Abstract
Study Objectives:
To characterize elderly persons into sleep/rest groups based on their self-reported habitual total sleeping time (TST) and habitual time in bed (TIB) and to examine the prospective association between sleep/rest behavior on physical function decline.
Design:
Population-based InCHIANTI study with 6 years follow-up (Tuscany, Italy).
Setting:
Community.
Participants:
Men and women aged ≥ 65 years (n = 751).
Measurements and Results:
At baseline, participants were categorized into 5 sleep/rest behavior groups according to their self-reported TST and TIB, computed from bedtime and wake-up time. Physical function was assessed at baseline and at 3- and 6-year follow-ups as walking speed, the Short Physical Performance Battery (SPPB), and self-reported mobility disability (ability to walk 400 m or climb one flight of stairs). Both long (≥ 9 h) TST and long TIB predicted accelerated decline in objectively measured physical performance and greater incidence in subjectively assessed mobility disability, but short (≤ 6 h) TST did not. After combining TST and TIB, long sleepers (TST and TIB ≥ 9 h) experienced the greatest decline in physical performance and had the highest risk for incident mobility disability in comparison to mid-range sleepers with 7-8 h TST and TIB. Subjective short sleepers reporting short (≤ 6 h) TST but long (≥ 9 h) TIB showed a greater decline in SPPB score and had a higher risk of incident mobility disability than true short sleepers with short (≤ 6 h) TST and TIB ≤ 8 hours.
Conclusions:
Extended time in bed as well as long total sleeping time is associated with greater physical function decline than mid-range or short sleep. TIB offers important additive information to the self-reported sleep duration when evaluating the consequences of sleep duration on health and functional status.
Citation:
Stenholm S; Kronholm K; Bandinelli S; Guralnik JM; Ferrucci. Self-reported sleep duration and time in bed as predictors of physical function decline: results from the InCHIANTI study. SLEEP 2011;34(11):1583-1593.
Keywords: Aging, disability, epidemiology, follow-up studies, gerontology, physical performance, sleep, sleep duration, time in bed
INTRODUCTION
Sleep duration is associated with several health and functional consequences, with both short and long sleep duration increasing the risk for negative outcomes.1–4 A majority of the findings are based on subjective sleep duration assessments, and the interpretation may be confounded by the vagueness of the psychometric validity of self-reported sleep measures. Nevertheless, the use of self-reported assessments of sleep is almost unavoidable in large epidemiological studies due to practicality and simplicity of this method. One approach to define more accurately self-reported habitual sleep duration is to complement the sleep duration question with other questionnaire-based assessments related to sleep length. The most obvious one is time in bed (TIB), which in case of discrepancy with self-reported sleep duration, may reflect to some extent a resting state which is not experienced subjectively as sleep per se. Thus, self-reported total sleeping time (TST) and TIB may be considered complementary but partly independent measures.
In the context of self-reported sleep duration and health risks, little attention has been paid to functional capacity and its decline in older people, a problem which is increasingly relevant in many aging societies leading to higher need for help and care. Few previous studies have examined the association between sleep-related factors and physical function.5–7 Both short (≤ 6 h) and long (≥ 9 h) self-reported habitual sleep duration as well as increased sleep fragmentation are associated with slower walking speed and functional limitation compared to persons with mid-range sleep duration.6 In addition, insomnia-related symptoms and daytime consequences of poor sleep, such as weakness and tiredness, are associated with physical performance in older men and women.5,6 Most previous studies are cross-sectional and cannot truly address the question of whether sleep-related factors explain physical function decline or vice versa. However, the findings are consistent with the suggestions that short self-reported sleep duration tends to cause sleep deprivation and is associated with adverse health effects.1,4 On the other hand, it is well known that in older persons severe illness may be associated with excess sleepiness,8 and thus excessive sleep can be part of individual's subjective symptom formation.9 Consequently, self-reported TST and behavioral TIB may be interpreted as indicators of sleep's functional integrity which is part of the broader notion of body integrity (including the brain). Especially long TST and long TIB irrespective of sleep time may reflect body's need to work harder and longer to reset a homeostatic and energetic balance.3,10 If this hypothesis is correct, long TST and TIB should be predictive of adverse health outcomes, especially in the elderly who may lack effective compensatory mechanisms when facing disease and stress.
To our knowledge, there have been no prior studies of the prospective association between self-reported TST and TIB and physical function decline. The InCHIANTI study provides a unique opportunity to examine these questions in a large cohort of community-dwelling older adults with self-reported information on TST and TIB as well as 6-year follow-up data on physical function. The specific aims for this study were to characterize persons into sleep/rest groups based on their self-reported habitual TST and habitual TIB and secondly, to examine the prospective association of sleep/rest behavior on physical function decline.
METHODS
Study Design and Participants
InCHIANTI (Invecchiare in Chianti, aging in the Chianti area) is an epidemiological study of factors contributing to loss of mobility in late life and was carried out in 2 Italian towns located in the Chianti geographic area (Tuscany, Italy). The baseline data were collected in 1998-2000; the 3-year follow-up took place in 2001-2003 and the 6-year follow-up in 2004-2006. The design of the study and data collection methods have been described earlier in detail.11 The study population consisted of a random sample of 1,260 persons aged 65 years and older selected from the population registries of 2 municipalities: 1,155 older adults agreed to participate in the study (participation rate 91.7%), and 923 had information regarding sleep duration and physical performance at baseline. Of these, 172 did not have either 3- or 6-year follow-up data of physical performance: 153 died, 14 refused or were unable to participate in the study, and 5 moved from the area during the 6-year follow-up. Thus, the final analytical sample was 751 participants.
Participants received a full description of the study and provided a written informed consent. The Italian National Institute of Research and Care on Aging Ethical Committee approved the study protocol, which complied with the principles stated in the Declaration of Helsinki.
Sleep/Rest Behavior
Self-reported habitual TST was inquired about with a question “During the past month, how many hours of actual sleep did you get on average at night?” The responses were recorded in whole numbers. TIB was determined based on the questions: “During the past month, what time have you usually gone to bed at night?” and “During the past month, what time have you usually gotten up in the morning?” The difference was rounded up in whole numbers. For the purposes of this study, we combined information from the self-reported TST and TIB and categorized persons into 5 groups (partly) based on previous reports dividing persons into short sleepers (≤ 6 h), mid-range sleepers (7-8 h), and long sleepers (≥ 9 h).2,8,12 Since TIB and TST 7-8 h can be considered normal, i.e., not harmful, TST 7-8 h and TIB 7-8 h was chosen as a reference group. Four other categories were created in order to tease out different combinations of TST and TIB. Sleep/rest behavior was categorized as following: (1) TST 7-8 h and TIB 7-8 h (true mid-range sleepers; reference group); (2) TST ≤ 6 h and TIB ≤ 8 h (true short sleepers); (3) TST ≤ 6 h and TIB ≥ 9 h (subjective short sleepers); (4) TST 7-8 h and TIB ≥ 9 h (subjective mid-range sleepers); and (5) TST ≥ 9 h and TIB ≥ 9 h (long sleepers).
