Abstract
Background
Patients with ovarian cancer usually present to a family physician with nonspecific symptoms, most often abdominal pain. The outcome depends above all on the stage of the disease when it is diagnosed and on the quality of treatment.
Methods
This article is based on a review of selected publications from 2000 to 2010 that were retrieved by an automated search in Medline on the terms “ovarian cancer,” “screening,” “diagnosis,” “treatment,” and “prognosis,” as well as the interdisciplinary S2k guideline Diagnostik und Therapie maligner Ovarialtumoren (the diagnosis and treatment of malignant ovarian tumors) issued in 2007 by the Ovarian Tumor Committee of the German Consortium of Gynecologic Oncology (AGO) and the Committee’s updated recommendations of 2009.
Results
The proper treatment of early ovarian cancer involves resection of the primary tumor and all macroscopically visible tumor mass as well as meticulous inspection of the entire abdominal cavity for staging. Platinum-based chemotherapy is indicated for women with ovarian cancer in FIGO stage I to IIA (except stage IA, G1). For women with advanced ovarian cancer, the prognosis largely depends on the extent of tumor mass reduction on initial surgery. Complete resection confers significantly longer survival (median 5 years) than incomplete resection. After surgery, the standard adjuvant chemotherapy consists of a combination of carboplatin and paclitaxel. Treatment that conforms to published guidelines significantly improves survival (60% versus 25% at 3 years).
Conclusion
The possibility of ovarian cancer must be considered for any woman who presents with new, persistent, nonspecific abdominal pain. Ovarian cancer should always be treated in accordance with published guidelines.
Every year in Germany approximately 9600 women develop malignant ovarian tumors. 5500 women die of ovarian cancer every year (1). This makes ovarian cancer the fifth most common cancer among women in Germany, after breast, colorectal, lung, and endometrial cancer, with 4.8% of cases. 70% of cases of ovarian cancer are not diagnosed until the cancer has reached an advanced stage, FIGO Stages IIB to IV (spread of tumor within the pelvis or elsewhere in the abdomen). In these cases, the five-year survival rate is less than 40%. In contrast, the five-year survival rate for tumors diagnosed at early stages, FIGO Stages I to IIA, is much better: more than 80% (2). This makes it very important to provide diagnosis as early as possible. In classifying tumor stages, the FIGO classification corresponds to the TNM classification.
Patients with ovarian cancer have no specific symptoms. Possible symptoms range from diffuse abdominal complaints, newly occurred meteorism, changes in bowel habits, and unexplained weight loss to massive abdominal swelling and usually lead patients to consult a family physician first. As these complaints are fairly nonspecific, early diagnosis is difficult (case illustration). In view of this, it is crucial to patients’ survival that they undergo surgery according to guidelines, with the aim of achieving the maximum possible reduction in tumor size, followed by combined chemotherapy with carboplatin and paclitaxel. Quality of treatment and compliance with treatment standards varies greatly in Germany. This has severe consequences: If treated according to guidelines, more than 60% of patients are still alive after three years, whereas with “suboptimum” treatment the corresponding figure is only 25%. This difference is significant (3). Precisely because clinical symptoms are nonspecific, it is vital for patients that ovarian cancer be considered even by physicians other than gynecologists during differential diagnosis. This article is intended to provide family physicians and other interested colleagues with data that are relevant to everyday practice.
Case Illustration. A 60-year-old patient complains of a bloated feeling, tympanites, and constipation that began three months ago. Ultrasound of the upper abdomen, gastroscopy, and colonoscopy reveal no abnormal findings. Two months later, the patient consults again with massive abdominal swelling. Ultrasound reveals abundant ascites throughout the abdomen. Gynecological examination shows a tumor in the region of the left ovary. Ascites puncture is performed. Cytological examination of the puncture material yields adenocarcinoma cells. A chest X-ray shows a small right-side pleural effusion. Transfer to a gynecological institution is followed by laparotomy. Advanced epithelial ovarian cancer is revealed intraoperatively, with an enlarged left ovary, extensive disseminated peritoneal carcinomatosis, diaphragmatic carcinomatosis, and tumorous thickening of the omentum majus.
