Abstract
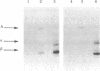
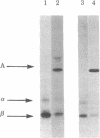
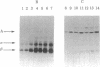
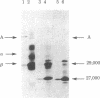
Ia.W39 is a private specificity of the I-Ab subregion of the H-2 complex. It is selectively expressed on a subset of B lymphocytes that is absent in newborn normal and adult mutant mice carrying the xid gene. Immunoprecipitation and one-dimensional NaDodSO4/polyacrylamide gel electrophoresis showed that the molecule bearing Ia.W39 consists of two noncovalently linked glycoproteins of apparent Mr 33,000 and 28,000. Anti-Ia.W39 serum did not preclear the Iab molecule; however, the conventional allo-anti-I-Ab serum cleared Ia.W39 completely. In view of the identical two-dimensional gel pattern generated by the Ia.W39 and the conventional Iab immunoprecipitates, we believe that all Ia molecules bear the conventional specificities and only a subset would in addition express Ia.W39. Ia.W39 is probably not a carbohydrate antigen, because the antibiotic tunicamycin had no influence on its expression. It may be a conformational determinant on the A alpha and A beta complex induced by the association of an unknown molecule with these chains.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed A., Scher I., Sharrow S. O., Smith A. H., Paul W. E., Sachs D. H., Sell K. W. B-lymphocyte heterogeneity: development and characterization of an alloantiserum which distinguishes B-lymphocyte differentiation alloantigens. J Exp Med. 1977 Jan 1;145(1):101–110. doi: 10.1084/jem.145.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berning A. K., Eicher E. M., Paul W. E., Scher I. Mapping of the X-linked immune deficiency mutation (xid) of CBA/N mice. J Immunol. 1980 Apr;124(4):1875–1877. [PubMed] [Google Scholar]
- Charron D. J., McDevitt H. O. Analysis of HLA-D region-associated molecules with monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6567–6571. doi: 10.1073/pnas.76.12.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen S. E., David C. S., Cone J. L., Sachs D. H. Evidence for more than one Ia antigenic specificity on molecules determined by the I-A subregion of the mouse major histocompatibility complex. J Immunol. 1976 Feb;116(2):549–553. [PubMed] [Google Scholar]
- Cullen S. E., Freed J. H., Nathenson S. G. Structural and serological properties of murine Ia alloantigens. Transplant Rev. 1976;30:236–270. doi: 10.1111/j.1600-065x.1976.tb00222.x. [DOI] [PubMed] [Google Scholar]
- David C. S., Shreffler D. C., Frelinger J. A. New lymphocyte antigen system (Lna) controlled by the Ir region of the mouse H-2 complex. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2509–2514. doi: 10.1073/pnas.70.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David C., Meo T., McCormick J., Shreffler D. Expression of individual Ia specificities on T and B cells. I. Studies with mitogen-induced blast cells. J Exp Med. 1976 Jan 1;143(1):218–224. doi: 10.1084/jem.143.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber B. T. Antigenic marker on a functional subpopulation of B cells, controlled by the I-A subregion of the H-2 complex. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3460–3463. doi: 10.1073/pnas.76.7.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber B., Gershon R. K., Cantor H. Identification of a B-cell surface structure involved in antigen-dependent triggering: absence of this structure on B cells from CBA/N mutant mice. J Exp Med. 1977 Jan 1;145(1):10–20. doi: 10.1084/jem.145.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. P., Murphy D. B., Hewgill D., McDevitt H. O. Detection of a common polypeptide chain in I--A and I--E sub-region immunoprecipitates. Mol Immunol. 1979 Jan;16(1):51–60. doi: 10.1016/0161-5890(79)90027-0. [DOI] [PubMed] [Google Scholar]