Physical Function Outcomes
Physical function was assessed using performance-based tests of lower extremity and asking questions about the participants' ability to walk 400 m continuously and without developing symptoms. Similar methods were used at baseline and at the 3-year and 6-year follow-ups.11 To measure walking speed subjects were asked to walk 4 meters at their usual pace as if they were walking down the street, starting from a standing position. Use of a cane or walker was permitted. Walking speed is a valid and generally used measure of mobility limitation for both healthy and impaired older persons13 with high predictive validity for subsequent disability, hospitalization, and mortality.14,15
A Short Physical Performance Battery (SPPB) based on the lower-extremity performance tests16 was used to summarize physical performance. The SPPB consists of walking speed, ability to stand from a chair, and ability to maintain balance. Walking speed was determined based on the best performance (time in seconds) of two 4-meter walks at usual pace along a corridor. To test the ability to stand from a chair, participants were asked to stand up and sit down as quickly as possible in a chair 5 times with their hands folded across their chest; time (in seconds) to complete the test was recorded. For the standing balance test, participants were asked to stand in 3 progressively more difficult positions for 10 sec each: a side-by-side position, a semi-tandem position, and a full-tandem position. Each physical performance measure was categorized into a 5-level score, with 0 representing inability to do the test and 4 representing the highest level of performance. Both the walking and chair-rise time tasks were each scored from 1 to 4 based on quartile cut-points from normative data on community-dwelling older adults.14 The 3 measures were then added to create a summary physical performance measure ranging from 0 (worst) to 12 (best). SPPB has shown to have excellent 1-week test-retest reliability (intraclass correlation 0.88-0.92),17 and predict nursing home admission, disability, and mortality.13,14
As a part of the interview, participants were asked whether they had any difficulties in walking 400 meters or climbing a flight of stairs with the response options being (1) no difficulty, (2) with difficulty but without help, (3) with some help from another person, and (4) unable to perform the activity. Mobility-related disability was defined as need for help or inability to walk 400 meters or to climb a flight of stairs.13 Self-reported mobility disability predicts future disability, nursing home admission, and mortality.16,18 Participants with mobility disability at baseline (n = 12) were excluded from prospective analyses.
Covariates
Educational level, obesity, physical activity, smoking status, alcohol consumption, and chronic diseases, as well as inflammatory markers were all considered as possible confounders of the association between sleep/rest behavior and physical function. Education was recorded in years. Body mass index (BMI) was calculated as measured weight in kilograms divided by measured height in meters squared (kg/m2). Waist circumference was measured at the midpoint between the lower rib margin and the iliac crest. The level of physical activity in the 12 months prior to the interview was assessed through an interviewer-administered questionnaire19 and was coded as sedentary (inactivity or light-intensity activity less than 1 h/week), light physical activity (light-intensity activity 2-4 h/week), and moderate-high physical activity (light-intensity activity ≥ 5 h/wk or moderate activity ≥ 1-2 h/week). Smoking history was determined based on self-reports and participants were categorized into never smokers, former smokers, and current smokers. Daily alcohol (g) intake was estimated by the European Prospective Investigation Into Cancer and Nutrition Food Frequency Questionnaire (EPIC),20 and persons were classified as ≥ 30 g/day vs. < 30 g/day (30 g of alcohol corresponds to about ≥ 3 drinks/day).
Diseases were ascertained by a trained geriatrician according to standard, pre-established criteria and algorithms, based on those used in the Women's Health and Aging Study that combine information from self-reported physician diagnoses, current pharmacological treatment, medical records, clinical examinations, and blood tests.21 The following diseases were included in the current analyses: coronary heart disease, diabetes, asthma, chronic bronchitis, and knee osteoarthritis. Depressive symptoms were evaluated with the Center for Epidemiologic Studies Depression Scale (CES-D).22 The CES-D is a 20-item self-report scale, ranging from 0 to 60. The CES-D has been shown to have good psychometrics properties in assessing depressive symptoms, in older population-based studies23 as well as in an Italian sample.24 A score ≥ 16 was considered to indicate depressive symptoms. The use of sleeping medication was also inquired with a question “During the past month, how often have you taken sleeping pills to help you sleep?” Participants were categorized as never, once or twice a week, and ≥ 3 times a week.
Baseline blood samples were collected in the morning after a 12-h fast and after a 15-min rest. Serum and plasma were stored in a deep freezer at −80°C and were not thawed until analyzed. High sensitivity C-reactive protein levels (CRP) were measured by enzyme-linked immunoabsorbent assay using purified protein and polyclonal anti-CRP antibodies (Roche Diagnostics, GmbH, Mannheim, Germany). For high sensitivity CRP, the minimum detectable concentration (MDC) was 0.03 mg/L and the inter-assay coefficient of variation (CV) was 5%. Serum interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) concentrations were determined by high sensitivity enzyme-linked immunoabsorbent assays (ELISA), using commercial kits (Human Ultrasensitive, BioSource International, Camarillo, CA, USA). For IL-6, the MDC was 0.1 pg/mL and the inter-assay CV 4.5%. For TNF-α, the MDC was approximately 0.09 pg/mL and the inter-assay CV 7%. All cytokine assays were done in duplicate and were repeated if the second measure was > 10% or < 10% compared to the first. The average of the 2 measures was used in the analyses.
Statistical Analysis
Study population characteristics according to TST, TIB, and sleep/rest behavior groups are reported as mean and median values for continuous variables and proportions for categorical variables. Differences between groups were examined with χ2 test for categorical variables, Kruskal-Wallis test for skewed continuous variables and ANOVA post hoc test for normally distributed continuous variables. The association between TST and TIB was examined with Spearman correlation coefficient.
In this study, TST, TIB, and sleep/rest grouping are considered as statistical risk factors for physical function decline. The effect of TST, TIB, and sleep/rest behavior on the magnitude of physical performance decline over the follow-up time was calculated with linear mixed effect regression models by using compound symmetry as covariance structure. The analysis was completed with the MIXED procedure in SAS. The major advantage of using mixed models in longitudinal studies is that the technique takes into account the correlation between serial measures obtained in the same subjects as well as the missing observations, thus allowing all available data on each subject to be used.25,26 Thus in this study, we were able to utilize walking speed and SPPB information from baseline, 3-year follow-up, and 6-year follow-up measurements. The results are presented as beta estimates for each comparable group indicating the slope (change per year) as well as the baseline values for each group. TST 7-8 h, TIB 7-8 h, and true mid-range sleepers (TST 7-8 h and TIB 7-8 h) were defined as the reference groups in the analyses. Models were adjusted for baseline age, sex, education, physical activity, smoking, alcohol use, sleeping pill use, waist circumference, hypertension, coronary heart disease, diabetes, depressive symptoms, and inflammatory markers. The risk of developing mobility disability over 6 years of follow-up by TST, TIB, and sleep/rest behavior groups were estimated with logistic regression models, and above-mentioned covariates were used to adjust the models.
To examine whether sleep/rest behavior groups have different effect on physical performance decline in men and women, an interaction term sex*sleep/rest behavior was included in the linear mixed effect models. All the interaction terms were nonsignificant, and therefore men and women were combined in our analysis. The SAS 9.1 Statistical Package was used for all analyses (SAS Institute, Inc., Cary, NC, USA).
RESULTS
Individual Effects of TST and TIB on Physical Function Decline
At baseline, no differences in walking speed or SPPB scores were observed across TST and TIB groups. The only exception was significantly higher SPPB score among men with ≤ 6 h TIB compared to midrange TIB (P = 0.02). Detailed baseline characteristics of the study population by TST and TIB are shown in Table 1.