In this situation, it is crucial to the patient’s survival that she undergoes surgery according to guidelines, with the aim of achieving the maximum possible reduction in tumor size, followed by combined chemotherapy with carboplatin and paclitaxel.
The article is based on a selective search of the literature using the search terms “ovarian cancer,” “screening,” “diagnosis,” “treatment,” and “prognosis” between 2000 and 2010. The interdisciplinary S2k guideline Diagnostik und Therapie maligner Ovarialtumoren (“The diagnosis and treatment of malignant ovarian tumors”) issued in 2007 by the Ovarian Tumor Committee of the German Consortium of Gynecologic Oncology (AGO, Arbeitsgemeinschaft Gynäkologische Onkologie) for the German Cancer Society (DKG, Deutsche Krebsgesellschaft) and the German Gynecology and Obstetrics Society (DGGG, Deutsche Gesellschaft für Gynäkologie und Geburtshilfe) and the updated recommendations of the AGO’s Ovarian Tumor Committee of 2009 are also cited. The article therefore refers to the most relevant articles about ovarian cancer that had appeared before this publication.
Early detection and screening
As yet there is no approach that justifies a recommendation of general screening for ovarian cancer. The data from two large screening studies have been published to date: the PLCO Study (Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial of the US NIH) and the UKCTOCS Study (United Kingdom Collaborative Trial of Ovarian Cancer Screening). These evaluated regular transvaginal ultrasound and CA 125 testing, including evaluation that involved complex algorithms. However, it has not yet been shown whether these screening methods lead to a reduction in mortality. This must wait until the data from the control group and long-term follow-up are available (4). It is also impossible as yet to use the determination of protein patterns in blood serum or gene expression profiling for early detection.
Genetic risk and prevention
Approximately 10% of ovarian cancers are caused by genetic factors. The most common of these factors are germline mutations in genes BRCA1 or BRCA2. The risk of a woman with a BRCA1 mutation developing ovarian cancer is between 36% and 46%; that of a woman with a BRCA2 mutation is between 10% and 27% (5).
Prophylactic bilateral salpingo-oophorectomy (PBSO) in healthy mutation carriers, after they have completed their families, results in an 80% reduction in the risk of developing ovarian cancer (5). According to Kauff et al., three of 509 women with a BRCA1 or BRCA2 mutation following PBSO developed gynecological cancer, versus 12 of 283 women with a BRCA1 or BRCA2 mutation who had not undergone PBSO (6). However, even after PBSO there is still a risk of approximately 4% of developing primary peritoneal cancer. The peritoneum is ontogenetically related to the ovarian epithelium, and it too has an increased risk of malignant disease (7).
When prophylactic PBSO is performed, complete histological examination must be recommended, as early-stage tumors can be diagnosed in up to 8% of PBSOs (8).
Risk factors for the development of sporadic ovarian cancer are age, obesity, and polycystic ovary syndrome. Ovulation inhibitors have a protective effect (decrease in incidence from 1.2 to 0.8 per 100 users) (9). The effect of hormone replacement therapy (HRT) on the risk of ovarian cancer remains controversial. However, a recently published study showed a 40% increase for HRT users (one additional case of ovarian cancer for every 8300 users), thus corroborating the data of the Women’s Health Initiative. This risk returns to normal within two years of stopping HRT (10).
Diagnosis
Of all imaging procedures used to diagnose ovarian cancer, transvaginal ultrasound is the most valuable in determining whether lesions are benign or malignant. Computed tomography or magnetic resonance imaging may be used in particular cases, e.g. for differential diagnosis between ovarian cancer and a primary gastrointestinal tumor (11). However, both these procedures tend to underestimate peritoneal and mesenteric carcinomatosis, which are common in advanced ovarian cancer. There is currently no apparatus-based diagnostic procedure that can replace surgical staging of ovarian cancer and reliably assess the feasibility of surgery (12).
