Abstract
This study was undertaken to investigate the pregnancy outcomes in patients with systemic lupus erythematosus (SLE) and the appropriate timing of pregnancy. We performed a retrospective evaluation of 183 pregnancies with SLE at Catholic University Medical Center during the 13-year period from 1998 to 2010. Pregnancy outcomes were compared according to SLE characteristics. The predictive value of the different cut-off points of the stable period before conception on adverse pregnancy outcomes was calculated by ROC (Receiver operating characteristics) curve analysis. In multivariate analysis, the presence of antiphospholipid antibodies (aPLs) increased the risk of pregnancy loss (p<0.0001) and premature birth (p=0.0040). Active disease at conception increased the risk of premature birth (p< 0.0001) and complications (IUGR, PIH, or both) (p= 0.0078). The other predictor of complications was found to be lupus flare (p=0.0252). At a cut-off level of stable period of 4 months before conception, sensitivity and specificity were 70.8% and 53.2%, 71.4% and 61.5%, and 63.6 % and 59.8 %, respectively on reducing pregnancy loss, premature birth, and complications. Pregnancies with aPLs, active disease at conception and SLE flares are at a higher risk of adverse outcomes. It is essential that disease activity remains stable at least 4 months before conception, for favorable pregnancy outcomes.
Keywords: Systemic lupus erythematosus, Pregnancy outcomes, Disease activity
Introduction
Systemic lupus erythematosus (SLE) is a multisystem autoimmune connective tissue disorder that primarily affects women of childbearing age. It is recognized that the pregnancy may exacerbate SLE, and the SLE may increase the pregnancy complications, including spontaneous abortion, premature delivery, intrauterine growth restriction (IUGR), and preeclampsia 1.
However, the other studies found no difference in flares between pregnant and non-pregnant patients with SLE 2,3. The rates of SLE flares in pregnancies have been reported to range from 13-68 %, but rates have been reported to be reduced if pregnancy is delayed until disease is quiescent 2-7.
The timing of conception, management of pregnancy and treatments in women affected by SLE, as well as SLE inheritance, are challenging issues for obstetricians worldwide, in particular in Asian countries where there is lack of reports on these issues concerning Asian pregnant women. Therefore, it is mandatory to investigate the timing of pregnancy in women with complicating SLE, as well as the perinatal management, which is important for the reduction of the mortality of women and infants, and the improvement of neonatal survival rate in pregnancy complicating SLE. Even though several recommendations for the management of SLE have been developed and pregnancy recommended to be planned when SLE is in remission, there is lack of evidence regarding how many months the remission period should last before experiencing the trial of pregnancy in order to decrease adverse pregnancy outcomes 8-10.
This study was undertaken to investigate pregnancy outcomes in Asian women with complicating SLE, as well as the appropriate timing of pregnancy, according to disease activity to improve the pregnancy outcomes.
Materials and methods
We performed a retrospective study of 183 pregnancies occurring in 143 patients with SLE (as defined by the 1997 revised American College of Rheumatology [ACR] criteria) 11 managed in the department of internal medicine and department of obstetrics and gynecology at Catholic University Medical Center, Korea, during a period between 1 January 1998 and 31 December 2010. Fifteen pregnancies with SLE were excluded because we did not know pregnancy outcomes due to follow up loss.