Table 1.
Baseline characteristics by self-reported total sleeping time and time in bed (n = 751)
| Total Sleeping Time |
|||||
|---|---|---|---|---|---|
| ≤ 6 h | 7-8 h | ≥ 9 h | P for difference ≤ 6 h vs. 7–8 h | P for difference 7-8 h vs. ≥ 9 h | |
| N | 311 | 371 | 69 | ||
| Women, % | 63.67 | 51.48 | 39.13 | 0.06 | 0.001 |
| Physical activity, % | 0.25 | 0.02 | |||
| Sedentary | 14.56 | 9.73 | 15.94 | ||
| Moderate | 49.19 | 44.05 | 44.93 | ||
| Active | 36.25 | 46.22 | 39.13 | ||
| Smoking, % | 0.97 | 0.05 | |||
| Never | 63.02 | 54.45 | 53.62 | ||
| Former | 25.72 | 29.11 | 30.43 | ||
| Current | 11.25 | 16.44 | 15.94 | ||
| Alcohol use (≥ 3 drinks/day), % | 14.79 | 16.62 | 18.84 | 0.65 | 0.51 |
| Sleeping pill use, % | 0.47 | 0.01 | |||
| Never | 72.35 | 81.94 | 86.96 | ||
| Once or twice a week | 5.79 | 4.04 | 1.45 | ||
| Three or more times a week | 21.86 | 14.02 | 11.59 | ||
| Hypertension, % | 42.77 | 28.03 | 28.99 | 0.87 | < 0.0001 |
| Coronary heart disease, % | 7.31 | 6.94 | 8.70 | 0.61 | 0.86 |
| Diabetes, % | 11.25 | 11.05 | 15.94 | 0.25 | 0.93 |
| Bronchitis, % | 5.14 | 8.36 | 8.70 | 0.93 | 0.10 |
| Asthma, % | 3.22 | 5.66 | 7.25 | 0.61 | 0.13 |
| Depression, % | 37.94 | 25.55 | 22.06 | 0.54 | 0.001 |
| Knee osteoarthritis, % | 8.68 | 4.85 | 4.35 | 0.86 | 0.04 |
| Mean (SD) | Mean (SD) | Mean (SD) | P for difference ≤ 6 h vs. 7-8 h | P for difference 7-8 h vs. ≥ 9 h | |
| Age, years | 73.09 (5.86) | 72.57 (5.87) | 74.25 (6.80) | 0.08 | 0.50 |
| Education, years | 5.56 (3.41) | 5.75 (3.10) | 6.36 (3.95) | 0.34 | 0.72 |
| BMI, kg/m2 | 27.90 (4.12) | 27.37 (3.63) | 27.55 (4.75) | 0.94 | 0.19 |
| Waist circumference, cm | |||||
| Men | 95.64 (8.33) | 95.37 (8.36) | 94.33 (9.80) | 0.76 | 0.96 |
| Women | 91.59 (10.30) | 90.10 (10.64) | 91.26 (12.26) | 0.86 | 0.35 |
| Walking speed, m/s | |||||
| Men | 1.16 (0.21) | 1.17 (0.25) | 1.19 (0.25) | 0.81 | 0.94 |
| Women | 1.00 (0.21) | 1.05 (0.22) | 0.98 (0.29) | 0.28 | 0.11 |
| SPPB Score | |||||
| Men | 11.16 (1.63) | 11.30 (1.50) | 11.05 (1.53) | 0.61 | 0.73 |
| Women | 10.21 (2.06) | 10.44 (2.08) | 9.88 (2.96) | 0.43 | 0.53 |
| Median (IQR) | Median (IQR) | Median (IQR) | P for difference ≤ 6 h vs. 7-8 h | P for difference 7-8 h vs. ≥ 9 h | |
| C-reactive protein, mg/L | 2.37 (1.23-4.86) | 2.55 (1.29-5.01) | 2.46 (1.24-6.39) | 0.89 | 0.66 |
| Interleukin-6, pg/mL | 1.30 (0.80-1.95) | 1.37 (0.78-2.04) | 1.50 (0.95-2.52) | 0.25 | 0.51 |
| Tumor necrosis factor-α, pg/mL | 4.64 (3.39-5.97) | 4.22 (3.05-5.71) | 4.68 (3.52-5.99) | 0.04 | 0.02 |
| Time in Bed |
|||||
| ≤ 6 h | 7-8 h | ≥ 9 h | P for difference ≤ 6 h vs. 7-8 h | P for difference 7-8 h vs. ≥ 9 h | |
| N | 16 | 271 | 464 | ||
| Women, % | 50.00 | 55.72 | 55.39 | 0.93 | 0.65 |
| Physical activity, % | 0.01 | 0.07 | |||
| Sedentary | 20.00 | 7.75 | 14.72 | ||
| Moderate | 20.00 | 45.76 | 47.40 | ||
| Active | 60.00 | 46.49 | 37.88 | ||
| Smoking, % | 0.92 | 0.76 | |||
| Never | 50.00 | 59.04 | 57.54 | ||
| Former | 31.25 | 26.94 | 28.23 | ||
| Current | 18.75 | 14.02 | 14.22 | ||
| Alcohol use (≥ 3 drinks/day), % | 31.25 | 16.97 | 15.00 | 0.48 | 0.15 |
| Sleeping pill use, % | 0.65 | 0.18 | |||
| Never | 68.75 | 80.44 | 77.59 | ||
| Once or twice a week | 0.00 | 4.43 | 4.74 | ||
| Three or more times a week | 31.25 | 15.13 | 17.67 | ||
| Hypertension, % | 50.00 | 34.69 | 33.41 | 0.72 | 0.21 |
| Coronary heart disease, % | 6.25 | 7.92 | 6.90 | 0.61 | 0.81 |
| Diabetes, % | 18.75 | 11.44 | 11.42 | 0.99 | 0.38 |
| Bronchitis, % | 6.25 | 4.43 | 8.62 | 0.03 | 0.73 |
| Asthma, % | 0.00 | 4.06 | 5.39 | 0.42 | 0.41 |
| Depression, % | 43.75 | 27.34 | 31.74 | 0.21 | 0.16 |
| Knee osteoarthritis, % | 12.50 | 6.27 | 6.25 | 0.99 | 0.33 |
| Mean (SD) | Mean (SD) | Mean (SD) | P for difference ≤ 6 h vs. 7-8 h | P for difference 7-8 h vs. ≥ 9 h | |
| Age, years | 72.81 (5.80) | 71.99 (5.37) | 73.50 (6.24) | 0.003 | 0.85 |
| Education, years | 7.38 (4.95) | 6.20 (3.71) | 5.39 (2.94) | 0.004 | 0.35 |
| BMI, kg/m2 | 27.41 (4.19) | 27.47 (3.75) | 27.69 (4.07) | 0.76 | 0.99 |
| Waist circumference, cm | |||||
| Men | 97.88 (8.56) | 95.13 (8.67) | 95.35 (8.47) | 0.97 | 0.65 |
| Women | 91.13 (12.46) | 91.27 (10.62) | 90.65 (10.55) | 0.84 | 0.99 |
| Walking speed, m/s | |||||
| Men | 1.33 (0.26) | 1.18 (0.19) | 1.16 (0.24) | 0.62 | 0.18 |
| Women | 0.94 (0.23) | 1.06 (0.19) | 1.00 (0.24) | 0.06 | 0.31 |
| SPPB Score | |||||
| Men | 11.75 (0.46) | 11.50 (0.94) | 11.04 (1.81) | 0.02 | 0.90 |
| Women | 8.88 (2.75) | 10.43 (1.79) | 10.26 (2.29) | 0.70 | 0.11 |
| Median (IQR) | Median (IQR) | Median (IQR) | P for difference ≤ 6 h vs. 7-8 h | P for difference 7-8 h vs. ≥ 9 h | |
| C-reactive protein, mg/L | 1.54 (1.12-4.13) | 2.25 (1.20-4.63) | 2.60 (1.36-5.54) | 0.01 | 0.69 |
| Interleukin-6, pg/mL | 0.95 (0.73-1.52) | 1.22 (0.78-1.87) | 1.45 (0.82-2.07) | 0.04 | 0.38 |
| Tumor necrosis factor-α, pg/mL | 3.93 (2.76-5.62) | 4.48 (3.15-5.74) | 4.43 (3.21-5.94) | 0.90 | 0.35 |
Values are shown in mean (SD) for normally distributed and medians (IQR) for non-normally distributed continuous variables and N (%) for categorical variables.