Factors affecting the prognosis of ovarian cancer
Tumor stage, age, general health, and post-operative residual tumor are independent, significant parameters in the survival prognosis of patients with ovarian cancer.
Mucinous tumors have a significantly worse prognosis than serous papillary and endometrial cancers and respond less favorably to conventional platinum-based combined chemotherapy. Both the risk of recurrence and the risk of a fatal outcome are more than twice as high (13) (table).
Table. Factors in prognosis (13): multivariate Cox regression model for overall survival and progression-free survival.
| Overall survival | Multivariate analysis | ||
| Parameter | Hazard ratio | 95% CI | p-value |
| Age (10 years) | 1.13 | (1.08 to 1.18) | <0.0001 |
| ECOG 2 vs. 0 to 1 | 1.36 | (1.18 to 1.56) | <0.0001 |
| FIGO IIIC to IV vs. IIB to IIIB | 1.45 | (1.28 to 1.65) | <0.0001 |
| Grade 2/3 vs. Grade 1 | 1.74 | (1.37 to 2.21) | <0.0001 |
| Endometroid vs. serous histology | 0.94 | (0.79 to 1.13) | 0.5030 |
| Mucinous vs. serous histology | 2.38 | (1.94 to 2.93) | <0.0001 |
| Residual tumor 1 to 10 mm vs. 0 mm | 2.12 | (1.85 to 2.43) | <0.0001 |
| Residual tumor >10 mm vs. 1 to 10 mm | 1.20 | (1.08 to 1.33) | 0.0006 |
| Ascites >500 mL (intraoperatively) | 1.36 | (1.22 to 1.51) | <0.0001 |
| Progression-free survival | |||
| Age (10 years) | 1.07 | (1.02 to 1.11) | 0.0019 |
| ECOG 2 vs. 0 to 1 | 1.15 | (1.02 to 1.31) | 0.0280 |
| FIGO IIIC to IV vs. IIB to IIIB | 1.46 | (1.31 to 1.63) | <0.0001 |
| Grade 2/3 vs. Grade 1 | 1.66 | (1.36 to 2.01) | <0.0001 |
| Endometroid vs. serous histology | 0.91 | (0.78 to 1.06) | 0.2165 |
| Mucinous vs. serous histology | 2.02 | (1.67 to 2.44) | <0.0001 |
| Residual tumor 1 to 10 mm vs. 0 mm | 2.03 | (1.81 to 2.27) | <0.0001 |
| Residual tumor >10 mm vs. 1 to 10 mm | 1.25 | (1.14 to 1.37) | <0.0001 |
| Ascites >500 mL (intraoperatively) | 1.28 | (1.16 to 1.41) | <0.0001 |
Surgery
Epithelial ovarian cancer is characterized by intraperitoneal tumor extension in the abdomen as a whole, from the small true pelvis to the diaphragm. Lymphogenous dissemination takes place along the ovarian vascular bundles into the paraaortic lymph nodes and over the parametria into the pelvic lymph nodes.
Early ovarian cancer (FIGO Stages I to IIA)
In approximately 30% of patients with ovarian cancer, the disease is restricted to the true pelvis when it is diagnosed. In these early stages, there is a good chance of long-term cure. Crucial factors in survival are systematic examination of the whole abdomen with multiple peritoneal biopsies and complete removal of all macroscopically identifiable tumor manifestations. The main component of surgical staging is systematic pelvic and paraaortic lymph node dissection, as there is involvement of the retroperitoneal lymph nodes in 20% to 25% of cases of suspected Stage T1. The steps needed for surgical staging in early ovarian cancer are shown in Box.
Longitudinal laparotomy is the standard procedure for exploration of the abdominal cavity, as no studies with sufficient numbers of cases have yet shown that laparoscopy provides staging of equal value and comparable oncological safety.