Demographic data, SLE clinical manifestations and treatment, SLE disease activity index (SLEDAI) 12, maternal SLE status (flare or no flare), pregnancy data, its outcome and therapy were recorded from medical charts. Laboratory data included complete blood count, urinalysis, antinuclear antibodies (ANA), anti-Ro/SSA antibodies, anti-La/SSB antibodies, and antiphospholipid antibodies (aPLs). Positive aPLs were defined as more than one presence of anti-cardiolipin Antibodies, β2 glycoprotein, or lupus anticoagulant antibody. The pregnancy outcomes retrieved were live births including term and preterm births, pregnancy loss including miscarriages, stillbirths and neonatal deaths, as well as gestational age at birth in weeks, infant birth weight, delivery mode, lupus flare, oligohydramnios, preterm premature rupture of membrane, IUGR, and pregnancy induced hypertention (PIH), low Apgar score, and congenital anomaly. A flare was defined as onset of new signs of SLE disease activity during pregnancies in patients previously in remission. The criteria for relapse or flare-up of SLE included new evidence of acute synovitis, serositis, pruritis, typical skin lesions, new psychological or neurological symptoms (in the absence of eclampsia), haematological parameter revealing leucopenia, thrombocytopenia or active renal disease during pregnancy. Renal involvement was defined as the following: proteinuria [500 mg/day (in the absence of preeclampsia), cellular casts in urine and dysmorphic hematuria] 13. SLE flare including renal or CNS involvement and requiring hospitalization for management of flare up was classified as severe type. Premature birth was defined as a live birth occurring before 37 weeks of gestation, stillbirths as no signs of life in a fetus delivered after 24 weeks of gestation, and low birth weight as <2.5 kg birth weight of infant at term. Pregnancy outcomes were compared between SLE patients with and without different characteristics including lupus nephritis, ANA, anti-Ro/SSA antibodies, anti-La/SSB antibodies, aPLs, disease activity at pregnancy and lupus flares during pregnancy.
Statistical analysis
Statistical analysis was performed by t-test, chi-square test, or Fisher's exact test as appropriate. Stepwise multiple logistic regression analysis was employed to find the risk factors for adverse pregnancy outcomes. The predictive value of the different cut-off points of the stable time period of SLE before conception on adverse pregnancy outcomes including pregnancy loss, premature birth, and complications of IUGR, PIH, or both was calculated by ROC (Receiver operating characteristics) curve analysis. Statistical calculations were performed with SPSS version 19.0 (SPSS, Chicago, IL, USA). A p-value <0.05 was considered significant.
Results
Patient characteristics of pregnant women
Mean age at the time of conception was 30.4±3.2 years, and mean SLE duration was 69.8±47.8 months. Mean numbers of pregnancies and deliveries per patient were 1.1±1.3 and 0.35±0.5, respectively. Mean gestational age at delivery was 37.2±3.5 weeks. Of the 183 women who conceived, the disease status of SLE was active in 56 (30.6%) and stable in 127 (69.4%) patients. 37 (20.3%) had a history of lupus nephritis and 47 (25.8%) were positive of aPLs.
ANA was positive in 124 of 178 tested (69.7 %), anti-Ro in 50 of 134 tested (37.3%), anti-La in 15 of 115 tested (13.0%). Of the 183 women, 137 (76.5%) received treatment for SLE during pregnancy including glucocorticoid, hydroxychloroquine, aspirin, intravenous gamma globulin, heparin, or plasmapheresis (Table 1).
Table 1.
Baseline Characteristics
| Age at conception (years) | 30.0 ± 3.2 (22-39) |
| Previous pregnancies | 1.1 ± 1.3 (0-7) |
| Previous deliveries | 0.35 ± 0.51 (0-2) |
| Gestational age at delivery (weeks) | 37.2 ± 3.5 (16.0-41.6) |
| Disease duration before conception(months) | 69.8±47.8 (0-240) |
| SLE status at conception | |
| Active | 56 (30.6) |
| Stable | 127 (69.4) |
| Lupus Nephritis | 37 (20.3) |
| aPLs, positive | 47 (25.8) |
| Autoantibody profile, positive | |
| Antinuclear antibody | 124 (69.7) |
| Anti-SSA(Ro) antibody | 50 (37.3) |
| Anti-SSB(La) antibody | 15 (13.0) |
| Medication during pregnancy | 137 (76.5) |
| Glucocorticoid (less than 15 mg per day) | 128 (73.1) |
| Glucocorticoid pulse therapy | 16 (9.1) |
| Hydroxychloroquine | 8 (4.6) |
| Low dose aspirin | 39 (22.4) |
| IVGV | 10 (5.7) |
| Heparin | 8 (4.6) |
| Plasmapheresis | 6 (3.4) |
Values are presented as mean±SD (min.-max.) or n(%)
aPLs: Antiphospholipid antibodies; IVGV: Intravenous gammaglobulin
Pregnancy outcomes in different SLE characteristics including lupus flares
There was no significant difference in pregnancy loss (miscarriage, still birth, neonatal death), premature birth, IUGR with and without PIH in patients with and without lupus nephritis. The presence of ANA was related with pregnancy loss (p=0.0154). Pregnancy loss and premature birth were significantly increased in patients with aPLs (p<0.0001). Pregnancy loss, premature birth, and IUGR with PIH were significantly increased in patients with active status of SLE at conception (p< 0.0001, each). Lupus flare was related with premature birth and IUGR with PIH (p=0.0142 and p< 0.0001, respectively) (Table 2).