SPPB, Short Physical Performance Battery; IQR, inter quartile range.
The individual effects of TST and TIB on physical performance decline are shown in Table 2. Participants who reported ≥ 9 h of TST or TIB ≥ 9 h experienced greater decline in walking speed than persons who reported 7-8 h of TST or TIB (P = 0.04 and P = 0.03, respectively). In addition, TIB ≥ 9 h was associated with greater decline in SPPB score compared to participants who reported 7-8 h of TIB (P < 0.0001). Short TST and TIB (≤ 6 h) were not associated with accelerated decline in walking speed or SPPB score. After adding TST and TIB to the same model, both remained statistically significantly associated with walking speed and SPPB, score and no interaction was found between TST and TIB on walking speed or SPPB score.
Table 2.
Annual change in walking speed and Short Physical Performance Battery by total sleeping time and time in bed
| Walking Speed |
Short Physical Performance Battery |
||||||
|---|---|---|---|---|---|---|---|
| N | β Estimate* | SE | P† | β Estimate* | SE | P† | |
| Total Sleeping Time | |||||||
| ≤ 6 h | 311 | −0.023 | 0.002 | 0.435 | −0.289 | 0.023 | 0.211 |
| 7-8 h | 371 | −0.021 | 0.002 | ref | −0.249 | 0.022 | ref |
| ≥ 9 h | 69 | −0.032 | 0.005 | 0.035 | −0.335 | 0.051 | 0.119 |
| Time in Bed | |||||||
| ≤ 6 h | 16 | −0.027 | 0.010 | 0.440 | −0.092 | 0.111 | 0.363 |
| 7-8 h | 271 | −0.019 | 0.002 | ref | −0.195 | 0.024 | ref |
| ≥ 9 h | 464 | −0.025 | 0.002 | 0.027 | −0.333 | 0.020 | < 0.0001 |
Beta estimates for different groups are derived from linear mixed effect models and they indicate the average change in walking speed/SPPB score per year in that specific category.
P value indicates the significance of the difference between groups. SE, standard error. Models are adjusted for age, sex, education, physical activity, smoking, alcohol use, sleeping pills use, hypertension, coronary heart disease, diabetes, waist circumference, depression, C-reactive protein, interleukin-6, and tumor necrosis factor-α.
The effects of TST and TIB on subcomponents of SPPB score, chair stand and standing balance, were also examined. Long TST (≥ 9 h) was associated with accelerated decline in chair stand score compared to 7-8 h of TST, but no difference in balance score across TST groups was observed. For TIB the results related to walking speed, chair score, and balance score were comparable, i.e., ≥ 9 h TIB was associated with accelerated decline in walking speed, chair stand, and balance compared to 7-8 hours of TIB (data not shown).
The risk of developing mobility disability was also examined by adjusting for age, sex, lifestyle factors, sleeping pill use, waist circumference, diseases, and inflammatory markers. Both long (≥ 9 h) TST and TIB were associated with increased odds for mobility disability in a separate models (odds ratio, 95% confidence limit (OR, 95% CI) 2.94, 1.15-7.50 and 2.55, 1.25-5.17, respectively). After adding them to the same model, ≥ 9 h TST and TIB remained independently associated with incident mobility disability (OR = 2.52, 95% CI 1.03-6.20 and OR = 2.11, 95% CI 1.08-4.13), and no interaction was found between these 2 variables.
Effect of Sleep/Rest Behavior on Physical Function Decline
Self-reported TST and TIB were moderately correlated with each other (Spearman correlation coefficient r = 0.31, P < 0.001), indicating that TST and TIB should be considered related but partially independent measures. In Table 3 the percentages of different sleep/rest behavior groups are shown. Overall, 18% of the participants were considered as true mid-range sleepers (TST 7-8 h and TIB 7-8 h), 20% as true short sleepers (TST ≤ 6 h and TIB ≤ 8 h), 21% as subjective short sleepers (TST ≤ 6 h and TIB ≥ 9 h), 31% as subjective mid-range sleepers (TST 7-8 h and TIB ≥ 9 h), and 9% as long sleepers (TST ≥ 9 h and TIB ≥ 9 h).
Table 3.
Sleep/rest behavior categories by distribution of total sleeping time (TST) and time in bed (TIB) (n = 751)
| Time in Bed |
Total n | |||
|---|---|---|---|---|
| ≤ 6 h | 7-8 h | ≥ 9 h | ||
| Total Sleeping Time | ||||
| ≤ 6 h (n) | 2.1% (16)b | 18.0% (135)b | 21.3% (160)c | 311 |
| 7-8 h (n) | 0 | 18.1% (136)a | 31.3% (235)d | 371 |
| ≥ 9 h (n) | 0 | 0 | 9.2% (69)e | 69 |
| Total n | 16 | 271 | 464 | 751 |
True mid-range sleepers (TST 7-8 h and TIB 7-8 h);
True short sleepers (TST ≤ 6 h and TIB ≤ 8 h);
Subjective short sleepers (TST ≤ 6 h and TIB ≥ 9 h);
Subjective mid-range sleepers (TST 7-8 h and TIB ≥ 9 h);
Long sleepers (TST ≥ 9 h and TIB ≥ 9 h).
Baseline characteristics of the study population by sleep/rest behavior groups are shown in Table 4. Compared to true mid-range sleepers (reference group), true and subjective short sleepers were more often women than men (P < 0.05), and the mean age was lowest among the true mid-range sleepers (P < 0.05). Physically sedentary behavior was more common in all other groups compared to true mid-range sleepers, with subjective mid-range sleepers and long sleepers having the highest prevalence (P < 0.05). Use of sleeping pills, presence of hypertension, and depressive symptoms were more prevalent among true and subjective short sleepers compared to true mid-range sleepers (P < 0.05). In addition, depressive symptoms were more common in subjective mid-range sleepers than true mid-range sleepers (P < 0.05), and CRP level was higher in subjective mid-range sleepers than true mid-range sleepers (P < 0.05). TNF-α level was higher in all groups than in the true mid-range sleepers, (P < 0.05) except for the subjective mid-range sleepers. There were no differences in walking speed or SPPB at baseline in men across sleep/rest behavior groups (Table 4). In women, true mid-range sleepers had significantly higher walking speed than subjective short sleepers (1.09 vs. 0.98 m/s, P = 0.02).