Following surgery, patients with early ovarian cancer—FIGO Stages I to IIA, except Stage IA, Grade 1—benefit from 3 to 6 cycles of platinum-based chemotherapy, in terms of both overall survival (increase in five-year survival rate from 74% to 82%; p<0.008) and disease-free survival (increase from 65% to 76%; p<0.001) (14). The optimum number of cycles that should be performed cannot be deduced from currently available data. The protocols of most of the available studies established six cycles of platinum-based chemotherapy. It is also unclear whether combined chemotherapy with carboplatin and paclitaxel is superior to monotherapy with carboplatin AUC 5 in early stages (Box 2).
Box 1. Ovarian cancer: FIGO Stages I to II (staging/surgery).
Longitudinal laparotomy
Examination and palpation of abdominal cavity as a whole
Peritoneal cytology
Biopsies from all sites with abnormal findings
Bilateral adnexal extirpation with high ligation of the ovarian vascular bundles
Hysterectomy, extraperitoneally if appropriate
Omentectomy, at least infracolic
Appendectomy (for mucinous/unclear tumor types)
Systematic pelvic and infrarenal paraaortic lymph node dissection
Fertility-preserving surgery is possible in cases of confirmed FIGO Stage IA, Grade 1.
Source: Current recommendations of the AGO’s Ovarian Tumor Committee, www.ago-online.org
Advanced ovarian cancer (FIGO Stages IIB to IV)
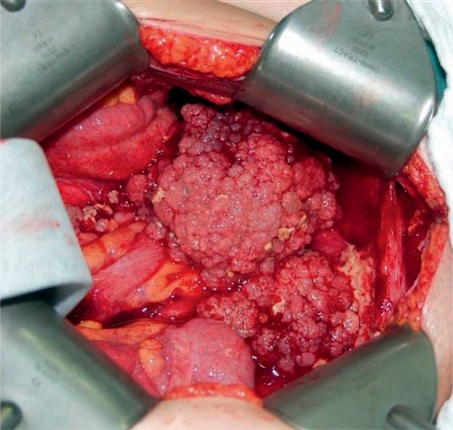
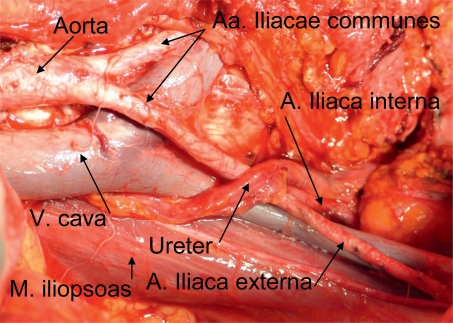
Post-operative residual tumor is the strongest independent parameter in prognosis after disease stage. It is currently the only factor that can be effectively influenced. A so-called optimum residual tumor of less than 1 cm can be achieved in 50% to 85% of patients with advanced ovarian cancer who are operated on by specialists in gynecological oncology (15). Current data from the analysis of three large treatment studies by the AGO, involving more than 3000 patients, showed that complete tumor reduction is the strongest factor in prognosis. Patients with complete tumor resection survived a median of five years longer than patients with post-operative residual tumor. In the analysis, residual tumor of less than 1 cm was a more favorable factor in prognosis than residual tumor of more than 1 cm. At 11 months, however, the increase in survival was much less than the increase in survival with complete tumor resection. The aim of every operation must therefore be complete tumor resection (13) (Figures 1 and 2).
Figure 1:
Typical site of ovarian cancer
Figure 2:
Site following surgery
The steps required for this in cases of advanced ovarian cancer are shown in Box. Intestinal surgery is necessary in approximately 30% to 50% of cases of advanced ovarian cancer. Studies have shown a significant improvement in survival following surgery on the upper abdomen such as partial resection of the liver or pancreas, splenectomy, cholecystectomy, diaphragm stripping, or tumor resection in the region of the porta hepatis if the total tumor burden could be reduced to less than 1 cm. This is also true for Stage IV patients, who benefit more from complete tumor reduction or from tumor reduction to tumor residual of less than 1 cm than patients with larger residual tumor (16– 19).