- Jones P. P., Murphy D. B., McDevitt H. O. Two-gene control of the expression of a murine Ia antigen. J Exp Med. 1978 Oct 1;148(4):925–939. doi: 10.1084/jem.148.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Marrack P. The role of H-2 linked genes in helper T-cell function. IV. Importance of T-cell genotype and host environment in I-region and Ir gene expression. J Exp Med. 1978 Dec 1;148(6):1510–1522. doi: 10.1084/jem.148.6.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D. H., Graves M., Dorf M. E., Dimuzio H., Benacerraf B. Cell interactions between histoincompatible T and B lymphocytes. VII. Cooperative responses between lymphocytes are controlled by genes in the I region of the H-2 complex. J Exp Med. 1975 Jan 1;141(1):263–268. doi: 10.1084/jem.141.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney J. F., Cooper M. D., Klein J., Abney E. R., Parkhouse R. M., Lawton A. R. Ontogeny of Ia and IgD on IgM-bearing B lymphocytes in mice. J Exp Med. 1977 Jul 1;146(1):297–301. doi: 10.1084/jem.146.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehle L., Tanner W. The specific site of tunicamycin inhibition in the formation of dolichol-bound N-acetylglucosamine derivatives. FEBS Lett. 1976 Nov 15;72(1):167–170. doi: 10.1016/0014-5793(76)80922-2. [DOI] [PubMed] [Google Scholar]
- McDevitt H. O., Chinitz A. Genetic control of the antibody response: relationship between immune response and histocompatibility (H-2) type. Science. 1969 Mar 14;163(3872):1207–1208. doi: 10.1126/science.163.3872.1207. [DOI] [PubMed] [Google Scholar]
- McDevitt H. O., Deak B. D., Shreffler D. C., Klein J., Stimpfling J. H., Snell G. D. Genetic control of the immune response. Mapping of the Ir-1 locus. J Exp Med. 1972 Jun 1;135(6):1259–1278. doi: 10.1084/jem.135.6.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. B., Herzenberg L. A., Okumura K., Herzenberg L. A., McDevitt H. O. A new I subregion (I-J) marked by a locus (Ia-4) controlling surface determinants on suppressor T lymphocytes. J Exp Med. 1976 Sep 1;144(3):699–712. doi: 10.1084/jem.144.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa Y., Yamamoto Y., Onodera K., Tamura G., Mitsui H. Altered molecular structure of HLA-DR antigens synthesized in the presence of tunicamycin. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1235–1242. doi: 10.1016/s0006-291x(79)80039-x. [DOI] [PubMed] [Google Scholar]
- Rosenwasser L. J., Huber B. T. The xid gene controls Ia.W39-associated immune response gene function. J Exp Med. 1981 May 1;153(5):1113–1123. doi: 10.1084/jem.153.5.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford D. A., Strominger J. L. Demonstration of structural polymorphism among HLA-DR light chains by two-dimensional gel electrophoresis. J Exp Med. 1980 Jan 1;151(1):144–165. doi: 10.1084/jem.151.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach E. M., Paul W. E., Green I. Histocompatibility-linked immune response gene function in guinea pigs. Specific inhibition of antigen-induced lymphocyte proliferation by alloantisera. J Exp Med. 1972 Nov 1;136(5):1207–1221. doi: 10.1084/jem.136.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreffler D. C., David C. S., Cullen S. E., Frelinger J. A., Niederhuber J. E. Serological and functional evidence for further subdivision of the I regions of the H-2 gene complex. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):477–487. doi: 10.1101/sqb.1977.041.01.055. [DOI] [PubMed] [Google Scholar]
- Sprent J. Restricted helper function of F1 leads to parent bone marrow chimeras controlled by K-end of H-2 complex. J Exp Med. 1978 Jun 1;147(6):1838–1842. doi: 10.1084/jem.147.6.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Kaplan G., Witmer M. D., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. V. Purification of spleen dendritic cells, new surface markers, and maintenance in vitro. J Exp Med. 1979 Jan 1;149(1):1–16. doi: 10.1084/jem.149.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao B., Mosier D. E., Ahmed A., Mond J. J., Scher I., Paul W. E. Role of a nonimmunoglobulin cell surface determinant in the activation of B lymphocytes by thymus-independent antigens. J Exp Med. 1979 Feb 1;149(2):495–506. doi: 10.1084/jem.149.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Uhr J. W., Capra J. D., Vitetta E. S., Cook R. G. Organization of the immune response genes. Science. 1979 Oct 19;206(4416):292–297. doi: 10.1126/science.113876. [DOI] [PubMed] [Google Scholar]
- von Boehmer H., Shortman K. The separation of different cell classes from lymphoid organs. IX. A simple and rapid method for removal of damaged cells from lymphoid cell suspensions. J Immunol Methods. 1973 Apr;2(3):293–301. doi: 10.1016/0022-1759(73)90055-0. [DOI] [PubMed] [Google Scholar]