Abstract
Study Objectives:
Chronic fatigue syndrome (CFS) and fibromyalgia (FM) are medically unexplained conditions that often have overlapping symptoms, including sleep-related complaints. However, differences between the 2 conditions have been reported, and we hypothesized that dynamic aspects of sleep would be different in the 2 groups of patients.
Participants:
Subjects were 26 healthy control subjects, 14 patients with CFS but without FM (CFS alone), and 12 patients with CFS and FM (CFS+FM)—all women.
Measurements and Results:
We studied transition probabilities and rates between sleep stages (waking, rapid eye movement [REM] sleep, stage 1 [S1], stage 2 [S2], and slow-wave sleep [SWS]) and duration distributions of each sleep stage. We found that the probability of transition from REM sleep to waking was significantly greater in subjects with CFS alone than in control subjects, which may be the specific sleep problem for people with CFS alone. Probabilities of (a) transitions from waking, REM sleep, and S1 to S2 and (b) those from SWS to waking and S1 were significantly greater in subjects with CFS+FM than in control subjects; in addition, rates of these transitions were also significantly increased in subjects with CFS+FM. Result (a) might indicate increased sleep pressure in subjects with CFS+FM whereas result (b) may be the specific sleep problem of subjects with CFS+FM. We also found that shorter durations of S2 sleep are specific to patients with CFS+FM, not to CFS alone.
Conclusions:
These results suggest that CFS and FM may be different illnesses associated with different problems of sleep regulation.
Citation:
Kishi A; Natelson BH; Togo F; Struzik ZR; Rapoport DM; Yamamoto Y. Sleep-stage dynamics in patients with chronic fatigue syndrome with or without fibromyalgia. SLEEP 2011;34(11):1551-1560.
Keywords: Sleep dynamics, transition probability, duration distribution, chronic fatigue syndrome, fibromyalgia, unrefreshing sleep
INTRODUCTION
Chronic fatigue syndrome (CFS) is a medically unexplained condition characterized by persistent or relapsing fatigue lasting at least 6 months, which substantially reduces normal activity.1 In addition to severe fatigue, one of the symptoms used for diagnosing CFS is “unrefreshing sleep,” and this sleep-related problem is the most common complaint among patients with CFS.2,3 Fibromyalgia (FM) is a medically unexplained illness characterized by 4-quadrant pain and multiple tender points4 and frequently occurs in conjunction with CFS.5 In fact, CFS and FM often have similar symptoms, including sleep-related complaints; this has led some researchers to suggest that they are essentially the same illness.6 However, differences between CFS and FM exist.7–9 Therefore, rather than focus on similarities between the 2 disorders, it seems more appropriate to focus on differences between them. Finding differences would suggest that the pathophysiologic processes responsible for the 2 illnesses could differ.
Polysomnographic studies have shown that sleep problems in patients with CFS and FM are quite similar (e.g., alpha-delta sleep, more arousals, reduced sleep efficiency, prolonged sleep onset, increased stage 1 sleep [S1], reduced slow-wave sleep [SWS]).10–18 However, these observations are not consistent between studies for both CFS and FM, and there are even studies reporting no statistical differences in traditional sleep parameters between healthy humans and those with CFS or FM2,19–22 or between patients with CFS, with or without FM.23
Historically, most sleep studies have been performed based upon sleep staging according to the traditional standardized scoring criteria established by Rechtschaffen and Kales in 1968.24 While this methodology has been extremely useful, sleep-stage analysis has been limited to simple descriptive statistics (e.g., total sleep time, sleep efficiency, and total duration of each sleep stage), providing static information about whole-night sleep. Recently, we investigated dynamic aspects of sleep for patients with CFS and revealed that they had altered patterns of sleep-stage transitions, compared with healthy control subjects.25 This kind of information could not be obtained using the traditional analysis based on Rechtschaffen and Kales criteria; moreover, the pattern of sleep-stage transitions across a night of sleep could be a novel way of determining the quality of sleep.
Therefore, we hypothesized that dynamic aspects of sleep, a new focus of sleep researchers,25–28 would be different between the 2 groups of patients. We thus studied transition probabilities between sleep stages and duration distributions of each sleep stage of patients with CFS, with or without FM.
Methods
Subjects
The subjects were 52 women—26 healthy control subjects (age: 38 ± 8 years; mean ± SD), 14 patients with CFS without FM (CFS alone; age: 37 ± 9 years), and 12 patients with CFS and FM (CFS+FM; age: 41 ± 6 years). None of these subjects had clinically evident sleep disorders in the form of restless leg syndrome or obstructive sleep apnea (see next subsection). An extensive health screening was used to exclude patients taking antidepressants, opiates, steroids, hypnotics, and other sedatives, including benzodiazepines. The patients all fulfilled the 1994 case definition for CFS and thus had neither any medical explanation for their symptoms based on history, physical examination, and blood tests to rule out other disorders nor did they have any serious psychiatric diagnoses, including schizophrenia, eating disorders, substance abuse or bipolar disorder,1 as determined by using the computerized version of the Diagnostic Interview Schedule (DIS-IV)29; of these patients, 14 also fulfilled the American College of Rheumatology criteria (1990) for FM.4 Controls all reported their health to be excellent or good and had normal findings upon examination and normal results from blood tests. Because sleep-electroencephalographic changes are frequent symptoms of major depressive disorder,30 we also used the psychiatric diagnostic interview to confirm that no subject with major depressive disorder was included. To further reduce variability, menstruating subjects were all studied in the follicular phase of their menstrual cycles.
All the subjects gave their informed consent, approved by the New Jersey Medical School's Institutional Review Board, to participate in this research. Following instructions to refrain from alcohol and caffeine ingestion and avoid engaging in prolonged and/or strenuous exercise in the daytime before study nights, the subjects underwent 1 night of polysomnographic recording in a quiet, darkened, hospital room. The subjects went to bed at their usual bedtime and awoke the next morning between 07:15 and 08:00.
