Abstract
Study Objectives:
Obstructive sleep apnea (OSA) is known as a major cardiovascular risk factor, and high prevalence of OSA has been reported in patients with thoracic aortic dissection. The aim of our study was to assess the relationship between OSA, its vascular consequences, and aortic root size.
Design/Patients:
156 newly diagnosed apneic patients free of cardiovascular disease and medication were included. Patients underwent cardiac ultrasound for measuring aortic root diameter, polysomnography, office and 24-h ambulatory blood pressure (BP) measurements, baroreflex sensitivity (BRS), and arterial stiffness evaluation by carotid-to-femoral pulse wave velocity (PWV).
Measurements and Results:
In univariate analysis, greater aortic root size was associated with older age (P = 0.03) and severity of OSA as expressed by mean nocturnal oxygen saturation (SpO2) (P = 0.015). Moreover, greater aortic root size was associated with higher diastolic BP, measured both clinically (P = 0.0005) or by 24-h ambulatory BP monitoring (P = 0.02), and PWV (P = 0.03). Mean nocturnal SpO2 was correlated with BRS (P = 0.0008), thus potentially influencing BP values and arterial stiffness. In multivariate stepwise regression analysis, diastolic BP was the only significant factor for aortic root size (P = 0.0003).
Conclusions:
In OSA patients, nocturnal hypoxemia decreased BRS and increased diastolic BP, which was the main factor influencing aortic root size.
Citation:
Baguet JP; Minville C; Ramisier R; Roche F; Barone-Rochette G; Ormezzano O; Levy P; Pepin JL. Increased aortic root size is associated with nocturnal hypoxia and diastolic blood pressure in obstructive sleep apnea. SLEEP 2011;34(11):1605-1607.
Keywords: Aorta, arterial stiffness, blood pressure, hypoxia, obstructive sleep apnea
In the September 2010 issue of SLEEP, Lee et al. reported a substudy on thoracic aorta size in patients included in a prospective study on obstructive sleep apnea (OSA) in patients referred for acute myocardial infarction (MI).1 They found a positive correlation between thoracic aortic size and older age, body mass index (BMI) and hypertension. Severity of OSA as defined by apnea-hypopnea index (AHI) was a significant factor in univariate analysis. Although interesting, this study has some limitations that must be considered, the main one being high rate of comorbidities and acute phase of MI at inclusion.
We have addressed a similar question but in a prospective cohort of OSA patients without metabolic and cardiovascular comorbidities (i.e., without confounders). One hundred fifty-six newly diagnosed, otherwise healthy, overweight OSA patients (mean BMI: 27 ± 3 kg/m2) were recruited. OSA was defined by AHI > 10/h of sleep. Aortic root diameter was measured by ultrasound at Valsalva sinus. Patients underwent clinical blood pressure (BP) measurements, 24-h ambulatory BP monitoring (ABPM), baroreflex sensitivity (BRS), and carotid-to-femoral pulse wave velocity (PWV) measurements. The relationships between these parameters and aortic root size were examined in univariate analysis. Then, a multivariate stepwise regression was done including OSA severity parameters, age, systolic and diastolic BP (both clinical and ABPM values), PWV, and BRS. The data are presented as mean ± SD or frequencies.
Patients' mean age was 49 ± 10 y; 136 (87%) were men and their mean BMI was 27 ± 3 kg/m2. Mean systolic/diastolic BP measured by ABPM was 123 ± 12/80 ± 7 mmHg. Patients exhibited moderate-to-severe OSA with a mean AHI of 42 ± 18/h and a mean Epworth Sleepiness Scale score of 9.7 ± 5.2. They were free of known cardiovascular disease and vasoactive medication.
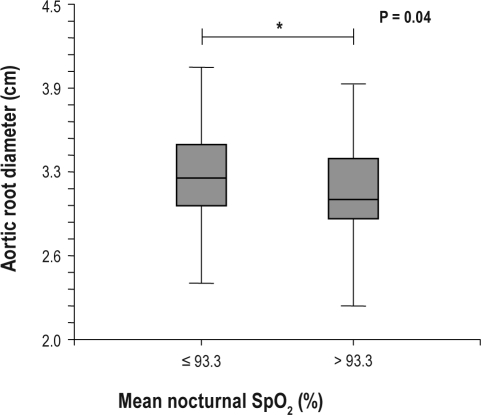
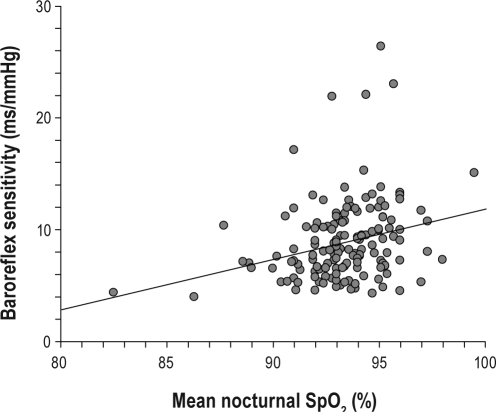
In univariate analysis, greater aortic root size was associated with older age (P = 0.03) and severity of OSA as expressed by mean nocturnal SpO2 (rhospearman = −0.20; P = 0.015; Figure 1A), whereas AHI was not. Moreover, greater aortic root size was associated with higher diastolic BP, both measured by clinic (P = 0.0005) or 24-h ABPM (P = 0.02), and PWV (P = 0.03). Mean nocturnal SpO2 was correlated with BRS (rhospearman = 0.28; P = 0.0008; Figure 1B), thus potentially influencing BP values and arterial stiffness. This association was significant, but mean nocturnal SpO2 only explained 8% of the BRS variance. In multivariate stepwise regression analysis, diastolic BP was the only significant factor for aortic root size (P = 0.0003).