Table 4.
Baseline characteristics by sleep/rest behavior (n = 751)
| True mid-range sleepers (TST 7-8 h and TIB 7-8 h) | True short sleepers (TST ≤ 6 h and TIB ≤ 8 h) (a) | Subjective short sleepers (TST ≤ 6 h and TIB ≥ 9 h) (b) | Subjective mid-range sleepers (TST 7-8 h and TIB ≥ 9 h (c) | Long sleepers (TST ≥ 9 h and TIB ≥ 9 h) (d) | Difference from true mid-range sleepers* | |
|---|---|---|---|---|---|---|
| N | 136 | 151 | 160 | 235 | 69 | |
| Women, % | 46.32 | 63.58 | 63.75 | 54.47 | 39.13 | a, b |
| Physical activity, % | a, b, c, d | |||||
| Sedentary | 5.15 | 11.33 | 17.61 | 12.39 | 15.94 | |
| Moderate | 41.18 | 47.33 | 50.94 | 45.73 | 44.93 | |
| Active | 53.68 | 41.33 | 31.45 | 41.88 | 39.13 | |
| Smoking, % | ||||||
| Never | 16.91 | 11.92 | 10.63 | 16.17 | 15.94 | |
| Former | 30.15 | 24.5 | 26.88 | 28.51 | 30.43 | |
| Current | 52.94 | 63.58 | 62.5 | 55.32 | 53.62 | |
| Alcohol use (≥ 3 drinks/day), % | 19.85 | 15.89 | 13.75 | 14.72 | 18.84 | |
| Sleeping pill use, % | a, b | |||||
| Never | 87.5 | 72.85 | 71.88 | 78.72 | 86.96 | |
| Once or twice a week | 2.21 | 5.96 | 5.63 | 5.11 | 1.45 | |
| Three or more times a week | 10.29 | 21.19 | 22.5 | 16.17 | 11.59 | |
| Hypertension, % | 25 | 45.03 | 40.63 | 29.79 | 28.99 | a, b |
| Coronary heart disease, % | 9.09 | 6.71 | 7.89 | 5.70 | 8.70 | |
| Diabetes, % | 11.76 | 11.92 | 10.63 | 10.64 | 15.94 | |
| Bronchitis, % | 5.15 | 3.97 | 6.25 | 10.21 | 8.7 | |
| Asthma, % | 5.88 | 1.99 | 4.38 | 5.53 | 7.25 | |
| Depression, % | 18.18 | 37.09 | 38.75 | 29.74 | 22.06 | a, b, c |
| Knee osteoarthritis, % | 4.41 | 8.61 | 8.75 | 5.11 | 4.35 | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age, years | 71.49 (5.19) | 72.53 (5.52) | 73.61 (6.12) | 73.20 (6.16) | 74.25 (6.80) | b, c, d |
| Education, years | 6.36 (3.95) | 5.47 (2.94) | 4.87 (2.25) | 6.28 (4.19) | 6.25 (3.31) | b |
| BMI, kg/m2 | 27.22 (3.46) | 27.70 (4.02) | 28.09 (4.22) | 27.46 (3.73) | 27.55 (4.75) | |
| Waist circumference, cm | ||||||
| Men | 94.28 (8.24) | 96.62 (9.05) | 94.70 (7.52) | 96.13 (8.39) | 94.33 (9.80) | |
| Women | 90.02 (10.76) | 92.07 (10.60) | 91.13 (10.05) | 90.14 (10.62) | 91.26 (12.26) | |
| Walking speed, m/s | ||||||
| Men | 1.18 (0.19) | 1.20 (0.21) | 1.15 (0.28) | 1.15 (0.22) | 1.19 (0.25) | |
| Women | 1.09 (0.19) | 1.02 (0.20) | 0.98 (0.22) | 1.02 (0.24) | 0.98 (0.29) | b |
| SPPB Score | ||||||
| Men | 11.67 (0.65) | 11.33 (1.16) | 11.00 (1.98) | 11.06 (1.83) | 11.05 (1.53) | |
| Women | 10.51 (1.93) | 10.25 (1.82) | 10.17 (2.28) | 10.41 (2.15) | 9.88 (2.96) | |
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | ||
| C-reactive protein, mg/L | 1.99 (1.20-4.46) | 2.35 (1.13-4.65) | 2.39 (1.32-4.98) | 2.74 (1.38-5.61) | 2.46 (1.24-6.39) | c |
| Interleukin-6, pg/mL | 1.26 (0.82-1.85) | 1.15 (0.75-1.86) | 1.37 (0.85-2.00) | 1.47 (0.78-2.12) | 1.50 (0.95-2.52) | |
| Tumor necrosis factor-α, pg/mL | 4.23 (3.03-5.54) | 4.78 (3.29-6.15) | 4.57 (3.46-5.90) | 4.19 (3.06-5.91) | 4.68 (3.52-5.99) | a, b, d |
Values are shown in mean (SD) for normally distributed and medians (IQR) for non-normally distributed continuous variables and N (%) for categorical variables.
Pair-wise comparison is made against true mid-range sleepers, P < 0.05. Comparisons across groups were examined with Chi-square test for categorical variables, Kruskal-Wallis test for skewed continuous variables and ANOVA post hoc test for normally distributed continuous variables.
SPPB, Short Physical Performance Battery.
Table 5 shows annual decline in walking speed and SPPB by sleep/rest behavior groups. In age- and sex-adjusted models, walking speed decline was lowest in the true mid-range sleepers (−0.016 m/s per year) and highest in the long sleepers (−0.031 m/s per year). After adjusting for lifestyle factors, sleeping pill use, waist circumference, diseases, and inflammatory markers, walking speed decline was significantly greater in all groups compared to true mid-range sleepers (P for all ≤ 0.05). There were no other significant differences between sleep/rest behavior groups except for the comparisons with true mid-range sleepers. In the fully adjusted model, in addition to sleep/rest behavior, age, sex, education, physical activity, use of sleeping pills, diabetes, waist circumference, and depressive symptoms remained independently associated with walking speed decline (P for all < 0.05).
Table 5.