Polysomnography
Subjects underwent full nocturnal polysomnography consisting of electroencephalogram (C3/A2, O1/A2 and FZ/A2), electrooculogram, submental electromyogram, anterior tibialis electromyography, a lead II electrocardiogram, thoracic and abdominal motion, airflow using a nasal cannula/pressure transducer and an oral thermistor, and pulse oximetry.
Sleep was scored every 30 seconds by a single scorer according to standard criteria of Rechtschaffen and Kales.24 Sleep stages were scored by dividing a sleep recording into nonoverlapping epochs of 30 sec duration, and a single stage assigned to each epoch. If more than 1 sleep stage occurred within an epoch, the sleep stage that occupied the greatest portion of the epoch was scored as the stage of the whole epoch. An arousal was defined according to standard American Academy of Sleep Medicine criteria31 as a return to alpha or fast-frequency electroencephalographic activity, well differentiated from the background, lasting at least 3 seconds but no more than 15 seconds. If alpha or fast-frequency electroencephalographic activity lasted at least 15 seconds within an epoch, the stage of the epoch was scored as awake.
Respiratory events were defined as any combination of apneas and hypopneas lasting at least 10 seconds or airflow suggesting flow limitation32 lasting at least 10 seconds associated with an arousal. Apnea was defined as a reduction in airflow to less than 10% of waking level in the nasal cannula and absent airflow in the oral thermistor, and hypopnea was defined as a decrease in inspiratory airflow to less than 50% of waking levels. Flow limitation was considered to occur when there were 2 or more consecutive breaths (for an event duration generally ≥ 10 sec) that had a flattened or nonsinusoidal appearance but had peak inspiratory amplitudes that did not meet the more than 50% reduction requirement of a hypopnea. These events were required to end abruptly, with a return to breaths with a sinusoidal shape. The respiratory disturbance index (RDI) was defined as the total number of apneas, hypopneas, and flow-limitation events per hour of sleep.32 The RDI including the flow limitation events terminated by arousal has been previously shown to be essentially identical to the number of the esophageal manometry events terminated by arousal, which have been called respiratory effort-related arousals.32 Our RDI is functionally equivalent to the “alternative definition” of the apnea-hypopnea index (AHI) proposed by the American Academy of Sleep Medicine in 2007.33 Based on results by Ayappa et al.,32 it was assumed that an RDI of at least 18 events per hour was sufficient to account for excessive daytime sleepiness on the basis of sleep disordered breathing, and the diagnosis of sleep disturbed breathing was then made for patients and healthy control subjects with this finding. Periodic leg movements were defined as 4 or more consecutive, involuntary leg movements per hour during sleep, lasting 0.5 to 5.0 seconds, with an intermovement interval of 5 to 90 seconds. Patients were labeled as having periodic leg movements in sleep syndrome when the number of periodic limb movements per hour of sleep was greater than 5 per hour. Using these criteria, we confirmed that none of the subjects in this study had either an RDI of 18 or greater or periodic leg movements in sleep syndrome.
Data Analysis
The dynamic aspects of sleep are composed of probabilities of sequential transitions of sleep stages and statistical distributions of duration of each stage. These 2 features are considered sufficient in describing the “path” to specific episodes that might lead to, for example, poor sleep quality, and are useful in studying underlying mechanisms responsible for their occurrences.
We used normalized transitions to characterize sleep-stage transitions. Each normalized transition probability between wake, rapid eye movement (REM), S1, stage 2 (S2), and SWS (stages 3 and 4) was calculated by dividing the number of transitions from a specific stage to one of the other stages by the total number of the transitions from that specific stage to another stage. For instance, when calculating the normalized probability of transition from S1 to S2, we divided the number of transitions from S1 to S2 by the sum of the number of transitions from S1 to wake, those from S1 to S2, those from S1 to SWS, and those from S1 to REM, and multiplied it by 100. In our previous paper,25 we used both global and normalized transition probabilities; the global transition probability was calculated by dividing the number of transitions between stages by the total number of all transitions. As this global probability can be obtained by calculating the product of the normalized transition probability and the number of continuous runs for each sleep stage (i.e., 1 of the traditional descriptive statistics), probabilities of global transitions will not be reported in this paper. In fact, results with global-transition probabilities were qualitatively the same as those with normalized data except for 1 occasion, which we do cite because the difference seemed to have an important implication. The mean ± SD of the number of continuous runs of each sleep stage analyzed per subject and group is shown in Table 1.
Table 1.
Mean ± SD of the number of continuous runs of each sleep stage analyzed per subject and group
| Stage | Healthy | CFS alone | CFS+FM |
|---|---|---|---|
| Awake | 21.8 ± 6.5 | 21.7 ± 8.2 | 19.1 ± 5.4 |
| REM | 13.4 ± 7.0 | 6.5 ± 4.8 | 8.3 ± 3.8 |
| S1 | 43.9 ± 17.1 | 36.7 ± 17.4 | 29.3 ± 8.9 |
| S2 | 50.2 ± 16.0 | 43.1 ± 11.9 | 52.7 ± 14.8 |
| SWS | 18.4 ± 10.2 | 15.6 ± 6.6 | 25.1 ± 12.0 |
Values are presented as means ± SD.
Normalization sometimes makes it difficult to interpret the results, mostly because the probability does not provide information about the number of transitions. In the present paper, we supplied the “transition rate”34 per minute, which was calculated by dividing the number of transitions between sleep stages by the total duration (minutes) of the origin stage of that transition, to complement the interpretation of the result of differences between groups.