Box 3. Ovarian cancer: FIGO Stages IIB to IV (surgery).
Longitudinal laparotomy
Infragastric resection of the omentum including the part close to the spleen, examination of the bursa omentalis
Salpingo-oophorectomy, hysterectomy by retroperitoneal access; hysterectomy; high ligation of the ovarian vascular bundles
Resection of tumor infiltrated (parietal) peritoneum, including the diaphragmatic peritoneum (deperitonealization)
Resection of infiltrated portions of the small and large intestines
Upper abdominal surgery if this reduces the overall tumor burden
Appendectomy if there are macroscopic findings (routine for mucinous histology or histology that is unclear intraoperatively)
If lymph node removal is indicated, pelvic and paraaortic lymph node removal should be performed systematically up to the vena renalis. The greatest benefit is expected for complete intra-abdominal tumor resection. With residual tumor of less than 1 cm, “only” an effect on progression-free survival is observed; with a larger residual tumor outside the lymph nodes, lymph node removal does not seem to be beneficial.
Source: Current recommendations of the AGO’s Ovarian Tumor Committee, www.ago-online.org
A therapeutic benefit of systematic pelvic and paraaortic lymph node removal in cases of advanced ovarian cancer can be deduced from only one prospective study to date. In this study, patients with residual tumor of less than 1 cm and systematic lymph node removal enjoyed a significantly prolonged progression-free survival of seven months (median: 22.4 versus 29.4 months) but did not benefit in terms of overall survival, when compared to patients who underwent removal of enlarged lymph nodes only (20). A prospective study by the AGO Study Group for Genital Tumors is currently investigating whether lymph node removal in cases of advanced ovarian cancer with complete tumor resection and clinically non-suspicious lymph nodes has a therapeutic effect.
Following surgery, the standard treatment for advanced ovarian cancer is six cycles of platinum- and taxane-based chemotherapy. According to the results of a meta-analysis of the available studies on the subject, the combination of these two substances is superior to platinum monotherapy (21). The best available data on efficacy, adverse effects, and mode of application are for the use of paclitaxel (175 mg/m2 IV over three hours) and carboplatin (AUC 5) (Box 4).
It has not yet been possible to demonstrate an advantage either for the addition of further cytostatics as part of a triplet or as sequential or maintenance therapy, or for treatment prolongation or dose escalation over conventional combined treatment with six cycles of platinum and taxane. To date, the same is also true of molecular biological approaches. The efficacy of these treatments, e.g. treatments involving inhibition of signal transduction, angiogenesis, and immune and gene therapy, as primary treatment for ovarian cancer is currently being investigated in clinical studies. In Germany, the AGO’s study group and the North-East German Society of Gynecologic Oncology (NOGGO, Nord-Ostdeutsche Gesellschaft für Gynäkologische Onkologie) provide several studies on this subject. These can be consulted at www.ago-ovar.de and www.noggo.de (21, 22).
Because ovarian cancer usually spreads within the peritoneum, intraperitoneal (IP) chemotherapy seems to be a useful alternative to intravenous, systemic chemotherapy. There are seven randomized Phase III studies available on the use of IP platinum; three showed improved survival for IP administration when compared to intravenous administration. The main problems of IP therapy are its marked toxicity and complications associated with catheters. As yet, none of the intraperitoneal treatment regimens have been compared to standard IV combined chemotherapy involving carboplatin and paclitaxel.
There are few data available to date on hyperthermic intraperitoneal chemotherapy (HIPEC). Those there are describe its feasibility and high toxicity. As yet there are no studies comparing HIPEC to standard treatment, i.e., radical surgery followed by intravenous chemotherapy. Therefore, HIPEC cannot be recommended outside clinical studies (23).