Table 2.
Comparison of pregnancy outcomes according to different SLE characteristics
| No. of pregnancies | Pregnancy Outcomes | delivery | Complications | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Miscarriage | Stillbirths | Neonatal deaths | Live births | p | N | Preterm | Term | p | N* | IUGR | PIH+IUGR | p | ||
| Nephritis | 0.4748 | 0.0594 | 0.1473 | |||||||||||
| Yes | 37(20.4) | 4(10.8) | 2(5.4) | 2(5.4) | 29(78.4) | 33 | 14(42.4) | 19(57.6) | 35 | 4(11.4) | 7(20.0) | |||
| No | 144(79.6) | 13(9.0) | 3(2.1) | 5(3.5) | 123(85.4) | 132 | 34(25.8) | 98(74.2) | 117 | 20(17.1) | 10(8.6) | |||
| ANA | 0.0154 | 0.8180 | 0.9992 | |||||||||||
| Yes | 124(70.1) | 16(12.9) | 4(3.2) | 5(4.0) | 99(79.8) | 109 | 31(28.4) | 78(71.6) | 105 | 17(16.3) | 12(11.6) | |||
| No | 53(29.9) | 0(0.0) | 1(1.9) | 2(3.8) | 50(94.3) | 53 | 16(30.2) | 37(69.8) | 43 | 7(15.9) | 5(11.4) | |||
| aPLs | <0.0001 | <0.0001 | 0.2225 | |||||||||||
| Yes | 47(26.0) | 15(31.9) | 4(8.5) | 5(10.6) | 23(48.9) | 33 | 19(57.6) | 14(42.4) | 33 | 3(9.1) | 6(18.2) | |||
| No | 134(74.0) | 2(1.5) | 1(0.8) | 2(1.5) | 129(96.3) | 132 | 29(22.0) | 103(78.0) | 119 | 21(17.7) | 11(9.2) | |||
| Activity | <0.0001 | <0.0001 | <0.0001 | |||||||||||
| Yes | 56(30.8) | 7(12.5) | 6(10.7) | 6(10.7) | 37(66.1) | 49 | 27(55.1) | 22(44.9) | 47 | 6(12.8) | 14(29.8) | |||
| No | 126(69.2) | 10(7.9) | 0(0.0) | 1(0.8) | 115(91.3) | 117 | 22(18.8) | 95(81.2) | 106 | 18(17.0) | 3(2.8) | |||
| Lupus flare | 0.2278 | 0.0142 | <0.0001 | |||||||||||
| Yes | 92(50.6) | 8(8.7) | 4(4.3) | 6(6.5) | 74(80.4) | 84 | 32(38.1) | 52(61.9) | 83 | 11(13.3) | 17(20.5) | |||
| No | 90(49.5) | 9(10.0) | 2(2.2) | 1(1.1) | 78(86.7) | 82 | 17(20.7) | 65(79.3) | 70 | 13(18.6) | 0(0.0) | |||
By chi-square test or Fisher's exact test
ANA: antinuclear antibody; aPLs: antiphospholipid antibodies
* N, not equal to the sum of IUGR and PIH+IUGR due to the omission of other complications
Pregnancy outcomes in patients with disease status of SLE at conception
There were 115 live births (91.3%), 10 miscarriages (7.9%), no stillbirth and 1 neonatal death (0.8%) in a group with stable status of SLE at conception and 37 live births (66.1%), 7 miscarriages (12.5%), 6 stillbirths (10.7%) and 6 neonatal death (10.7%) in the other group with active status of SLE at conception. There were significant differences in gestational age at delivery (p=0.0003), neonatal birth weight (p<0.0001), low birth weight less than 2.5 kg (p<0.0001), the presence of lupus flare during pregnancy (p<0.0001), severe type of lupus flare (p<0.0001), pregnancy loss (p<0.0001), PIH (p=0.0422), IUGR with PIH(p<0.0001), premature birth (p<0.0001), and low Apgar score (less than 7) at 1 min (p<0.0001) and 5 min (p=0.0002), between two groups (Table 3).