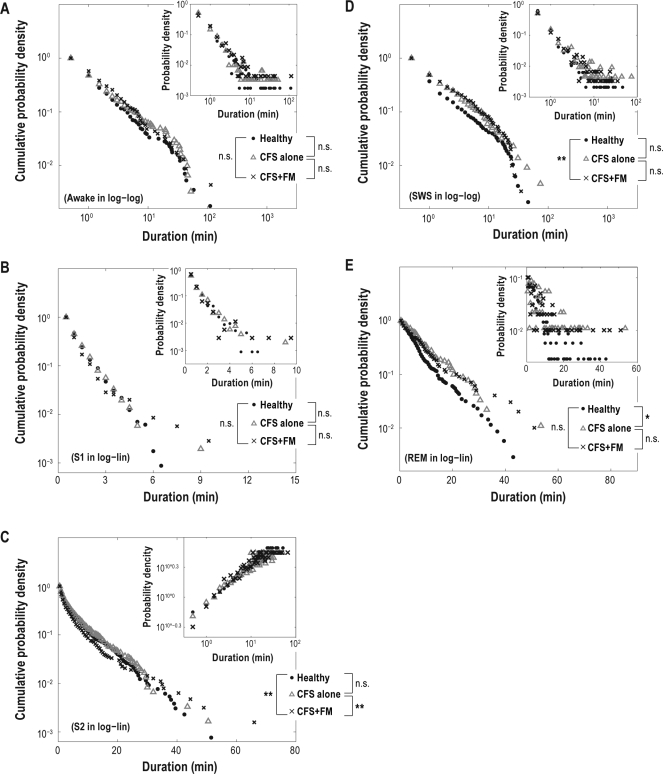
Our previous paper reported that duration distributions take a different form for each sleep stage25: duration distributions for S1 and REM sleep follow an exponential function p(t) ∼ e–t/τ, where the p(t) is a probability distribution of durations t of a stage and the τ is a constant (i.e., linear on the log-lin plots); duration distributions for S2 sleep follow a stretched exponential function p(t) ∼ e–(t/τ)β, where β is a constant (i.e., linear on the loglog-log plot); and duration distributions for wake and SWS follow a power-law p(t) ∼ t–α, where the α is a constant (i.e., linear on the log-log plots). Longer durations, that is, heavy-tailed distributions, are observed as the distribution form is changed from a simple exponential to complex power-law distribution, and, in such a case, transitions to the next stage are statistically more delayed. A stretched exponential distribution lies between the exponential and power-law distribution.35 In this study, duration distributions were presented in log-lin, loglog-log, or log-log axis, in which distributions most seem to follow a straight line (Figure 3, insets), for the purpose of making the differences of distributions more visible. The probability densities for durations for each sleep stage were analyzed by pooling those of all the individuals in each group. Cumulative duration distributions were calculated as ∫t∞ p(x)dx, where p(t) is the probability density function of durations t.
Figure 3.
Cumulative distributions for durations of sequential runs for wake and slow-wave sleep (SWS) in double logarithmic plots (A and D), and stage 1 sleep (S1), stage 2 sleep (S2), and rapid eye movement sleep (REM) in semilogarithmic plot (B, C, and E) for healthy control subjects (•), subjects with chronic fatigue syndrome (CFS) alone (Δ), and those with CFS+ fibromyalgia (FM) (×). Insets: Distributions for durations of sequential runs for wake and SWS in double logarithmic plot (A and D), S1 and REM sleep in semilogarithmic plot (B and C), and S2 in loglog-log plot (E) for healthy control subjects (•), those with CFS alone (Δ), and subjects with CFS+FM (×). Cumulative duration distributions for S2 sleep are presented not in loglog-log but in log-lin axis because cumulative distribution of stretched exponential function does not follow a straight line in loglog-log axis. **P < 0.01 and *P < 0.05 by Kolmogorov-Smirnov test with Bonferroni corrections.
Statistical Analysis
Differences among groups on traditional sleep variables were assessed using a Tukey-Kramer procedure for multiple comparisons. Differences in transition probabilities for subjects who had non-zero transition probabilities and transition rates per minute were assessed using a Mann-Whitney U test, with statistical significance level adjusted using Bonferroni corrections; we used nonparametric tests for these data because the variables were not normally distributed, as assessed by the Kolmogorov-Smirnov test. Differences of duration distributions for each sleep stage were assessed using Kolmogorov-Smirnov tests, with statistical significance level adjusted using Bonferroni corrections. To assess interindividual differences in distributions, we also used the Kolmogorov-Smirnov test for all the pairs of individuals. Statistical significance was accepted when P was less than 0.05.
Results
Descriptive Statistics of Traditional Sleep Variables
Comparisons of traditional sleep variables among the 3 groups (healthy control subjects, CFS alone, and CFS+FM) are shown in Table 2. Total sleep time and total duration of REM sleep were significantly longer in healthy control subjects than in subjects with CFS alone. Total durations of S1 and S2 were significantly longer in healthy control subjects than in subjects with CFS+FM, and, conversely, the total duration of SWS was significantly longer in subjects with CFS+FM than in healthy control subjects.
Table 2.
Descriptive statistics of traditional sleep variables
| Variable | Healthy | CFS alone | CFS+FM | Tukey-Kramer |
|---|---|---|---|---|
| Number | 26 | 14 | 12 | |
| Time in bed (min) | 453.0 ± 32.5 | 440.0 ± 40.0 | 435.9 ± 46.4 | n.s. |
| Total sleep time (min) | 385.0 ± 38.1 | 345.9 ± 60.1 | 357.6 ± 49.0 | Healthy > CFS alone (*) |
| Sleep efficiency (%) | 85.1 ± 8.2 | 78.4 ± 10.2 | 82.3 ± 9.3 | n.s. |
| Wakefulness (min) | 51.7 ± 38.3 | 63.0 ± 27.1 | 58.7 ± 39.4 | n.s. |
| S1 (min) | 44.8 ± 18.7 | 38.3 ± 20.3 | 27.3 ± 10.5 | Healthy > CFS+FM (*) |
| S2 (min) | 222.4 ± 34.5 | 203.4 ± 35.1 | 191.8 ± 30.4 | Healthy > CFS+FM (*) |
| SWS (min) | 35.0 ± 26.2 | 44.6 ± 34.5 | 69.8 ± 28.1 | CFS+FM > Healthy (*) |
| REM sleep (min) | 82.8 ± 24.2 | 59.6 ± 22.2 | 68.7 ± 30.0 | Healthy > CFS alone (*) |
| Sleep latency (min) | 16.3 ± 18.6 | 31.2 ± 27.3 | 19.5 ± 24.1 | n.s. |
| REM latency (min) | 107.3 ± 49.1 | 149.2 ± 76.5 | 101.3 ± 37.3 | n.s. |
Values of traditional sleep variables for healthy controls, CFS alone, CFS+FM are presented as means ± SD.