Annual change in walking speed and Short Physical Performance Battery by sleep/rest behavior groups
| True mid-range sleepers (TST 7-8 h and TIB 7-8 h) | True short sleepers (TST ≤ 6 h and TIB ≤ 8 h) | Subjective short sleepers (TST ≤ 6 h and TIB ≥ 9 h) | Subjective mid-range sleepers (TST 7-8 h and TIB ≥ 9 h) | Long sleepers (TST ≥ 9 h and TIB ≥ 9 h) | |
|---|---|---|---|---|---|
| β Estimate* (SE) | β Estimate* (SE) | β Estimate* (SE) | β Estimate* (SE) | β Estimate* (SE) | |
| Walking Speed | |||||
| Model 1 | −0.016 (0.003) | −0.023 (0.003) | −0.025 (0.003) | −0.026 (0.002) | -0.031 (0.005) |
| P† | ref | 0.077 | 0.043 | 0.015 | 0.006 |
| Model 2 | −0.014 (0.003) | −0.024 (0.003) | −0.023 (0.003) | −0.026 (0.003) | -0.032 (0.005) |
| P† | ref | 0.023 | 0.050 | 0.006 | 0.002 |
| Short Physical Performance Battery | |||||
| Model 1 | −0.160 (0.030) | −0.220 (0.030) | −0.380 (0.030) | −0.310 (0.030) | −0.350 (0.050) |
| P† | ref | 0.164 | < 0.0001 | 0.001 | 0.001 |
| Model 2 | −0.155 (0.035) | −0.218 (0.031) | −0.365 (0.033) | −0.308 (0.028) | −0.335 (0.050) |
| P† | ref | 0.182 | < 0.0001 | 0.001 | 0.003 |
Beta estimates for different sleep/rest groups are derived from linear mixed effect models and they indicate the average change in walking speed/SPPB score per year in that specific category. For the comparison the decline in walking speed and SPPB score with 1 year of advancing age in the whole study population was −0.021 and −0.198, respectively.
P value indicates the significance of the difference between sleep/rest groups.
SE, standard error.
Model 1: Adjusted for age and sex. Model 2: Additionally adjusted for education, physical activity, smoking, alcohol use, sleeping pills use, hypertension, coronary heart disease, diabetes, waist circumference, depression, C-reactive protein, interleukin = 6, and tumor necrosis factor-α.
The results for the SPPB were nearly comparable with walking speed, the decline being lowest in true mid-range sleepers and greatest in those participants reporting TIB ≥ 9 h independent of reported sleep duration (subjective short sleepers, subjective mid-range sleepers, and long sleepers). These same participants had significantly greater decline in SPPB scores compared to true mid-range sleepers and true short sleepers (P for all < 0.05). After including all the confounding variables in the model, in addition to sleep/rest behavior, age, sex, physical activity, and depressive symptoms remained independently associated with SPPB decline (P for all < 0.05).
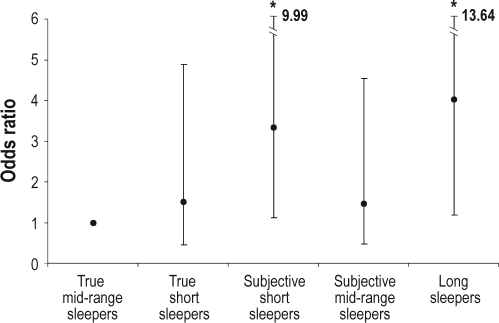
Finally, we examined the predictive role of sleep/rest behavior on risk of developing mobility disability over 6 years of follow-up (Figure 1). After adjusting for potential confounding factors, the odds for incident mobility disability were 1.50 (95% CL 0.46-4.89) for true short sleepers; 3.33 (95% CL 1.11-9.99) for subjective short sleepers; 1.47 (95% CL 0.48-4.55) for subjective mid-range sleepers; and 4.02 (95% CI 1.18-13.64) for long sleepers in comparison to true mid-range sleepers.
Figure 1.
Risk of developing mobility disability over 6 years of follow-up by sleep/rest behavior groups. Logistic regression models are adjusted for age, sex, education, physical activity, smoking, alcohol use, sleeping pills use, hypertension, coronary heart disease, diabetes, waist circumference, depression, C-reactive protein, interleukin-6, and tumor necrosis factor-α. *Statistically significant difference compared to true mid-range sleeper, P < 0.05.
DISCUSSION
The results of this study provide new evidence in the field of epidemiological sleep research by demonstrating that TIB offers important additive information to the self-reported habitual sleep duration when evaluating the consequences of sleep behavior on physical function decline. We found that both long (≥ 9 h) TST and long TIB independently predicted accelerated decline in objectively measured physical performance (walking speed and SPPB score) and greater incidence in subjectively assessed mobility disability, but short (≤ 6 h) TST and short TIB did not. After combining self-reported TST and TIB, we observed that long sleepers (TST ≥ 9 h and TIB ≥ 9 h), experience the greatest decline in physical performance and have the highest risk for incident mobility disability compared to true mid-range sleepers (TST 7-8 h and TIB 7-8 h). Importantly, we found that the level of physical performance decline and incident mobility disability varied between the persons reporting equal sleep durations but differing in their TIB. For example, subjective short sleepers (TST ≤ 6 h and TIB ≥ 9 h) showed a significantly greater decline in SPPB score and had higher risk of incident mobility disability than true short sleepers (TST ≤ 6 h and TIB ≤ 8 h). Consequently, by relying solely on self-reported sleep duration, an additive risk related to long TIB among persons reporting short sleep would be partially masked.
To our knowledge this is the first study to combine self-reported TST and TIB when examining their association with physical function decline in older adults. Based on this study two critical groups in terms of functional decline can be distinguished: long sleepers and subjective short sleepers. In this study, long sleepers were more often older, but did not report on average more chronic conditions or sleeping pill use when compared with true mid-range sleepers. On the other hand, subjective short sleepers constituted of group of persons, who more often used sleeping pills and more often had hypertension and depression in comparison to the reference group. A common feature for these two groups was a physically sedentary behavior. It can be argued that persons with long TIB may have less time (or energy) to engage in physically active behavior. Indeed, several studies have found that long self-reported sleep duration is associated with a sedentary way of life or low levels of daytime physical activity,10,27,28 suggesting that sedentary lifestyle may account for part of the increased risks for physical function decline in this group. Since there were no major differences in the physical performance at baseline across the groups, low physical performance alone is an unlikely explanation for lower physical activity in persons with long TIB. Moreover, in experimental studies, extended bed rest, irrespective of sleeping, is shown to accompany a marked decrease in muscle strength29 as well as insulin resistance and cardiac atrophy,30 linking long TIB to low physical performance. The key question is whether long TIB is only a proxy for sedentary lifestyle or if it could have a direct causal effect on physical activity behavior. Further research is needed to examine factors characterizing persons with long TIB but different TST and their role in physical functional decline.
There are few potential explanations for increased risk of physical function decline among long sleepers in our study. In addition to sedentary or passive lifestyle,28 the most obvious ones are sleep fragmentation, fatigue, and underlying disease.3 It has been reported that in terms of tiredness and fatigue, long sleepers do not benefit from their longer sleep duration when compared with mid-range sleepers or even with short sleepers.10 This may indicate that self-reported long TST is associated with the failure of the restorative function(s) of sleep.9 Additionally, the key problem in this context is the uncertainty about how indicative long TST is of physiologic sleep or TIB.31
Accumulating data from population-based studies indicates that persons tend to overestimate their sleep time when compared with objectively measured sleep duration.32–34 Importantly, the overestimation may depend on the quality of sleep. Objectively defined poor sleep was found to be associated with longer subjective estimates of TST, whereas subjectively poor sleepers tended to report shorter TST when compared with objective measurements.34 This may indicate that among self-reported long sleepers, objective changes in physiological sleep structure may be more prominent than subjectively reported sleep disturbances. We are inclined to think that our differentiation between subjective and true short and mid-range sleepers reflects the underlying failure in sleep function, in response to which a substantially longer TIB (as a compensatory effort to restore sleep homeostasis) is associated with shorter subjective sleep duration. In a recent study among 35 self-reported long sleepers aged 50-70 years, it was found that they reported much longer sleep durations than was verified by actigraphic measurements.31 The important conclusion was that because self-reported long sleepers sleep physiologically less than they claim, the increased health risks in this group may be related more to long TIB than to long physiological sleep duration in itself.31 Thus, the main findings of our study may be interpreted in a way that failures in restorative sleep function(s) associate with long TST and long TIB, which, in turn, predispose individuals to sedentary life style, which, in turn, predispose individuals to decrease in physical function and incident mobility disability.