Time of surgery
The requirement for optimum chemotherapy efficacy is complete removal of all macroscopically visible and palpable tumor manifestations. Standard treatment is therefore primary surgery with the aim of removing as much of the tumor as possible, followed by chemotherapy.
The data from a prospective randomized study comparing neoadjuvant chemotherapy followed by surgery with primary surgery followed by chemotherapy show comparable survival rates in both treatment arms, with lower morbidity rates for neoadjuvant therapy. The patients recruited into the study had very advanced tumors with unfavorable tumor resection rates: only 46% of the patients in the control arm had residual tumor of less than 1 cm, and the progression-free survival rate was low, at just 12 months. Because of this, the results of this study cannot be extrapolated to all patients with advanced ovarian cancer. It has not yet been possible to select appropriate patients. Neoadjuvant chemotherapy should therefore only be used within clinical studies (23).
Psycho-oncology, follow-up care, rehabilitation, and palliative treatment
Psycho-oncological care of patients with ovarian cancer is an integral part of oncological diagnosis, treatment, and follow-up care. Patients’ quality of life during treatment and follow-up care must also be assessed regularly. Patients should be informed early on of the options and the legal entitlement to rehabilitation in hospitals or departments that specialize in oncology.
Routine laboratory and apparatus-based diagnostic tests should not be performed on asymptomatic patients (with the exception of germ cell and sex-cord stromal tumors). They can lead to earlier diagnosis of recurrence. This shortens the disease-free and treatment-free period without any identifiable effects on overall survival. The results of the studies MRC OV05 and EORTC 55955 showed unambiguously that the survival rates of patients in whom treatment is begun early when there is an increase in tumor markers are no better than those of patients in whom treatment is begun later following clinical symptoms and objective evidence of a tumor. Further diagnostics are indicated when there is clinical suspicion of recurrent disease.
When quality of life is impaired by symptoms of estrogen deficiency, hormone therapy (HT) using sex steroids may be administered, following risk/benefit analysis. The estrogen doses used should be as low as possible.
In very advanced cases, palliative care for patients must be guaranteed.
Borderline cases
Borderline tumors (BOTs) of the ovary are defined by atypical nuclei, mitotic activity, and a pseudostratified epithelium but no stromal invasion. There is molecular genetic evidence that BOTs have different characteristics from epithelial high-grade ovarian cancers. The procedure for surgical staging of borderline tumors is the same as that for invasive ovarian cancer, with the following exception concerning how radical surgery is: if a patient wishes to have children, organ-preserving surgery is usually possible if the contralateral ovary or ovary remnant is tumor-free. The risk of recurrence is higher if organs are preserved, but this seems to have no effect on overall survival. With a BOT it is possible not to perform lymph node removal if lymph nodes are normal on palpation (24). No benefit of adjuvant chemotherapy has yet been proved for borderline tumors.
Box 2. Early ovarian cancer: FIGO Stages I to IIA (adjuvant therapy).
Patients with Stage IA, Grade 1 ovarian cancer do not require adjuvant chemotherapy. Appropriate surgical staging must be performed.
Patients with Stages I to IIA other than Stage IA, Grade 1 require platinum-based adjuvant chemotherapy.
This improves recurrence-free and overall survival.
Chemotherapy should be platinum-based and consist of six cycles.
Source: Current recommendations of the AGO’s Ovarian Tumor Committee, www.ago-online.org
Box 4. Advanced ovarian cancer (chemotherapy).
For patients with advanced ovarian cancer, a combination of state-of-the-art surgery and state-of-the-art chemotherapy is crucial to maximum survival.
A total of six cycles, every three weeks, of carboplatin AUC 5 and paclitaxel 175 mg/m2 IV over three hours is currently the standard regimen.
There are no data on prolonging treatment for more than six cycles, dose escalation, or the addition of other drugs outside clinical trials.
Source: Current recommendations of the AGO’s Ovarian Tumor Committee, www.ago-online.org
Key Messages.