P < 0.05 by Tukey-Kramer procedure for multiple comparisons.
Transition Probabilities
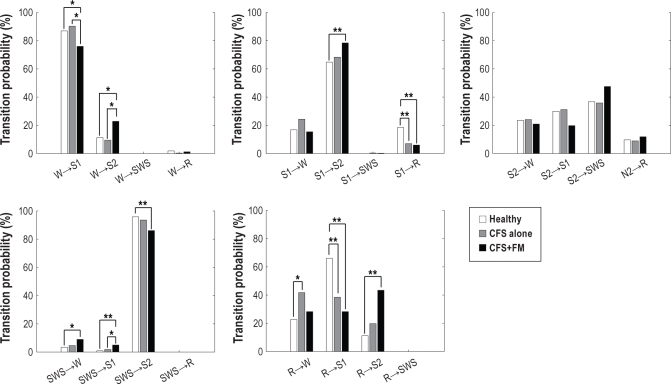
Normalized probabilities of transitions among the 5 sleep stages (wake, REM, S1, S2, and SWS) of healthy control subjects, those with CFS alone and those with CFS+FM are shown in Figure 1.
Figure 1.
Normalized transition probabilities between wake (W), rapid eye movement sleep (R), stage 1 sleep (S1), stage 2 sleep (S2), and slow-wave sleep (SWS) for healthy control subjects (white), those with chronic fatigue syndrome (CFS) alone (gray), and subjects with CFS+fibromyalgia (FM) (black). **P < 0.01 and *P < 0.05 by Mann-Whitney U test with Bonferroni corrections.
Transitions that were greater for healthy control subjects
Both transition probabilities from S1 to REM sleep (S1 → R) and REM sleep to S1 (R → S1) were significantly greater for healthy control subjects than for those with CFS alone or for subjects with CFS+FM.
Transitions that were greater for subjects with CFS alone
The transition probability from REM sleep to wake (R → W) was significantly greater for subjects with CFS alone than for healthy control subjects.
Transitions that differed for subjects with CFS+FM
Several of the transition probabilities for CFS+FM suggested increased sleep pressure and supported the finding of increased total time in SWS found in subjects with CFS+FM: (1) the transition probability from wake to S2 (W → S2) was significantly greater for subjects with CFS+FM than for healthy control subjects or for those with CFS alone, with an almost commensurate decrease in the transition probability from wake to S1, and (2) transition probabilities from S1 to S2 (S1 → S2) and REM sleep to S2 (R → S2) for subjects with CFS+FM were significantly increased compared with those of healthy control subjects.
Although the normalized transition probability from S2 to SWS (S2 → SWS) did not differ significantly between subjects with CFS+FM and control subjects, a significant increase in the global-transition probability was found (12.4% for control subjects and 18.5% for subjects with CFS+FM; P < 0.05); these results indicate that the CFS+FM group shows a tendency for higher likelihood of transitions from S2 to SWS (S2 → SWS) than does the control group.
The actual rates of transition between stages are shown in Figure 2 and indicate that subjects with CFS+FM had an increased rate of transition from wake/S1 to deeper sleep and a decreased rate of transition from REM to S1; these results suggest that sleep pressure is greater in subjects with CFS+FM than in normal control subjects and those with CFS alone.
Figure 2.
Transition rates per unit minute between wake (W), rapid eye movement sleep (R), stage 1 sleep (S1), stage 2 sleep (S2), and slow-wave sleep (SWS) for healthy control subjects (white), subjects with chronic fatigue syndrome (CFS) alone (gray), and subjects with CFS+fibromyalgia (FM) (black). **P < 0.01 and *P < 0.05 by Mann-Whitney U test with Bonferroni corrections.
Although subjects with CFS+FM showed less total S2 sleep than did the healthy control subjects or those with CFS alone, neither the actual rate of transitions (Figure 2) nor the normalized probability of transition (Figure 1) out of S2 was significantly altered; this result suggests that the reduction in total duration of S2 is not abnormal and may simply be a consequence of the increased amount of SWS.
Given the greater total time in SWS in subjects with CFS+FM, it is noteworthy that transition probabilities from SWS were significantly greater to S1 (SWS → S1) than in healthy control subjects and in subjects with CFS alone and also greater to wake (SWS → W) than in healthy control subjects. Also, the actual rates of these 2 transitions were significantly greater for subjects with CFS+FM than for healthy control subjects. These results are consistent with disrupted sleep in this group of patients.
Duration Distributions
Distributions of durations of the 5 sleep stages (wake, REM, S1, S2, and SWS) of healthy control subjects, those with CFS alone and subjects with CFS+FM are shown in the insets of panels of Figure 3. Since the number of durations of each of these classes of transition was small due to the brevity of a normal night of sleep, we considered pooling the data. Prior to doing this, however, we had to be sure that data from individual recordings for wake, REM sleep, S1, S2, and SWS periods were respectively drawn from the same probability distribution. To determine this, we applied the nonparametric Kolmogorov-Smirnov test and compared the probability densities pairwise among subjects for all individual sleep stages. In most cases, we were not able to reject the null hypothesis that duration distributions for each subject were drawn from the same distribution for each stage (Table 3). This analysis supported our pooling of the data from all subjects in each group in the analysis of duration distributions for each sleep stage.