Figure 1A.
Patients were separated by the median value of mean nocturnal SpO2. Aortic root size was increased in those exhibiting the most severe nocturnal hypoxemia.
Figure 1B.
Correlation between BRS and mean nocturnal SpO2 showing significant decrease in BRS in patients with more severe hypoxia
In comparison with the cohort of Lee et al.,1 our OSA patients were younger (49 vs 54 years) and were mainly Caucasians. Women were included in our study while excluded from the study by Lee et al.,1 but they represented only 13% of the group. Mean aortic root diameter was equivalent between our cohort and that of the Lee et al.1 study (31.6 ± 3.8 vs 31.2 ± 3.4 mm, respectively). We report on a larger group (156 vs 94) of otherwise healthy OSA patients without cardiovascular history or medication. This represents a major difference when comparing to the patients of Lee et al.1 which were included in post acute MI period and had multiple comorbidities, including hyperlipidemia (84%), diabetes mellitus (39%), and hypertension (51%), representing multiple possible confounding factors. Importantly, 46% of their patients used antihypertensive medication prior to admission, and probably all patients had vasoactive medication after MI (i.e., when polysomnography, echocardiography, and BP measurements were done). Moreover, only clinic BP was taken and measurement conditions are not specified (timing after MI, number of measures). This high hypertension prevalence was also a limitation in the report of Serizawa et al., in which 59% of patients were hypertensive, and it was unclear how many suffered from coronary heart disease and diabetes.2 In comparison, all our patients had 24-h ABPM in addition to clinic BP measurements. Moreover, all echocardiographic examinations were done by the same cardiologist, assuring standardisation of the data. This methodological aspect was not detailed in the paper by Lee et al.1 However, the findings of Lee et al.1 and our results are not contradictory; both show that OSA severity (measured by mean SpO2 or AHI) is associated with aortic root size in “healthy” subjects as well as subjects with multiple comorbidities, even at the time of an MI. However, in both studies OSA was not related to aortic size after adjustment of other covariates.
Owing to the absence of comorbidities, our cohort demonstrates more convincingly than previous studies a significant relationship between aortic root size, nocturnal oxygen desaturations, and diastolic BP. Moreover, our set of subjects was cautiously characterized in terms of vascular phenotype by clinical BP, 24-h ABPM, and arterial stiffness, thus allowing hypothesizing on the underlying mechanisms. Oxygen desaturations induced increased sympathetic activation that may have raised arterial stiffness and blunted BRS, both of which may contribute to the elevation of BP.3 Hence, we showed a decrease in BRS related to nocturnal hypoxemia. This altered BRS probably favored the occurrence of diastolic hypertension, which is the most frequent pattern of hypertension in the early evolution of OSA patients.4 Accordingly, in our population of OSA patients, diastolic BP was the main determinant of aortic root size. We5 and others6 have previously described an increased carotid diameter in patients with OSA, which is in accordance with an enlargement in aortic size.
From a clinical point of view, our results raise the question of a systematic OSA screening in patients with increased aortic thoracic diameter. It also raises the question whether optimal treatment of OSA episodes, by reducing diastolic BP, correcting hypoxemia and avoiding important negative intrathoracic pressures, could then protect against a continuous development of aortic dilatation. This “protecting effect” of OSA treatment remains to be established in further studies.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENT
This work was supported by a grant from the Regional Research Council (PHRC 2006, Grenoble University Hospital, France). These funding sources had no involvement in the collection, analysis, interpretation of data, writing the report or in the decision to submit the paper for publication.
Abbreviations
- OSA
Obstructive sleep apnea
- MI
Myocardial infarction
- BMI
Body mass index
- AHI
Apnea-hypopnea index
- ABPM
Ambulatory blood pressure monitoring
- BRS
Baroreflex sensitivity
- PWV
Pulse wave velocity
- BP
Blood pressure
- SpO2
Oxygen saturation
REFERENCES
- 1.Lee LC, Torres MC, Khoo SM, et al. The relative impact of obstructive sleep apnea and hypertension on the structural and functional changes of the thoracic aorta. Sleep. 2010;33:1173–6. doi: 10.1093/sleep/33.9.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serizawa N, Yumino D, Takagi A, et al. Obstructive sleep apnea is associated with greater thoracic aortic size. J Am Coll Cardiol. 2008;52:885–6. doi: 10.1016/j.jacc.2008.05.039. [DOI] [PubMed] [Google Scholar]
- 3.Kohler M, Pepperell JC, Casadei B, et al. CPAP and measures of cardiovascular risk in males with OSAS. Eur Respir J. 2008;32:1488–96. doi: 10.1183/09031936.00026608. [DOI] [PubMed] [Google Scholar]
- 4.Baguet JP, Hammer L, Levy P, et al. Night-time and diastolic hypertension are common and underestimated conditions in newly diagnosed apnoeic patients. J Hypertens. 2005;23:521–7. doi: 10.1097/01.hjh.0000160207.58781.4e. [DOI] [PubMed] [Google Scholar]
- 5.Lefebvre B, Pepin JL, Baguet JP, et al. Leukotriene B4: early mediator of atherosclerosis in obstructive sleep apnoea? Eur Respir J. 2008;32:113–20. doi: 10.1183/09031936.00137107. [DOI] [PubMed] [Google Scholar]
- 6.Drager LF, Bortolotto LA, Lorenzi MC, Figueiredo AC, Krieger EM, Lorenzi-Filho G. Early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:613–8. doi: 10.1164/rccm.200503-340OC. [DOI] [PubMed] [Google Scholar]