Abstract
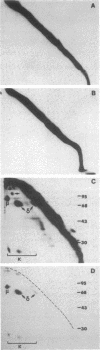
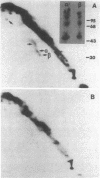
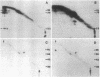
The surface proteins of lymphocytes from spleen and thymus and several cultured lymphoid tumor lines were radioiodinated in situ, solubilized with Triton X-100, and examined for the presence of disulfide-bonded subunits by two-dimensional (intact, reduced) NaDodSO4/polyacrylamide gel electrophoresis. [Hynes, R. O. & Destree, A. (1977) Proc. Natl. Acad. Sci. USA 74, 2855-2859]. Few lymphocyte surface proteins were found to consist of disulfide-bonded subunits, and the most prominent of these could be identified. In normal B lymphocytes and B-lymphoma cells, IgD or IgM (or both) were the major disulfide-bonded proteins, and these were easily detectable, even without immunoprecipitation. In contrast, analysis of thymocytes and T-lymphoma cells did not reveal any protein resembling immunoglobulin in its chain structure. The major labeled thymocyte membrane protein consisting of disulfide-bonded subunits was identified as the Ly-2/3 antigen. It appeared to contain disulfide-bonded homodimers of Mr 35,000 (alpha 2) noncovalently associated with a second pair of homodimers of Mr 30,000 (beta 2). Peptide mapping showed these polypeptides to be homologous. A third disulfide-bonded homodimer, which was heterogeneous in apparent Mr, appeared to be part of the Ly-2/3 complex. All cultured T- and B-lymphoma lines examined were found to possess a major surface protein that appeared to be a disulfide-bonded homodimer of a polypeptide of Mr 95,000. This protein was identified as the receptor for transferrin. It is suggested that the presence of two or more subunits in cell surface receptors renders their ligand functionally bivalent, making ligand-induced receptor aggregation possible.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binz H., Wigzell H. Shared idiotypic determinants on B and T lymphocytes reactive against the same antigenic determinants. V. Biochemical and serological characteristics of naturally occurring, soluble antigen-binding T-lymphocyte-derived molecules. Scand J Immunol. 1976;5(5):559–571. doi: 10.1111/j.1365-3083.1976.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Bramwell M. E., Harris H. An abnormal membrane glycoprotein associated with malignancy in a wide range of different tumours. Proc R Soc Lond B Biol Sci. 1978 Apr 13;201(1142):87–106. doi: 10.1098/rspb.1978.0034. [DOI] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Regulation of cellular and humoral immune responses by T-cell subclasses. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):23–32. doi: 10.1101/sqb.1977.041.01.006. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- De Lean A., Munson P. J., Rodbard D. Multi-subsite receptors for multivalent ligands. Application to drugs, hormones, and neurotransmitters. Mol Pharmacol. 1979 Jan;15(1):60–70. [PubMed] [Google Scholar]
- Ecarot-Charrier B., Grey V. L., Wilczynska A., Schulman H. M. Reticulocyte membrane transferrin receptors. Can J Biochem. 1980 May;58(5):418–426. doi: 10.1139/o80-055. [DOI] [PubMed] [Google Scholar]
- Fukuda M., Fukuda M. N., Papayannopoulou T., Hakomori S. Membrane differentiation in human erythroid cells: unique profiles of cell surface glycoproteins expressed in erythroblasts in vitro from three ontogenic stages. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3474–3478. doi: 10.1073/pnas.77.6.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goding J. W. Structural studies of murine lymphocyte surface IgD. J Immunol. 1980 May;124(5):2082–2088. [PubMed] [Google Scholar]
- Goding J. W., Walker I. D. Allelic forms of beta 2-microglobulin in the mouse. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7395–7399. doi: 10.1073/pnas.77.12.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb P. D. Genetic correlation of a mouse light chain variable region marker with a thymocyte surface antigen. J Exp Med. 1974 Nov 1;140(5):1432–1437. doi: 10.1084/jem.140.5.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Destree A. Extensive disulfide bonding at the mammalian cell surface. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2855–2859. doi: 10.1073/pnas.74.7.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd W., Poodry C. A., Strominger J. L. Novel surface antigen expressed on dividing cells but absent from nondividing cells. J Exp Med. 1980 Nov 1;152(5):1430–1435. doi: 10.1084/jem.152.5.