Table 3.
Percentage of individual pairs with positive Kolmogorov-Smirnov test
| Stage | Healthy | CFS alone | CFS+FM |
|---|---|---|---|
| Awake (%) | 5.8 | 0.0 | 6.1 |
| REM (%) | 24.0 | 18.7 | 13.6 |
| S1 (%) | 12.3 | 5.5 | 0.0 |
| S2 (%) | 21.2 | 8.8 | 16.7 |
| SWS (%) | 7.7 | 5.5 | 3.0 |
The percentage of individual pairs for which the null hypothesis is rejected for each stage for healthy controls, CFS alone and CFS+FM.
For healthy control subjects, those with CFS alone and the subjects with CFS+FM, the probability densities for each sleep-stage duration exhibited the expected stage-specific behavior, as we previously have reported.25 Duration distributions for wake and SWS followed a power-law (i.e., linear on the log-log plots; Figure 3A, D, insets); duration distributions for S1 and REM sleep followed an exponential function (i.e., linear on the log-lin plots; Figure 3B, E, insets); and those for S2 sleep obeyed a stretched exponential function (i.e., linear on the loglog-log plot; Figure 3C, inset).
Duration distributions for S2 differed significantly between healthy control subjects and those with CFS+FM and also between subjects with CFS alone and those with CFS+FM (Figure 3C, inset). The cumulative duration distributions for S2 showed shorter durations of S2 sleep for subjects with CFS+FM, compared with healthy control subjects and subjects with CFS alone (t < 25 min; Figure 3C). Duration distributions for SWS differed significantly between healthy control subjects and those with CFS+FM (Figure 3D, inset). The cumulative duration distributions for SWS showed longer durations of SWS for subjects with CFS+FM compared to healthy control subjects (Figure 3D). Duration distributions for REM sleep differed significantly between healthy control subjects and those with CFS alone (Figure 3E, inset). The cumulative duration distributions for REM sleep showed longer durations of REM sleep for subjects with CFS alone, compared with healthy control subjects (Figure 3E). Duration distributions for waking and S1 did not differ significantly among healthy control subjects, those with CFS alone, and subjects with CFS+FM (Figure 3A and B, insets).
DISCUSSION
Conventional methods to characterize human sleep have used simple descriptive statistics of traditional (static) sleep variables. However, those indexes have not shown consistent differences among patients with CFS, patients with FM and control subjects, and studies comparing CFS and FM—illnesses with symptoms that often coexist—have not been previously performed.
In the present study, we did find some differences between illness groups and control subjects: specifically, the total duration of REM sleep was shorter in subjects with CFS alone than in healthy control subjects. Also, total durations of S1 and of S2 were shorter and, commensurately, that of SWS was longer in patients with CFS+FM than in healthy control subjects. These results might lead to the surprising conclusion that sleep quality in patients with CFS+FM is better than that in healthy control subjects, which is not consistent with the common complaint of patients with CFS+FM of “unrefreshing sleep.”23 An alternate explanation would be that the increase in deep sleep in patients with CFS+FM may be a response to some process that is generating a greater need to sleep in people with CFS+FM. These changes were not seen in patients with CFS alone.
In addition, our transition analysis of sleep stages and duration distributions for each sleep stage revealed striking differences between patients with CFS alone compared with those with CFS+FM. Figure 4 shows a way of depicting the normalized probability of transitions occurring from each sleep state to all of the other sleep states for the 3 study groups—those with CFS only, those with CFS+FM, and healthy control subjects. The thickness of the arrow represents the probability of that particular transition; the legend provides information to the reader as to significant differences. This analysis showed that subjects with CFS alone (open arrow) had a greater probability of transitioning from REM to wake (R → W) than did healthy control subjects. We have previously reported this sleep disruption as the specific sleep problem for CFS alone.25 We interpret this result to mean that patients with CFS alone have reduced sleep pressure. Supporting this interpretation is a report that sleep latency of patients with CFS is not shortened by sleep deprivation.36
Figure 4.
Transition diagrams among waking (W), rapid eye movement sleep (R), stage 1 sleep (S1), stage 2 sleep (S2), and slow-wave sleep (SWS) stages showing significantly increased transition probabilities for subjects with chronic fatigue syndrome (CFS) alone and those with CFS+fibromyalgia (FM), compared with healthy control subjects (A); significantly decreased transition probabilities for subjects with CFS alone and those with CFS+FM, compared with healthy control subjects (B); and significantly different transition probabilities between subjects with CFS alone and those with CFS+FM (C). (A) Open arrow depicts significantly greater transition probability for subjects with CFS alone compared with healthy control subjects. Closed arrows depict significantly greater transition probability for subjects with CFS+FM compared with healthy control subjects. (B) Closed arrows depict significantly decreased transition probabilities for subjects with CFS+FM compared with healthy control subjects. Arrows with cross-hatching depict significantly decreased transition probabilities for both subjects with CFS alone and those with CFS+FM, compared with healthy control subjects. (C) Open arrow depicts significantly greater transition probability for subjects with CFS alone compared with subjects with CFS+FM. Closed arrows depict significantly greater transition probability for subjects with CFS+FM compared with those with CFS alone. In all 3 panels, arrow diameter is proportional to the transition probability for that particular transition. A circle, triangle, or square indicates that distributions follow a power-law, an exponential, and a stretched exponential function, respectively.25
In contrast, patients with CFS+FM (closed arrows) have higher probabilities of having transitions from waking, REM, and S1 to S2 (W/R/S1 → S2) than do healthy control subjects. Transitions from waking and REM to S2 occur only rarely in healthy humans.25 Thus, the pattern of transition probabilities suggests increased sleep pressure in patients with CFS+FM, and this interpretation was supported by increased rates of those transitions in patients with CFS+FM relative to control subjects. In addition, patients with CFS+FM had greater probabilities of having transitions from SWS to waking and S1 (SWS → W/S1); although this may have been due to the increase in total time spent in SWS, it may also reflect sleep disruption or a specific sleep problem. The latter interpretation was supported by increased rates of those transitions in patients with CFS+FM and is consistent with an earlier report showing that disruption of SWS in healthy control subjects results in feeling “unrefreshed” after awakening and the appearance of FM-like symptoms, such as reduced pain thresholds and subjective reports of musculoskeletal symptoms.37 The combination of increased sleep pressure and sleep disruption leads us to hypothesize that patients with CFS+FM may have a pathologic need for increased sleep as well as an intrinsic sleep-disrupting process.