-
Early ovarian cancer: FIGO Stages I to IIA
Complete removal of all macroscopically identifiable tumor manifestations is associated with longer survival and a higher cure rate.
Patients with Stages I to IIA other than Stage IA, Grade 1 require platinum-based adjuvant chemotherapy.
-
Advanced ovarian cancer: FIGO Stages IIB to IV
Prognosis depends essentially on how much of the tumor is removed during primary surgery. To date, residual tumor is the only factor in prognosis that can be effectively influenced.
Patients with no residual tumor following surgery have the best prognosis.
A total of six cycles, every three weeks, of carboplatin AUC 5 and paclitaxel 175 mg/m2 IV over three hours is currently the standard regimen.
Acknowledgments
Translated from the original German by Caroline Devitt, MA.
AGO Ovarian Tumor Committee:
A. du Bois, Essen; A. Burges, München; G. Emons, Göttingen; D. Fink, Zürich; M. Gropp, Ravensburg; P. Harter, Essen; A. Hasenburg, Freiburg; S. Hauptmann, Wangen; F. Hilpert, Kiel; R. Kimmig, Essen; F. Kommoss, Mannheim; R. Kreienberg, Ulm; W. Kuhn, Bonn; C. Kurzeder, Essen; S. Mahner, Hamburg; W. Meier, Düsseldorf; K. Münstedt, Giessen; O. Ortmann, Regensburg; J. Pfisterer, Solingen; M. Pölcher, Bonn; I. Runnebaum, Jena; B. Schmalfeldt, München; W. Schröder, Bremen; J. Sehouli, Berlin; B. Tanner, Oranienburg; U. Wagner, Marburg; P. Wimberger, Essen.
Footnotes
Conflict of interest statement
Dr. Burges declares that no conflict of interest exists.
Prof. Schmalfeldt has received lecture fees from Essex, Glaxo Smith Kline, Fresenius Biotech, Amgen, Lilly Deutschland, Roche International, and Boehringer.
References
- 1.Krebs in Deutschland 2003 - 2004. Berlin: 2008. Häufigkeiten und Trends. 6th revised edition. Robert-Koch-Institut (ed.) und die Gesellschaft der epidemiologischen Krebsregister in Deutschland e. V. (ed.) [Google Scholar]
- 2.Heintz AP, et al. Carcinoma of the ovary FIGO 6th anual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):161–192. doi: 10.1016/S0020-7292(06)60033-7. [DOI] [PubMed] [Google Scholar]
- 3.du Bois, A Rochon, J Lamparter, C Pfisterer. J für die AGO-Organkommission Ovar: Ovarialkarzinom - Versorgungsstruktur und -qualität in Deutschland 2001-2004. Der Frauenarzt. 2005;46(7):60–567. [Google Scholar]
- 4.Partridge E, et al. Results from four rounds of ovarian cancer screening in a randomized trial. Obstet Gynecol. 2009;113(4):775–782. doi: 10.1097/AOG.0b013e31819cda77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101(2):80–87. doi: 10.1093/jnci/djn442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kauff ND, et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: a multicenter, prospective study. J Clin Oncol. 2008;26(8):1331–1337. doi: 10.1200/JCO.2007.13.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casey MJ, et al. Intra-abdominal carcinomatosis after prophylactic oophorectomy in women of hereditary breast ovarian cancer syndrome kindreds associated with BRCA1 and BRCA2 mutations. Gynecol Oncol. 2005;97(2):457–467. doi: 10.1016/j.ygyno.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 8.Olivier RI, et al. Clinical outcome of prophylactic oophorectomy in BRCA1/BRCA2 mutation carriers and events during follow-up. Br J Cancer. 2004;90(8):1492–1497. doi: 10.1038/sj.bjc.6601692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collaborative Group on Epidemiological Studies of Ovarian Cancer, ovarian cancer and oral contraceptives collaborative reanalysis of data from 45 epidemiological studies including 23 257 women with ovarian cancer and 87 303 controls. Lancet. 2008;(371):303–314. doi: 10.1016/S0140-6736(08)60167-1. [DOI] [PubMed] [Google Scholar]