Table 3.
Pregnancy outcomes in SLE patients with disease status of SLE at conception
| Activity at conception | p-value | ||||
|---|---|---|---|---|---|
| Stable state | Active state | ||||
| Gestational age at delivery | 38.1±1.8 | 35.0±5.3 | 0.0003 | ||
| Birth weight | 2.85±0.50 | 2.15±0.82 | <0.0001 | ||
| Low birth weight (<2.5 kg) | 28 (24.1) | 26 (57.8) | <0.0001 | ||
| Delivery mode | |||||
| Vaginal delivery | 61 (52.1) | 23 (47.9) | 0.6224 | ||
| Cesarean delivery | 56 (47.9) | 25 (52.1) | |||
| Lupus flare | Yes | Mild | 38 (80.9) | 20 (42.6) | <.0001 |
| Severe | 8 (17.0) | 26 (55.3) | |||
| No | 81 (63.8) | 10 (17.6) | |||
| Pregnancy loss | 11 (8.7) | 19 (33.9) | <.0001 | ||
| Oligohydramnios | 4 (3.8) | 1 (2.1) | 1.0000 | ||
| PPROM | 5 (4.7) | 0 (0.0) | 0.3253 | ||
| PIH | 13 (12.3) | 12 (25.5) | 0.0422 | ||
| IUGR | 18 (17.0) | 6 (12.8) | 0.5230 | ||
| PIH+IUGR | 3 (2.8) | 14 (29.8) | <.0001 | ||
| Preterm birth | 22 (18.8) | 27 (55.1) | <0.0001 | ||
| Low apgar score (< 7 at 1 min) | 10 (8.6) | 17 (37.0) | <0.0001 | ||
| Low apgar score (< 7 at 5 min) | 4 (3.5) | 11 (23.9) | 0.0002 | ||
| Congenital anomaly | 3 (4.8) | 3 (10.3) | 0.3750 | ||
By t-test, chi-square test, or Fisher's exact test
Pregnancy loss includes miscarriage, still birth, and neonatal death; PPROM: Preterm premature rupture of membrane; PIH: Pregnancy induced hypertension; IUGR: Intrauterine growth restriction
Analysis of predictors of adverse pregnancy outcome by stepwise multiple logistic regression analysis
Factors that were analyzed to identify the predictors of adverse pregnancy outcome (pregnancy loss, preterm births, IUGR) included antinuclear antibody, aPLs, disease activity at conception, and lupus flare. Among these, aPLs (OR 21.35; 95% CI 6.50-70.17; p<0.0001) was found to be the predictors of pregnancy loss in patients with SLE. The remaining factors were not associated with pregnancy loss (p>0.05). The predictors of preterm delivery in our SLE patients were active status of SLE at conception (OR 5.52; 95% CI 2.38-12.83; p< 0.0001) and aPLs (OR 3.64; 95% CI 1.51-8.79; p=0.0040). The predictors of complications of IUGR, PIH, or both were found to be active status of SLE at conception (OR 3.23; 95% CI 1.36-7.67; p= 0.0078) and lupus flare during pregnancy (OR 2.44; 1.12-5.34; p=0.0252) (Table 4).