One prior study investigated the dynamic aspects of sleep in patients with FM. Burns et al.38 studied duration distributions of patients with FM and, similar to our data in patients with CFS+FM, found that durations of S2 were shorter in patients with FM than in healthy control subjects. In contrast to our data, these authors did not find an increase in SWS duration. Although the earlier study provided no information as to whether these findings are specific to patients with FM or are shared with patients with CFS, we found the differences to be specific to patients with CFS+FM and not occurring in patients with CFS alone. Specifically, distributions of durations of S2 in patients with CFS+FM were significantly different from those in healthy control subjects and in patients with CFS alone—being shorter in patients with CFS+FM than in healthy control subjects and in patients with CFS alone (duration t < 25 min).
One explanation for this finding may be an impairment in the thalamocortical mechanism for generating sleep spindles in patients with FM, seen characteristically in S2—an effect that could lead to altered sensory processing and vulnerability to external stimuli. Data exist showing that sleep in individuals who generate more sleep spindles is more stable and more resistant to disruptive stimuli.39 Moreover, earlier work reported sleep-spindle density and power to be decreased in patients with FM.40 It is possible that the increase in SWS shown by patients with CFS+FM, which suggests an increased sleep pressure, is, in fact, a result of the decrease in stability of S2. Further research will be needed to confirm this notion and the idea that the thalamocortical mechanism responsible for generating sleep spindles is dysfunctional in patients with FM but not in those with CFS.
For the duration distributions for S2 sleep, the stretching exponent β was estimated at approximately 0.4 for all groups, which is consistent with our previous result25 but somewhat different from the result of Burns et al.38 The stretching exponent β ranges between 0 and 1, where the power-law function is the special case β → 0 and the exponential function is the special case β = 1.35 Therefore, the result of β = 0.4 obtained in this study suggests that the functional form of duration distributions for S2 sleep is different from the simple exponential distribution; this result suggests a multifactorial decay for survival times of S2 sleep durations.35
The tendency for patients with CFS+FM to have a higher probability of having transitions from S2 to SWS (S2 → SWS), as compared with control subjects, is of interest in view of our earlier finding that a central serotonergic and dopaminergic antagonist significantly increases the probability of this transition.41 Therefore, finding such increased transitions might indicate downregulation of central serotonergic systems that, importantly, has been reported in patients with FM.42–46 It is possible, therefore, that the tendency for an increased probability of transitions from S2 to SWS occurring (S2 → SWS) might also be a specific feature for FM. We plan to further evaluate this idea in future research.
One limitation of our work was related to the necessity of pooling the duration-distribution data of all subjects in each group, due to limited sample size at each sleep stage for any given subject. Using such a group analysis means that we are not able to use duration distributions to define norms for clinical application at this time. We are currently working on statistical sampling methods to deal with this problem; another possibility might be to collect data on multiple sleep nights for individual subjects.
Another possible limitation is related to our studying subjects on their first night in the sleep laboratory. If differences in rates of habituation to being in a sleep lab existed among the 3 study groups, that might have had an impact on our results. Our use of 30-second sleep-stage scoring in accordance with Rechtschaffen and Kales criteria is another possible limitation. Although the advantage of doing this is that this scoring regimen is widely and easily applicable to existing sleep-stage data, the time resolution is probably not good enough to capture fine microstructural changes of sleep, such as arousals, K-alpha complexes, and the cyclic alternating pattern. A previous study reported an increase in the cyclic alternating pattern rate, a condition of sleep instability, in patients with FM, with a strong correlation with the severity of clinical symptoms.16 Also, the total number of continuous runs of each sleep stage—that is, the data length—of the subjects studied here might not have been long enough to reliably estimate the transition-probability matrix, given the results of the previous computational study.47 Finally, another limitation has to do with the clinical population studied—one in which the diagnosis was made using clinical criteria, leading to a clinically heterogeneous population. We have tried to reduce heterogeneity by studying women only at a fixed time in their menstrual cycle and who did not have evidence of major depressive disorder. These choices obviously limit the generalizability of the data presented. We have also tried to control for pain by further stratifying the sample into those with CFS alone and those with CFS+FM. Despite these efforts, however, it is possible that differences in symptom severity might still be playing a role in producing the differences we found between CFS subgroups.
In conclusion, we have clearly demonstrated that dynamic transition statistics differentiate patients with CFS alone from those with CFS+FM. The fact that the coexistence of FM alters the dynamic sleep-stage transitions of patients with CFS suggests that CFS and FM may have different pathophysiologic underpinnings associated with different aspects of sleep regulation. Despite the differences, however, both sets of abnormalities could lead to unrefreshing sleep. Improving sleep quality would be quite important for the patients with FM, particularly because it appears they need more delta sleep. Patients with CFS, on the other hand, require treatments to increase sleep pressure or tactics to maintain REM sleep. We believe that the data reported here could lead to targeted sleep treatments that depend on the specific symptom-based diagnosis for any individual patient.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Natelson has received research support from Tempur-Pedic Corporation and Forest Laboratories. Dr. Rapoport has received research support from Fisher & Paykel Healthcare, Ventus Medical, Apnex Medical and Mannkind (Sanofi-Aventis). Dr. Rapoport holds multiple US and foreign patents covering techniques and analysis algorithms for the diagnosis of sleep disordered breathing and for administrating CPAP. These have been licensed and pay royalties to NYU from Biologics, Inc, Fisher & Paykel Healthcare, Covidian (now Health C'Aire) and Advanced Brain Monitoring (now Watermark). The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported in part by NIH #AI-54478 to Dr. Natelson and Grant-in-Aid for JSPS fellows #21-10313 to Dr. Kishi.
