Ishikawa S, Gotoh T, Kayaba K, Nakamura Y, Kajii E. Sleep duration and incidence of cardiovascular events in a Japanese population: the Jichi Medical School cohort study. J Epidemiol. 2010;20:106–10. doi: 10.2188/jea.JE20090053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrie JE, Shipley MJ, Cappuccio FP, et al. A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort. Sleep. 2007;30:1659–66. doi: 10.1093/sleep/30.12.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meisinger C, Heier M, Lowel H, Schneider A, Doring A. Sleep duration and sleep complaints and risk of myocardial infarction in middle-aged men and women from the general population: the MONICA/KORA Augsburg cohort study. Sleep. 2007;30:1121–7. doi: 10.1093/sleep/30.9.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eguchi K, Pickering TG, Schwartz JE, et al. Short sleep duration as an independent predictor of cardiovascular events in Japanese patients with hypertension. Arch Intern Med. 2008;168:2225–31. doi: 10.1001/archinte.168.20.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qureshi AI, Giles WH, Croft JB, Bliwise DL. Habitual sleep patterns and risk for stroke and coronary heart disease: a 10-year follow-up from NHANES I. Neurology. 1997;48:904–11. doi: 10.1212/wnl.48.4.904. [DOI] [PubMed] [Google Scholar]
- 21.Mallon L, Broman JE, Hetta J. Sleep complaints predict coronary artery disease mortality in males: a 12-year follow-up study of a middle-aged Swedish population. J Intern Med. 2002;251:207–16. doi: 10.1046/j.1365-2796.2002.00941.x. [DOI] [PubMed] [Google Scholar]
- 22.Smolensky MH, Hermida RC, Castriotta RJ, Portaluppi F. Role of sleep-wake cycle on blood pressure circadian rhythms and hypertension. Sleep Med. 2007;8:668–80. doi: 10.1016/j.sleep.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 23.King CR, Knutson KL, Rathouz PJ, Sidney S, Liu K, Lauderdale DS. Short sleep duration and incident coronary artery calcification. JAMA. 2008;300:2859–66. doi: 10.1001/jama.2008.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merry AH, Boer JM, Schouten LJ, et al. Validity of coronary heart diseases and heart failure based on hospital discharge and mortality data in the Netherlands using the cardiovascular registry Maastricht cohort study. Eur J Epidemiol. 2009;24:437–47. doi: 10.1007/s10654-009-9335-x. [DOI] [PubMed] [Google Scholar]
- 25.Harteloh P, de Bruin K, Kardaun J. The reliability of cause-of-death coding in The Netherlands. Eur J Epidemiol. 2010;25:531–8. doi: 10.1007/s10654-010-9445-5. [DOI] [PubMed] [Google Scholar]
- 26.Youngstedt SD, Kripke DF. Long sleep and mortality: rationale for sleep restriction. Sleep Med Rev. 2004;8:159–74. doi: 10.1016/j.smrv.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19:838–45. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van den Berg JF, Van Rooij FJ, Vos H, et al. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. J Sleep Res. 2008;17:295–302. doi: 10.1111/j.1365-2869.2008.00638.x. [DOI] [PubMed] [Google Scholar]