Abstract
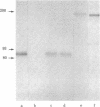
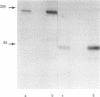
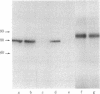
A murine monoclonal antibody (OKT9) raised against human leukemic cells binds to a wide variety of leukemia and tumor cell lines and to a minority of leukemia cells taken directly from patients. Fetal thymus and liver are strongly reactive as are some normal, immature hemopoietic cells and activated lymphocytes. Reactivity with OKT9 appears to correlate with proliferation status in both normal and malignant populations. Biochemical analysis indicates that this structure is a approximately equal to 180,000-dalton glycoprotein with two disulfide-bonded subunits of approximately equal to 90,000-daltons. Isolation of the transferrin receptor from a T-cell line (MOLT-4) indicates that it also has a dimeric approximately equal to 180,000-dalton structure. Radio-labeled transferrin bound to its receptors can be specifically precipitated by the monoclonal OKT9, although the latter does not bind transferrin itself, indicating that the antigenic structure defined by this antibody is likely to be the transferrin receptor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boss M. A., Delia D., Robinson J. B., Greaves M. F. Differentiation-linked expression of cell surface markers on HL-60 leukemic cells. Blood. 1980 Nov;56(5):910–916. [PubMed] [Google Scholar]
- Bramwell M. E., Harris H. An abnormal membrane glycoprotein associated with malignancy in a wide range of different tumours. Proc R Soc Lond B Biol Sci. 1978 Apr 13;201(1142):87–106. doi: 10.1098/rspb.1978.0034. [DOI] [PubMed] [Google Scholar]
- Bramwell M. E., Harris H. Some further information about the abnormal membrane glycoprotein associated with malignancy. Proc R Soc Lond B Biol Sci. 1978 Nov 20;203(1150):93–99. doi: 10.1098/rspb.1978.0094. [DOI] [PubMed] [Google Scholar]
- Breard J., Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody reactive with human peripheral blood monocytes. J Immunol. 1980 Apr;124(4):1943–1948. [PubMed] [Google Scholar]
- Brodsky F. M., Parham P., Barnstable C. J., Crumpton M. J., Bodmer W. F. Monoclonal antibodies for analysis of the HLA system. Immunol Rev. 1979;47:3–61. doi: 10.1111/j.1600-065x.1979.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dippold W. G., Lloyd K. O., Li L. T., Ikeda H., Oettgen H. F., Old L. J. Cell surface antigens of human malignant melanoma: definition of six antigenic systems with mouse monoclonal antibodies. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6114–6118. doi: 10.1073/pnas.77.10.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Pol J. A., Klos D. J. Isolation and characterization of normal rat kidney cell membrane proteins with affinity for transferrin. Biochemistry. 1980 Aug 19;19(17):3904–3912. doi: 10.1021/bi00558a003. [DOI] [PubMed] [Google Scholar]
- Galbraith G. M., Galbraith R. M., Faulk W. P. Transferrin binding by human lymphoblastoid cell lines and other transformed cells. Cell Immunol. 1980 Jan;49(1):215–222. doi: 10.1016/0008-8749(80)90072-6. [DOI] [PubMed] [Google Scholar]
- Galbraith G. M., Galbraith R. M., Temple A., Faulk W. P. Demonstration of transferrin receptors on human placental trophoblast. Blood. 1980 Feb;55(2):240–242. [PubMed] [Google Scholar]
- Gallagher R., Collins S., Trujillo J., McCredie K., Ahearn M., Tsai S., Metzgar R., Aulakh G., Ting R., Ruscetti F. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979 Sep;54(3):713–733. [PubMed] [Google Scholar]
- Hakomori S. Structures and organization of cell surface glycolipids dependency on cell growth and malignant transformation. Biochim Biophys Acta. 1975 Mar 20;417(1):55–89. doi: 10.1016/0304-419x(75)90008-6. [DOI] [PubMed] [Google Scholar]
- Hamilton T. A., Wada H. G., Sussman H. H. Identification of transferrin receptors on the surface of human cultured cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6406–6410. doi: 10.1073/pnas.76.12.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmaplardh D., Morgan E. H. Transferrin and iron uptake by human cells in culture. Exp Cell Res. 1974 Jul;87(1):207–212. doi: 10.1016/0014-4827(74)90543-6. [DOI] [PubMed] [Google Scholar]