Another interesting finding from this study is that unlike subjective short sleepers, true short sleepers (TST ≤ 6 h and TIB ≤ 8 h) do not show significantly greater decline in SPPB or incidence for self-reported mobility disability than true mid-range sleepers. This result suggests that the functional integrity of sleep may be compromised in subjective short sleepers, possibly due to insomnia or other sleep disorders; the sleeper then tries to compensate for this by spending more time in bed. In other words, the discrepancy between TST and TIB reflects behavioral response to the perception of the quality of sleep and probably also to the sedentary lifestyle. On the contrary in true short sleepers, the functional integrity of sleep may be compromised to lesser degree or not at all. Taken together, our results emphasize the importance of the discrepancy between self-reported sleep duration and calculated TIB when predicting physical function decline. Consequently, in epidemiological studies, it is important to include both self-reported TST and the time of getting into and out of the bed for calculating the estimate of TIB in order to yield maximally sensitive information to predict future health risks.
In this study, three different outcome variables were used: objectively measured lower extremity performance in terms of walking speed and SPPB as well as self-reported mobility disability. Some discrepancies in the findings between walking speed and SPPB were observed. True short sleepers had significantly greater decline in walking speed compared to true mid-range sleepers, but this difference was not observable with SPPB. On the other hand, subjective short sleepers experienced greater decline in SPPB, but not so much in walking speed compared to true mid-range sleepers. Similarly, risk of incident mobility disability was increased in subjective short sleepers but not in true short sleepers. The same pattern was observed in subcomponents of SPPB, chair stand, and standing balance. One plausible explanation for these findings is that walking speed is more sensitive for changes in physical performance, and greater changes in performance would be required to observe differences in SPPB score or mobility status across our sleep/rest behavior groups. Therefore, true short sleepers also seem to show accelerated decline in physical performance, but it is only observable with walking speed as an outcome measure.
Based on previous research the size of the differences observed between sleep/rest behavior groups in the present study can be considered clinically meaningful. For example, the difference in annual decline in walking speed between true mid-range sleepers (−0.014 m/s) and long sleepers (−0.032 m/s) corresponds to a 0.11 m/s change over 6 years, when change of 0.08 m/s or more has been considered substantial meaningful change.35,36 Similarly, the difference in annual decline in SPPB score between true mid-range sleepers (−0.155) and long sleepers (−0.335) corresponds to a 1.08 point change over 6 years, when change of 1.0 points or more has been considered substantial meaningful change.35,36
The major strength of this study is that we had concurrent dual information about sleep behavior, and by utilizing the information from the self-reported TST and TIB, we were able to constitute sleep/rest behavior grouping. Previous studies have reported that long sleep is associated with poor functioning and health,2,3,5 and there is some evidence that short sleep duration is associated with higher likelihood of mobility disability,6 hypertension,37,38 diabetes,39 and mortality.8 The fundamental problem in interpreting the findings of these studies is that they may partially mask the real risk associated with the sleep/rest behavior. Practically all epidemiological studies on self-reported sleep duration associated with health risks have defined the reference group solely by self-reported 7-8 h sleep duration. Consequently, true and subjective mid-range sleepers as well as true and subjective short sleepers have been pooled together, possibly leading to biased risk estimates. Therefore, to obtain as comprehensive a picture of sleep behavior as possible, it is recommended to include questions about TIB in addition to self-reported sleep duration in future studies or inquire about it in the clinical practice. In addition, further studies are needed to define if the distinction between subjective TST and TIB is also important in regards to risk for adverse health and mortality.
Another strength of this study is the age of the target group. From the point of view of physical function decline, persons aged 65 years and older create a feasible target group. Furthermore, since the majority of our participants were retired (89%), they had fewer social restrictions in choosing individual TIB than in individuals still working. Therefore, we believe that TIB in this study reflects each participant's needs in their homeostatic sleep regulation to a much stronger extent than in younger individuals.
The limitations of the study also need to be addressed. Although the longitudinal design with repeated walking speed and SPPB measurement is one of its major strengths, longitudinal studies in older adults, especially those with long follow-up, also produce natural limitations. Compared to the 751 participants included in the longitudinal analysis, those who were lost to follow-up (n = 172) were older (P < 0.0001), had lower education (P < 0.0001), were more often physically sedentary (P < 0.0001), and had more coronary heart disease (P = 0.03), hypertension (P = 0.04), and knee osteoarthritis (P = 0.02). In addition, they had significantly lower walking speed (0.87 vs. 1.08 m/s) and SPPB score (8.29 vs. 10.67) at baseline (P for both < 0.0001) compared to those who remained in the study. Thus, the oldest and most disabled persons were lost to follow-up, and this may have weakened our findings.
Second, TST was based on self-reports, which leaves open the question of its relationship to physiological sleep duration. However, it has been shown that among middle-aged adults, subjective reports of habitual sleep moderately correlate with actigraph-measured sleep.33 Future studies are needed to confirm our sleep/rest classification and its association with physical function decline by using objective instruments. Also, since the aim of this study was to examine the possible predictive role of sleep/rest behavior on physical function decline, only data from the baseline sleep/rest behavior were utilized. Based on additional analysis, we observed small changes in long and short sleep based on TST and TIB at 3 and 6 years of follow-up, and the prevalence of sleep/rest groups varied also in each data point, although relatively slightly. If self-reported TST and TIB are direct causal risk factors for physical function decline, the changes in their prevalence during the follow-up would have resulted in a slight underestimation of their real risk estimates. If they are only indirect or noncausal (surrogates) statistical risk factors, it is more difficult to estimate how changes in their prevalence may have influenced the results.
Finally, in the present study several confounding factors were included in the multivariable models, including sleeping pill use, which can be considered as an indicator of insomnia. However, we did not have information on other primary sleep disorders such as sleep apnea or restless legs, which can be considered as a limitation of our study. However, we feel that the sleep/rest grouping based on the combination of self-reported sleep duration and TIB creates in integrative indicator of the functional integrity of sleep, which, in turn, is an important health predictor in epidemiological studies. Future studies are needed to decipher different causal mechanisms behind this sleep/rest typology
In conclusion, our study confirms that information on TIB complements self-reported sleep duration in evaluating the consequences of sleep duration on health and functional status. The biological mechanisms leading from different sleep/rest behavior to functional decline and also to other health outcomes should be further studied.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.M
ACKNOWLEDGMENTS
The InCHIANTI study baseline (1998-2000) was supported as a “targeted project” (ICS110.1/RF97.71) by the Italian Ministry of Health and in part by the U.S. National Institute on Aging (Contracts: 263 MD 9164 and 263 MD 821336); the InCHIANTI Follow-up 1 (2001-2003) was funded by the U.S. National Institute on Aging (Contracts: N.1-AG-1-1 and N.1-AG-1-2111); the InCHIANTI Follow-up 2 study (2004-2006) was financed by the U.S. National Institute on Aging (Contract: N01-AG-5-0002); supported in part by the Intramural research program of the National Institute on Aging, National Institutes of Health, Baltimore, Maryland. The authors have no financial or personal conflicts of interest. None of the sponsoring institutions interfered with the design, methods, subject recruitment, data collections, analysis and preparation of paper.