Table 4.
Relation of pregnancy outcomes with different SLE features and SLE flares
| Pregnancy loss | Preterm | Complications | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Factors | OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p |
| ANA | 4.81 | 0.97-23.79 | 0.0544 | 0.51 | 0.22-1.20 | 0.1244 | |||
| aPLs | 21.35 | 6.50-70.17 | <0.0001 | 3.64 | 1.51-8.79 | 0.0040 | |||
| Activity | 5.52 | 2.38-12.83 | <0.0001 | 3.23 | 1.36-7.67 | 0.0078 | |||
| Lupus-flare | 2.44 | 1.12-5.34 | 0.0252 | ||||||
We presented only the values which are significant at 0.15 level in stepwise multiple logistic regression.
The cut-off stable time period of SLE before conception for adverse pregnancy outcomes
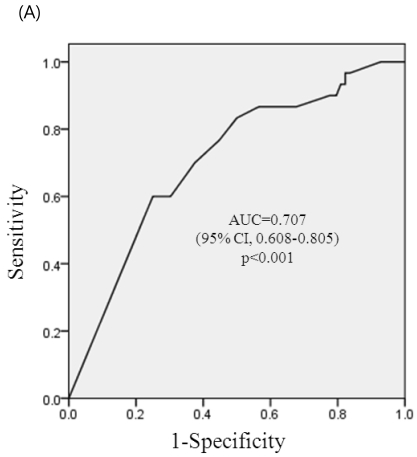
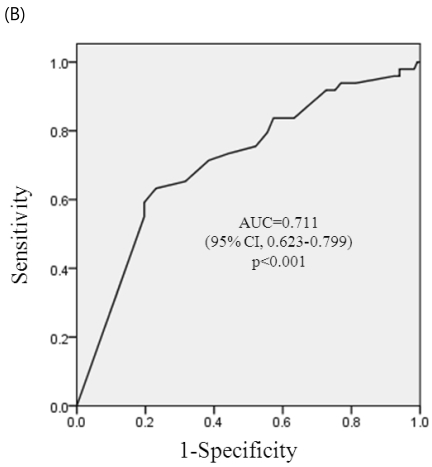
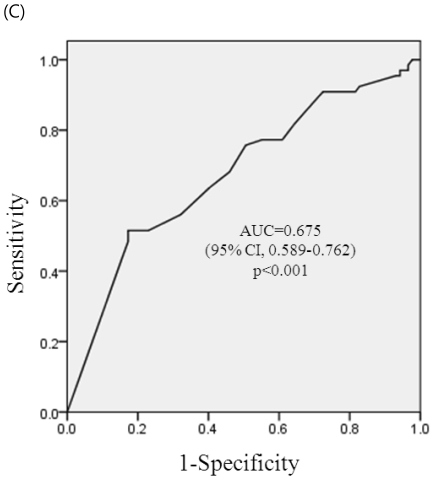
ROC curve analysis of a stable period of SLE for adverse pregnancy outcomes (pregnancy loss, premature birth, and complications of IUGR, PIH or both) are shown in Figure 1a-1c. The area below the graph did reach statistical significance of pregnancy loss, premature birth and complications of IUGR, PIH, or both (p< 0.001, each) and area under the curves (AUCs) were 0.707 (95% CI, 0.608-0.805), 0.711(95% CI, 0.623-0.799), and 0.675 (96%CI, 0.589-0.762), respectively. At a cut-off level of stable period of 4 months before conception, sensitivity was 70.8% and specificity 53.2% on reducing pregnancy loss, sensitivity was 71.4% and specificity 61.5% on reducing premature birth, and sensitivity was 63.6 % and specificity was 59.8 % on reducing complications of IUGR, PIH, or both.