- Hu H. Y., Aisen P. Molecular characteristics of the transferrin-receptor complex of the rabbit reticulocyte. J Supramol Struct. 1978;8(3):349–360. doi: 10.1002/jss.400080312. [DOI] [PubMed] [Google Scholar]
- Hutchings S. E., Sato G. H. Growth and maintenance of HeLa cells in serum-free medium supplemented with hormones. Proc Natl Acad Sci U S A. 1978 Feb;75(2):901–904. doi: 10.1073/pnas.75.2.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Cell surface proteins and malignant transformation. Biochim Biophys Acta. 1976 Apr 30;458(1):73–107. doi: 10.1016/0304-419x(76)90015-9. [DOI] [PubMed] [Google Scholar]
- Iscove N. N., Guilbert L. J., Weyman C. Complete replacement of serum in primary cultures of erythropoietin-dependent red cell precursors (CFU-E) by albumin, transferrin, iron, unsaturated fatty acid, lecithin and cholesterol. Exp Cell Res. 1980 Mar;126(1):121–126. doi: 10.1016/0014-4827(80)90476-0. [DOI] [PubMed] [Google Scholar]
- Judd W., Poodry C. A., Strominger J. L. Novel surface antigen expressed on dividing cells but absent from nondividing cells. J Exp Med. 1980 Nov 1;152(5):1430–1435. doi: 10.1084/jem.152.5.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung P. C., Talle M. A., DeMaria M. E., Butler M. S., Lifter J., Goldstein G. Strategies for generating monoclonal antibodies defining human t-lymphocyte differentiation antigens. Transplant Proc. 1980 Sep;12(3 Suppl 1):141–146. [PubMed] [Google Scholar]
- Larrick J. W., Cresswell P. Modulation of cell surface iron transferrin receptors by cellular density and state of activation. J Supramol Struct. 1979;11(4):579–586. doi: 10.1002/jss.400110415. [DOI] [PubMed] [Google Scholar]
- Larrick J. W., Cresswell P. Transferrin receptors on human B and T lymphoblastoid cell lines. Biochim Biophys Acta. 1979 Apr 3;583(4):483–490. doi: 10.1016/0304-4165(79)90065-5. [DOI] [PubMed] [Google Scholar]
- Minowada J., Janossy G., Greaves M. F., Tsubota T., Srivastava B. I., Morikawa S., Tatsumi E. Expression of an antigen associated with acute lymphoblastic leukemia in human leukemia-lymphoma cell lines. J Natl Cancer Inst. 1978 Jun;60(6):1269–1277. doi: 10.1093/jnci/60.6.1269. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Trans-membrane control of the receptors on normal and tumor cells. II. Surface changes associated with transformation and malignancy. Biochim Biophys Acta. 1976 Apr 30;458(1):1–72. doi: 10.1016/0304-419x(76)90014-7. [DOI] [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S., Minowada J. Human cell-surface glycoprotein with unusual properties. Nature. 1980 Aug 28;286(5776):888–891. doi: 10.1038/286888a0. [DOI] [PubMed] [Google Scholar]
- Phillips J. L. Specific binding of zinc transferrin to human lymphocytes. Biochem Biophys Res Commun. 1976 Sep 20;72(2):634–639. doi: 10.1016/s0006-291x(76)80087-3. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman P. A., Schleicher R. B., Allen R. H. Isolation and characterization of the transferrin receptor from human placenta. J Biol Chem. 1979 Oct 25;254(20):9943–9946. [PubMed] [Google Scholar]
- Sullivan A. L., Weintraub L. R. Identification of 125I-labeled rat reticulocyte membrane proteins with affinity for transferrin. Blood. 1978 Aug;52(2):436–446. [PubMed] [Google Scholar]
- Trowbridge I. S., Omary M. B. Human cell surface glycoprotein related to cell proliferation is the receptor for transferrin. Proc Natl Acad Sci U S A. 1981 May;78(5):3039–3043. doi: 10.1073/pnas.78.5.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoef N. J., Noordeloos P. J. Binding of transferrin and uptake of iron by rat erythroid cells in vitro. Clin Sci Mol Med. 1977 Jan;52(1):87–96. doi: 10.1042/cs0520087. [DOI] [PubMed] [Google Scholar]
- Wada H. G., Hass P. E., Sussman H. H. Transferrin receptor in human placental brush border membranes. Studies on the binding of transferrin to placental membrane vesicles and the identification of a placental brush border glycoprotein with high affinity for transferrin. J Biol Chem. 1979 Dec 25;254(24):12629–12635. [PubMed] [Google Scholar]
- Woodbury R. G., Brown J. P., Yeh M. Y., Hellström I., Hellström K. E. Identification of a cell surface protein, p97, in human melanomas and certain other neoplasms. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2183–2187. doi: 10.1073/pnas.77.4.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]