REFERENCES
- 1.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–92. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Youngstedt SD, Kripke DF. Long sleep and mortality: rationale for sleep restriction. Sleep Med Rev. 2004;8:159–74. doi: 10.1016/j.smrv.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Grandner MA, Drummond SP. Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep Med Rev. 2007;11:341–60. doi: 10.1016/j.smrv.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res. 2009;18:148–58. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 5.Goldman SE, Stone KL, Ancoli-Israel S, et al. Poor sleep is associated with poorer physical performance and greater functional limitations in older women. Sleep. 2007;30:1317–24. doi: 10.1093/sleep/30.10.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stenholm S, Kronholm E, Sainio P, et al. Sleep-related factors and mobility in older men and women. J Gerontol A Biol Sci Med Sci. 2010;65:649–57. doi: 10.1093/gerona/glq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dam TT, Ewing S, Ancoli-Israel S, Ensrud K, Redline S, Stone K. Association between sleep and physical function in older men: the osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2008;56:1665–73. doi: 10.1111/j.1532-5415.2008.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration associated with mortality in elderly, but not middle-aged, adults in a large US sample. Sleep. 2008;31:1087–96. [PMC free article] [PubMed] [Google Scholar]
- 9.Kronholm E, Sallinen M, Suutama T, Sulkava R, Era P, Partonen T. Self-reported sleep duration and cognitive functioning in the general population. J Sleep Res. 2009;18:436–46. doi: 10.1111/j.1365-2869.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 10.Kronholm E, Harma M, Hublin C, Aro AR, Partonen T. Self-reported sleep duration in Finnish general population. J Sleep Res. 2006;15:276–90. doi: 10.1111/j.1365-2869.2006.00543.x. [DOI] [PubMed] [Google Scholar]
- 11.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–25. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 12.Chami H, Gottlieb D. Sleep duration and cardiovascular health. Int J Sleep Wakefulness. 2008;1:156–65. [Google Scholar]
- 13.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower Extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55A:M221–31. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people - results from the health, aging and body composition study. J Am Geriatr Soc. 2005;53:1675–80. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 16.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 17.Ostir GV, Volpato S, Fried LP, Chaves P, Guralnik JM. Reliability and sensitivity to change assessed for a summary measure of lower body function: results from the Women's Health and Aging Study. J Clin Epidemiol. 2002;55:916–21. doi: 10.1016/s0895-4356(02)00436-5. [DOI] [PubMed] [Google Scholar]
- 18.Reuben DB, Seeman TE, Keeler E, et al. Refining the categorization of physical functional status: The added value of combining self-reported and performance-based measures. J Gerontol A Biol Sci Med Sci. 2004;59A:M1050–61. doi: 10.1093/gerona/59.10.m1056. [DOI] [PubMed] [Google Scholar]
- 19.Wareham NJ, Jakes RW, Rennie KL, Mitchell J, Hennings S, Day NE. Validity and repeatability of the EPIC-Norfolk Physical Activity Questionnaire. Int J Epidemiol. 2002;31:168–74. doi: 10.1093/ije/31.1.168. [DOI] [PubMed] [Google Scholar]
- 20.Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol. 1997;26(Suppl 1):S152–60. doi: 10.1093/ije/26.suppl_1.s152. [DOI] [PubMed] [Google Scholar]
- 21.Guralnik J, Fried L, Simonsick E, Kasper J, Lafferty M. Bethesda, MD: National Institute on Aging; 1995. The Women's Health and Aging Study: health and social characteristics of older women with disability. NIH Publication No. 95-4009. [Google Scholar]
- 22.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 23.Beekman AT, Deeg DJ, Van Limbeek J, Braam AW, De Vries MZ, Van Tilburg W. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): results from a community-based sample of older subjects in The Netherlands. Psychol Med. 1997;27:231–5. doi: 10.1017/s0033291796003510. [DOI] [PubMed] [Google Scholar]
- 24.Fava GA. Assessing depressive symptoms across cultures: Italian validation of the CES-D self-rating scale. J Clin Psychol. 1983;39:249–51. doi: 10.1002/1097-4679(198303)39:2<249::aid-jclp2270390218>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 25.Verbeke G, Molenberghs G. Linear mixed models for longitudinal data. 2nd ed. New York: Springer-Verlag; 2001. [Google Scholar]
- 26.Edwards LJ. Modern statistical techniques for the analysis of longitudinal data in biomedical research. Pediatr Pulmonol. 2000;30:330–44. doi: 10.1002/1099-0496(200010)30:4<330::aid-ppul10>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 27.Basner M, Fomberstein K, Razavi F, et al. American time use survey: Sleep time and its relationship to waking activities. Sleep. 2007;30:1085–95. doi: 10.1093/sleep/30.9.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgan K. Long sleep duration and all cause mortality in later life: a possible consequence of sedentary death syndrome. Sleep. 2007;30:A115. [Google Scholar]
- 29.Evans WJ. Skeletal muscle loss: cachexia, sarcopenia, and inactivity. Am J Clin Nutr. 2010;91:1123S–7S. doi: 10.3945/ajcn.2010.28608A. [DOI] [PubMed] [Google Scholar]
- 30.Hamburg NM, McMackin CJ, Huang AL, et al. Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler Thromb Vasc Biol. 2007;27:2650–6. doi: 10.1161/ATVBAHA.107.153288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kline CE, Zielinski MR, Devlin TM, Kripke DF, Bogan RK, Youngstedt SD. Self-reported long sleep in older adults is closely related to objective time in bed. Sleep Biol Rhythms. 2009;8:42–51. doi: 10.1111/j.1479-8425.2009.00422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva GE, Goodwin JL, Sherill D, et al. Relationship between reported and measured sleep times: The Sleep Heart Health Study (SHHS) J Clin Sleep Med. 2007;3:622–30. [PMC free article] [PubMed] [Google Scholar]
- 33.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19:838–45. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Den Berg JF, Van Rooij FJA, Vos H, et al. Disagreement between subjective and actigraphic measures of sleep duration in a population-based study of elderly persons. J Sleep Res. 2008;17:295–302. doi: 10.1111/j.1365-2869.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- 35.Kwon S, Perera S, Pahor M, et al. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study) J Nutr Health Aging. 2009;13:538–44. doi: 10.1007/s12603-009-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–9. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 37.Stranges S, Dorn JM, Cappuccio FP, et al. A population-based study of reduced sleep duration and hypertension: the strongest association may be in premenopausal women. J Hypertens. 2010;28:896–902. doi: 10.1097/HJH.0b013e328335d076. [DOI] [PubMed] [Google Scholar]
- 38.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 39.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2007;30:1667–73. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]