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn C. R., Baird K. L., Jarrett D. B., Flier J. S. Direct demonstration that receptor crosslinking or aggregation is important in insulin action. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4209–4213. doi: 10.1073/pnas.75.9.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp D. J., Harris A. W., Cory S., Adams J. M. Expression of the immunoglobulin C mu gene in mouse T and B lymphoid and myeloid cell lines. Proc Natl Acad Sci U S A. 1980 May;77(5):2876–2880. doi: 10.1073/pnas.77.5.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang U., Kahn C. R., Harrison L. C. Subunit structure of the insulin receptor of the human lymphocyte. Biochemistry. 1980 Jan 8;19(1):64–70. doi: 10.1021/bi00542a010. [DOI] [PubMed] [Google Scholar]
- Larrick J. W., Cresswell P. Modulation of cell surface iron transferrin receptors by cellular density and state of activation. J Supramol Struct. 1979;11(4):579–586. doi: 10.1002/jss.400110415. [DOI] [PubMed] [Google Scholar]
- Laskov R., Scharff M. D. Synthesis, assembly, and secretion of gamma globulin by mouse myeloma cells. I. Adaptation of the Merwin plasma cell tumor-11 to culture, cloning, and characterization of gamma globulin subunits. J Exp Med. 1970 Mar 1;131(3):515–541. doi: 10.1084/jem.131.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Rouse R. V., Micklem H. S., Herzenberg L. A. T cell subsets defined by expression of Lyt-1,2,3 and Thy-1 antigens. Two-parameter immunofluorescence and cytotoxicity analysis with monoclonal antibodies modifies current views. J Exp Med. 1980 Aug 1;152(2):280–295. doi: 10.1084/jem.152.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lineback-Zins J., Brew K. Preparation and characterization of an NH2-terminal fragment of human serum transferrin containing a single iron-binding site. J Biol Chem. 1980 Jan 25;255(2):708–713. [PubMed] [Google Scholar]
- Marchalonis J. J. Molecular biology of T-cell receptors: introduction. Mol Immunol. 1980 Jul;17(7):795–801. doi: 10.1016/0161-5890(80)90028-0. [DOI] [PubMed] [Google Scholar]
- Rajewsky K., Eichmann K. Antigen receptors of T helper cells. Contemp Top Immunobiol. 1977;7:69–112. doi: 10.1007/978-1-4684-3054-7_3. [DOI] [PubMed] [Google Scholar]
- Reilly E. B., Auditore-Hargreaves K., Hämmerling U., Gottlieb P. D. Lyt-2 and Lyt-3 alloantigens: precipitation with monoclonal and conventional antibodies and analysis on one- and two-dimensional polyacrylamide gels. J Immunol. 1980 Nov;125(5):2245–2251. [PubMed] [Google Scholar]
- Schechter Y., Hernaez L., Schlessinger J., Cuatrecasas P. Local aggregation of hormone-receptor complexes is required for activation by epidermal growth factor. Nature. 1979 Apr 26;278(5707):835–838. doi: 10.1038/278835a0. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Van Obberghen E., Kahn C. R. Insulin and antibodies against insulin receptor cap on the membrane of cultured human lymphocytes. Nature. 1980 Aug 14;286(5774):729–731. doi: 10.1038/286729a0. [DOI] [PubMed] [Google Scholar]
- Shinohara N., Sachs D. H. Mouse alloantibodies capable of blocking cytotoxic T-cell function. I. Relationship between the antigen reactive with blocking antibodies and the Lyt-2 locus. J Exp Med. 1979 Sep 19;150(3):432–444. doi: 10.1084/jem.150.3.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland R., Delia D., Schneider C., Newman R., Kemshead J., Greaves M. Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4515–4519. doi: 10.1073/pnas.78.7.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S., Omary M. B. Human cell surface glycoprotein related to cell proliferation is the receptor for transferrin. Proc Natl Acad Sci U S A. 1981 May;78(5):3039–3043. doi: 10.1073/pnas.78.5.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker P. W., Liu C. P., Mushinski J. F., Blattner F. R. Mouse immunoglobulin D: messenger RNA and genomic DNA sequences. Science. 1980 Sep 19;209(4463):1353–1360. doi: 10.1126/science.6968091. [DOI] [PubMed] [Google Scholar]
- Wada H. G., Hass P. E., Sussman H. H. Transferrin receptor in human placental brush border membranes. Studies on the binding of transferrin to placental membrane vesicles and the identification of a placental brush border glycoprotein with high affinity for transferrin. J Biol Chem. 1979 Dec 25;254(24):12629–12635. [PubMed] [Google Scholar]
- Woodbury R. G., Brown J. P., Yeh M. Y., Hellström I., Hellström K. E. Identification of a cell surface protein, p97, in human melanomas and certain other neoplasms. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2183–2187. doi: 10.1073/pnas.77.4.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]