REFERENCES
- 1.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A International Chronic Fatigue Syndrome Study Group. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Ann Intern Med. 1994;121:953–9. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 2.Reeves WC, Heim C, Maloney EM, et al. Sleep characteristics of persons with chronic fatigue syndrome and non-fatigued controls: results from a population-based study. BMC Neurol. 2006;6:41. doi: 10.1186/1471-2377-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unger ER, Nisenbaum R, Moldofsky H, et al. Sleep assessment in a population-based study of chronic fatigue syndrome. BMC Neurol. 2004;4:6. doi: 10.1186/1471-2377-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum. 1990;33:160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 5.Ciccone DS, Natelson BH. Comorbid illness in women with chronic fatigue syndrome: a test of the single syndrome hypothesis. Psychosom Med. 2003;65:268–75. doi: 10.1097/01.psy.0000033125.08272.a9. [DOI] [PubMed] [Google Scholar]
- 6.Wessely S, Nimnuan C, Sharpe M. Functional somatic syndromes: one or many? Lancet. 1999;354:936–9. doi: 10.1016/S0140-6736(98)08320-2. [DOI] [PubMed] [Google Scholar]
- 7.Evengard B, Nilsson CG, Lindh G, et al. Chronic fatigue syndrome differs from fibromyalgia. No evidence for elevated substance P levels in cerebrospinal fluid of patients with chronic fatigue syndrome. Pain. 1998;78:153–5. doi: 10.1016/S0304-3959(98)00134-1. [DOI] [PubMed] [Google Scholar]
- 8.Russell IJ, Orr MD, Littman B, et al. Elevated cerebrospinal fluid levels of substance P in patients with the fibromyalgia syndrome. Arthritis Rheum. 1994;37:1593–601. doi: 10.1002/art.1780371106. [DOI] [PubMed] [Google Scholar]
- 9.Weaver SA, Janal MN, Aktan N, Ottenweller JE, Natelson BH. Sex differences in plasma prolactin response to tryptophan in chronic fatigue syndrome patients with and without comorbid fibromyalgia. J Womens Health. 2010;19:951–8. doi: 10.1089/jwh.2009.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer B, Le Bon O, Hoffmann G, Cluydts R, Kaufman L, De Meirleir K. Sleep anomalies in the chronic fatigue syndrome.A comorbidity study. Neuropsychobiology. 1997;35:115–22. doi: 10.1159/000119331. [DOI] [PubMed] [Google Scholar]
- 11.Krupp LB, Jandorf L, Coyle PK, Mendelson WB. Sleep disturbance in chronic fatigue syndrome. J Psychosom Res. 1993;37:325–31. doi: 10.1016/0022-3999(93)90134-2. [DOI] [PubMed] [Google Scholar]
- 12.Morriss R, Sharpe M, Sharpley AL, Cowen PJ, Hawton K, Morriss J. Abnormalities of sleep in patients with the chronic fatigue syndrome. BMJ. 1993;306:1161–4. doi: 10.1136/bmj.306.6886.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharpley A, Clements A, Hawton K, Sharpe M. Do patients with “pure” chronic fatigue syndrome (neurasthenia) have abnormal sleep? Psychosom Med. 1997;59:592–6. doi: 10.1097/00006842-199711000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Van Hoof E, De Becker P, Lapp C, Cluydts R, De Meirleir K. Defining the occurrence and influence of alpha-delta sleep in chronic fatigue syndrome. Am J Med Sci. 2007;333:78–84. doi: 10.1097/00000441-200702000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Moldofsky H. The significance of the sleeping-waking brain for the understanding of widespread musculoskeletal pain and fatigue in fibromyalgia syndrome and allied syndromes. Joint Bone Spine. 2008;75:397–402. doi: 10.1016/j.jbspin.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Rizzi M, Sarzi-Puttini P, Atzeni F, et al. Cyclic alternating pattern: a new marker of sleep alteration in patients with fibromyalgia? J Rheumatol. 2004;31:1193–9. [PubMed] [Google Scholar]
- 17.Landis CA, Lentz MJ, Tsuji J, Buchwald D, Shaver JLF. Pain, psychological variables, sleep quality, and natural killer cell activity in midlife women with and without fibromyalgia. Brain Behav Immun. 2004;18:304–13. doi: 10.1016/j.bbi.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Harding SM. Sleep in fibromyalgia patients: subjective and objective findings. Am J Med Sci. 1998;315:367–76. doi: 10.1097/00000441-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Afari N, Buchwald D. Chronic fatigue syndrome: a review. Am J Psychiatry. 2003;160:221–36. doi: 10.1176/appi.ajp.160.2.221. [DOI] [PubMed] [Google Scholar]
- 20.Chervin RD, Teodorescu M, Kushwaha R, et al. Objective measures of disordered sleep in fibromyalgia. J Rheumatol. 2009;36:2009–16. doi: 10.3899/jrheum.090051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer B. Review of clinical and psychobiological dimensions of the chronic fatigue syndrome: differentiation from depression and contribution of sleep dysfunctions. Sleep Med Rev. 1999;3:131–46. doi: 10.1016/s1087-0792(99)90020-5. [DOI] [PubMed] [Google Scholar]
- 22.Neu D, Mairesse O, Hoffmann G, et al. Sleep quality perception in the chronic fatigue syndrome: correlations with sleep efficiency, affective symptoms and intensity of fatigue. Neuropsychobiology. 2007;56:40–6. doi: 10.1159/000110727. [DOI] [PubMed] [Google Scholar]