Figure 1.
ROC curve for prediction of adverse pregnancy outcomes. (A) ROC curve for prediction of pregnancy loss by the stable period of SLE. At a cut-off of 4 month, sensitivity was 70.8% and specificity 53.2% on reducing pregnancy loss. (B) ROC curve for prediction of premature birth by the stable period of SLE. At a cut-off of 4 month, sensitivity was 71.4% and specificity 61.5% on reducing premature birth. (C) ROC curve for prediction of complications during pregnancy (PIH, IUGR, or both) by the stable period of SLE. At a cut-off of 4 month, sensitivity was 63.6 % and specificity was 59.8 % on reducing complications of IUGR, PIH, or both.
Discussion
Because SLE is a multi-system, complex illness, pregnancy is a challenge for lupus patients and their physicians. It is recognized that pregnancies in women with SLE show high rates of SLE flare, hypertension, nephritis, and preeclampsia, although some reports show different results. Fetal complications included spontaneous abortion, stillbirth, neonatal death, and IUGR 14. These risks are increased in the presence of anticardiolipin antibodies or lupus anticoagulant, lupus nephritis or hypertension and if there is either active disease at the time of conception or first presentation of SLE during pregnancy.
In our study, the history of lupus nephritis was not related with adverse pregnancy outcomes including pregnancy loss, premature birth, IUGR, and PIH. Antinuclear antibody was related with pregnancy loss in univariate analysis, but not in multivariate analysis. Active status of SLE at conception was related with all adverse outcomes including pregnancy loss, PIH, IUGR with PIH, lupus flare, premature birth, neonatal low birth weight, low Apgar score at 1 and 5 min.
Several studies have suggested that nephritis may contribute to adverse maternal and fetal outcomes 15,16. However, studies of the associations of SLE and lupus nephritis with pregnancy outcomes showed significant variation with respect to study design, definitions, statistical methods, bias and outcomes. Early studies reported poor clinical outcomes, but a number of recent papers have shown that outcomes are better than previously thought. These differences may reflect the changing clinical environment and the emergence of new therapeutic options. We did not find association of adverse pregnancy outcomes with lupus nephritis.
It has been proposed that SLE patients with quiescent renal disease do not have adverse pregnancy outcomes 17, and our results support this finding. It seems that adverse pregnancy outcomes associated with active renal disease at conception and not quiescent renal disease, even though we need to monitor the SLE patients with lupus nephritis, closely.
In univariate and multivariate analysis, lupus flare in pregnancy was found to be related to adverse pregnancy complications of IUGR, PIH, or both in our patients, consistent with previous reports 5. Most studies have shown that lupus tends to flare during pregnancy and after pregnancy; in some patients flares were mild such as arthritis, constitutional and cutaneous manifestations, and in others they were more serious problems affecting the kidneys and central nervous system 5,6,18. The severe types of lupus flare were occurred more frequently when women with SLE were at active disease status before conception in our study.
Our study demonstrated that aPLs were related with pregnancy loss and premature birth in univariate and multivariate analysis. The aPLs are the major risk factor for pregnancy loss in patients with SLE and in those with primary antiphospholid antibody syndrome. These antibodies play a direct pathogenic role not only by aPL-mediated thrombophilia of the placenta, but also by the direct effect of antibodies on trophoblast possibly through exposed anionic phospholipids and/or adherent β2 glycoprotein, resulting in altered trophoblast intercellular fusion, gonadotropin secretion and trophoblast invasiveness 19. A previous study has shown that positive aPLs are predictive of both premature births and pre-eclampsia, but not of pregnancy loss 20. We can speculate that aPLs are stronger risk factors for pregnancy loss than disease activity at conception, because disease activity did not reach statistical significance on pregnancy loss in multivariate analysis.
Over the last decades, improvement of survival rate and quality of life in SLE patients has led to an increased number of pregnancies observed during the course of the disease.