- 10.Morch LS, et al. Hormone therapy and ovarian cancer. JAMA. 2009;302(3):298–305. doi: 10.1001/jama.2009.1052. [DOI] [PubMed] [Google Scholar]
- 11.Kinkel K, et al. Indeterminate ovarian mass at US: incremental value of second imaging test for characterization - meta-analysis and Bayesian analysis. Radiology. 2005;236(1):85–94. doi: 10.1148/radiol.2361041618. [DOI] [PubMed] [Google Scholar]
- 12.Salani R, et al. Limited utility of conventional criteria for predicting unresectable disease in patients with advanced stage epithelial ovarian cancer. Gynecol Oncol. 2008;108(2):271–275. doi: 10.1016/j.ygyno.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 13.du Bois A, et al. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO) Cancer. 2009;115(6):1234–1244. doi: 10.1002/cncr.24149. [DOI] [PubMed] [Google Scholar]
- 14.Trimbos JB, et al. Impact of adjuvant chemotherapy and surgical staging in early-stage ovarian carcinoma: European Organisation for Research and Treatment of Cancer-Adjuvant Chemotherapy in Ovarian Neoplasm Trial. J Natl Cancer Inst. 2003;95(2):113–125. [PubMed] [Google Scholar]
- 15.Bristow RE, et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20(5):1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 16.Eisenhauer EL, et al. The addition of extensive upper abdominal surgery to achieve optimal cytoreduction improves survival in patients with stages IIIC-IV epithelial ovarian cancer. Gynecol Oncol. 2006;103(3):1083–1090. doi: 10.1016/j.ygyno.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 17.Aletti GD, et al. Surgical treatment of diaphragm disease correlates with improved survival in optimally debulked advanced stage ovarian cancer. Gynecol Oncol. 2006;100(2):283–287. doi: 10.1016/j.ygyno.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 18.Bristow RE, et al. Survival impact of surgical cytoreduction in stage IV epithelial ovarian cancer. Gynecol Oncol. 1999;72(3):278–287. doi: 10.1006/gyno.1998.5145. [DOI] [PubMed] [Google Scholar]
- 19.Dowdy SC, et al. Assessment of outcomes and morbidity following diaphragmatic peritonectomy for women with ovarian carcinoma. Gynecol Oncol. 2008;109(2):303–307. doi: 10.1016/j.ygyno.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panici PB, et al. Systematic aortic and pelvic lymphadenectomy versus resection of bulky nodes only in optimally debulked advanced ovarian cancer: a randomized clinical trial. J Natl Cancer Inst. 2005;97(8):560–566. doi: 10.1093/jnci/dji102. [DOI] [PubMed] [Google Scholar]
- 21.Covens A, et al. Systematic review of first-line chemotherapy for newly diagnosed postoperative patients with stage II, III, or IV epithelial ovarian cancer. Gynecol Oncol. 2002;85(1):71–80. doi: 10.1006/gyno.2001.6552. [DOI] [PubMed] [Google Scholar]
- 22.Robinson WR, et al. Clinical trial participation is associated with improved outcome in women with ovarian cancer. Int J Gynecol Cancer. 2009;19(1):124–128. doi: 10.1111/IGJ.0b013e31819a1ce8. [DOI] [PubMed] [Google Scholar]
- 23.Piso P, Ghali N, Dahlke MH, et al. Die intraoperative hypertherme intraperitoneale Chemotherapie als Therapieoption beim Ovarialkarzinom - Entwicklungen der letzten Jahre. Geburtshilfe und Frauenheilkunde. 2007;67(12):1317–1323. [Google Scholar]
- 24.du Bois A, Ewald-Riegler N, du Bois O, Harter P. Borderline tumors of the ovary - a systematic review. GebFra. 2009;69(9):807–833. [Google Scholar]