- 23.Togo F, Natelson BH, Cherniack NS, FitzGibbons J, Garcon C, Rapoport DM. Sleep structure and sleepiness in chronic fatigue syndrome with or without coexisting fibromyalgia. Arthritis Res Ther. 2008;10:R56. doi: 10.1186/ar2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep states of human subjects. Washington, DC: US Government Printing Office; 1968. [Google Scholar]
- 25.Kishi A, Struzik ZR, Natelson BH, Togo F, Yamamoto Y. Dynamics of sleep stage transitions in healthy humans and patients with chronic fatigue syndrome. Am J Physiol Regul Integr Comp Physiol. 2008;294:1980–7. doi: 10.1152/ajpregu.00925.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Comte JC, Ravassard P, Salin PA. Sleep dynamics: a self-organized critical system. Phys Rev E. 2006;73:056127. doi: 10.1103/PhysRevE.73.056127. [DOI] [PubMed] [Google Scholar]
- 27.Lo CC, Chou T, Penzel T, et al. Common scale-invariant patterns of sleep-wake transitions across mammalian species. Proc Natl Acad Sci U S A. 2004;101:17545–8. doi: 10.1073/pnas.0408242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lo CC, Nunes Amaral LA, Havlin S, et al. Dynamics of sleep-wake transitions during sleep. Europhys Lett. 2002;57:625–31. [Google Scholar]
- 29.Robbins LN, Cottler LB, Bucholz KK, Compton WM, North CS, Rourke KM. St. Louis, MO: Washington University School of Medicine; 2000. Diagnostic Interview Schedule for the DSM-IV (DIS-IV) [Google Scholar]
- 30.Maes M, Lambrechts J, Bosmans E, et al. Evidence for a systemic immune activation during depression: results of leukocyte enumeration by flow cytometry in conjunction with monoclonal antibody staining. Psychol Med. 1992;12:45–53. doi: 10.1017/s0033291700032712. [DOI] [PubMed] [Google Scholar]
- 31.American Sleep Disorders Association. EEG arousals: scoring rules and examples. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 32.Ayappa I, Norman RG, Krieger AC, Rosen A, O'Malley RL, Rapoport DM. Non-invasive detection of respiratory effort-related arousals (RERAs) by a nasal cannula/pressure transducer system. Sleep. 2000;23:763–71. doi: 10.1093/sleep/23.6.763. [DOI] [PubMed] [Google Scholar]
- 33.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 34.Laffan A, Caffo B, Swihart BJ, Punjabi NM. Utility of sleep stage transitions in assessing sleep continuity. Sleep. 2010;33:1681–6. doi: 10.1093/sleep/33.12.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sornette D. Critical Phenomena in Natural Sciences: Chaos, Fractals, Self-organization and Disorder: Concepts and Tools. 2nd ed. Berlin, Germany: Springer; 2004. [Google Scholar]
- 36.Nakamura T, Togo F, Cherniack NS, Rapoport DM, Natelson BH. A subgroup of patients with chronic fatigue syndrome may have a disorder of arousal. Open Sleep J. 2010;3:6–11. [Google Scholar]
- 37.Moldofsky H, Scarisbrick P, England R, Smythe H. Musculoskeletal symptoms and non-REM sleep disturbance in patients with “fibrositis syndrome” and healthy subjects. Psychosom Med. 1975;37:341–51. doi: 10.1097/00006842-197507000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Burns JW, Crofford LJ, Chervin RD. Sleep stage dynamics in fibromyalgia patients and controls. Sleep Med. 2008;9:689–96. doi: 10.1016/j.sleep.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 39.Dang-Vu TT, McKinney SM, Buxton OM, Solet JM, Ellenbogen JM. Spontaneous brain rhythms predict sleep stability in the face of noise. Curr Biol. 2010;20:R626–7. doi: 10.1016/j.cub.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 40.Landis CA, Lentz MJ, Rothermel J, Buchwald D, Shaver JLF. Decreased sleep spindles and spindle activity in midlife women with fibromyalgia and pain. Sleep. 2004;27:741–50. doi: 10.1093/sleep/27.4.741. [DOI] [PubMed] [Google Scholar]
- 41.Kishi A, Yasuda H, Matsumoto T, et al. Sleep stage transitions in healthy humans altered by central monoaminergic antagonist. Methods Inf Med. 2010;49:458–61. doi: 10.3414/ME09-02-0032. [DOI] [PubMed] [Google Scholar]
- 42.Alnigenis MN, Barland P. Fibromyalgia syndrome and serotonin. Clin Exp Rheumatol. 2001;19:205–10. [PubMed] [Google Scholar]
- 43.Wolfe F, Russell IJ, Vipraio G, Ross K, Anderson J. Serotonin levels, pain threshold, and fibromyalgia symptoms in the general population. J Rheumatol. 1997;24:555–9. [PubMed] [Google Scholar]
- 44.Bazzichi L, Giannaccini G, Betti L, et al. Alteration of serotonin transporter density and activity in fibromyalgia. Arthritis Res Ther. 2006;8:R99. doi: 10.1186/ar1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yunus MB, Dailey JW, Aldag JC, Masi AT, Jobe PC. Plasma tryptophan and other amino acids in primary fibromyalgia: a controlled study. J Rheumatol. 1992;19:90–4. [PubMed] [Google Scholar]
- 46.Moldofsky H, Warsh JJ. Plasma tryptophan and musculoskeletal pain in non-articular rheumatism (“fibrositis syndrome”) Pain. 1978;5:65–71. doi: 10.1016/0304-3959(78)90025-8. [DOI] [PubMed] [Google Scholar]
- 47.Jansen BH, Cheng WK. Structural EEG analysis: an explorative study. Int J Biomed Comput. 1988;23:221–37. doi: 10.1016/0020-7101(88)90016-5. [DOI] [PubMed] [Google Scholar]