However, it has been reported that if SLE is active at conception, then the patient has a high risk of having a disease flare during pregnancy; if the disease is in remission then the risk is reduced 21. Pregnancy should therefore be planned when SLE is in remission 9. So, some rheumatologists say that the risk of flare seems to depend on the level of maternal disease activity in the 6-12 months before conception 10.
However, there is lack of reports about the stable or remission period before the trial of pregnancy to decrease adverse pregnancy outcome. Even though fertility is generally conserved in SLE patients, the timing for the trial of pregnancy can be challenge, because maternal age at marriage and first birth in general population is getting older not only in high-income countries but also middle-income countries in Asia where social activity of women is getting higher and social supporting system is not enough yet. Older women are more likely to experience fertility problems and require assisted reproductive technology (ART) to conceive. Impact of assisted fertilization on disease activity has to be evaluated also 8. A trial and achievement of pregnancy is associated not only disease status but also social, cultural, economical factors, and so on. So, it is important when the women with SLE can start the trial of pregnancy.
Based on our results, pregnancy loss, premature birth and pregnancy complications including IUGR, PIH, or both were reduced significantly if pregnancy occurs after more than 4 months of stable period in women with SLE.
Our study has several limitations. We evaluated observational data, and therefore the treatment strategy was not based on randomized assignment. Also, we could not observe all immunologic data we planned from the all patients. However, this is a large observational data in the East Asian pregnant women with SLE and we found cut-off stable time period of disease activity before the trial of pregnancy to decrease adverse pregnancy outcomes.
In women with SLE, the present study showed that the stable group according to disease activity at conception was superior to the active group in terms of success rate of fetal survival, full-term delivery, body weight of neonate, complication of IUGR or PIH, and SLE flare of the mother, indicating the important role of the physicians in consulting patients with SLE for the timing of pregnancy.
We conclude that in order to achieve favorable pregnancy outcomes it is essential that disease activity remains stable at least 4 months at the time of conception, and that pregnancy is managed by experienced rheumatologists and obstetricians.
References
- 1.Cunningham FG, Gant NF, Leveno I, et al. Diabetes. In: Cunningham FG, Gant NF, Leveno KJ, et al., editors. Williams obstetrics (21st ed) NY: Appleton-Century-Crosfts; 2002. pp. 1385–1389. [Google Scholar]
- 2.Lockshin MD, Reinitz E, Druzin ML, Murrman M, Estes D. Lupus pregnancy: a case control prospective study demonstrating absence of lupus exacerbation during or after pregnancy. Am J Med. 1984;77:893–898. doi: 10.1016/0002-9343(84)90538-2. [DOI] [PubMed] [Google Scholar]
- 3.Urowitz MB, Gladman DD, Farewell VT, Stewart J, McDonald J. Lupus and pregnancy studies. Arthritis Rheum. 1993;36:1392–1397. doi: 10.1002/art.1780361011. [DOI] [PubMed] [Google Scholar]
- 4.Petri M. Prospective study of systemic lupus erythematosus pregnancies. Lupus. 2004;13:688–689. doi: 10.1191/0961203303lu2006oa. [DOI] [PubMed] [Google Scholar]
- 5.Chakravarty EF, Colon I, Langen ES, Nix DA, El-Sayed YY, Genovese MC. et al. Factors that predict prematurity and preeclampsia in pregnancies that are complicated by systemic lupus erythematosus. Am J Obstet Gynecol. 2005;192:1897–1904. doi: 10.1016/j.ajog.2005.02.063. [DOI] [PubMed] [Google Scholar]
- 6.Georgiou PE, Politi EN, Katsimbri P, Sakka V, Drosos AA. Outcome of lupus pregnancy: a controlled study. Rheumatology. 2000;39:1014–1019. doi: 10.1093/rheumatology/39.9.1014. [DOI] [PubMed] [Google Scholar]
- 7.Georgiou PE F, Font J, Cervera R, Muñoz F, Cararach V, Balasch J. Obstetrical outcome of pregnancy in patients with systemic lupus erythematosus. A study of 60 cases. Eur J Obstet Gynecol Reprod Biol. 1999;83:137–142. doi: 10.1016/s0301-2115(98)00312-1. [DOI] [PubMed] [Google Scholar]
- 8.Bertsias G, Ioannidis JP, Boletis J, Bombardieri S, Cervera R, Dostal C. et al. EULAR recommendations for the management of systemic lupus erythematosus. Report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. Ann Rheum Dis. 2008;67(2):195–205. doi: 10.1136/ard.2007.070367. [DOI] [PubMed] [Google Scholar]
- 9.Moroni G, Ponticelli C. Pregnancy after lupus nephritis. Lupus. 2005;14:89–94. doi: 10.1191/0961203305lu2066oa. [DOI] [PubMed] [Google Scholar]
- 10.Doria A, Tincani A, Lockshin MD. Challenges of lupus pregnancies. Rheumatology. 2008;47:9–12. doi: 10.1093/rheumatology/ken151. [DOI] [PubMed] [Google Scholar]
- 11.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 12.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The committee on prognosis studies in SLE. Arthritis Rheum. 1992;35:630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 13.Hughes EC. Obstetric-gynaecologic terminology. Philadelphia: FA Davis; 1972. pp. 423–442. [Google Scholar]
- 14.Smyth A, Oliveira GH, Lahr BD, Bailey KR, Norby SM, Garovic VD. A systematic review and meta-analysis of pregnancy outcomes in patients with systemic lupus erythematosus and lupus nephritis. Clin J Am Soc Nephrol. 2010;5(11):2060–2068. doi: 10.2215/CJN.00240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huong DL, Wechsler B, Vauthier-Brouzes D, Beaufils H, Lefebvre G, Piette JC. Pregnancy in past or present lupus nephritis: a study of 32 pregnancies from a single center. Ann Rheum Dis. 2001;60:599–604. doi: 10.1136/ard.60.6.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johns KR, Morand EF, Littlejohn GO. Pregnancy outcome in systemic lupus erythematosus. (SLE): a review of 54 cases. Aust N Z J Med. 1998;28:18–22. doi: 10.1111/j.1445-5994.1998.tb04453.x. [DOI] [PubMed] [Google Scholar]
- 17.Tandon A, Ibanez D, Gladman DD, Urowitz MB. The effect of pregnancy on lupus nephritis. Arthritis Rheum. 2004;50:3941–3946. doi: 10.1002/art.20638. [DOI] [PubMed] [Google Scholar]
- 18.Cortes-Hernandez J, Ordi-Ros J, Paredes F, Casellas M, Castillo F, Vilardell-Tarres M. Clinical predictors of fetal and maternal outcome in systemic lupus erythematosus: a prospective study of 103 pregnancies. Rheumatology (Oxford) 2002;41:643–650. doi: 10.1093/rheumatology/41.6.643. [DOI] [PubMed] [Google Scholar]
- 19.Di Simone N, Raschi E, Testoni C, Castellani R, D'Asta M, Shi T. et al. Pathogenic role of anti-ß2-glycoprotein I antibodies in antiphospholipid associated fetal loss: characterisation of ß 2-glycoprotein I binding to trophoblast cells and functional effects of anti- ß 2-glycoprotein I antibodies in vitro. Ann Rheum Dis. 2005;64:462–467. doi: 10.1136/ard.2004.021444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tincani A, Faden D, Biasini-Rebaioli C. et al. Pregnancy in SLE (2): is it possible to predict gestational outcome? Athritis Rheum. 2002;812:322. [Google Scholar]
- 21.Mintz G, Nitz J, Gutierrez G, Karchmer S. Prospective study of pregnancy in systemic lupus erythematosus: results of a multi-disciplinary approach. J Rheumatol. 1986;13:732–739. [PubMed] [Google Scholar]